Abstract
Objective
The number of patients with hepatitis C virus (HCV)-related cirrhosis is increasing, leading to a rising risk of complications and death. Prognostic stratification in patients with early-stage cirrhosis is still challenging. We aimed to develop and validate a clinically useful prognostic index based on genomic and clinical variables to identify patients at high risk of disease progression.
Design
We developed a prognostic index, comprised of a 186-gene signature validated in our previous genome-wide profiling study, bilirubin (>1mg/dL), and platelet count (<100,000/mm3), in an Italian HCV cirrhosis cohort (training cohort, n=216, median follow-up 10 years). The gene signature test was implemented utilizing a digital transcript counting (nCounter) assay specifically developed for clinical use, and the prognostic index was evaluated using archived specimens from an independent cohort of HCV-related cirrhosis in the U.S. (validation cohort, n=145, median follow-up 8 years).
Results
In the training cohort, the prognostic index was associated with hepatic decompensation (HR=2.71, p=0.003), overall death (HR=6.00, p<0.001), hepatocellular carcinoma (HR=3.31, p=0.001), and progression of Child-Turcotte-Pugh class (HR=6.70, p<0.001). The patients in the validation cohort were stratified into high (16%), intermediate (42%), or low (42%) risk group by the prognostic index. The high-risk group had a significantly increased risk of hepatic decompensation (HR=7.36, p<0.001), overall death (HR=3.57, p=0.002), liver-related death (HR=6.49, p<0.001), and all liver-related adverse events (HR=4.98, p<0.001).
Conclusion
A genomic and clinical prognostic index readily available for clinical use was successfully validated, warranting further clinical evaluation for prognostic prediction, and clinical trial stratification and enrichment for preventive interventions.
Keywords: hepatitis C, cirrhosis, gene expression
INTRODUCTION
Liver cirrhosis affects 1% to 2% of the world’s population, and leads to more than one million deaths every year worldwide.1,2 Chronic hepatitis C virus (HCV) infection is one of the major etiologies of cirrhosis in developed countries. More than one million individuals in the U.S., representing the "baby boomer" population, are projected to develop HCV-related cirrhosis, hepatic decompensation, or hepatocellular carcinoma (HCC) by 2020.3,4 HCV superseded HIV as a cause of death by 2007, and costs for patient management are estimated to reach $8.6 billion by 2015 (excluding drug costs) in the U.S. and increase by 60% by 2032 in Canada.4–6 Despite the emergence of direct-acting anti-viral agents, eradication of HCV reduces but does not eliminate the risk of the lethal complications of cirrhosis, especially when more advanced fibrosis is present.7 In addition, cost of the direct-acting anti-virals will limit their wider use to prevent disease progression.8,9
Cirrhosis is the major driver in the development of HCC, the second leading cause of cancer mortality worldwide and the fastest and only rising cancer death in the U.S.10,11 The extremely high HCC incidence in HCV-related cirrhosis (up to 7% per year) justifies regular HCC surveillance in the practice guidelines.12,13 However, only 12% of new HCV-related HCC cases are diagnosed through surveillance in the U.S., clearly indicating that current medical resources are challenged by this sizable patient population, and underscoring the urgent need for prognostic biomarkers to identify a subset of cirrhosis patients who most require surveillance and close follow-up.14 Recent emergence of non-invasive fibrosis assessment tests such as elastography may actually increase the burden of close follow-up and cancer screening by bringing a larger number of early-stage, asymptomatic cirrhotics to medical attention.15 Prognostic prediction in early-stage cirrhosis, the largest group among cirrhotics, is particularly challenging because of the lack of clinical prognostic indicators. The availability of an accurate prognostic biomarker will also help identify cirrhosis patients who will benefit from preventive intervention to reduce the incidence of cirrhosis complications.16
In previous studies, we identified and validated a prognostic 186-gene signature together with prognostic clinical variables in HCV-related early-stage cirrhosis patients from Asia, Europe, and the U.S.17–20 A cost-effectiveness analysis suggested that the signature enables personalized HCC surveillance with reduced net medical care cost and extended patient life expectancy.19 For clinical application of the finding, we implemented the 186-gene signature in an FDA-approved clinical diagnostic assay platform, constructed a genomic and clinical prognostic index, and externally validated the index in an independent cohort of HCV-related, early-stage cirrhotics with long-term follow-up.
