Abstract
Purpose
To develop a semi-quantitative MR-based hip osteoarthritis (OA) evaluation system (Scoring hip osteoarthritis with MRI, SHOMRI), and to test its reproducibility and face validity.
Material and Methods
The study involved 98 subjects with informed consent. Three-Tesla MR imaging of hip was performed in three planes with intermediate-weighted fat saturated FSE sequences. Two radiologists assessed cartilage loss, bone marrow edema pattern, subchondral cyst in 10 subregions, and assessed labrum in 4 subregions. In addition, presence or absence of ligamentum teres integrity, paralabral cysts, intraarticular body, and effusion in the hip joint were analyzed using the SHOMRI system. The reproducibility was assessed with intra-class correlation coefficient (ICC), Cohen’s Kappa values and percent agreement. SHOMRI scores were correlated with radiographic Kellgren-Lawrence (KL) and OARSI atlas gradings, and clinical parameters, the hip osteoarthritis outcome score (HOOS) and hip range of motion (ROM), using Spearman’s rank correlation and ordinal logistic regression.
Results
ICC values were in excellent ranging from 0.91 to 0.97. Cohen’s Kappa values and percent agreement ranged from 0.55 to 0.79 and 66 to 99 %, respectively. SHOMRI demonstrated significant correlations with KL and OARSI gradings as well as with clinical parameters, HOOS and ROM (P < 0 .05). Among the SHOMRI features, subchondral cyst and bone marrow edema pattern showed the highest correlation with HOOS and ROM.
Conclusion
SHOMRI demonstrated moderate to excellent reproducibility and significant correlation with radiographic gradings and clinical parameters.
Keywords: Hip osteoarthritis, classification system; Hip osteoarthritis, diagnosis; Hip osteoarthritis, pathology; Hip osteoarthritis, Magnetic Resonance Imaging
INTRODUCTION
Osteoarthritis (OA) is a common debilitating joint pathology that is more prevalent in the elderly. The reported radiographic and symptomatic hip OA prevalence is 28% and 10% in subjects over age 45 (1). Hip osteoarthritis contributes significantly to the cost of osteoarthritis that is estimated to be over 185.5 billion dollars/year, due to its effects on ambulation and associated disability (2). Given the high morbidity associated with hip OA there has been vigorous research to find means to prevent progression, provide treatment, and relieve symptoms (3, 4).
To assess the disease burden, monitor progression, and estimate the efficacy of preventive efforts or treatments of hip OA, an effective, sensitive and objective means for disease grading is crucial. For radiographs the current reference standards are Kellgren-Lawrence (KL) and OARSI (Osteoarthritis Research Society International) grading systems (5). They allow evaluation of osteoarthritis based on the severity of joint space narrowing and osteophyte development.
In recent decades, magnetic resonance imaging (MRI) has emerged as an important tool in evaluation of hip and other joint change (6). It has allowed evaluation of abnormalities of the bone marrow, articular cartilage, ligaments, labrum and synovium thus enabling an earlier and more differentiated diagnosis compared to simply analyzing osteophyte formation and joint space narrowing. Hip MR imaging, however, is challenging due to its thin articular cartilage, the distance from the body surface and the complexity of labral assessment.
In addition to new developments in imaging, management of early OA has changed with more emphasis on joint preservation. Debridement of chondral lesions, microfracture, labrum refixation and rim trimming are being performed more frequently in hopes of preventing or delaying joint replacement, which was once considered an inevitable outcome (7). As the numbers of hip procedures to prevent and manage hip osteoarthritis at earlier stages have increased, the need for a practical and reliable systemic MRI assessment of early hip osteoarthritis to assess their efficacy is becoming evident.
Previously, two 1.5-T MRI based hip scoring systems have been proposed (8, 9). The first hip scoring system used direct arthrography, but was not compared to any OA parameters for validation (8, 9). The second hip scoring system, HOAMS, had a detailed cartilage scoring system and included features that did not correlate with radiographic OA severity or clinical symptoms. A more detailed scoring scheme increases the demand of score effort and time and also increases the risk of misclassification when cut-off point is subjective. The goals of our study therefore were (i) to develop a practical, semi-quantitative MR-based hip osteoarthritis (OA) evaluation system (Scoring hip osteoarthritis with MRI, SHOMRI) with subregion divisions that are based on the geographic zone method, introduced by the Arthroscopy Society of North America (10), (ii) to test its reproducibility and (iii) to correlate it with radiographic and clinical scores.
MATERIALS AND METHODS
Subjects
The study involved 98 subjects who were recruited using media and internet posting from September 2010 to November 2012 for a multidisciplinary hip study encompassing routine clinical and research imaging sequences, clinical information and functional performance correlation with emphasis in evaluation of progression of OA. The data included in this study is the baseline cross-sectional assessment.
In all 98 subjects MRI and radiographs of the hip were performed; in 7 subjects of the pilot phase, no Hip osteoarthritis outcome score (HOOS) and range of motion (ROM) assessment were made. Of the 91 subjects, 91.2% (n=83) completed the HOOS questionnaire and 80.2% (n=73) were available for ROM measurement. The study protocol was approved by the Committee of Human Research and informed consents were acquired from all individuals before participation.
The inclusion criteria for this study were subject with (i) age 18 years old or older, and (ii) no known systemic disease that would alter hip joint use. Patients were excluded from the study if they had: (i) hip surgery or hip injury in the previous year, (ii) inflammatory arthritis, (iii) hematochromatosis, (iv) sickle cell disease, (v) hemoglobinopathy, (vi) knee or ankle osteoarthritis with KL score greater than 2, (vii) hip KL score 4, (viii) any condition other than osteoarthritis which would limit lower extremity function and mobility, such as history of stroke, lower extremity joint replacement or amputation, (ix) positive pregnancy test, and (x) MRI contraindications (e.g. implanted pacemaker or claustrophobia) or (xi) if the acquired MRI images were suboptimal in quality. Those with significant knee or ankle OA were excluded to ensure that lower extremity mobility evaluation were not affected by non-hip lower extremity joint.
