Abstract
This study describes skeletal, neuromuscular and fitness impairments among 109 children (median age 10 (range 4–18) years, 65.1% male, 63.3% white) with acute lymphoblastic leukemia (ALL), enrolled on a physical activity trial from 2009 to 2013. Outcomes were measured 7-10 days after diagnosis and compared to age- and sex-specific expected values. Associations between function and HRQL were evaluated with logistic regression. Children low values for BMD z-scores/height (mean±standard error: −0.53±0.16 vs. 0.00±0.14, p <0.01), body mass index percentile (57.6±3.15 vs. 50.0±3.27%, p=0.02), quadriceps strength (201.9±8.3 vs. 236.1±5.4 Newtons, p<0.01), six minute walk distance (385.0±13.1 vs. 628.2±7.1 meters, p < 0.001), and Bruininks-Oseretsky Test of Motor Proficiency (23±2.5 vs. 50±3.4%, p < 0.001). Quadriceps weakness was associated with a 20.9-fold (95% CI 2.5–173.3) increase in poor physical HRQL. Children with newly diagnosed ALL have weakness and poor endurance and may benefit from early rehabilitation that includes strengthening and aerobic conditioning.
Keywords: Acute lymphoblastic leukemia, pediatric, muscle strength, physical endurance, quality of life
INTRODUCTION
Children with acute lymphoblastic leukemia (ALL) are at risk for skeletal, neuromuscular and cardiopulmonary impairments that interfere with physical function. Low bone mineral density [1–5], loss of lean muscle mass [6,7], motor neuropathy [8–10], muscle weakness [7] and impaired fitness [11–15] are possible during and after therapy, and have been attributed to both leukemia and its treatment. Even though correlations between acute impairment and long-term functional loss have not been evaluated, early initiation of exercise or physical activity interventions have been proposed to restore function or to prevent impairments for children with newly diagnosed ALL [1,16–18].
Individual differences in bone density, lean muscle mass, neuromuscular function and fitness are likely at diagnosis among children with ALL. Knowledge of the prevalence and magnitude of common problems at diagnosis will inform the development of comprehensive, modifiable interventions that can be tailored to meet the individual rehabilitation needs of these children.
This study evaluates skeletal health, body composition, neuromuscular function, general fitness and self- and parent-reported health-related quality of life (HRQL) among children newly diagnosed with ALL and compares the results with age-, sex- and race-expected normative values. In addition, associations between physical function measures and self- and parent-reported HRQL are reported.
METHODS
Study Population
Participants eligible for these analyses were children with newly diagnosed ALL who enrolled in and completed baseline physical performance testing for a single blind, randomized, intervention trial (Physical Activity to Modify Sequelae and Quality of Life in Childhood Acute Lymphoblastic Leukemia - NCT00902213) at St. Jude Children's Research Hospital, the Hospital for Sick Children, MD Anderson Cancer Center, or Children's Healthcare of Atlanta. The trial was designed to evaluate the impact of an autonomy-supportive behavioral intervention, combined with a standard but individually tailored set of seven physical therapy exercises, on bone health, physical function and HRQL. Children eligible for this study were diagnosed with B- or T-cell ALL between 4 and 18 years of age, were within 7-10 days of initiation of chemotherapy for ALL, and had at least one parent 18 years of age or older. Children with diagnoses of cerebral palsy or Down syndrome, previous malignant neoplasms, chromosome breakage syndromes or severe congenital immunodeficiency were not eligible. Study processes and documents were approved by institutional review boards. Parent consent and child assent, as appropriate, were obtained prior to enrollment.
Measures
We selected bone and body composition, physical function and self- or parent-report HRQL measures that were documented as valid and reliable among children 4 to 18 years of age.
Bone mineral density (BMD)
Dual X-ray absorptiometry (DXA) was used to determine BMD in grams per centimeter squared (g/cm2) anteriorly in the first four lumbar vertebrae (L1-L4), the most common fracture site among children with newly diagnosed ALL [19], with either a GE Lunar Prodigya or Hologic 1000Wb DXA scanner. Calibration and quality control for comparability of data were conducted by BioClinica Technologiesc with their own spine phantoms to assure site calibration and quality control between the two different manufacturers and models of equipment [20–24]. Height adjusted age-, race - and sex-specific Z-scores [25] of L1-L4 lumbar vertebrae were used for analysis and are reported separately by machine type.
Body mass index (BMI)
Weight in kilograms (kg) and height in centimeters (cm) without shoes were measured on an electric scaled and a stadiometer,d respectively. Weight in kg was divided by height in meters (m) squared to determine BMI values, which were converted to age- and sex-specific percentiles using data from the Centers for Disease Control and Prevention growth charts [26].
Ankle range of motion
Active and passive ankle dorsi- and plantar-flexion range of motion were measured with a Baseline® 360° goniometere in degrees; the child was assessed in a sitting position with the hip and knee in approximately 90 degrees of flexion. Measurements for children with ALL have intra-rater and inter-rater reliability, and intra-class correlation coefficients of 0.97 and 0.84, respectively [27], Each motion was measured twice and the maximum used for analysis comparing participants to age- and sex- specific reference values [28].
Lower extremity strength
Hand-Held Myometry (Chatillion-Ametek DFE Series IIf) was used to measure isometric ankle (distal lower extremity) dorsiflexion and knee extension (proximal lower extremity) strength. For both muscle groups, children were in a sitting position with the spine, hips and contralateral thigh stabilized. For knee extension, the feet were off the floor and the examiner applied force on the anterior lower leg 5–8 cm above the malleoli until the child could no longer hold the contraction (“break test”). For dorsiflexion, the child's heel was in contact with the floor and force was applied over the dorsum of the foot, again until the child could no longer sustain the muscle contraction. Among children as young as age four years and as old as age 17 years, these strength measures have intra-rater and inter-rater reliability and intra-class correlation coefficients ranging from 0.92–0.99 and 0.85–0.97, respectively.[27] Each test was repeated twice on each lower limb and the peak force was used in analyses comparing participants to age- and sex-specific reference values [29].
