Abstract
Background
We investigated the pattern of disease progression in the asymptomatic, mild cognitive impairment (MCI) and dementia stage of Alzheimer's Disease (AD).
Methods
We selected 284 subjects with AD pathology, defined as abnormal levels of amyloid beta 1-42 (Aß1-42) in cerebrospinal fluid (CSF). Disease outcome measures included six biomarkers and five cognitive markers. We compared differences in baseline measures and decline over 4 years between the AD stages and tested whether these changes differed from subjects, without AD pathology (N=132).
Results
CSF Aß1-42 reached the maximum abnormality level in the asymptomatic stage and tau in the MCI stage. The imaging and cognitive markers started to decline in the asymptomatic stage, and decline accelerated with advancing clinical stage.
Conclusion
This study provides further evidence for a temporal evolution of AD biomarkers. Our findings may be helpful to determine stage specific outcome measures for clinical trials.
Keywords: Longitudinal, Observational, Biomarkers, Cognitive markers, Alzheimer's disease, Asymptomatic, MCI, Dementia
1. Introduction
Alzheimer's disease (AD) is a neurodegenerative disease, hypothesized to be initiated by abnormal amyloid processing, followed by neuronal dysfunction and structural brain changes which ultimately lead to cognitive impairment and dementia [1]. According to the new NIA-AA/IWG research criteria, AD can be subdivided in 3 stages: an asymptomatic or preclinical stage, a stage of mild cognitive impairment (MCI) and the dementia stage [2-4]. The pattern of disease progression in each of these stages is not fully understood yet. This limits trial design, in particular in the predementia stage where intervention is believed to be most effective because neuronal injury and cognitive impairment are still limited. The aim of the present study is to investigate biomarker and cognitive changes in the asymptomatic stage, MCI stage and dementia stage of AD. We also investigated whether these changes differed from subjects with normal cognition, MCI or dementia but without AD pathology. We selected subjects from Alzheimer's Disease Neuroimaging Initiative (ADNI) with AD pathology, defined as abnormal amyloid beta 1-42 (Aß1-42) in cerebrospinal fluid (CSF), who had normal cognition, MCI or dementia. We examined change for up to four years on six key biomarkers for AD (CSF Aß1-42, CSF tau, Fludeoxyglucose Positron Emission Tomography (FDG-PET) and hippocampal, whole brain and ventricular volume on Magnetic Resonance Imaging (MRI), and five cognitive markers (Clinical Dementia Rating scale sum of boxes (CDR-SOB) [5], Mini-mental state examination (MMSE) [6], Alzheimer's Disease Assessment Scale-cognitive (ADAS-Cog) [7], and composite scores for executive function and composite scores for memory [8, 9]. We compared baseline scores and slope of decline on each measure between the AD stages and with subjects who had normal cognition, MCI or dementia but no AD pathology.
2. Methods
2.1 ADNI study
We selected subjects from ADNI (adni.loni.ucla.edu). ADNI was initiated by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies and non-profit organizations and launched in 2003. The initial goal of ADNI was to recruit 800 adults, ages 55 to 90, to participate in the research, approximately 200 cognitively normal older individuals to be followed for 3 years, 400 people with MCI to be followed for 3 years and 200 people with early AD to be followed for 2 years. For up-to-date information, see www.adni-info.org. The institutional review boards of all participating institutions approved the procedures for this study. Written informed consent was obtained from all participants or surrogates.
2.2 Participants
The ADNI inclusion criteria for participants with normal cognition were absence of memory complaints, a MMSE score of 24-30, a CDR score of 0 and no MCI or dementia diagnosis. The inclusion criteria for subjects with MCI were memory complaints, objective memory loss, a MMSE score between 24 and 30 and a CDR of 0.5. The inclusion criteria for subjects with AD were memory complaints, objective memory loss, a MMSE score between 20-26, a CDR of 0.5-1.0 and a diagnosis of probable AD according to NINCDS-ADRDA criteria [10]. Exclusion criteria were absence of an informant, a score of >4 on the modified Hachinski scale [11] and score of >5 on the Geriatric Depression Scale [12], diseases expected to interfere with the study, use of investigational agents, neurological disease, psychiatric disorders, alcohol abuse and neuroimaging abnormalities showing other reasons for cognitive problems. Permitted medication had to be stable for at least four weeks prior to screening. We downloaded ADNI data on May 2012. Of the 800 subjects included in ADNI-1 we selected all cognitively normal, MCI and demented participants (N=416) with available baseline CSF Aß1-42.
2.3 Definition of diagnostic groups
We defined AD pathology as a CSF Aß1-42 level below 192 pg/ml. Subjects were classified as AD-asymptomatic (n=44) if cognition was normal, AD-MCI (n=148) if subjects had MCI, and AD-dementia (n=92) if subjects were demented. Subjects with CSF Aß1-42 levels >192 pg/ml were classified as control (n=72) if cognition was normal, MCI-other (n=51) if subjects had MCI, or dementia-other (n=9) if subjects were demented.
2.4 Baseline assessment and longitudinal assessment
At baseline all subjects underwent a standardized assessment, which included neurological examination, physical examination and neuropsychological assessments. Furthermore, CSF and blood samples were taken and MRI and FDG-PET scans were obtained. The protocols for cognitive testing, CSF, MRI and PET are described in detail at http://www.loni.ucla.edu/ADNI/Data/ADNI_Data.shtml. Assessments were repeated at 6 or 12 months intervals up to 6 years. For the present study we used results from the baseline and annually assessments for up to four years for cognitive measures, CSF Aß1-42 and tau, FDG-PET and MRI volumetric measures.