METHODS
Patient cohorts
A cohort of 216 Italian HCV-related, early-stage cirrhosis patients was used to develop a prognostic index based on a Cox regression model reported in our previous study (training cohort).19 The cohort was prospectively enrolled and followed for a median of 10 years to evaluate clinical utility of HCC surveillance.19,21,22 A subset of the training cohort (n=90) who had poor- or good- prognosis prediction and had leftover RNA samples were reanalyzed by the digital transcript counting assay (see “Gene expression profiling” for details) to compare the gene signature-based prediction with the genome-wide profiles from the previous study (NCBI Gene Expression Omnibus, accession number GSE15654).
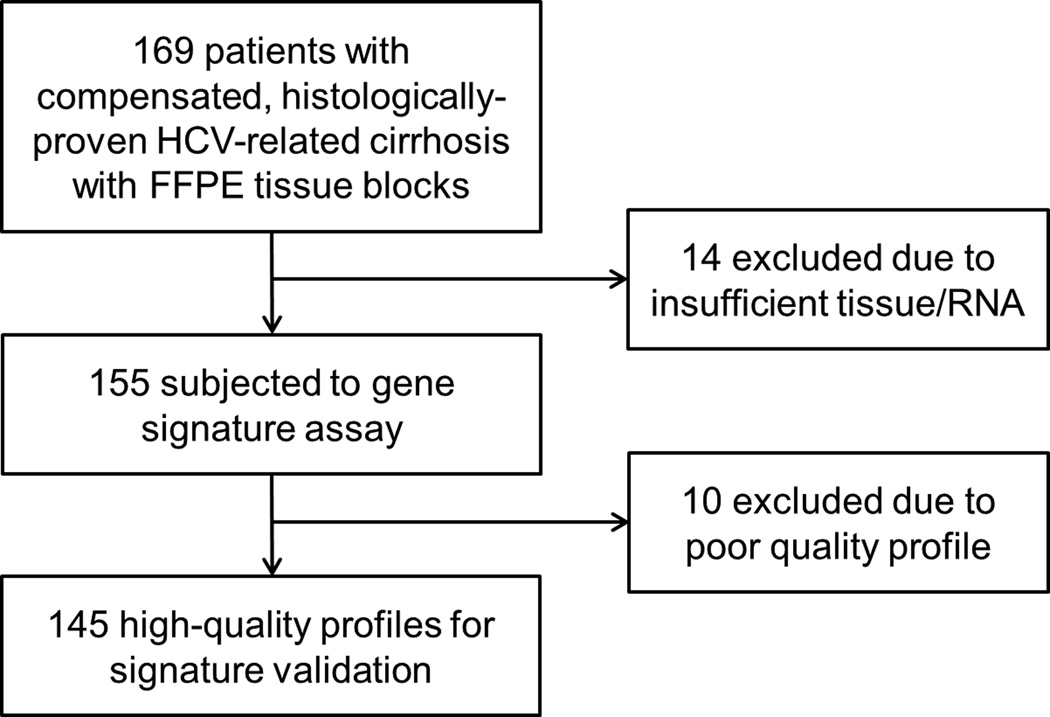
External validation of the prognostic index was performed by using archived liver biopsy specimens from an independent cohort of 145 HCV-related, compensated cirrhosis patients who had a liver biopsy between 1990 and 2007 and were followed at Massachusetts General Hospital (validation cohort). Enrolled patients were age >18 years at the time of biopsy. HCV infection was confirmed by serum HCV antibody and/or RNA. A diagnosis of cirrhosis was defined histologically as having Ishak23 fibrosis stage 5/6 or Metavir24 fibrosis stage 4. No co-infection of human immunodeficiency virus or hepatitis B virus was present. Patients with prior history of ascites, variceal bleeding, hepatic encephalopathy, HCC, and liver transplantation were excluded. One hundred sixty nine patients were identified as having formalin-fixed paraffin-embedded (FFPE) tissue blocks. Analyzed clinical endpoints include overall death (primary endpoint) development of hepatic decompensation (ascites, variceal bleeding, hepatic encephalopathy, spontaneous bacterial peritonitis, and hepatorenal syndrome), HCC, liver-related death (deaths related to complications of end-stage liver disease, HCC, hepatorenal syndrome, portopulmonary hypertension), and a composite of all liver-related adverse events (hepatic decompensation, HCC, and liver-related death). No patient was lost from follow-up for overall death. Serial biopsy specimens were available for 3 patients for longitudinal analysis. Liver tissue from right and left lobes of an explanted liver were probed in one patient to assess sampling variability. The study was approved and the requirement for written informed consent was waived by the institutional review board based on the condition that all samples were anonymous.