Grip strength
Bilateral grip (distal arm) strength in kilograms was evaluated with a Jamar® hand held dynamometer.g The child was assessed in a sitting position, with the shoulder in neutral, the elbow flexed and the forearm midway between pronation and supination. The ranges of intra- and inter-rater reliability for grip strength are 0.92–0.97 and 0.80–0.95, respectively [30–32]. Two measurements were taken for each hand and the peak used in analysis comparing participants to age- and sex-specific reference values [32].
Motor development
The Bruininks-Oseretsky Test of Motor Proficiency Version 2 Short Form (BOT2-SF)h total motor composite was used to characterize overall motor function. This norm-referenced instrument is designed for children and adolescents 4–21 years of age. The composite score describes overall motor abilities and includes fine motor control, manual coordination, body coordination, strength and agility. Internal consistency reliability coefficients for the BOT-2 total motor composite are 0.95, 0.95 and 0.96 for 4–7, 8–11, and 12–21 year olds, respectively. Test-retest reliability correlation coefficients are 0.83, 0.82, and 0.77 for the three age groups. Inter-rater reliability is extremely high with an overall correlation of 0.98. The items on the BOT-2 were administered and scored according to the standardized procedures in the manual. Age-specific percentile ranks were used for analysis [33].
Fitness
The modified Six Minute Walk Test was used to measure cardio-respiratory fitness [34]. Since it is self-paced, this test represents fitness for usual daily activities. The child was instructed to walk as far as possible in a 6-minute time period (not running or jogging) along the 40 meter course. Children were allowed to stop, slow down, and rest against the wall during the test if necessary. Encouragement was given in 1-minute intervals. Rate of perceived exertion was recorded at 0, 2, 4, and 6 minutes, and after several minutes of recovery. Distances walked in six minutes were compared to age- and sex-specific reference data provided by Geiger [34].
Health-related quality of life
The Child Health Questionnaire in parallel parent (CHQ-PF50) and child (CHQ-CF44) formsi was used to measure HRQL. This instrument has reliability coefficients for parent-report subscales ranging from 0.70–0.93 and for child-report subscales ranging from 0.59–0.95. Lower alpha values reflect the small number of items on some scales. Comparison of a pediatric oncology population with healthy children on the parallel parent and child CHQ outcomes demonstrate significant parent-child correlation for all the CHQ scales, with correlation being greater in the cancer group than in controls [35]. Scores on this instrument were converted to age-specific T-scores for analysis [36].
Statistical Methods
Means and standard deviations, frequencies and percentages were calculated to describe the demographics and the outcome measures in the study population. Mean values on measures of bone density and body composition, physical performance, and HRQL were compared between survivors and age- and sex-specific expected values with one sample t-tests. Associations between HRQL (physical function and family activities) and measures of physical performance (ankle dorsiflexion range of motion, ankle dorsiflexion strength, knee extension strength, handgrip strength, fitness) were evaluated in multivariable logistic regression models adjusted for sex, age and race. Each multivariable model evaluated the impact of one measure of physical performance. All analyses were completed using SAS version 9.2.j
Role of the Funding Sources
The funding sources had no role in the study design; in the collection, analysis and interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication.
RESULTS
Study Population
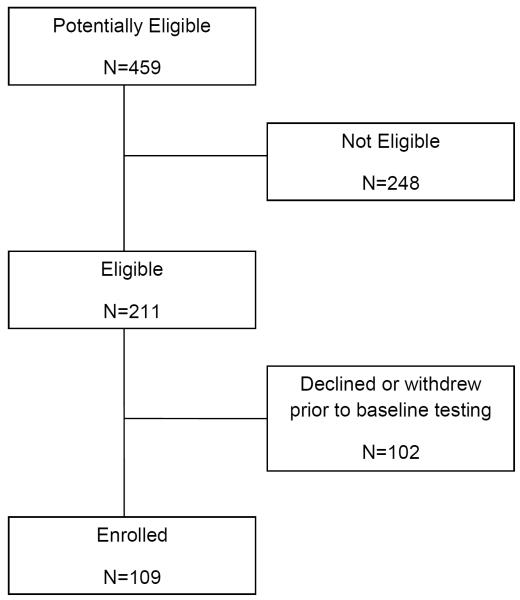
Between 2009 and 2013, 211 (46%) of 459 children with newly diagnosed ALL at the four participating institutions were eligible for this study. Participants for these analyses included 109 children who completed baseline testing. Non-participants included 89 who declined participation and 13 who withdrew prior to baseline testing (Figure 1). The primary reasons cited for study refusal were feeling overwhelmed and having inadequate time to complete study activities. Participants did not differ from non-participants by age or sex, but were slightly less likely to be white than non-participants (Table I). Participants had a median age of 10 (range 4–18) years at diagnosis. Nearly two-thirds (65.1%) were male and 63.3% were white.
Figure 1.
Consort diagram
Table I.