MRI Protocol
Hip MRI examinations were performed on a 3.0-Tesla scanner (GE MR750; GE Healthcare, Waukesha, WI) using an 8-channel cardiac coil (GE Healthcare, Waukesha, WI). The hip joint with higher KL grading was chosen if gradings were different, otherwise the more symptomatic side was selected. The MRI protocol included intermediate-weighted fat-suppressed fast spin-echo (FSE) sequences in a sagittal, oblique coronal and oblique axial orientation with repetition time (TR) 2400 – 3700 ms, echo time (TE) 60 ms, slice thickness 4 mm, echo time (TE) 60 ms, field of view 14 – 20 cm, matrix 288 × 224, slice thickness 3 – 4 mm and acquisition time 3’50” - 4’40” per sequence. The entire examination took approximately 30 minutes. To achieve a reproducible position in all hip joints the feet were internally rotated and forefeet were taped together.
Radiographic Protocol
Standing anterior-posterior pelvis radiographs were performed in all patients. For positioning, the feet were aligned with slight internal rotation. Settings included focus-film distance of 40 inches, voltage of 80 kVp with automatic exposure using a GE Discovery 650 x-ray system (GE healthcare, Waukesha, WI).
Image Analysis – MRI (11)
All images were analyzed using a standard clinical PACS system (Agfa, Ridgefield Park, NJ). The radiologists performing SHOMRI scoring were blinded to both radiographic osteoarthritis scores and clinical and functional information, other than age. Initially, consensus training sessions were performed by 3 board certified radiologists (SL, LN and TML) with 7, 5 and 25 years of musculoskeletal imaging expertise respectively. The training sessions served to calibrate and standardize readings and included 26 studies, which were read in three sessions separated by 2 weeks. Subsequently the remainder of the studies (n=72) was scored by 2 radiologists (SL, LN) independently. Both radiologists analyzed the studies two times with time separation greater than two weeks to calculate intra- and inter-reader reproducibility.
Development Of The Scoring System
Based on a literature review of hip osteoarthritis MRI findings, eight features were identified (12, 13), to reflect process cardinal to hip joint osteoarthritis, and most optimally evaluated by MR imaging: (i) articular cartilage loss, (ii) bone marrow edema pattern, (iii) subchondral cysts, (iv) labral abnormality, (v) paralabral cysts, (vi) intraarticular bodies, (vii) effusion/synovitis and (viii) ligamentum teres abnormality. Features that were excluded include (i) OA features that were better evaluated on radiograph such as osteophytes, subchondral sclerosis and bone attrition, (ii) features that may have association with hip OA, however, may also be present without OA, such as (a) femoral head/neck junction bump, (b) synovial herniation pit or fibrocystic change, (c) acetabular overcoverage and (d) acetabular undercoverage, and (iii) features that did not show significant relationship with OA such as greater trochanter tendinopathy.
Scoring Hip Osteoarthritis Using MRI (SHOMRI)
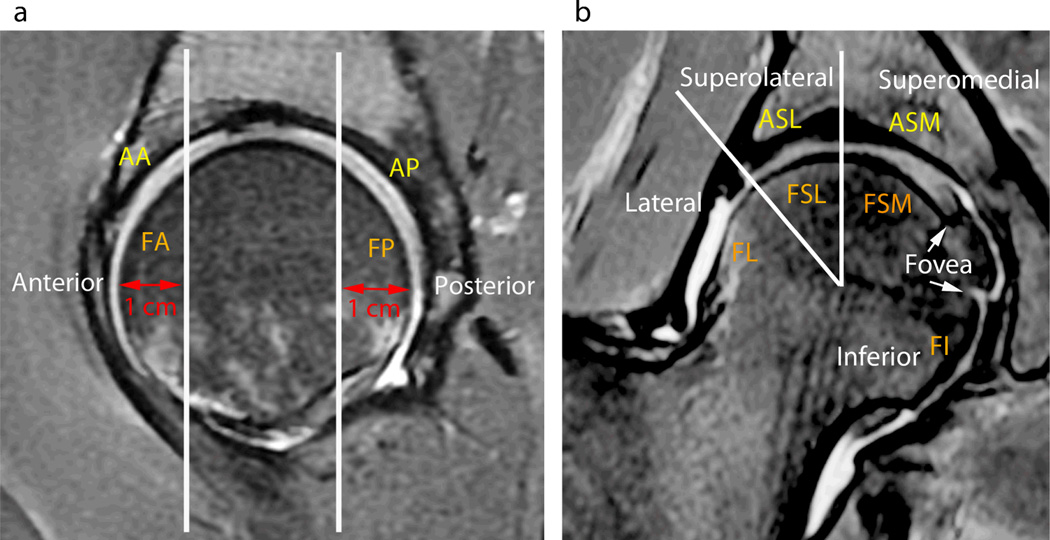
Articular cartilage loss, bone marrow edema pattern and subchondral cysts were graded in 10 subregions as shown in Figure 1 a-d The femur was divided into 6 subregions (Fig. 1 b-d). The anterior subregion represented the anterior 1 cm of the femoral head shown on a sagittal MR study and was scored on the sagittal images (Fig. 1 b-c and 2 a). A mid portion of the femoral head was defined on the sagittal images (Fig. 2 a) and subdivided into four subregions on the coronal images, from lateral to medial as lateral, superolateral, superomedial and inferior subregions (Fig. 2 b). The landmark for division was lateral acetabular rim for lateral and superolateral, a vertical line from center of femoral head for superolateral and superomedial, and ligamentum teres for superomedial and inferior subregion. The posterior subregion represented the posterior 1 cm of the femoral head shown on a sagittal MR study and was scored on the sagittal images (Fig. 2 a). The acetabular articular surface was segmented into 4 subregions: The anterior subregion represented the anterior 1 cm of the acetabulum shown on a sagittal MR study and was scored on the sagittal images (Fig. 1 a and 2 a). A mid portion of the acetabulum was defined on the sagittal images (Fig. 2 a) and subdivided into two subregions on the coronal images (Fig. 2 b), these were defined moving from lateral to medial as superolateral and superomedial. The posterior subregion represented the posterior 1 cm of the acetabulum shown on a sagittal MR study and was scored on the sagittal images (Fig. 2 a).
Figure 1.