Tissue processing and RNA extraction
Total RNA was isolated from 3 to 5 10-µm-thick FFPE tissue sections by using High Pure RNA Paraffin kit (Roche) according to manufacturer’s instruction. RNA fragmentation was evaluated by qRT-PCR of a housekeeping gene RPL13A as previously described18 and all samples were confirmed to have crossover threshold (Ct) value <33. One hundred fifty five samples with RNA concentration > 20ng/µl were subjected to gene expression profiling with the nCounter assay.
Gene expression profiling
The 186-gene signature was implemented in the digital transcript counting (nCounter) assay (NanoString). Expression profiling was performed with 100ng to 400ng total RNA by using nCounter Digital Analyzer system (NanoString) according to manufacturer's instruction. For the analysis of the training cohort, the first generation of reagent plate (“white” Prep Plate) was used. For the validation cohort, newer version (“green” New Prep Plate) with improved sensitivity for signal detection was used. Poor quality profiles were detected based on maximum signal intensity from positive control probes <8,000U for the older reagent plate, and median signal intensity >100U for the newer reagent plate according to manufacturer’s recommendation. Raw transcript count data were log-transformed and scaled by geometric mean of control probe data by using NanoString normalizer module implemented in GenePattern genomic analysis toolkit (www.broadinstitute.org/genepattern). Genome-wide expression profiling for paired biopsies and explanted liver was performed by using whole-genome DASL assay (Illumina) according to manufacturer's instruction. Scanned data were extracted by Genome Studio software ver.3 (Illumina), and normalized by cubic spline algorithm implemented in GenePattern Illumina Normalizer module as previously described.18
Bioinformatics and statistical analysis
The 186-gene signature-based clinical outcome prediction was performed based on previously reported prediction model and algorithm without making any modification18 by using nearest template prediction algorithm25 implemented in GenePattern. A prediction of poor or good outcome was determined based on prediction p<0.05, and the rest of the samples with intermediate expression level of the poor or good prognosis-correlated genes in the signature were classified as having intermediate prognosis as previously reported.19 Reduction of signature genes was performed based on a series of cut-offs of absolute Cox scores calculated in the original training dataset (DASL)18 as well as recalculated Cox scores in the nCounter data of the training cohort. Gene expression datasets are available at NCBI Gene Expression Omnibus database (accession number GSE54102). All bioinformatics analyses were performed by using GenePattern and R statistical language (www.r-project.org).
For the validation cohort, the date of the liver biopsy documenting HCV-related cirrhosis was defined as the time of enrollment. Prognostic association of the gene signature-based prediction with clinical outcome was evaluated by Kaplan-Meier curves, log-rank test, and Cox regression modeling. In the analysis of liver-related death, competing risk was additionally adjusted for non-liver-related death by using proportional subdistribution hazards regression modeling,26 Clinical outcomes that occurred in more than 20% of the cohort were analyzed in the Cox regression analysis to achieve a statistical power of 0.8 to detect hazard ratio of 3.0 (alpha=0.05). Annual incidence rate for each clinical outcome was calculated using Declining Exponential Approximation of Life Expectancy (DEALE)27 based on cumulative 5-year incidence. A prognostic index was calculated using the following formula based on our previously reported multivariable Cox regression model19: 0.848 × gene-signature-based prediction of poor prognosis (0: no, 1: yes) + 0.998 × serum bilirubin (0: ≤ 1.0 mg/dL, 1: > 1.0 mg/dL) + 0.905 × platelet count (0: ≥ 100,000/mm3, 1: < 100,000/mm3). The tertile of the score in the training cohort (0.848, 1.846) was used as the cut-off values to classify the patients into high, intermediate, and low risk groups. A subgroup analysis within Child-Turcotte-Pugh class A patients was performed to evaluate robustness of outcome association for the prognostic index. Correction for multiple hypothesis testing was performed using Bonferroni’s method when needed. A two-tailed p-value <0.05 was regarded as statistically significant. R statistical language and SAS (SAS Institute, Cary, NC) version 9.3 were used for statistical analyses.