Characteristics of the study participants and non-participants
| Participants (N=109) | Nonparticipants (N=102) | ||||
|---|---|---|---|---|---|
| Frequency | % | Frequency | % | p-value* | |
| Age | |||||
| 4–9 years | 53 | 48.6 | 53 | 52.0 | 0.89 |
| 10–14 years | 38 | 34.9 | 33 | 32.4 | |
| 15–18 years | 18 | 16.5 | 16 | 15.7 | |
| Sex | 0.13 | ||||
| Male | 71 | 65.1 | 56 | 54.9 | |
| Female | 38 | 34.9 | 46 | 45.1 | |
| Race | 0.02 | ||||
| White-non Hispanic | 69 | 63.3 | 77 | 76.2 | |
| Black-non Hispanic | 16 | 14.7 | 16 | 15.8 | |
| Other | 24 | 22.0 | 8 | 7.9 | |
| Mother's educational attainment | |||||
| < High school | 14 | 12.8 | |||
| High school graduate | 39 | 35.8 | |||
| College graduate | 51 | 46.8 | |||
| Not reported | 5 | 4.6 | |||
From Chi-square statistic
Bone Density and Body Composition
Mean values for bone density, anthropometric and physical performance measures among study participants are shown in Table II along with age-, race- and sex-specific expected values for each measure of performance and the percentage of patients whose values or scores were at least 1.5 and two standard deviations below their age-and sex- expected means. Based on a standard normal distribution, population expected percentages of persons with values below these standard deviations for each outcome are 6.7% and 2.3% respectively. A higher than expected percentage of children with newly diagnosed ALL had low lumbar bone mineral density values at diagnosis (p=0.01 at sites 1 and 3, p <0.001 at sites 2 and 4). BMI in the study population was slightly higher than the expected value (p=0.02). None of the children had BMI values less than two standard deviations below the expected mean value.
Table II.
Mean (standard deviation) values for bone density, body mass index, and motor performance, and percentage with scores < −1.5 and < −2.0 standard deviations among children with newly diagnosed ALL
| Measure | Children with ALL (N=109) | Age- and sex-specific expected value | < −1.5 SD | < −2.0 SD | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SE of Mean | Mean | SE of Mean | p-value | % | [95% CI] | % | [95% CI] | |
| BMDL1-L4(g/cm2)* Site 1 and 3 |
−0.45 | 0.17 | 0.00 | 0.14 | 0.01 | 19.0 | [8.6–34.1] | 11.9 | [4.0–25.6] |
| Site 2 and 4 | −0.60 | 0.16 | 0.00 | 0.15 | <0.001 | 18.6 | [8.4–33.4] | 14.0 | [5.3–27.9] |
| BMI percentile | 57.6 | 3.15 | 50.0 | 3.27 | 0.02 | 0.0 | [0.0–3.3] | 0.0 | [0.0–3.3] |
| Active ankle dorsiflexion (°) | |||||||||
| Right | 13.6 | 0.7 | 13.0 | 0.5 | 0.47 | 13.9 | [8–21.9] | 7.4 | [3.3–14.1] |
| Left | 12.2 | 0.8 | 13.0 | 0.5 | 0.33 | 19.4 | [12.5–28.2] | 8.3 | [3.9–15.2] |
| Both | 12.9 | 0.7 | 13.0 | 0.5 | 0.88 | 14.8 | [8.7–22.9] | 8.3 | [3.9–15.2] |
| Passive ankle dorsiflexion (°) | |||||||||
| Right | 18.6 | 0.8 | 19.7 | 0.6 | 0.18 | 10.3 | [5.2–17.7] | 5.6 | [2.1–11.8] |
| Left | 17.3 | 0.8 | 19.7 | 0.6 | 0.01 | 17.8 | [11–26.3] | 11.2 | [5.9–18.8] |
| Both | 18.0 | 0.8 | 19.7 | 0.6 | 0.03 | 13.1 | [7.3–21] | 8.4 | [3.9–15.4] |
| Knee extension strength (N) | |||||||||
| Right | 205.1 | 8.4 | 236.1 | 5.4 | <0.01 | 36.4 | [26.9–46.6] | 30.3 | [21.5–40.4] |
| Left | 198.6 | 8.4 | 236.1 | 5.4 | <001 | 43.4 | [33.5–53.8] | 34.3 | [25.1–44.6] |
| Both | 201.9 | 8.3 | 236.1 | 5.4 | <0.01 | 37.4 | [27.9–47.7] | 30.3 | [21.5–40.4] |
| Ankle dorsiflexion strength (N) | |||||||||
| Right | 165.5 | 7.5 | 161.2 | 3.4 | 0.25 | 25.3 | [17.1–35] | 13.1 | [7.2–21.4] |
| Left | 163.1 | 7.1 | 161.2 | 3.4 | 0.52 | 19.2 | [12–28.3] | 14.1 | [8–22.6] |
| Both | 164.3 | 7.2 | 161.2 | 3.4 | 0.36 | 22.2 | [14.5–31.7] | 14.1 | [8–22.6] |
| Hand grip strength (kg) | |||||||||
| Right | 17.5 | 0.8 | 19.1 | 0.4 | 0.01 | 30.5 | [21.9–40.2] | 21.0 | [13.6–30] |
| Left | 15.9 | 0.8 | 19.1 | 0.4 | <001 | 38.1 | [28.8–48.1] | 26.7 | [18.5–36.2] |
| Both | 16.7 | 0.8 | 19.1 | 0.4 | <001 | 35.2 | [26.2–45.2] | 22.9 | [15.2–32.1] |
| BOTSF-2 | |||||||||
| Percentile rank | 23.2 | 2.5 | 50.0 | 3.4 | <0.01 | 33.3 | [24.3–43.4] | 14.7 | [8.5–23.1] |
| Six minute walk distance (m) | 385.0 | 13.1 | 628.2 | 7.1 | <001 | 89.0 | [80.7–94.6] | 79.1 | [69.3–86.9] |
SE=Standard Error, BMD L1-L4=Average bone mineral density of the first through the fourth lumbar vertebrae adjusted for height Z-score, BMI=body mass index, SD=Standard Deviation, CI=Confidence Interval, BOTSF-2=Bruininks-Oseretsky Test of Motor Proficiency Short Form (version 2.0), g/cm2=grams per centimeter squared, °=degrees, N=Newtons, kg=kilograms, m-meters
Expected values also specific to race for BMD
Range of Motion and Strength
On average, active ankle dorsiflexion range of motion and isometric strength were within normal limits. However, patients had significantly less passive ankle dorsiflexion (p=0.03), less knee extension strength (p<0.01), and less handgrip strength than expected values.