Illustration of hip joint subregion subdivisions with color coding. (A) Acetabulum joint surface subregions seen from lateral aspect. (B) Femur joint surface subregions seen from medial aspect. Foveal attachment is noted in the medial center of femoral head. Dotted crescent line represents outline of acetabular fossa. (C) Femur joint surface subregions seen from anterior aspect. (D) Femur joint surface subregions seen from posterior aspect.
Figure 2.
Hip MRI images with subregion subdivision illustration. (A) Sagittal MR images were used to evaluate the acetabular anterior (AA), femoral anterior (FA) and acetabular posterior (AP) and femoral posterior (FP) subregions. White line outlines the anterior and posterior 1 cm division. (B) Coronal MR images were used to evaluate acetabular superolateral (ASL), acetabular superomedial (ASM), and femoral lateral (FL), femoral superolateral (FSL), femoral superomedial (FSM) and femoral inferior (FI) subregions. Two white lines extend from center of the femoral head, one a vertical line dividing acetabular and femoral superolateral and superomedial subregions and the other extending to the lateral edge of acetabulum dividing superolateral and lateral subregion of femur.
This approach represented a modified version of the geographic zone method described by Ilizaliturri et al. for hip arthroscopy which showed superior inter-observer reproducibility compared to the clock-face method (10). It divides the acetabulum and femoral surface into 6 zones, by two vertical imaginary lines of the acetabular fossa and a horizontal line at the superior limit of the fossa dividing the joint into superior and inferior half.
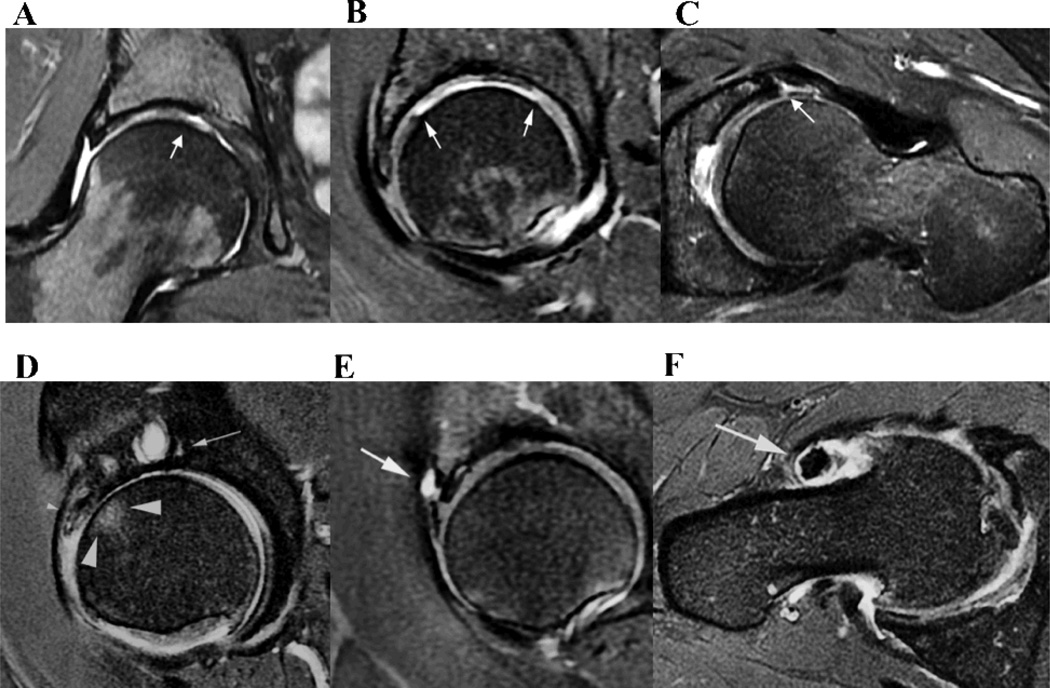
Articular cartilage lesions were scored in each of the 10 subregions using a 3-point scale: 0 for no loss, 1 for partial thickness loss (Fig. 3 A) and 2 for full thickness loss (Fig. 3 B). For large lesions that spanned more than one region, if it was greater than 1 cm in maximal diameter, it was scored in both subregions, and if it is was less than 1 cm it was scored in the subregion where more than 50 % of the lesion was located. Each of the 10 subregions was scored separately and added for a total cartilage score.
Figure 3.
Examples of SHOMRI grading. (A) On the coronal MR image, a small partial thickness articular cartilage loss is noted at the femoral superomedial subregion (white arrow), which would be scored as 1. (B) On the sagittal MR image, a full thickness large articular cartilage lesion is noted at femoral anterior and superomedial subregions with white arrows denoting the anterior-posterior extent. As full thickness cartilage loss is greater than 1 cm, it is scored in both subregions, a score of 2 in each subregion. (C) On the axial MR image, a labral tear with labrocartilage separation is demonstrated at the anterior aspect (white arrow), which is scored as 3. (D) The sagittal MR image demonstrates bone marrow edema pattern with a size larger than 0.5 cm and smaller than 1.5 cm, that was scored as 2 in the femoral anterior subregion (large arrow head), a subchondral cyst larger than 0.5 cm that was scored as 2 (arrow) in the superomedial subregion and a labrum maceration in the anterosuperior region, that was scored as 5 (small arrowhead). Full thickness articular cartilage loss is also noted in the acetabular and femoral anterior subregions that was scored as 2 in each subregion. (E) The sagittal MR image shows a paralabral cyst (arrow) with a score of 1 at the anterosuperior aspect of the labrum. (F) An intraarticular body and effusion, both scored as1 each, are shown on the axial MR image.
Bone marrow edema pattern was defined as an ill-defined subchondral lesion hyperintense on fluid-sensitive sequences (Fig. 3 D). These lesions were scored in the subregions using a 4 point scale : 0 if no lesion was present, 1 if equal to or less than 0.5 cm in size, 2 if greater than 0.5 cm but equal to or less than 1.5 cm, and 3 if greater than 1.5 cm in size (Fig. 3 D). Measurements were made perpendicular to the articular surface on the longest dimension. Each 10 subregions were scored separately and added for a total bone marrow lesion score.
Subchondral cysts were defined as well-defined fluid-signal bone lesion (Fig. 3 D). They were scored in the 10 subregions using a 3 point scale: 0 for absent, 1 for size equal to or less than 0.5 cm, and 2 if size greater than 0.5 cm. All the measurements were taken from the maximum diameter.