RESULTS
Technical evaluation of 186-gene-signature assay
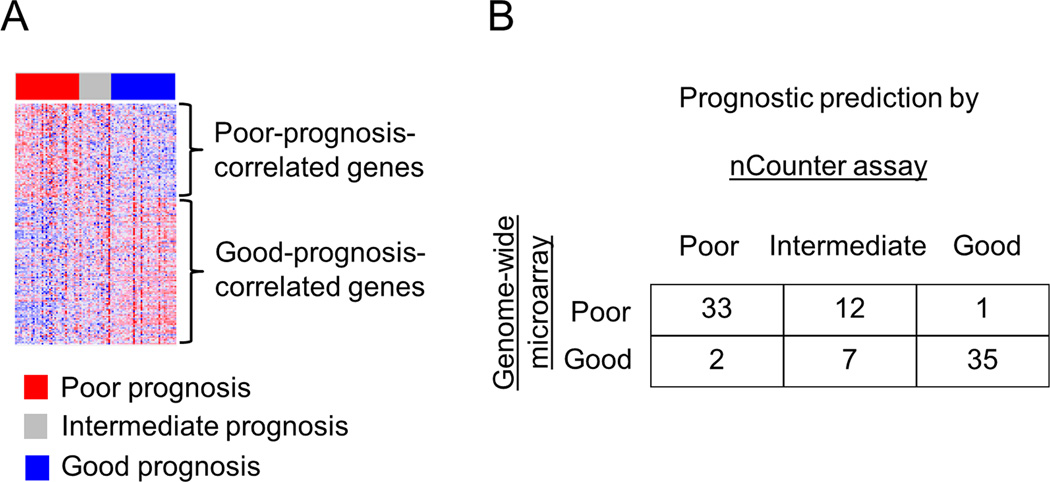
To assess the prognostic index in a clinically relevant setting, we implemented the 186-gene signature in an FDA-approved clinical diagnostic assay platform, the nCounter system, and we re-assayed a subset of the training cohort previously analyzed by genome-wide DNA microarray.19 Among the 106 samples with leftover RNA that were re-assayed by the nCounter assay, 90 (85%) passed the predetermined quality threshold. Prognostic prediction was performed by applying the same prediction model and algorithm reported in the original studies18 without making any modification. Thirty five (39%), 19 (21%), and 36 (40%) samples presented poor-, intermediate-, and good-prognosis signatures, respectively (Figure 1A). Inconsistent poor or good prediction between the nCounter assay and genome-wide microarray was observed in only 3 samples (3%) (Figure 1B). Stability of the gene signature-based prediction in longitudinal and multi-site liver sampling was assessed in paired core needle liver biopsy specimens obtained with the time interval ranging from 1 month to 7.5 years from three individuals, and liver tissues from right and left lobes of explanted liver with chronic HCV infection. Histologically, necroinflammatory grade and fibrosis stage23 were comparable within each pair. There was no instance of inconsistent poor or good prediction between the paired samples (Supplementary table 1). Changes of prediction were observed only between poor/good and intermediate prognosis, which may reflect subtle changes in molecular status of the liver unrecognizable by histological assessment.
Figure 1.