Motor Performance and Fitness
Average performance on the Bruninks-Oseretsky Test of Motor Proficiency Short Form, Version 2.0 (BOT2-SF) was in the twenty-third (±25) percentile (p<0.001). The average distance walked in six minutes was 385.0±13.1 meters, significantly less than the expected distance of 628.2±7.1 meters (p<0.001).
Health-Related Quality of Life
Parent and child reported HRQL values are reported in Table III. Scores on every subscale except for the parent-reported behavior subscale were significantly lower than expected. Physical functioning, role emotional/behavioral, role physical, bodily pain, and family activities subscale scores were more than two standard deviations below the mean for over 30% of the children when children's HRQL was reported by parents. Physical functioning and family activities were the only subscales with scores more than two standard deviations below the mean for more than 30% of children when HRQL was reported by the children themselves.
Table III.
Health related quality of life reported by parents and children on the Child Health Questionnaire
| Children with ALL | Age- and sex-specific expected value | < −1.5 SD | < −2.0 SD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHQ Subscale | Report | Mean | SE of Mean | Mean | SE of Mean | p-value | % | [95% CI] | % | [95% CI] |
| Physical functioning | Parent | 56.6 | 3.1 | 100 | 1.3 | <.001 | 72.0 | [62.5–80.2] | 62.6 | [52.7–71.8] |
| Child | 69.2 | 2.7 | 100 | 1.4 | <.001 | 55.7 | [45.2–65.8] | 39.2 | [29.4–49.6] | |
| Role emotional/behavior | Parent | 69.4 | 3.5 | 100 | 1.8 | <.001 | 45.7 | [36–55.7] | 37.1 | [27.9–47.1] |
| Child | 81.6 | 2.8 | 100 | 1.9 | <.001 | 30.8 | [21.5–41.3] | 22.0 | [14–31.9] | |
| Role physical | Parent | 62.1 | 3.7 | 100 | 1.8 | <.001 | 53.3 | [43.4–63] | 43.0 | [33.5–52.9] |
| Child | 73 | 3 | 100 | 2.0 | <.001 | 47.8 | [37.1–58.6] | 27.8 | [18.9–38.2] | |
| Bodily pain | Parent | 48.3 | 2.3 | 80 | 1.8 | <.001 | 61.7 | [51.8–70.9] | 48.6 | [38.8–58.5] |
| Child | 58 | 2.8 | 80 | 1.9 | <.001 | 43.2 | [33–53.7] | 26.3 | [17.8–36.4] | |
| Behavior | Parent | 77.4 | 1.6 | 79.2 | 1.6 | 0.25 | 8.4 | [3.9–15.4] | 3.7 | [1.0–9.3] |
| Child | 75 | 1.8 | 79.2 | 1.7 | 0.02 | 11.7 | [6.0–20.0] | 6.4 | [2.4–13.4] | |
| Mental health | Parent | 69 | 1.7 | 80 | 1.3 | <.001 | 37.4 | [28.2–47.3] | 18.7 | [11.8–27.4] |
| Child | 70.2 | 2.1 | 80 | 1.4 | <.001 | 27.7 | [18.9–37.8] | 14.9 | [8.4–23.7] | |
| Self-esteem | Parent | 72.1 | 2.4 | 83.3 | 1.7 | <.001 | 22.6 | [15.1–31.8] | 17.9 | [11.2–26.6] |
| Child | 72.3 | 2.2 | 83.3 | 1.8 | <.001 | 23.9 | [15.6–33.9] | 13.0 | [6.9–21.7] | |
| General health | Parent | 66.5 | 1.7 | 76.7 | 1.7 | <.001 | 20.6 | [13.4–29.5] | 7.5 | [3.3–14.2] |
| Child | 56.8 | 1.5 | 76.7 | 1.8 | <.001 | 27.8 | [18.9–38.2] | 10.0 | [4.7–18.1] | |
| Family activities | Parent | 56.8 | 2.6 | 100 | 1.8 | <.001 | 71.0 | [61.5–79.4] | 64.5 | [54.6–73.5] |
| Child | 66.4 | 2.3 | 100 | 2.0 | <.001 | 62.5 | [51.5–72.6] | 40.9 | [30.5–51.9] | |
| Family cohesion | Parent | 80.3 | 2.0 | 85 | 2.1 | 0.02 | 6.6 | [2.7–13.1] | 6.6 | [2.7–13.1] |
| Child | 75.2 | 2.8 | 85 | 2.3 | <0.001 | 10.3 | [4.8–18.7] | 10.3 | [4.8–18.7] | |
SE=Standard Error, SD=Standard Deviation, CI=Confidence Interval
Strength, Motor Performance and HRQL
In multivariable logistic regression analysis (Table IV), impaired knee extension was associated with child report of poor HRQL on the physical function subscale and with parent report of poor HRQL on the family activities subscale.
Table IV.