Labral abnormalities were scored on three planes in 4 different subregions: anterior and posterior on the axial plane, anterosuperior on the sagittal plane, and superior on the coronal plane, respectively (Figure 3 C and E). Labral lesions were graded as 0 for normal or normal variant such as aplasia or hypoplasia, 1 for abnormal signal and/or fraying, 2 for simple tear, 3 for labrocartilage separation, 4 for complex tear and 5 for maceration.
The presence or absence of paralabral cysts and intraarticular bodies was scored as 1 or 0 (Figure 3 E and F). Joint effusion was interpreted as a sign of synovitis and the presence of fluid signal at the femoral neck region greater 0.7 cm in thickness was scored as 1 (14). Ligamentum teres abnormalities were graded as 0 for normal, 1 for signal abnormalities or fraying, 2 for partial tear and 3 for complete tear.
The time required to score was measured using a set of 10 cases composed of 3 KL0, 3 KL1, 2 KL2 and 2 KL 3 randomly selected from each KL group, more than 3 weeks after the 3rd calibration session. Average time per case and standard deviation were calculated.
Image Analysis – Radiographs
Initially all radiographs were analyzed using the Kellgren-Lawrence score (5), as part of the inclusion and exclusion criteria. KL score equal to or greater than 2 was used to define radiographic OA. Kellgren-Lawrence classification is composed of grade 0, normal, grade 1, doubtful narrowing of joint space and possible osteophytic lipping, grade 2, definite definite osteophytes, definite narrowing of joint space, grade 3, moderate multiple osteophytes, definite narrowing of joints space, some sclerosis and possible deformity of bone contour, grade 4, large osteophytes, marked narrowing of joint space, severe sclerosis and definite deformity of bone contour (15).
Subsequently we graded all radiographs using the OARSI hip osteoarthritis score (16), as this provided a more detailed approach to classify degenerative disease. Revised OARSI atlas was used to scored marginal osteophytes of joint margin at superior acetabular, superior femoral and inferior femoral regions on the scale from 0 to 3 on the severity, and inferior acetabular region, either absent or present, and joint space narrowing in the superior and medial region on the scale from 0 to 3, acetabular subchondral cyst, acetabular subchondral cyst, femoral subchondral sclerosis and flattening of the femoral head (11). The OARSI scoring was performed greater than 2 weeks apart from KL or SHOMRI scoring to prevent recall bias.
Clinical Assessment
Self-reported functional assessment was performed using the hip disability and osteoarthritis outcome score (HOOS) (17–19). Among the HOOS 5 subscales, three subscales most relevant in diagnosis of OA (pain, other symptoms such as stiffness and grinding, and activity of daily living) were selected to simplify the analysis and minimize multiple comparisons. Responses to each item were scored from 0 to 4. The scores were then transformed to a percentage score of 0 to 100, with 0 representing no hip problems and 100 representing extreme hip problems. Active Range of motion (ROM) in flexion, abduction, adduction, external rotation and internal rotation was measured by physical therapist (DK, 12 years experience) using a goniometer (20).
Statistical Analysis
Descriptive statistics were calculated for all subjects: age, gender, height, weight and body mass index (BMI). Intra and inter-reader reproducibility, intra-class correlation coefficients (ICC) were calculated for SHOMRI features scored in multiple subregions for which we regarded it to be best to treat the values as a numerical outcome (21), whereas linearly weighted Cohen’s kappa and percent agreement were calculated for individual observations, which are ordered categorical outcomes (22). In order to assess the face validity of the MRI evaluation system, total scores of each feature were correlated with radiographic assessment with KL scores and OARSI scores and also with the clinical parameters from HOOS and ROM evaluations using Spearman’s Rank correlation coefficients. The association between the SHOMRI features and OA symptoms was also assessed by logistic regression model with binary outcome to predict the odds of having significant clinical symptoms defined by HOOS subscale maximum score equal to or greater than 2. Face validity assessment was also performed in the subset of patients who are older (age greater than 50) and those with KL ≥1 to test SHOMRI performance in those who meet classic diagnostic definition of osteoarthritis. P-value adjustments for tied values were assessed for features with small point-scale and low disease prevalence. Statistical Analysis was performed using SAS® (Version 9.1 SAS institute, Cary, NC) and JMP10 (SAS institute, Cary, NC). The level of statistical significance was defined at P < 0.05, and statistical trend was defined as P < 0.10.
RESULTS
Subject characteristics
Characteristics of the study population and the distribution and ranges of scores of the SHOMRI system are presented in Table 1 and Table 2. Distribution of the hip radiographic KL scores 0, 1, 2 and 3 were 27 % (26/98), 50 % (49/98), 13 % (13/98) and 10 % (10/98).
Table 1.
Population characteristics
| Characteristics | Values |
|---|---|
| Age (year)* | 44 ±13 (23–72) |
| Weight (kg)* | 71 ± 13 (44–104) |
| Height (cm)* | 170 ±11 (145–193) |
| Body Mass Index* | 24 ± 3 (15–31) |
| Sex (Male/Female) | 52/48 |
| Radiographic OA, KL ≥ 2 | 23/98 (23 %) |
| OARSI hip score* | 1.4 ±2.4 (0–13) |
| Hip pain | 42/83 (51 %) |
| Hip symptoms other than pain | 45/83 (54 %) |
| Hip discomfort effecting ADL | 35/83 (42 %) |
Values are mean ± standard deviation (range)
Unless otherwise specified, data are numbers of patients with percentages in parentheses.
Table 2.