2.5 Cognitive assessment
We used the MMSE, ADAS Cog, CDR-SOB, and composite scores for executive function and memory. The composite executive function measure consisted of 7 subtests and the memory composite measure of 8 subtests as described in detail elsewhere [8, 9]. We selected scores from the annual assessment up to four years.
2.6 CSF analyses
CSF was collected by lumbar puncture and shipped on dry ice to the Penn ADNI Biomarker Core Laboratory at the University of Pennsylvania, Philadelphia for storage until further analysis. CSF was analysed using a multiplex xMAP Luminex platform (Luminex Corp) with immunoassay kit-based reagents (INNO-BIA Alzbio3; Innogenetics; www.adni-info.org) as described elsewhere [13]. Follow-up was performed annually up to four years.
2.7 MRI analyses
We used scans made on a 1.5 Tesla MRI scanner. We selected measures for whole brain, ventricular and hippocampal volume. For measurement of whole brain and ventricular volume boundary shift integral (BSI) was used [14, 15]. Whole brain and ventricles were first semi-automatically delineated from T1-weighted MRI. The repeat scans were then registered to the baseline scans using 9-degree-of-freedom registration. The intensity inhomogeneity between baseline and registered repeat scans was corrected using the differential bias correction. Hippocampal volumes were measured, using FreeSurfer version 4.3 on T1 weighted images which were pre-processed (gradient warping, scaling, B1 correction and N3 inhomogeneity correction)[16]. For measurements, an unbiased within-subject template space and average image was created using robust, inverse consistent registration. Information from each subject's template was used to initialize the longitudinal image processing in several locations to increase reliability and statistical power when measuring brain change over time [17]. Hippocampal volume was measured bilateral and averaged. We used BSI data from baseline and the first two annual visits and FreeSurfer of the annual visits up to four years. To correct for intracranial volume (ICV), we used the estimated ICV measure from FreeSurver.
2.8 FDG-PET analyses
FDG-PET was available in a subgroup of 207 subjects. FDG image data were acquired 30 to 60 minutes post-injection. After prepocessing (frames were averaged, spatially aligned, interpolated to a standard voxel size, and smoothed to a common resolution of 8 mm full width at half maximum) images were spatially normalized in SPM5 to MNI PET template. MetaROI's were calculated that includes FDG uptake in bilateral angular gyrus, posterior cingular and bilateral inferior temporal gyrus. Each MetaROI was normalised to a reference region composed of the pons and vermis. Total FDG uptake was calculated as a mean of the five individual MetaROI's [18]. Follow-up was annually for 4 years for cognitively normal subjects and MCI subjects for 2 years for subjects with dementia.
2.9 Statistical analyses
Analyses were performed with SPSS version 19.0 for the Macintosh. In order to compare cognitive markers and biomarkers at baseline and over time, raw scores were converted into z-scores, relative to the baseline scores of the cognitively normal controls. The z-score is the number of standard deviations from which the score deviates from the expected score given age, sex, education, and apolipoprotein E (APOE) genotype. In the control group we performed multiple linear regression with age, sex, education, APOE genotype, and ICV (MRI measurements only) entered in the first step, using P<.05 as the criterion for remaining in the model. On the basis of the resulting model, an expected test score for each subject was calculated. This score was subtracted from the observed score. The residual was divided by the standard deviation of the residual in the reference population to give the z-score. Z-scores were expressed such that a negative score indicated a performance worse than the control group at baseline. For each variable and assessment z-scores were calculated relative to the control group at baseline.
Change in biomarkers and cognitive scores over time were assessed by slope analyses with mixed models using an unstructured covariance matrix (which assumes a random intercept and random slope), with age, education and gender as covariates and follow-up time as repeated measure. We assumed a linear change in time, as time coded with a quadratic term was not a statistically significant predictor. Analyses were performed in the total group using contrasts to calculate baseline differences and slopes for individual groups and to compare them between groups. The analyses of the slopes included baseline score and available follow-up scores. We tested whether slopes were different from 0 and whether they differed between groups. A difference with a p-value <0.05, without correction for multiple testing, was considered statistically significant. In table 2 we indicate which differences would not be statistically significant after correction for multiple testing according to Benjamini-Hochberg [19].
Table 2.