Risk of Reduced HRQL subscales (Physical Function and Family Activities) associated with impairment
| Physical function subscale* | Family activities subscale* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Impairment† | Parent | Child | Parent | Child | ||||||||
| OR‡ | 95% CI | p-value | OR‡ | 95% CI | p-value | OR‡ | 95% CI | p-value | OR‡ | 95% CI | p-value | |
| Ankle dorsiflexion range of motion | 2.4 | [0.5–12.5] | 0.28 | 3.5 | [0.7–17.7] | 0.13 | 1.1 | [0.3–4.3] | 0.92 | 6.8 | [0.8–58.1] | 0.08 |
|
| ||||||||||||
| Ankle dorsiflexion strength | 1.5 | [0.4–6.1] | 0.54 | 1.4 | [0.3–5.6] | 0.66 | 0.7 | [0.2–2.7] | 0.65 | 1.3 | [0.3–5.6] | 0.76 |
|
| ||||||||||||
| Knee extension strength | 2.3 | [0.8–6.7] | 0.13 | 20.9 | [2.5–173.3] | <0.001 | 4.3 | [1.2–16.3] | 0.03 | 2.5 | [0.7–8.4] | 0.15 |
|
| ||||||||||||
| Handgrip strength | 1.0 | [0.4–2.7] | 0.97 | 3.3 | [1.0–10.8] | 0.05 | 1.7 | [0.6–5.2] | 0.33 | 2.1 | [0.6–7.2] | 0.24 |
|
| ||||||||||||
| Fitness | 1.7 | [0.5–5.5] | 0.4 | 2.1 | [0.6–7.2] | 0.26 | 0.7 | [0.2–2.9] | 0.64 | 1.1 | [0.2–5.0] | 0.88 |
OR=Odds ratio, CI=Confidence Interval
Poor HRQL defined as scoring at least one standard deviation below the population mean for that subscale
Impairment defined as scoring at least 2 standard deviations below the mean on that measure
Adjusted for age, sex and race
DISCUSSION
The results of our analysis uniquely characterize bone mineral density, body composition, motor performance and HRQL soon after a diagnosis of childhood ALL, just after initiation of a multi-agent chemotherapy regimen. It appears that distal muscle strength, albeit often cited as a common concern for children during and after treatment for ALL is, on average, not initially impaired. However, low bone mineral density, increased body mass, proximal muscle weakness, poor overall motor performance and low endurance are problematic for a significant number of these children. This indicates that children with newly diagnosed ALL either present with obesity or have early weight gain, and that low bone mineral density, weakness and poor fitness are prevalent, perhaps related to the disease process, illness related deconditioning, or to initial treatment with glucocorticoids during induction therapy. These physical impairments, particularly muscle weakness, are associated with early reports of poor quality of life, both among parents and among the children themselves.
The results of our bone density evaluation in children with ALL are similar to those previously reported. Other studies have indicated that low bone mineral density is prevalent in this population at diagnosis [37–41]. A recent report from the Canadian Steroid-Associated Osteoporosis in the Pediatric Population research program reported a mean height adjusted lumbar BMD Z-score of −1.2±1.3 among 186 children newly diagnosed with ALL (median age 5.3 years, 108 male) [42]. Our average BMD values (−0.45 and −0.60) were slightly higher. There are several possible reasons for this higher average value. First, our study population was evaluated for BMD very soon after diagnosis (7-10 days). Others who have reported low BMD at diagnosis in children with ALL included values from DXA scans completed up to 30 days after initiation of chemotherapy [42]. Previous reports indicate that vitamin D metabolism and bone turnover impairments, present at diagnosis among children with ALL, are further escalated during induction therapy [43]. Second, consistent with two recent reports [44,45], our population had slightly higher than expected BMI values when compared to age- and sex-referenced normative data. While previous research indicates a positive correlation between body weight and BMD in children [46], it also indicates that obese children have lower levels of 25-hydroxyvitamin D than non-obese children [47], with the potential to compound the adverse effects of treatment for ALL on bone during the course of curative therapy Our findings indicate that early interventions designed to both prevent bone loss and preserve muscle mass are important for children with newly diagnosed ALL.
Interestingly, reduced hand grip strength, active range of motion and ankle muscle weakness were not highly prevalent among children with ALL at diagnosis, indicating that these problems have later onset and are, as previously documented, associated with administration of chemotherapy agents, such as vincristine and intrathecal methotrexate, that are neurotoxic [48]. Documentation of the trajectory of development of these neuromuscular problems during treatment will be important so the timing of appropriate interventions can be determined. Like BMD deficits, distal range of motion and strength impairments may be amenable to preventive strategies.
Our data documenting higher than expected BMI at diagnosis in children with ALL is supported by a recent study by Tan et al [49]. These investigators examined anthropometrics among 53 children (ages 3–12, 31 males) with newly diagnosed ALL and acute myeloid leukemia and compared them to healthy controls. Children with acute leukemia were more likely to be overweight than controls. Another report by Chow et al [50] reported higher than expected BMI among 165 children (median age 4.6 years, 94 males) with newly diagnosed ALL (BMI Z-score 0.24±1.24). Obesity and initial weight gain are concerning in this patient population as higher BMI levels are associated with decreased insulin sensitivity and increased beta-cell function [51], and if enduring, with inferior prognosis [52]. Childhood ALL survivors are at elevated risk for insulin resistance [53–56]. Our results indicate that the risk for this outcome may be pre-existing or begin very early in the disease and treatment process. Nutrition and physical activity discussions, counseling and interventions should be initiated early rather than later in the course of treatment to have the greatest impact.
Impairments of passive ankle range of motion, proximal muscle strength, motor performance and overall fitness appear to be present at diagnosis in children with ALL. Joint stiffness, muscular weakness and associated fatigue may be due to either the disease process or related to the initial administration of glucocorticoids in this patient population. Rayar and colleagues [57] recently reported that lean muscle mass is reduced at diagnosis in this patient population, and that mass declines over the course of therapy. Others have reported paraneoplastic myopathy as a presenting symptom of ALL [58], and knee extension strength deficits and poor performance early during therapy [59]. The leukemic process induces muscular atrophy in mouse models which is associated with abnormal protein synthesis, protein breakdown and potentially with inflammation [60]. Glucocorticoid administration is also associated with muscle wasting [61], compounding the insult conferred initially by the disease process. Because early deficits in proximal strength is associated with both parent and child reports of poor HRQL, interventions designed to minimize loss of muscle mass and preserve strength have promise to improve function in daily life for these children whose therapy spans two to three years during important developmental periods.