Technical assessment of the 186-gene signature nCounter assay in a subset of the training cohort (n=90). (A) Expression pattern of the 186-gene signature. Red and blue colors indicate high and low gene expression, respectively. (B) Prediction concordance between the nCounter assay and genome-wide microarray used in our previous study.19
Prognostic index in the training cohort
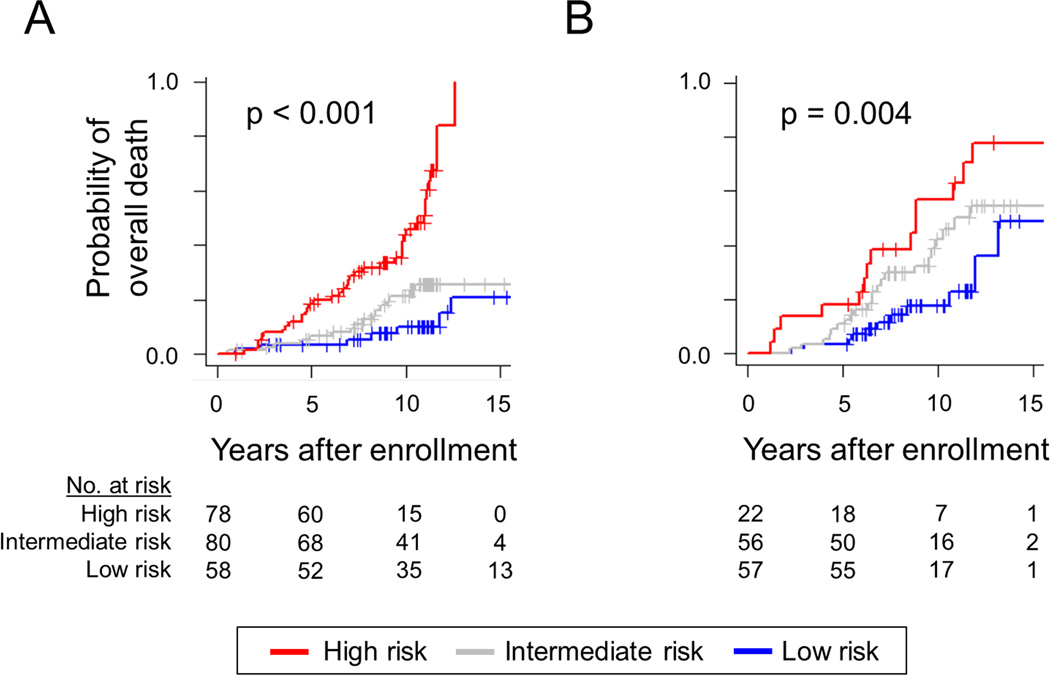
The prognostic index, developed using the entire training cohort (n=216), classified patients in the training cohort into high (n=78, 36%), intermediate (n=80, 37%), and low (n=58, 27%) risk groups. The high risk group showed significantly more frequent development of hepatic decompensation (hazard ratio [HR]=2.71, p=0.003), overall death (HR=6.00, p<0.001), HCC development (HR=3.31, p=0.001), and progression of Child-Turcotte-Pugh class (HR=6.70, p<0.001) (Supplementary table 2). The probabilities of overall death at 5- and 10-years were 19% and 44% in high risk group, 7% and 21% in intermediate risk group, and 3% and 10% in low risk group, respectively. Clinical demographics of the training cohort were reported in our previous study19 and summarized in Supplementary table 3.
Validation of the prognostic index
We next assessed the prognostic index in an external independent cohort of HCV-related compensated cirrhosis patients in the U.S. using the latest version of nCounter assay. One hundred fifty five samples with sufficient tissue to isolate more than 100ng total RNA were subjected to the nCounter assay, among which 145 samples (94%) yielded high-quality profiles (Figure 2). Table 1 summarizes clinical demographics of the 145 patients. Compared to retrospective or prospective cohorts of HCV-related compensated cirrhosis in literature and the training cohort in the current study, patients in the validation cohort were approximately 10 years younger and more predominantly male (Supplementary table 3). The median follow-up time was 8.0 years (IQR: 6.3 to 11.1 years, range: 1.2 to 22.9 years). Forty-five patients (31%) developed at least one episode of hepatic decompensation. The first decompensation events were as follows: ascites, n=19; variceal hemorrhage n=11; hepatic encephalopathy, n=8; ascites and hepatic encephalopathy, n=4; ascites and variceal hemorrhage, n=1, variceal hemorrhage and hepatic encephalopathy, n=1; and ascites, variceal hemorrhage, and hepatic encephalopathy, n=1. The annual incidence rate of hepatic decompensation (3.9%) was comparable, whereas annual incidence rates of death (1.7%) and HCC development (1.3%) were lower than reported in the published cohorts and the training cohort of the current study, possibly due to the younger age. HCC was analyzed together with all liver-related adverse events because only 21 patients (14%) had HCC during the follow-up presumably due to the younger age. The probabilities of hepatic decompensation at 5- and 10-years were 18% and 37%, respectively (Supplementary figure 1). The probabilities of overall death at 5- and 10-years were 8% and 35%, respectively, and the probabilities of liver-related death at 5- and 10-years were 7% and 27%, respectively. The probabilities of all liver-related adverse events at 5- and 10-years were 24% and 51%, respectively.