Baseline z-scores and slopes according to diagnostic group at baseline
| Normal |
MCI |
Dementia |
Difference between cognitive stages$ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (N=72) |
AD- asymptomatic (N=44) |
p-value | MCI-other (N=51) |
AD-MCI (N=148) |
p-value | Dementia-other (N=9) |
AD-dementia (N=92) |
p- value |
No AD pathology |
AD pathology |
|
| Baseline | |||||||||||
| CSF aβ 1-42 | 0.0 (1.0) | –3.84 (1.24) | 0.0001 | –0.06 (1.03) | –4.61 (1.68)# | 0.0001 | 0.18 (0.96) | –4.81 (1.39)# | 0.0001 | ns | N>M,D |
| CSF tau | 0.0 (1.0) | –0.73 (1.21) | 0.001 | –0.16 (0.89) | –1.54 (1.29)# | 0.0001 | –0.37 (1.25) | –1.75 (1.25)# | 0.002 | ns | N>M,D |
| FDG-PET | 0.0 (1.0) | –0.34 (1.14) | 0.38 | –0.33 (0.93) | –1.27 (1.32)# | 0.003 | –1.58 (0.47)# | –2.13 (1.44)# | 0.99 | N, M>D | N>M>D |
| Hippocampal volume | 0.0 (1.0) | –0.08 (0.85) | 0.70 | –1.3 (1.76)# | –1.83 (1.37)# | 0.023 | –1.96 (2.15)# | –2.63 (1.51)# | 0.22 | N>M,D | N>M>D |
| Ventricular volume | 0.0 (1.0) | –0.35 (0.94) | 0.04 | –0.52 (1.09)# | –1.11 (0.99)# | 0.001 | –0.48 (0.82) | –1.55 (1.03# | 0.03 | N>M | N>M>D |
| Whole brain volume | 0.0 (1.0) | –0.11 (1.05) | 0.46 | –0.43 (1.01)# | –0.68 (0.89)# | 0.11 | –0.68 (1.59)# | –1.08 (0.93)# | 0.36 | N>M,D^ | N>M>D |
| CDR-SOB | 0.0 (1.0) | 0.18 (0.59) | 0.64 | –8.11 (1.68)# | –8.82 (1.85)# | 0.009 | –12.58 (2.22)# | –12.98 (1.87)# | 0.58 | N>M>D | N>M>D |
| MMSE | 0.0 (1.0) | 0.13 (1.06) | 0.69 | –1.73 (1.92)# | –2.32 (2.08)# | 0.07 | –5.27 (3.15)# | –6.21 (2.51)# | 0.16 | N>M>D | N>M>D |
| ADAS-Cog | 0.0 (1.0) | –0.35 (0.98) | 0.05 | –1.12 (0.95)# | –1.56 (0.87)# | 0.002 | –1.95 (0.57)# | –2.49 (0.72)# | 0.08 | N>M>D | N>M>D |
| Memory | 0.0 (1.0) | –0.06 (1.15) | 0.90 | –1.95 (1.99)# | –3.68 (2.99)# | 0.0001 | –7.06 (7.96)# | –5.16 (3.02)# | 0.13 | N>M>D | N>M>D |
| Executive function | 0.0 (1.0) | –1.24 (2.37) | 0.004 | –1.02 (1.75)# | –2.39 (2.39)# | 0.0001 | –2.19 (1.33) | –3.47 (2.55)# | 0.36 | N>M,D | N>M>D |
| Annual decline | |||||||||||
| CSF aβ 1-42 | –0.14 (0.04)* | –0.09 (0.06) | 0.49 | –0.10 (0.06) | –0.06 (0.03) | 0.56 | 0.48 (0.48) | 0.07 (0.06) | 0.49 | ns | N^>D |
| CSF tau | –0.08 (0.02)* | –0.12 (0.03)* | 0.34 | 0.02 (0.03) | –0.08 (0.02)# | 0.015 | –0.22 (0.27) | 0.01 (0.03) | 0.27 | N>M | N,M^>D |
| FDG-PET | –0.13 (0.05)* | –0.12 (0.06)*^ | 0.92 | –0.16 (0.07)* | –0.34 (0.04)* | 0.019 | - | –0.70 (0.09)* | - | ns | N<M<D |
| Hippocampal volume | –0.13 (0.02)* | –0.16 (0.03)* | 0.31 | –0.21 (0.03)* | –0.36 (0.02)* | 0.0001 | –0.68 (0.29)* | –0.52 (0.04)* | 0.57 | N<M | N<M<D |
| Ventricular volume | –0.07 (0.01)* | –0.11 (0.01)* | 0.05^ | –0.09 (0.01)* | –0.17 (0.01)* | 0.0001 | –0.14 (0.09) | –0.23 (0.01)* | 0.34 | ns | N<M<D |
| Whole brain volume | –0.12 (0.02)* | –0.17 (0.03)* | 0.22 | –0.17 (0.03)* | –0.27 (0.02)* | 0.014 | –0.54 (0.28) | –0.36 (0.04)* | 0.53 | ns | N<M=<D |
| CDR-SOB | –0.31 (0.13)* | –0.76 (0.18)* | 0.04^ | –0.02 (0.17) | –1.27 (0.10)* | 0.0001 | –1.16 (1.26) | –1.49 (0.21)* | 0.79 | ns | N<M,D |
| MMSE | 0.20 (0.33) | –0.28 (0.43) | 0.37 | 0.00 (0.43) | –1.63 (0.25)* | 0.001 | –0.69 (2.55) | –4.50 (0.45)* | 0.15 | ns | N<M<D |
| ADAS-Cog | 0.04 (0.04) | –0.05 (0.05) | 0.13 | –0.02 (0.05) | –0.25 (0.03)* | 0.0001 | –0.33 (0.51) | –0.38 (0.07)* | 0.92 | ns | N<M,D |
| Memory | –0.03 (0.08) | –0.05 (0.10) | 0.86 | 0.18 (0.12) | –0.58 (0.09)* | 0.0001 | - | –1.09 (0.51)*^ | – | ns | N<M,D^ |
| Executive function | –0.001 (0.06) | 0.02 (0.08) | 0.85 | –0.08 (0.09) | –0.46 (0.06)* | 0.01 | - | 0.18 (0.24) | - | ns | N<M>D |
All scores are z-scores (see methods). Data are mean (SD) for baseline scores and mean (SE) for slopes.
AD= Alzheimer type pathology; MCI= mild cognitive impairment; APOE4= apolipoprotein E4; CSF= cerebrospinal fluid; abeta 1-42= amyloid beta 1-42; FDG-PET= Fludeoxyglucose Positron Emission Tomography; CDR-SOB= Clinical Dementia Rating scale sum of boxes; MMSE= Mini-Mental State Examination; ADAS-Cog= Alzheimer's Disease Assessment Scale-Cognitive, ns= not significant, N= normal cognition, M=MCI, D=dementia.
p-value<0.05 compared to control
p-value slope different from 0 at p-value<0.05; - not estimated due to small sample size.