Our study results should be considered in the context of some potential study limitations. Not every child who was eligible for our study elected to enroll. Even though our study participants did not differ from non-participants by age or sex, it is possible that children who were sicker or who had more severe musculoskeletal symptoms at presentation of their disease elected not to participate. This would result in an underestimation of the degree and magnitude of musculoskeletal problems at diagnosis in this patient population. However, results from a sensitivity analysis evaluating the impact of deviations from observed proportions among non-participants (from 50% fewer to 50% more) on percentage estimates for the most extreme outcome category (two standard deviations below expected) for each measure indicate biases ranging from a low of ±1.0% for BMD to a high of ±16.4% for six minute walk distances. Additionally, children younger than age 4 years were not eligible for our study. Our results are not generalizable to the youngest children with newly diagnosed ALL.
Nevertheless, the results of our study indicate that children ages 4 to 18 years of age with ALL have bone density, body composition, strength and fitness deficits at diagnosis that adversely impact HRQL. Interventions for these deficits initiated early during the course of treatment for ALL need to be tested, and should include muscle strengthening and fitness components to address presenting skeletal and motor impairments.
ACKNOWLEDGEMENTS
This work [NCT00902213] was supported by National Institutes of Health grants R01 CA129384 (CL Cox, PI) and CA21765 (RJ Gilbertson, PI) and by the American Lebanese Syrian Associated Charities (ALSAC).
Clinical Trials Registration Number: NCT00902213
Footnotes
GE Healthcare Lunar, P.O. Box 7550, Madison, WI 53707
Hologic Inc., 35 Crosby Drive, Bedford, MA 01730
BioClinica Technologies, 826 Newtown-Yardley Rd, Newtown, PA 18940
Scale-Tronix, 200 East Post Road, White Plains, NY 10601
AliMed, 287 High Street, Dedham, MA 01026
Chatillon Ametek Test & Calibration Instruments, 8600 Comerset Drive, Largo, FL 33773
Patterson Medical Holdings, 1000 Remington Boulevard, Sutie 210, Bolingbrook, IL 60440
Pearson, 19500 Bulverde Road, San Antonio, TX 78259
HealthActCHQ, 800 Boylston Street, 16th Floor, Boston, MA 02199
SAS Institute Inc., 100 SAS Campus Drive, Cary, NC 27513
References
- 1.Kaste SC, Rai SN, Fleming K, et al. Changes in bone mineral density in survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2006;46:77–87. doi: 10.1002/pbc.20553. [DOI] [PubMed] [Google Scholar]
- 2.Le Meignen M, Auquier P, Barlogis V, et al. Bone mineral density in adult survivors of childhood acute leukemia: impact of hematopoietic stem cell transplantation and other treatment modalities. Blood. 2011;118:1481–1489. doi: 10.1182/blood-2011-01-332866. [DOI] [PubMed] [Google Scholar]
- 3.Makitie O, Heikkinen R, Toiviainen-Salo S, Henriksson M, Puukko-Viertomies LR, Jahnukainen K. Long-term skeletal consequences of childhood acute lymphoblastic leukemia in adult males: a cohort study. Eur J Endocrinol. 2013;168:281–288. doi: 10.1530/EJE-12-0702. [DOI] [PubMed] [Google Scholar]
- 4.Mandel K, Atkinson S, Barr RD, Pencharz P. Skeletal morbidity in childhood acute lymphoblastic leukemia. J Clin Oncol. 2004;22:1215–1221. doi: 10.1200/JCO.2004.04.199. [DOI] [PubMed] [Google Scholar]
- 5.Maniadaki I, Stiakaki E, Germanakis I, Kalmanti M. Evaluation of bone mineral density at different phases of therapy of childhood all. Pediatr Hematol Oncol. 2006;23:11–18. doi: 10.1080/08880010500313272. [DOI] [PubMed] [Google Scholar]
- 6.Janiszewski PM, Oeffinger KC, Church TS, et al. Abdominal obesity, liver fat, and muscle composition in survivors of childhood acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2007;92:3816–3821. doi: 10.1210/jc.2006-2178. [DOI] [PubMed] [Google Scholar]
- 7.Ness KK, Baker KS, Dengel DR, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:975–981. doi: 10.1002/pbc.21091. [DOI] [PubMed] [Google Scholar]
- 8.Harila-Saari AH, Huuskonen UE, Tolonen U, Vainionpaa LK, Lanning BM. Motor nervous pathway function is impaired after treatment of childhood acute lymphoblastic leukemia: a study with motor evoked potentials. Med Pediatr Oncol. 2001;36:345–351. doi: 10.1002/mpo.1084. [DOI] [PubMed] [Google Scholar]
- 9.Lehtinen SS, Huuskonen UE, Harila-Saari AH, Tolonen U, Vainionpaa LK, Lanning BM. Motor nervous system impairment persists in long-term survivors of childhood acute lymphoblastic leukemia. Cancer. 2002;94:2466–2473. doi: 10.1002/cncr.10503. [DOI] [PubMed] [Google Scholar]
- 10.Ramchandren S, Leonard M, Mody RJ, et al. Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. J Peripher Nerv Syst. 2009;14:184–189. doi: 10.1111/j.1529-8027.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar G, Black PC, Gutjahr P, Stopfkuchen H. Recovery kinetics of heart rate and oxygen uptake in long-term survivors of acute leukemia in childhood. Eur J Pediatr. 2007;166:1135–1142. doi: 10.1007/s00431-006-0394-7. [DOI] [PubMed] [Google Scholar]
- 12.Jarvela LS, Niinikoski H, Lahteenmaki PM, et al. Physical activity and fitness in adolescent and young adult long-term survivors of childhood acute lymphoblastic leukaemia. J Cancer Surviv. 2010;4:339–345. doi: 10.1007/s11764-010-0131-0. [DOI] [PubMed] [Google Scholar]