SHOMRI features, frequency, range of scores and distribution of abnormalities
| SHOMRI features | Range of score |
Distribution of scores (N total = 98) |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| Articular cartilage | 0–2 | 35 | 36 | 27 | |||
| Bone marrow edema pattern | 0–3 | 80 | 7 | 6 | 5 | ||
| Subchondral cysts | 0–2 | 80 | 5 | 13 | |||
| Labrum | 0–5 | 7 | 4 | 9 | 33 | 31 | 14 |
| Paralabral cysts | 0,1 | 84 | 14 | ||||
| Intraarticular bodies | 0,1 | 93 | 5 | ||||
| Effusion/synovitis | 0,1 | 86 | 12 | ||||
| Ligamentum teres | 0–3 | 58 | 22 | 11 | 7 | ||
Reproducibility
ICCs of intra- and inter-reader per feature were excellent (ICC > 0.9) (23). It ranged from 0.93–0.98 and 0.91–0.94. Bone marrow edema pattern had the highest value in both intra-reader and inter-reader ICC of 0.98 and 0.94. Labrum had the lowest ICC in both intra- and inter-reader ICC of 0.93 and 0.91. The Cohen Kappa and percent agreement results are demonstrated in Table 3. Intra-reader kappa values were between 0.65 and 0.79; lowest values were obtained for paralabral cysts and highest values for bone marrow edema pattern. Inter-reader kappa values were between 0.55 and 0.79; lowest values were seen for effusion/synovitis and highest values for intraarticular bodies. The percent agreement ranged from 70.5 to 98.4 % for intra-reader and from 66.3 to 99.0 % for inter-reader reproducibility. It was highest in subchondral cysts at 98 and 97%, and lowest in labral changes at 74 % and 66 % for intra and inter-reader. The average percent agreements of all observations were 90.5% and 86.5% for intra and inter-reader respectively.
Table 3.
Reproducibility in weighted Cohen’s Kappa and percent agreement
| SHOMRI features | Frequency of scores > 0 |
Intra-reader | Inter-reader | ||
|---|---|---|---|---|---|
| Cohen’s kappa (95% CI) |
Percent Agreement (95% CI) |
Cohen’s kappa (95%CI) |
Percent agreement (95% CI) |
||
| Articular cartilage | 31 % | 0.70 (0.67–0.74) | 85 (83–86) % | 0.57 (0.52–0.62) | 78 (75–80) % |
| Bone marrow edema pattern | 12 % | 0.79 (0.74–0.84) | 96 (95–97) % | 0.55 (0.46–0.64) | 91 (89–93) % |
| Subchondral cysts | 4 % | 0.78 (0.71–0.85) | 98 (97–99) % | 0.71 (0.60–0.81) | 97 (96–98) % |
| Labrum | 54 % | 0.73 (0.69–0.77) | 74 (71–77) % | 0.65 (0.60–0.71) | 66 (61–71) % |
| Paralabral cyst | 19 % | 0.65 (0.50–0.80) | 91 (86–95) % | 0.63 (0.42–0.84) | 90 (82–95) % |
| Effusion/synovitis | 12 % | 0.68 (0.53–0.82) | 92 (87–94) % | 0.55 (0.33–0.76) | 87 (78–92) % |
| Intraarticular bodies | 3 % | 0.76 (0.50–1.00) | 98 (95–100) % | 0.79 (0.40–1.00) | 99 (94–100) % |
| Ligamentum teres | 39 % | 0.72 (0.63–0.80) | 79 (73–84) % | 0.72 (0.60–0.84) | 81 (71–88) % |
The time required to score a single hip were ‘9’06” ± 4’28” for reader 1 and 7’46” ± 3’36” for reader 2.
Correlation With Radiographic And Clinical Scores
To obtain a measure of face validity, the correlation of SHOMRI with the typical hip OA evaluation standards of reference was investigated, which included radiographic scores and clinical parameters. Significant correlations between the MRI scores versus both KL and OARSI radiographic scores were found (Table 4). All eight MRI features showed a statistically significant correlation with KL classification (P range <0.001–0.03) and either significant correlation or a trend towards significance with OARSI scores. The correlations between MRI features and radiographic features were highest for articular cartilage and subchondral cysts and lowest for paralabral cysts and effusion.
Table 4.
Spearman rank correlation coefficient (P-value) between SHOMRI and radiographic OA scores, Hip osteoarthritis outcome score, and Range of motion
| Radiographic OA scores |
Hip osteoarthritis outcome score |
Range of motion measurements (°) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kellgren Lawrence grading |
OARSI hip OA score |
Pain | Symptoms† | Activity of Daily Living |
Flexion | Abduction | Adduction | External rotation |
Internal rotation |
|
|
SHOMRI Features |
||||||||||
| Articular cartilage |
0.52 (<0.001*) |
0.51 (<0.001*) |
0.17 (0.12) |
0.24 (0.03*) |
0.27 (0.01*) |
−0.03 (1.0) |
−0.29 (0.02*) |
−0.15 (0.21) |
−0.01 (0.93) |
−0.27 (0.03*) |
| Bone marrow edema |
0.36 (<0.001*) |
0.28 (0.005*) |
0.27 (0.01*) |
0.34 (0.002*) |
0.31 (0.004*) |
−0.10 (0.40) |
−0.37 (0.002*) |
−0.37 (0.002*) |
−0.19 (0.12) |
−0.07 (0.56) |
| Subchondral cyst | 0.44 (<0.001*) |
0.49 (<0.001*) |
0.40 (<0.001*) |
0.35 (0.001*) |
0.44 (<0.001*) |
−0.17 (0.16) |
−0.35 (0.003*) |
−0.30 (0.01*) |
−0.21 (0.09) |
−0.32 (0.009)* |
| Labrum | 0.41 (<0.001*) |
0.43 (<0.001*) |
0.13 (0.25) |
0.24 (0.03*) |
0.08 (0.45) |
−0.006 (0.96) |
−0.18 (0.14) |
−0.10 (0.43) |
−0.18 (0.13) |
−0.10 (0.43) |
| Paralabral cyst | 0.21 (0.034*) |
0.18 (0.072) |
0.10 (0.38) |
0.24 (0.03*) |
0.22 (0.05*) |
−0.05 (0.71) |
−0.04 (0.74) |
−0.15 (0.22) |
−0.06 (0.65) |
−0.04 (0.71) |
| Intraarticular body |
0.31 (0.002*) |
0.29 (0.004*) |
0.20 (0.09) |
0.11 (0.33) |
0.09 (0.43) |
−0.03 (0.80) |
−0.03 (0.79) |
−0.00 (1.0) |
−0.27 (0.03*) |
−0.12 (0.31) |
| Effusion/synovit is |
0.21 (0.033*) |
0.20 (0.052) |
0.18 (0.11) |
0.20 (0.07) |
0.16 (0.16) |
−0.08 (0.51) |
−0.26 (0.04*) |
−0.03 (0.79) |
−0.07 (0.57) |
−0.02 (0.86) |
| Ligamentum teres |
0.44 (<0.001*) |
0.38 (<0.001*) |
0.13 (0.23) |
0.03 (0.78) |
0.16 (0.17) |
−0.11 (0.36) |
−0.24 (0.05*) |
−0.12 (0.32) |
−0.11 (0.38) |
−0.33 (0.006*) |
|
Radiographic OA gradings |
||||||||||
| KL classification | 0.23 (0.04*) |
0.19 (0.09) |
0.28 (0.02*) |
−0.09 (0.47) |
−0.31 (0.01*) |
−0.04 (0.78) |
−0.09 (0.46) |
−0.23 (0.06) |
||
| OARSI hip OA score |
0.34 (0.002*) |
0.28 (0.02*) |
0.28 (0.01*) |
−0.09 (0.45) |
−0.28 (0.03*) |
−0.09 (0.48) |
−0.01 (0.92) |
−0.22 (0.08) |
||
Symptoms other than pain