Differences between cognitive groups with same amyloid status are indicated if p<0.05
Not statistically significant after correction for multiple testing (baseline analyses 11 comparisons for each variable, annual decline analyses 15 comparisons for each variable)
3. Results
3.1 Baseline characteristics
Table 1 shows the baseline characteristics according to diagnostic groups. Age, gender and APOE-ε4 status differed between groups. Age was higher in subjects with dementia-other compared to the other subjects, except for subjects with AD-asymptomatic, and subjects were more often female in the dementia-other group compared to the MCI-other group. APOE-ε4 was more frequently positive in subjects with abnormal amyloid levels than in subjects with normal amyloid, regardless of clinical status. AD-asymptomatic subjects were less often APOE-ε4 positive (45%) than subjects with AD-MCI (65 %) and APOE-ε4 carriership tended to be lower in AD-MCI compared to AD-dementia (77%). Among ε4 carriers, ε4 homozygosity was least common in AD-asymptomatic (10%) and highest in AD-dementia (32%). The unadjusted biomarker and cognitive scores are shown in table 1 and the z-scores relative to controls in table 2 and in figures 1, 2 and 3 and will be discussed below.
Table 1.
Baseline characteristics
| Normal cognition |
MCI |
Dementia |
Differences between cognitive stages* |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls (N=72) |
AD- asymptomatic (N=44) |
p-value | MCI-other (N=51) |
AD-MCI (N=148) |
p-value | Dementia-other (N=9) |
AD-dementia (N=92) |
p-value | No AD pathology |
AD pathology |
|
| Age (years) | 75 (5.2) | 76 (5.1) | 0.31 | 74 (8.7) | 74 (7.0) | 0.86 | 81 (7.5) | 74 (7.8) | 0.007 | N,M<D | ns |
| Females (%) | 51 | 45 | 0.67 | 27 | 35 | 0.37 | 50 | 41 | 0.46 | N>M | ns |
| Years of education | 15.7 (2.7) | 15.9 (3.1) | 0.74 | 15.9 (3.0) | 15.8 (3.0) | 0.87 | 14.8 (3.7) | 15.2 (3.3) | 0.84 | ns | ns |
| 1/2 APOE-ε4 alleles (%carrier) | 7/0 (9.7%) | 18/2 (45.0%) | 0.0001 | 12/0 (23.5%) | 74/22 (65.0%) | 0.0001 | 0/0 (0.0%) | 48/23 (77.0%) | 0.0001 | ns | N<M,D |
| CSF aß 1-42 (pg/ml) | 250 (31) | 153 (25) | 0.0001 | 247 (31) | 140 (28) | 0.0001 | 255 (31) | 135 (23) | 0.0001 | ns | N>M,D |
| CSF tau (pg/ml) | 61 (23) | 83 (38) | 0.001 | 64 (23) | 116 (63) | 0.0001 | 73.3 (38) | 124 (56) | 0.009 | ns | N<M,D |
| FDG PET (SUVr) | 1.30 (0.12) | 1.27 (0.15) | 0.41 | 1.26 (0.11) | 1.17 (0.13) | 0.006 | 1.08 (0.08) | 1.08 (0.11) | 0.96 | N,M>D | N>M>D |
| Whole brain volume (cm3) | 1044 (108) | 1066 (110) | 0.31 | 1075 (123) | 1048 (108) | 0.18 | 1024 (144) | 1010 (118) | 0.11 | ns | N>M>D |
| Hippocampal volume (mm3) | 3620 (425) | 3598 (393) | 0.78 | 3330 (659) | 3092 (479) | 0.024 | 2932 (768) | 2832 (534) | 0.27 | N>M, D | N>M>D |
| Ventricular volume (cm3) | 35 (21) | 38 (16) | 0.96 | 46 (23) | 48 (26) | 0.29 | 64 (43) | 53 (26) | 0.40 | N<M,D | N<M<D |
| CDR sum of boxes | 0.03 (0.13) | 0.01 (0.08) | 0.97 | 1.80 (0.72) | 2.14 (0.90) | 0.059 | 4.69 (1.90) | 5.0 (1.80) | 0.49 | N<M<D | N<M<D |
| MMSE score | 29.1 (1.1) | 29.2 (0.9) | 0.67 | 27.3 (1.8) | 26.8 (1.8) | 0.057 | 24.3 (2.2) | 23.5 (1.9) | 0.17 | N>M>D | N>M>D |
| ADAS-Cog | 6.0 (2.8) | 7.0 (3.0) | 0.27 | 10.1(4.4) | 12.2 (4.5) | 0.004 | 14.0 (3.8) | 18.5 (6.3) | 0.054 | N<M<D | N<M<D |
| Memory score | 0.97 (0.52) | 0.93 (0.47) | 0.79 | 0.12 (0.60) | −0.21 (0.54) | 0.0001 | −0.43 (0.46) | −0.85 (0.54) | 0.027 | N>M>D | N>M>D |
| Executive score | 0.77 (0.57) | 0.52 (0.66) | 0.03 | 0.28 (0.77) | −0.17 (0.72) | 0.0001 | −0.64 (0.58) | −0.93 (0.83) | 0.17 | N>M>D | N>M>D |
Data are mean (SD), unless otherwise specified. MRI values are not corrected for intracranial volume.
AD= Alzheimer type pathology; MCI= mild cognitive impairment; APOE= apolipoprotein E genotype; CSF = cerebrospinal fluid; abeta 1-42= amyloid beta 1-42; FDG-PET= Fludeoxyglucose Positron Emission Tomography; SUV= standardized uptake values; MRI= Magnetic Resonance Imaging; CDR sum of boxes= Clinical Dementia Rating scale sum of boxes; MMSE= Mini-Mental State Examination, ADAS-Cog= Alzheimer's Disease Assessment Scale-Cognitive; ns= not significant, N= normal cognition, M=MCI, D=dementia.