- 13.Tonorezos ES, Snell PG, Moskowitz CS, et al. Reduced cardiorespiratory fitness in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013 doi: 10.1002/pbc.24492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Brussel M, Takken T, van der Net J, et al. Physical function and fitness in long-term survivors of childhood leukaemia. Pediatr Rehabil. 2006;9:267–274. doi: 10.1080/13638490500523150. [DOI] [PubMed] [Google Scholar]
- 15.Warner JT, Bell W, Webb DK, Gregory JW. Daily energy expenditure and physical activity in survivors of childhood malignancy. Pediatr Res. 1998;43:607–613. doi: 10.1203/00006450-199805000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Jarfelt M, Fors H, Lannering B, Bjarnason R. Bone mineral density and bone turnover in young adult survivors of childhood acute lymphoblastic leukaemia. Eur J Endocrinol. 2006;154:303–309. doi: 10.1530/eje.1.02092. [DOI] [PubMed] [Google Scholar]
- 17.Joyce ED, Nolan VG, Ness KK, et al. Association of muscle strength and bone mineral density in adult survivors of childhood acute lymphoblastic leukemia. Arch Phys Med Rehabil. 2011;92:873–879. doi: 10.1016/j.apmr.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tillmann V, Darlington AS, Eiser C, Bishop NJ, Davies HA. Male sex and low physical activity are associated with reduced spine bone mineral density in survivors of childhood acute lymphoblastic leukemia. J Bone Miner Res. 2002;17:1073–1080. doi: 10.1359/jbmr.2002.17.6.1073. [DOI] [PubMed] [Google Scholar]
- 19.Alos N, Grant RM, Ramsay T, et al. High incidence of vertebral fractures in children with acute lymphoblastic leukemia 12 months after the initiation of therapy. J Clin Oncol. 2012;30:2760–2767. doi: 10.1200/JCO.2011.40.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diessel E, Fuerst T, Njeh CF, et al. Evaluation of a new body composition phantom for quality control and cross calibration of DXA devices. J Appl Physiol. 2000;89:599–605. doi: 10.1152/jappl.2000.89.2.599. [DOI] [PubMed] [Google Scholar]
- 21.Kalender WA. A phantom for standardization and quality control in spinal bone mineral measurements by QCT and DXA: design considerations and specifications. Med Phys. 1992;19:583–586. doi: 10.1118/1.596899. [DOI] [PubMed] [Google Scholar]
- 22.Kalender WA, Felsenberg D, Genant HK, Fischer M, Dequeker J, Reeve J. The European Spine Phantom - a tool for standardization and quality control in spinal bone mineral measurements by DXA and QCT. Eur J Radiol. 1995;20:83–92. doi: 10.1016/0720-048x(95)00631-y. [DOI] [PubMed] [Google Scholar]
- 23.Molgaard C, Thomsen BL, Prentice A, Cole TJ, Michaelsen KF. Whole body bone mineral content in healthy children and adolescents. Arch Dis Child. 1997;76:9–15. doi: 10.1136/adc.76.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orwoll ES, Oviatt SK, Biddle JA. Precision of dual-energy x-ray absorptiometry: development of quality control rules and their application in longitudinal studies. J Bone Miner Res. 1993;8:693–699. doi: 10.1002/jbmr.5650080607. [DOI] [PubMed] [Google Scholar]
- 25.Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention . A SAS Program for the CDC Growth Charts. Atlanta, GA: Mar 26, 2014. [cited 2014 May 8]. Available from: http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. [Google Scholar]
- 27.Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;42:127–133. doi: 10.1002/pbc.10481. [DOI] [PubMed] [Google Scholar]
- 28.Soucie JM, Wang C, Forsyth A, et al. Range of motion measurements: reference values and a database for comparison studies. Haemophilia. 2011;17:500–507. doi: 10.1111/j.1365-2516.2010.02399.x. [DOI] [PubMed] [Google Scholar]
- 29.Eek MN, Kroksmark AK, Beckung E. Isometric muscle torque in children 5 to 15 years of age: normative data. Arch Phys Med Rehabil. 2006;87:1091–1099. doi: 10.1016/j.apmr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Merlini L, Mazzone ES, Solari A, Morandi L. Reliability of hand-held dynamometry in spinal muscular atrophy. Muscle Nerve. 2002;26:64–70. doi: 10.1002/mus.10166. [DOI] [PubMed] [Google Scholar]
- 31.Hager-Ross C, Rosblad B. Norms for grip strength in children aged 4–16 years. Acta Paediatr. 2002;91:617–625. doi: 10.1080/080352502760068990. [DOI] [PubMed] [Google Scholar]
- 32.Mathiowetz V, Wiemer DM, Federman SM. Grip and pinch strength: norms for 6-to 19-year olds. Am J Occup Ther. 1986;40:705–711. doi: 10.5014/ajot.40.10.705. [DOI] [PubMed] [Google Scholar]
- 33.Bruininks RH, Bruininks BD. BOT2 Bruininks-Oseretsky Test of Motor Proficiency. AGS Publishing; Circle Pines, MN: 2005. [Google Scholar]
- 34.Geiger R, Strasak A, Treml B, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007;150:395–399. doi: 10.1016/j.jpeds.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 35.Russell KM, Hudson M, Long A, Phipps S. Assessment of health-related quality of life in children with cancer: consistency and agreement between parent and child reports. Cancer. 2006;106:2267–2274. doi: 10.1002/cncr.21871. [DOI] [PubMed] [Google Scholar]
- 36.Landgraf JM, Abetz L, Ware JE. Child Health questionnaire (CHQ): A User's Manual. HealthAct; Boston, MA: 1999. [Google Scholar]
- 37.Halton JM, Atkinson SA, Fraher L, et al. Altered mineral metabolism and bone mass in children during treatment for acute lymphoblastic leukemia. J Bone Miner Res. 1996;11:1774–1783. doi: 10.1002/jbmr.5650111122. [DOI] [PubMed] [Google Scholar]