The correlation with MRI scores and clinical features of hip osteoarthritis were also statistically significant (Table 4). SHOMRI bone marrow edema pattern and subchondral cysts scores showed a significant correlation with all three HOOS subscales (P range <0.001–0.01). Articular cartilage and paralabral cyst scores correlated with symptoms and activities of daily living (ADL) subscales (P range 0.01–0.05). Labrum scores correlated with the symptom subscale (P= 0.03). Effusion showed a statistical trend with symptoms and ADL and intraarticular bodies with pain (P <0.10). Correlations between SHOMRI scores and ROM are demonstrated in Table 4. The feature with the most significant correlation with ROM was subchondral cysts (P < 0.001), followed by bone marrow edema pattern. Of the ROM physical examination parameters, hip abduction showed statistically significant correlation with more SHOMRI features than internal rotation or flexion.
MRI features and clinical symptom severity was also assess by odds ratio. Presence of bone marrow edema greater than 0.5 cm, subchondral cyst of any size, labrum maceration, effusion and ligamentum teres complete tears were associated with clinical symptoms with statistical significance compared to those without such findings with P for trend ranging from less than 0.01 to 0.04.
Correlation of MRI score and radiographic assessment and clinical parameters performed in subset of those age ≥ 50 and those with KL ≥1 showed a pattern similar to the total study population, with statistically significant association seen between most SHOMRI features and at least one validation parameter (data not shown). Paralabral cyst showed association that did not meet the criteria of statistical significance or trend in those age ≥50 years (P=0.15) or those with KL ≥1 (P=0.14).
DISCUSSION
In conclusion, we developed a MRI hip osteoarthritis grading system that is practical in image acquisition and scoring; the system is based on a noncontrast MR protocol that can be acquired in a routine setting and scored within a practical timeframe. We demonstrated good intra- and inter-reader reproducibility and found a significant correlation with radiographic and clinical scores, which are the current standards of reference for hip OA and therefore were used as measures to test face validity of the new score. We believe that the new SHOMRI score will provide a detailed measure of disease burden, allow improved progression assessment of degenerative disease and will thus also allow better monitoring of therapeutic or preventive interventions.
Previously, two MR based hip scoring systems have been published (8, 9). Neumann et al. study published in 2007 focused on five features: cartilage, labrum, bone marrow, osteophytes and subchondral cysts (9). It included limited surgical correlation and no correlation to other OA parameters such as radiographs or clinical symptoms. The use of arthrography significantly limits its potential to be used in large clinical trials. The hip MRI scoring system by Roemer et al. was designed to evaluate OA (8). But it also included several features such as greater trochanter tendinopathy and bursitis that are generally not part of the standard osteoarthritis assessment (23–25). The reliability of this system varied widely from poor to very good (25). The standard protocol of HOAMS with intravenous contrast use limits its use in subjects with limited renal function, a relatively commonly encountered problem, especially in older population (26, 27). The study population was restricted to those with age over 50.
Our system evaluates eight OA features, which includes 4 out of 5 features of Neumann’s and 7 out of 14 of features of Roemer’s system (8, 9). Osteophyte was not included in the features, as MR osteophyte scoring added significant time, without much benefit over radiographic evaluation. Cortical bone lacks signal in MR, limiting evaluation of early osteophyte compared to radiograph, where bone formation or mineralization is easier to detect. Unlike the two systems that used 1.5-T with gadolinium, it was developed using noncontrast 3.0-T MR imaging. The articular cartilage evaluation is a 3-point scale compared to the 5 and 6 point scale of the other systems. Absolute measurement of size rather than proportion of area involved were used in evaluation of bone marrow edema and subchondral cysts to limit incorporation of subjective judgments into the score. The total score and all eight individual feature scores showed statistically significant association with radiographic hip OA scores. It also showed significant correlation with hip symptoms, assessed with HOOS and range of motion in a cohort of subjects with and without mild-moderate radiographic hip OA. The strength of correlations between clinical symptoms and MRI findings were not very high, but relatively modest. This may partly be related to low disease burden of our study population, or inherent low correlation between imaging manifestation of osteoarthritis and symptoms that have been described previously (28, 29). The highest correlation with the HOOS was seen with subchondral cyst, followed by bone marrow edema pattern. These findings are consistent with results from Taljanovic et al. that concluded that the amount of bone marrow edema in hip OA correlates with severity of pain and with the study of Crema et al. that showed strong association with bone marrow edema pattern and subchondral cystic lesions (30, 31). Labral abnormalities did not show a significant association with the HOOS suggesting that the contribution of labrum in generating hip symptoms may not be as large as commonly presumed. However, labral scores significantly correlated with radiographic hip OA scores, which confirms a study by McCarthy et al on early hip osteoarthritis and labrum (32).
Among the physical examination variables, the decrease in range of abduction, internal rotation and adduction showed significant correlation with more advanced OA features such as subchondral cysts. Hip flexion that is included in the American College of Rheumatology Clinical Classification Criteria for Hip OA along with internal rotation, did not show significant correlations with MRI-based features. This may be related to preponderance of minimal and mild hip OA rather than severe disease in our study population.