Differences between cognitive groups with same amyloid status are indicated if p<0.05.
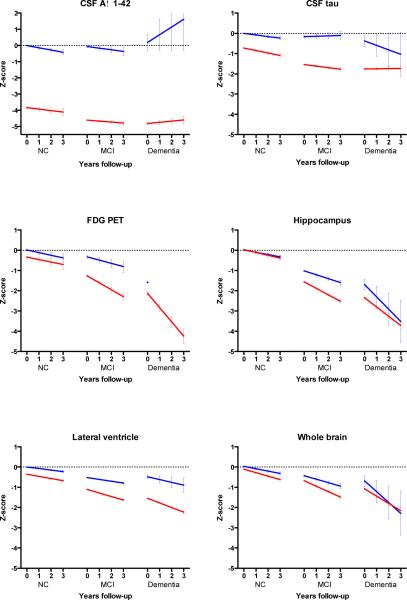
Figure 1. Estimated change in z-scores of the biomarkers according to clinical stage and AD biomarker status.
Change over time for biomarkers. CSF= cerebrospinal fluid; aß 1-42= amyloid beta 1-42; FDG-PET= Fludeoxyglucose Positron Emission Tomography; MRI= Magnetic Resonance Imaging; NC=normal cognition; MCI= Mild Cognitive Impairment. Slopes for change on FDG-PET could not be estimated in the dementia other group. A negative z-score indicates that the score is worse than that of the control group at baseline. Error bar indicates standard error of the mean.
 Subjects with AD pathology
Subjects with AD pathology
 Subjects without AD pathology
Subjects without AD pathology
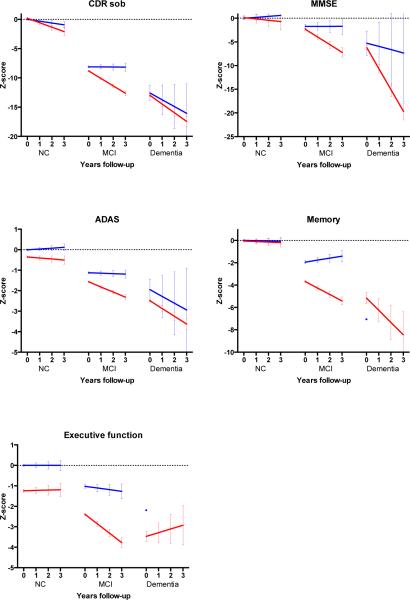
Figure 2. Estimated change in z-scores of the cognitive markers according to clinical stage and AD biomarker status.
Change over time for cognitive markers. CDR-SOB= Clinical Dementia Rating scale sum of boxes; MMSE= Mini-Mental State Examination; ADAS-Cog= Alzheimer's Disease Assessment Scale-Cognitive; NC=normal cognition; MCI= Mild Cognitive Impairment. Slopes for change on memory and executive scores could not be estimated in the dementia other group. A negative z-score indicates that the score is worse than that of the control group at baseline. Error bar indicates standard error of the mean.
 Subjects with AD pathology
Subjects with AD pathology
 Subjects without AD pathology
Subjects without AD pathology
Figure 3. Temporal evolution of biomarkers and cognitive markers from in AD from the asymptomatic to dementia stage.
Aß 1-42= amyloid beta 1-42; FDG-PET= Fludeoxyglucose Positron Emission Tomography; MRI= Magnetic Resonance Imaging; ADAS-Cog= Alzheimer's Disease Assessment Scale-Cognitive; NC= normal cognition; MCI= Mild Cognitive Impairment. A negative z-score indicates a score worse than that of the control.
3.2 AD-asymptomatic stage
At baseline, AD-asymptomatic subjects had, by definition, more abnormal CSF Aß1-42 compared to controls. In addition, they had more abnormal CSF tau levels, ventricular volume, ADAS-Cog scores and composite executive scores. At follow-up, AD-asymptomatic subjects tended to decline on CSF Aß1-42 (p=0.09) and significantly declined on CSF tau (p=0.0001), FDG-PET (p=0.046), hippocampal volume (p= 0.0001) ventricular volume (p=0.0001), whole brain volume (p=0.0001) and CDR-SOB (p=0.0001). Only the decline in ventricular volume (p=0.05) and CDR-SOB (p=0.04) exceeded the decline observed in the controls.
3.3 AD-MCI stage
AD-MCI subjects differed at baseline from controls on all measures. They also differed from MCI-other on all cognitive markers and biomarkers, except for whole brain volume. Subjects with AD-MCI declined on all biomarkers and cognitive markers, except on CSF Aß1-42. Decline on all these markers was larger than observed in MCI-other.
3.4 AD-dementia stage
At baseline subjects with AD-dementia were impaired on all biomarkers and cognitive markers compared to controls. Compared to the 9 subjects with dementia-other, only CSF tau and ventricular volume were more abnormal, in addition to CSF Aß1-42. AD-dementia subjects showed decline on all measures except CSF Aß1-42, CSF tau and the composite executive score. The decline was similar to that of dementia-other subjects, although in the latter group slopes for some markers could not be estimated, probably due to the small sample size.
3.5 Differences between AD stages
Baseline scores
AD-asymptomatic subjects had at baseline less abnormal CSF Aß1-42 and tau and less abnormal imaging markers and cognitive scores compared to AD-MCI and AD dementia subjects. AD-MCI differed from AD-dementia on all imaging markers and cognitive scores.