- 38.Rayar MS, Nayiager T, Webber CE, Barr RD, Athale UH. Predictors of bony morbidity in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59:77–82. doi: 10.1002/pbc.24040. [DOI] [PubMed] [Google Scholar]
- 39.Riccio I, Marcarelli M, Del Regno N, et al. Musculoskeletal problems in pediatric acute leukemia. J Pediatr Orthop B. 2013;22:264–269. doi: 10.1097/BPB.0b013e32835d731c. [DOI] [PubMed] [Google Scholar]
- 40.van der Sluis IM, van den Heuvel-Eibrink MM, Hahlen K, Krenning EP, de Muinck Keizer-Schrama SM. Altered bone mineral density and body composition, and increased fracture risk in childhood acute lymphoblastic leukemia. J Pediatr. 2002;141:204–210. doi: 10.1067/mpd.2002.125728. [DOI] [PubMed] [Google Scholar]
- 41.Swiatkiewicz V, Wysocki M, Odrowaz-Sypniewska G, Koltan A, Manysiak S, Dylewska K. Bone mass and bone mineral metabolism at diagnosis and after intensive treatment in children with acute lymphoblastic leukemia. Med Pediatr Oncol. 2003;41:578–580. doi: 10.1002/mpo.10415. [DOI] [PubMed] [Google Scholar]
- 42.Halton J, Gaboury I, Grant R, et al. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res. 2009;24:1326–1334. doi: 10.1359/jbmr.090202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atkinson SA, Halton JM, Bradley C, Wu B, Barr RD. Bone and mineral abnormalities in childhood acute lymphoblastic leukemia: influence of disease, drugs and nutrition. Int J Cancer Suppl. 1998;11:35–39. [PubMed] [Google Scholar]
- 44.Aldhafiri FK, McColl JH, Reilly JJ. Prevalence of being underweight and overweight and obesity at diagnosis in UK patients with childhood acute lymphoblastic leukaemia 1985–2002. J Hum Nutr Diet. 2013 doi: 10.1111/jhn.12112. [DOI] [PubMed] [Google Scholar]
- 45.Esbenshade AJ, Simmons JH, Koyama T, Koehler E, Whitlock JA, Friedman DL. Body mass index and blood pressure changes over the course of treatment of pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56:372–378. doi: 10.1002/pbc.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz JC, Mandel C, Garabedian M. Influence of spontaneous calcium intake and physical exercise on the vertebral and femoral bone mineral density of children and adolescents. J Bone Miner Res. 1995;10:675–682. doi: 10.1002/jbmr.5650100502. [DOI] [PubMed] [Google Scholar]
- 47.Au LE, Rogers GT, Harris SS, Dwyer JT, Jacques PF, Sacheck JM. Associations of vitamin D intake with 25-hydroxyvitamin D in overweight and racially/ethnically diverse US children. J Acad Nutr Diet. 2013;113:1511–1516. doi: 10.1016/j.jand.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ness KK, Hudson MM, Pui CH, et al. Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: associations with physical performance and chemotherapy doses. Cancer. 2012;118:828–838. doi: 10.1002/cncr.26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan SY, Poh BK, Nadrah MH, Jannah NA, Rahman J, Ismail MN. Nutritional status and dietary intake of children with acute leukaemia during induction or consolidation chemotherapy. J Hum Nutr Diet. 2013 doi: 10.1111/jhn.12074. [DOI] [PubMed] [Google Scholar]
- 50.Chow EJ, Pihoker C, Hunt K, Wilkinson K, Friedman DL. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110:2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 51.Chow EJ, Pihoker C, Friedman DL, et al. Glucocorticoids and insulin resistance in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:621–626. doi: 10.1002/pbc.24364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orgel E, Sposto R, Malvar J, et al. Impact on Survival and Toxicity by Duration of Weight Extremes During Treatment for Pediatric Acute Lymphoblastic Leukemia: A Report From the Children's Oncology Group. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.52.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 54.Oeffinger KC, Adams-Huet B, Victor RG, et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:3698–3704. doi: 10.1200/JCO.2008.19.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinberger J, Sinaiko AR, Kelly AS, et al. Cardiovascular risk and insulin resistance in childhood cancer survivors. J Pediatr. 2012;160:494–499. doi: 10.1016/j.jpeds.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tonorezos ES, Vega GL, Sklar CA, et al. Adipokines, body fatness, and insulin resistance among survivors of childhood leukemia. Pediatr Blood Cancer. 2012;58:31–36. doi: 10.1002/pbc.22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rayar M, Webber CE, Nayiager T, Sala A, Barr RD. Sarcopenia in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2013;35:98–102. doi: 10.1097/MPH.0b013e318279eea2. [DOI] [PubMed] [Google Scholar]
- 58.Kameda G, Vieker S, Duck C, Blaes F, Langler A. Paraneoplastic myopathy as a very rare manifestation of acute lymphoblastic leukemia. Klin Padiatr. 2010;222:386–387. doi: 10.1055/s-0030-1265137. [DOI] [PubMed] [Google Scholar]
- 59.Gocha Marchese V, Chiarello LA, Lange BJ. Strength and functional mobility in children with acute lymphoblastic leukemia. Med Pediatr Oncol. 2003;40:230–232. doi: 10.1002/mpo.10266. [DOI] [PubMed] [Google Scholar]
- 60.Bindels LB, Beck R, Schakman O, et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS One. 2012;7:e37971. doi: 10.1371/journal.pone.0037971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menconi M, Fareed M, O'Neal P, Poylin V, Wei W, Hasselgren PO. Role of glucocorticoids in the molecular regulation of muscle wasting. Crit Care Med. 2007;35:S602–608. doi: 10.1097/01.CCM.0000279194.11328.77. [DOI] [PubMed] [Google Scholar]