We acknowledge several limitations in our study: First, the predominance of subjects with less disease severity in the study population. Since the focus was a MR hip OA scoring system for clinical studies that investigate the different risk factors, prevention methods and treatments in early hip OA, severe radiographic hip OA (KL = 4) was excluded and therefore populations with severe disease were not tested. Though the study population was skewed toward those with no, minimal or mild disease burden based on radiographs, 95 % of subjects showed MR changes related to degenerative joint pathology, such as cartilage loss and labral tears. The pathology was seen commonly across the age range, although the severity and prevalence was higher with increase of age. Although osteoarthritis is more prevalent in older age, we consider inclusion of younger subjects critical as we hope this scoring system to be used in early onset osteoarthritis studies involving femoroacetabular impingement, hip dysplasia and post-trauma, to assess OA progression and efficacy of treatment in those with greatest potential gain in early intervention. The MRI evaluation system performed in a similar pattern in analysis performed after exclusion of those ages less than 50 and those with KL 0. Another limitation is the lack of surgical or pathologic correlation. Since the study was predominantly performed in those with mild to moderate disease burden, surgical correlation was not possible. It was difficult to ethically justify invasive procedure in research subjects who did not meet clinical indication for surgery, which has inherent risk of complication. As the diagnosis of hip osteoarthritis is based on clinical parameters and radiographs, we consider correlation with these standards of reference as reasonable and clinically practical. There are several studies such as Mintz et al. that found high reliability and sensitivity in diagnosis of hip labral and chondral pathology using hip MR and surgical findings (33). Osteophytes, subchondral sclerosis and attrition were not included in our system, as they are more reliably, easily or efficiently evaluated on radiographs. Omission of these features reduced the score time and allowed the focus to be on features that are best demonstrated on MRI. The simplified articular cartilage of 3-point scale scored on ten regions, with minimal lesion measurement offered time efficiency and ease of scoring. The simplification was a necessity as we found it difficult to reproduce a satisfactory reliability using the detailed schemes based of published scoring system. However, the number of the point-scale increments and regions may affect the systems’ sensitivity to interval change. Another possible shortcoming of this study is the lack of contrast use. Early hip MR studies have reported significant diagnostic advantage in intra-articular contrast use (34). However, more recent studies reported comparable performance of optimized noncontrast hip MRI to those with intra-articular contrast in diagnosis of labral or chondral pathology (33, 35–37).
Although the parameters derived from SHOMRI analysis reveal significant correlation with radiographic and clinical indices of OA, longitudinal data is required for determining the weighting of these parameters in order to arrive at a single aggregate score that would best reflect the overall significant of the MR appearance of osteoarthritis.
The intra and inter-reader reliability ranged from modest to excellent, an overall improved compared to previously published system (25). ICC calculated for features scored in multiple subregions were all in excellent range. The modest kappa value of bone marrow edema and effusion/synovitis at 0.55, compared to that of ligamentum teres abnormality at 0.72, despite high percent agreement, may partly be related to the low frequency of abnormalities. Kappa values may underestimate agreement and thus lead to a paradox of high proportional agreement and low kappa in low prevalance abnormalities (38).Additionally, attention should be paid to measurement location to be placed on axial oblique images, mid femoral neck images rather than the superior or inferior joint capsule, to avoid overestimation of effusion/synovitis size.
In conclusion, we have developed a concise and reliable MRI hip osteoarthritis score, which all score features correlated significantly with current standards of reference used for hip OA diagnosis. We look forward to application of SHOMRI system to longitudinal data, comparison with other existing scoring systems and ultimately improving assessment of OA disease burden and its progression.
ACKNOWLEDGEMENTS
We thank Dr. Marco Zanetti for sharing his expertise in hip MR imaging and Dr. Thomas Sampson for providing insight as a hip surgeon with vast experience in arthroscopic repair of chondral and labral lesion in early hip osteoarthritis. In addition we would like to thank Peter K. Lee for the artful illustrations of the subregion division of the SHOMRI system.
Funding: NIH-NIAMS P50 AR06075 and K24-AR04884
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
REFERENCES
- 1.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2009;36(4):809–815. doi: 10.3899/jrheum.080677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60(12):3546–3553. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 3.Hochberg MC. Osteoarthritis year 2012 in review: clinical. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012 doi: 10.1016/j.joca.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Lohmander S. Osteoarthritis year 2012 in review. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012 doi: 10.1016/j.joca.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phan CM, Link TM, Blumenkrantz G, Dunn TC, Ries MD, Steinbach LS, et al. MR imaging findings in the follow-up of patients with different stages of knee osteoarthritis and the correlation with clinical symptoms. Eur Radiol. 2006;16(3):608–618. doi: 10.1007/s00330-005-0004-5. [DOI] [PubMed] [Google Scholar]
- 7.Sampson TG. Arthroscopic treatment for chondral lesions of the hip. Clin Sports Med. 2011;30(2):331–348. doi: 10.1016/j.csm.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Roemer FW, Hunter DJ, Winterstein A, Li L, Kim YJ, Cibere J, et al. Hip Osteoarthritis MRI Scoring System (HOAMS): reliability and associations with radiographic and clinical findings. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011;19(8):946–962. doi: 10.1016/j.joca.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Neumann G, Mendicuti AD, Zou KH, Minas T, Coblyn J, Winalski CS, et al. Prevalence of labral tears and cartilage loss in patients with mechanical symptoms of the hip: evaluation using MR arthrography. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;15(8):909–917. doi: 10.1016/j.joca.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Ilizaliturri VM, Jr, Byrd JW, Sampson TG, Guanche CA, Philippon MJ, Kelly BT, et al. A geographic zone method to describe intra-articular pathology in hip arthroscopy: cadaveric study and preliminary report. Arthroscopy. 2008;24(5):534–539. doi: 10.1016/j.arthro.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;(5 Suppl A):A1–A56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Horii M, Kubo T, Hirasawa Y. Radial MRI of the hip with moderate osteoarthritis. J Bone Joint Surg Br. 2000;82(3):364–368. doi: 10.1302/0301-620x.82b3.9923. [DOI] [PubMed] [Google Scholar]