Rate of decline
Decline in CSF Aß1-42 levels was larger in AD-asymptomatic than in AD-dementia. Decline in CSF tau was larger in AD-asymptomatic and AD-MCI subjects than in AD-dementia subjects. All imaging markers showed more decline with advancing clinical stage. The increase in decline between stages was largest for FDG-PET and hippocampal volume on MRI. Decline in CDR-SOB, ADAS-cog and in composite score for memory was larger in subjects with AD-MCI and AD-dementia than in AD-asymptomatic subjects. Decline on the MMSE and composite executive score was larger in AD-dementia than in AD-MCI and larger in AD-MCI than in AD-asymptomatic.
Figure 3 summarizes the baseline values and slopes according to clinical stage for CSF Aß1-42, CSF tau, FDG-PET, hippocampal volume and ADAS-Cog score.
3.6 Differences between controls, MCI-other and dementia-other
Baseline scores
Hippocampal atrophy and whole brain volume were more severe in MCI-other and dementia-other subjects compared to controls. Ventricular enlargement was significantly more abnormal in MCI-other subjects compared to controls and FDG uptake on PET more abnormal in demented-other subjects compared to controls and MCI-other subjects. Cognitive performance differed between the groups with worst performance in the demented group, as expected.
Rate of decline
CSF tau declined faster in controls and hippocampal volume less compared to MCI-other subjects. Other differences were not statistically significant or could not be tested (table 2).
4. Discussion
We found that CSF, imaging and cognitive markers show different rates of decline in subjects with AD-asymptomatic, AD-MCI and AD-dementia. The pattern of decline was distinct from that of subject without amyloid pathology.
Our observation that subjects in the AD-asymptomatic stage had abnormal CSF tau is in line with previous studies [20, 21]. Ventricular volume was abnormal in AD-asymptomatic subjects, indicating that this is a sensitive measure [22]. The finding of normal hippocampal volume, whole brain volume and FDG PET in AD-asymptomatic, is in line with previous studies [23, 24]. Other studies, however, reported cortical thinning in several cortical regions [25], and reduced whole brain and hippocampal volume [26, 27]. These discrepancies may be explained by differences in subject selection or image analysis techniques. All imaging measures showed decline at follow-up but only the increase of ventricular volume exceeded that of the control group. This finding is consistent with earlier studies [23, 28] and supports the observation that change in ventricular volume is better correlated with amyloid pathology in cognitively normal subjects than change in brain volume and hippocampal volume [22]. Subjects with AD-asymptomatic had impairments on the ADAS-Cog and executive functioning relative to controls while only the CDR-SOB declined at follow-up. Previous studies yielded conflicting results with some studies showing a relation between amyloid pathology and impairments or decline in memory, executive function, or global function, while others did not [27, 29-32]. These differences might, again, be explained by differences in tests used and in subject selection.
Subjects with AD-MCI differed at baseline from controls on all markers and from MCI-other on all markers, except whole brain volume. This finding, together with other studies, indicates that cross-sectionally measured whole brain volume, is not specific or sensitive for early AD in MCI subjects [33-35]. AD-MCI subjects declined more than MCI-other on CSF tau, imaging and cognitive markers, illustrating that AD pathology drives neurodegeneration in these subjects.
In AD-dementia subjects baseline cognitive scores and biomarkers did not differ from the dementia-other group, except for the CSF measures and ventricular volume. Over time, AD-demented subjects showed the same rate of decline as dementia-other subjects on CSF, MRI (except for ventricular volume) and cognitive markers, although the interpretation of these findings is limited by the small sample size of the dementia-other group.
We summarized the trajectory of change on 5 key markers for AD in figure 3 to make a comparison with previous modelling studies that hypothesized trajectories for these markers [32, 36]. As regards the rate of order of decline, our findings support the assumption that CSF Aß1-42 declines first, followed by tau, which is followed by the other markers. Unlike the proposed models, hippocampal volume, FDG PET and ADAS-Cog declined simultaneously in our analysis. As regards the form of the curves, our findings support the proposed flattening of the curves of Aß1-42 and tau in the AD-asymptomatic or AD-MCI stage. It also suggests that impairments on the imaging and cognitive markers will continue to increase in more advanced stages, as we did not observe flattening of these markers in the dementia stage. Because we used z-scores relative to controls rather than relative to end-stage dementia, we could also compare the severity of the impairments on each marker. We found that in the dementia stage impairment for CSF Aß1-42, FDG-PET, hippocampal volume and ADAS-Cog were similar and more severe than for tau. This would suggest that CSF tau levels reach a balance between tau release and tau metabolism, despite increasing neuronal cell death [37-39]. However, there are also other explanations such as the variability of the SD between measures which affected z-scores (see below), the possibility that tau is also abnormal in controls [40-42] or selective dropout of subjects with high tau, although this then would also apply to the other injury markers.
As regards the markers that were not taken into account in the summarized figure, whole brain volume followed the same pattern as FDG PET and hippocampal volume. Ventricular volume, was already abnormal in the asymptomatic stage. Besides ADAS–Cog, executive function was impaired in AD-asymptomatic but decline was observed only for the CDR-SOB. In the MCI and AD stage all markers were abnormal and showed further decline, and the rate of decline further increased in the dementia stage.
The APOE-ε4 allele distribution was lowest in AD-asymptomatic and highest in AD-dementia. Since the APOE-ε4 allele is strongly associated with age of onset, the difference in APOE-ε4 carriership between the AD stages could explain why subjects in each stage had a similar age despite differences in disease severity.