Figure 2.
The validation cohort used to assess the prognostic index.
Table 1.
Clinical characteristics of the validation cohort at enrollment.
| All patients (n=145) | |
|---|---|
| Age (y), median (IQR) | 49 (45–55) |
| Male, n, (%) | 107, (74) |
| Race, n, (%) | |
| White | 123, (85) |
| Black | 8, (6) |
| Hispanic | 10, (7) |
| Other | 4, (3) |
| Smoking history, n, (%) | 92, (69) |
| Diabetes, n, (%) | 20, (14) |
| HCV RNA >500,000 IU/ml, n, (%) | 70, (67) |
| HCV genotype 1, n, (%) | 93, (72) |
| Alanine aminotransferase (IU/L) , median (IQR) | 108 (64–165) |
| Aspartate aminotransferase (IU/L) , median (IQR) | 99 (67–136) |
| Albumin (g/dL), median (IQR) | 3.9 (3.4–4.2) |
| Total bilirubin (mg/dL), median (IQR) | 0.7 (0.5–1.0) |
| Creatinine (mg/dL), median (IQR) | 0.9 (0.8–1.0) |
| Platelet count (/mm3), median (IQR) | 198,000 (97,000–193,000) |
| Child-Turcotte-Pugh class, n, (%)* | |
| A | 121, (83) |