- 13.Stelzeneder D, Mamisch TC, Kress I, Domayer SE, Werlen S, Bixby SD, et al. Patterns of joint damage seen on MRI in early hip osteoarthritis due to structural hip deformities. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20(7):661–669. doi: 10.1016/j.joca.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Koski JM. Ultrasonographic evidence of hip synovitis in patients with rheumatoid arthritis. Scandinavian journal of rheumatology. 1989;18(3):127–131. doi: 10.3109/03009748909095409. [DOI] [PubMed] [Google Scholar]
- 15.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Annals of the rheumatic diseases. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;(15 Suppl A):A1–A56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Klassbo M, Larsson E, Mannevik E. Hip disability and osteoarthritis outcome score. An extension of the Western Ontario and McMaster Universities Osteoarthritis Index. Scand J Rheumatol. 2003;32(1):46–51. doi: 10.1080/03009740310000409. [DOI] [PubMed] [Google Scholar]
- 18.Satoh M, Masuhara K, Goldhahn S, Kawaguchi T. Cross-cultural adaptation and validation reliability, validity of the Japanese version of the Hip disability and Osteoarthritis Outcome Score (HOOS) in patients with hip osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(4):570–573. doi: 10.1016/j.joca.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Nilsdotter AK, Lohmander LS, Klassbo M, Roos EM. Hip disability and osteoarthritis outcome score (HOOS)--validity and responsiveness in total hip replacement. BMC Musculoskelet Disord. 2003;4:10. doi: 10.1186/1471-2474-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilsdotter A, Bremander A. Measures of hip function and symptoms: Harris Hip Score (HHS), Hip Disability and Osteoarthritis Outcome Score (HOOS), Oxford Hip Score (OHS), Lequesne Index of Severity for Osteoarthritis of the Hip (LISOH), and American Academy of Orthopedic Surgeons (AAOS) Hip and Knee Questionnaire. Arthritis Care Res (Hoboken) 2011;(63 Suppl 11):S200–S207. doi: 10.1002/acr.20549. [DOI] [PubMed] [Google Scholar]
- 21.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 22.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 23.Boutry N, Paul C, Leroy X, Fredoux D, Migaud H, Cotten A. Rapidly destructive osteoarthritis of the hip: MR imaging findings. AJR American journal of roentgenology. 2002;179(3):657–663. doi: 10.2214/ajr.179.3.1790657. [DOI] [PubMed] [Google Scholar]
- 24.Kumar D, Wyatt CR, Lee S, Nardo L, Link TM, Majumdar S, et al. Association of cartilage defects, and other MRI findings with pain and function in individuals with mild-moderate radiographic hip osteoarthritis and controls. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(11):1685–1692. doi: 10.1016/j.joca.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roemer FW, Hunter DJ, Winterstein A, Li L, Kim YJ, Cibere J, et al. Hip Osteoarthritis MRI Scoring System (HOAMS): reliability and associations with radiographic and clinical findings. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011;19(8):946–962. doi: 10.1016/j.joca.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Garg AX, Papaioannou A, Ferko N, Campbell G, Clarke JA, Ray JG. Estimating the prevalence of renal insufficiency in seniors requiring long-term care. Kidney international. 2004;65(2):649–653. doi: 10.1111/j.1523-1755.2004.00412.x. [DOI] [PubMed] [Google Scholar]
- 27.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA : the journal of the American Medical Association. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 28.Phan CM, Link TM, Blumenkrantz G, Dunn TC, Ries MD, Steinbach LS, et al. MR imaging findings in the follow-up of patients with different stages of knee osteoarthritis and the correlation with clinical symptoms. European radiology. 2006;16(3):608–618. doi: 10.1007/s00330-005-0004-5. [DOI] [PubMed] [Google Scholar]
- 29.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226(2):373–381. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 30.Crema MD, Roemer FW, Zhu Y, Marra MD, Niu J, Zhang Y, et al. Subchondral cystlike lesions develop longitudinally in areas of bone marrow edema-like lesions in patients with or at risk for knee osteoarthritis: detection with MR imaging--the MOST study. Radiology. 2010;256(3):855–862. doi: 10.1148/radiol.10091467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taljanovic MS, Graham AR, Benjamin JB, Gmitro AF, Krupinski EA, Schwartz SA, et al. Bone marrow edema pattern in advanced hip osteoarthritis: quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skeletal Radiol. 2008;37(5):423–431. doi: 10.1007/s00256-008-0446-3. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy JC, Noble PC, Schuck MR, Wright J, Lee J. The watershed labral lesion: its relationship to early arthritis of the hip. J Arthroplasty. 2001;16(8 Suppl 1):81–87. doi: 10.1054/arth.2001.28370. [DOI] [PubMed] [Google Scholar]
- 33.Mintz DN, Hooper T, Connell D, Buly R, Padgett DE, Potter HG. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy. 2005;21(4):385–393. doi: 10.1016/j.arthro.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Czerny C, Hofmann S, Neuhold A, Tschauner C, Engel A, Recht MP, et al. Lesions of the acetabular labrum: accuracy of MR imaging and MR arthrography in detection and staging. Radiology. 1996;200(1):225–230. doi: 10.1148/radiology.200.1.8657916. [DOI] [PubMed] [Google Scholar]
- 35.Sundberg TP, Toomayan GA, Major NM. Evaluation of the acetabular labrum at 3.0-T MR imaging compared with 1.5-T MR arthrography: preliminary experience. Radiology. 2006;238(2):706–711. doi: 10.1148/radiol.2382050165. [DOI] [PubMed] [Google Scholar]
- 36.Potter HG, Schachar J. High resolution noncontrast MRI of the hip. J Magn Reson Imaging. 2010;31(2):268–278. doi: 10.1002/jmri.22025. [DOI] [PubMed] [Google Scholar]
- 37.Robinson P. Conventional 3-T MRI and 1.5-T MR arthrography of femoroacetabular impingement. AJR Am J Roentgenol. 2012;199(3):509–515. doi: 10.2214/AJR.12.8672. [DOI] [PubMed] [Google Scholar]
- 38.Feinstein AR, Cicchetti DV. High agreement but low kappa: I The problems of two paradoxes. Journal of clinical epidemiology. 1990;43(6):543–549. doi: 10.1016/0895-4356(90)90158-l. [DOI] [PubMed] [Google Scholar]