Although the control group did not have amyloid pathology they still showed decline on the biomarkers and the CDR-SOB. This decline may result from normal aging, no-AD related neurodegeneration, or very early stage AD. Post-hoc analyses, however, made it less likely that in controls decline was driven by latent AD pathology because decline on the cognitive and biomarkers was very similar between subjects with a ‘low-normal’ CSF Aß1-42 (CSF Aß1-142 193- 250 pg/ml) and ‘high-normal’ CSF Aß1-42 levels (CSF Aß1-42 > 250 pg/ml).
Subjects with MCI-other had normal CSF Aß1-42 and tau at baseline and did not change over time in these measures. Relative to the control group, MCI-other subjects only showed increased decline in hippocampal volume, while change in other imaging markers was comparable to that of controls. Cognition was remarkably stable in MCI-other subjects suggesting a relatively benign underlying process [23, 31, 43-45].
Our data contained very few subjects with a clinical diagnosis of AD with normal CSF Aß, which were labelled as dementia-other. They were older and had less abnormal CSF tau levels than AD-dementia subjects. They were all APOE-ε4 negative and had CSF tau levels marginally increased compared to controls. These findings suggest non-AD pathology, but further studies with a larger sample size are needed to confirm this.
Our analyses expand those reported from other ADNI studies and other cohorts in several ways. We stratified clinical groups according to amyloid status, tested simultaneously a wide range of biomarkers and clinical markers and presented follow-up data for up to 4 years [32, 38, 46-50]. This enabled us to study trajectories of different markers in different AD stages and relative to amyloid negative subjects. We used z-scores, relative to control subjects, which enabled us to compare scores between different diagnostic groups and also between markers despite different units of measurements. A limitation of z-scores for comparison across different tests, however, is that the standard deviation (SD) could vary between different markers, which may influence the absolute z-scores. Variability in SD may be caused by biological variability, test characteristics and selection of subjects. For example, the CDR was used to define normal cognition, which resulted in a small SD in controls and large z-scores for diseased subjects. Still, when we repeated all analyses with raw scores similar results were obtained.
A possible limitation of our study is that our cognitive markers might not be sensitive enough to find abnormalities in the AD-asymptomatic stage although the tests used are well known and typically used in trials. FDG-PET was performed in only 50% of the subjects. This reduced statistical power to find changes compared to the other markers tested. Our subjects were relatively old and since rate of decline may depend on age, this might have resulted in an underestimation of decline in biomarkers and cognition and our findings may not apply to younger subjects [41]. We selected subjects with different AD stages cross-sectionally. Although our findings suggest a continuum between the stages (figure 3) findings need to be replicated in studies that follow subjects with asymptomatic AD until the dementia stage. A number of our findings were not statistically significant after correction for multiple testing.
Our study provided further evidence for a temporal evolution of AD. Our findings might be helpful to determine which marker can be used in each clinical stage of AD, for inclusion or outcome measure in clinical trials. For instance in AD-asymptomatic individuals, CSF Aß1-42, ventricular volume on MRI and CDR-SOB appear to be candidate outcome markers.
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129 and K01 AG030514. Data analysis was supported by the EU/EFPIA Innovative Medicines Initiative Joint Undertaking (EMIF grant no115372, DB and PJV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
D. Bertens, D.L Knol and PJ Visser report no disclosures.
P. Scheltens serves/has served on the advisory boards of Genentech, Novartis, Roche, Danone, Nutricia, Baxter, and Lundbeck. He has been a speaker at symposia organized by Lundbeck, Merz, Danone, Novartis, Roche, and Genentech. For all his activities he receives no personal compensation. He is a member of the scientific advisory board of the EU Joint Programming Initiative and the French National Plan Alzheimer. The Alzheimer Center receives unrestricted funding from various sources through the VUMC Fonds
References
- 1.Jack CR, Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 3.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visser PJ, Vos S, van Rossum I, Scheltens P. Comparison of International Working Group criteria and National Institute on Aging-Alzheimer's Association criteria for Alzheimer's disease. Alzheimers Dement. 2012;8:560–563. doi: 10.1016/j.jalz.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Berg L. Clinical Dementia Rating (CDR). Psychopharmacol Bull. 1988;24:637–639. [PubMed] [Google Scholar]
- 6.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 7.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 8.Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, et al. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav. 2012;6:502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, et al. A composite score for executive functioning, validated in Alzheimer's Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6:517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 11.Hachinski VC, Iliff LD, Zilhka E, Du Boulay GH, McAllister VL, Marshall J, et al. Cerebral blood flow in dementia. Archives of Neurology. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 12.Sheikh J, Yesavage J. Clinical Gerontology: A Guide to Assessment and Intervention. The Haworth Press; New York: 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. pp. 165–173. [Google Scholar]
- 13.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans Med Imaging. 1997;16:623–629. doi: 10.1109/42.640753. [DOI] [PubMed] [Google Scholar]
- 15.Leung KK, Clarkson MJ, Bartlett JW, Clegg S, Jack CR, Jr., Weiner MW, et al. Robust atrophy rate measurement in Alzheimer's disease using multi-site serial MRI: tissue-specific intensity normalization and parameter selection. Neuroimage. 2010;50:516–523. doi: 10.1016/j.neuroimage.2009.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 17.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamini YHY. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57:289–300. [Google Scholar]
- 20.Desikan RS, McEvoy LK, Thompson WK, Holland D, Brewer JB, Aisen PS, et al. Amyloid-beta--associated clinical decline occurs only in the presence of elevated P-tau. Arch Neurol. 2012;69:709–713. doi: 10.1001/archneurol.2011.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erten-Lyons D, Dodge HH, Woltjer R, Silbert LC, Howieson DB, Kramer P, et al. Neuropathologic basis of age-associated brain atrophy. JAMA Neurol. 2013;70:616–622. doi: 10.1001/jamaneurol.2013.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, et al. Brain atrophy in healthy aging is related to CSF levels of Abeta1-42. Cereb Cortex. 2010;20:2069–2079. doi: 10.1093/cercor/bhp279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack CR, Jr., Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, et al. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, et al. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, et al. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schott JM, Bartlett JW, Fox NC, Barnes J. Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Abeta1-42. Ann Neurol. 2010;68:825–834. doi: 10.1002/ana.22315. [DOI] [PubMed] [Google Scholar]
- 29.Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewers M, Insel P, Jagust WJ, Shaw L, Trojanowski JQ, Aisen P, et al. CSF biomarker and PIB-PET-derived beta-amyloid signature predicts metabolic, gray matter, and cognitive changes in nondemented subjects. Cereb Cortex. 2012;22:1993–2004. doi: 10.1093/cercor/bhr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 33.Jack CR, Jr., Shiung MM, Weigand SD, O'Brien PC, Gunter JL, Boeve BF, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sluimer JD, van der Flier WM, Karas GB, Fox NC, Scheltens P, Barkhof F, et al. Whole-brain atrophy rate and cognitive decline: longitudinal MR study of memory clinic patients. Radiology. 2008;248:590–598. doi: 10.1148/radiol.2482070938. [DOI] [PubMed] [Google Scholar]
- 35.Evans MC, Barnes J, Nielsen C, Kim LG, Clegg SL, Blair M, et al. Volume changes in Alzheimer's disease and mild cognitive impairment: cognitive associations. Eur Radiol. 2010;20:674–682. doi: 10.1007/s00330-009-1581-5. [DOI] [PubMed] [Google Scholar]
- 36.Jack CR, Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andreasen N, Hesse C, Davidsson P, Minthon L, Wallin A, Winblad B, et al. Cerebrospinal fluid beta-amyloid(1-42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999;56:673–680. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- 38.Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Trojanowski JQ, Shaw LM, et al. Serial MRI and CSF biomarkers in normal aging, MCI, and AD. Neurology. 2010;75:143–151. doi: 10.1212/WNL.0b013e3181e7ca82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattsson N, Portelius E, Rolstad S, Gustavsson M, Andreasson U, Stridsberg M, et al. Longitudinal cerebrospinal fluid biomarkers over four years in mild cognitive impairment. J Alzheimers Dis. 2012;30:767–778. doi: 10.3233/JAD-2012-120019. [DOI] [PubMed] [Google Scholar]
- 40.Burger nee Buch K, Padberg F, Nolde T, Teipel SJ, Stubner S, Haslinger A, et al. Cerebrospinal fluid tau protein shows a better discrimination in young old (<70 years) than in old old patients with Alzheimer's disease compared with controls. Neurosci Lett. 1999;277:21–24. doi: 10.1016/s0304-3940(99)00845-9. [DOI] [PubMed] [Google Scholar]
- 41.Holland D, Desikan RS, Dale AM, McEvoy LK. Rates of decline in Alzheimer disease decrease with age. PLoS One. 2012;7:e42325. doi: 10.1371/journal.pone.0042325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattsson N, Rosen E, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. Age and diagnostic performance of Alzheimer disease CSF biomarkers. Neurology. 2012;78:468–476. doi: 10.1212/WNL.0b013e3182477eed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Rossum IA, Vos SJ, Burns L, Knol DL, Scheltens P, Soininen H, et al. Injury markers predict time to dementia in subjects with MCI and amyloid pathology. Neurology. 2012;79:1809–1816. doi: 10.1212/WNL.0b013e3182704056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vos S, van Rossum I, Burns L, Knol D, Scheltens P, Soininen H, et al. Test sequence of CSF and MRI biomarkers for prediction of AD in subjects with MCI. Neurobiol Aging. 2012;33:2272–2281. doi: 10.1016/j.neurobiolaging.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Fagan AM, Shah AR, Beg MF, Csernansky JG, Morris JC, et al. Cerebrospinal fluid proteins predict longitudinal hippocampal degeneration in early-stage dementia of the Alzheimer type. Alzheimer Dis Assoc Disord. 2012;26:314–321. doi: 10.1097/WAD.0b013e31823c0cf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caroli A, Frisoni GB. The dynamics of Alzheimer's disease biomarkers in the Alzheimer's Disease Neuroimaging Initiative cohort. Neurobiol Aging. 2010;31:1263–1274. doi: 10.1016/j.neurobiolaging.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nettiksimmons J, Harvey D, Brewer J, Carmichael O, DeCarli C, Jack CR, Jr, et al. Subtypes based on cerebrospinal fluid and magnetic resonance imaging markers in normal elderly predict cognitive decline. Neurobiol Aging. 2010;31:1419–1428. doi: 10.1016/j.neurobiolaging.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jack CR, Jr., Vemuri P, Wiste HJ, Weigand SD, Aisen PS, Trojanowski JQ, et al. Evidence for ordering of Alzheimer disease biomarkers. Arch Neurol. 2011;68:1526–1535. doi: 10.1001/archneurol.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo RY, Hubbard AE, Shaw LM, Trojanowski JQ, Petersen RC, Aisen PS, et al. Longitudinal change of biomarkers in cognitive decline. Arch Neurol. 2011;68:1257–1266. doi: 10.1001/archneurol.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jack CR, Jr., Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Lowe V, et al. Shapes of the trajectories of 5 major biomarkers of Alzheimer disease. Arch Neurol. 2012;69:856–867. doi: 10.1001/archneurol.2011.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]