| B | 9, (6) |
IQR, interquartile range; HCV, hepatitis C virus. Some data were not available for all patients.
Child-Turcotte-Pugh score could not be calculated in 15 patients due to missing laboratory data.
Clinical characteristics of the training cohort were published in our previous study19 and summarized in Supplementary table 3.
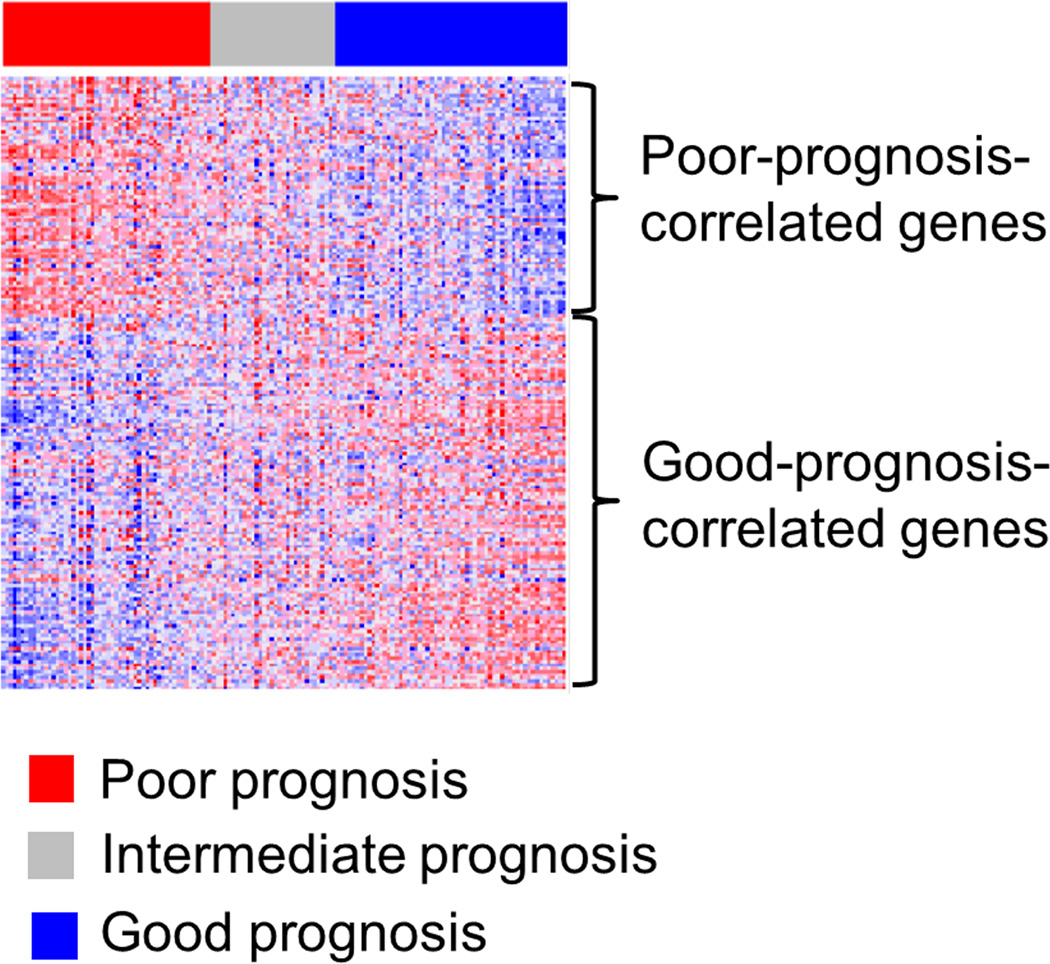
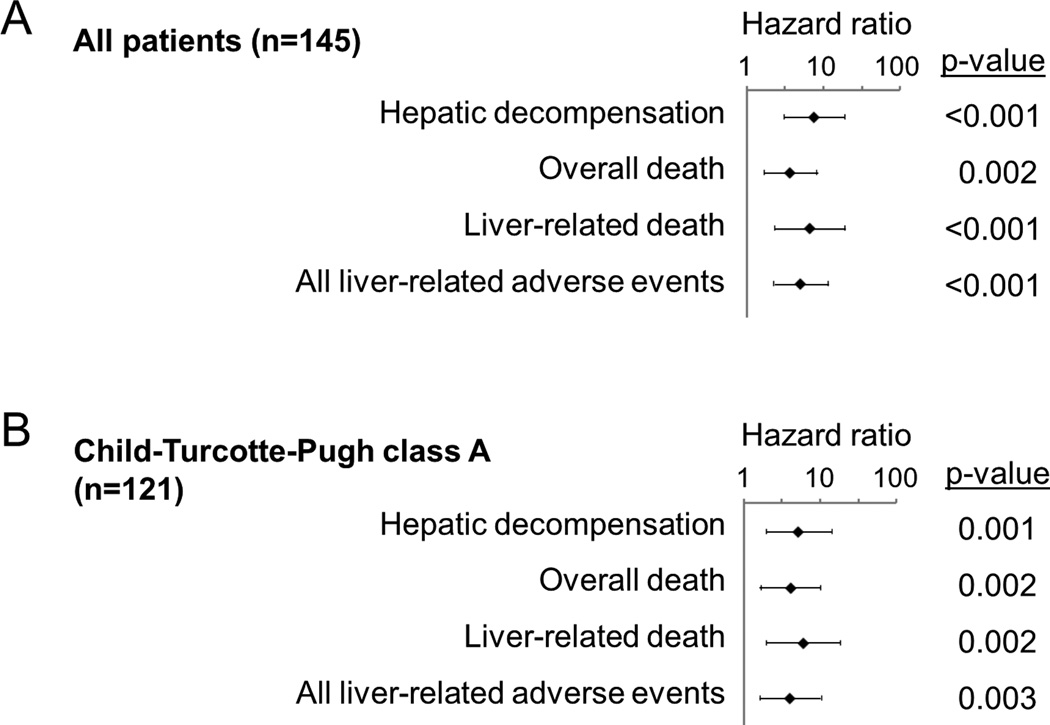
Gene signature-based prognostic prediction was performed using the nCounter assay, and 53 (37%), 32 (22%), and 60 (41%) patients had poor-, intermediate-, and good-prognosis signature, respectively (Figure 3), which was significantly associated with overall survival (p=0.02) (Supplementary figure 2). The prognostic index classified the patients in the validation cohort into high (n=22, 16%), intermediate (n=56, 42%), and low (n=57, 42%) risk groups. The high-risk group experienced significantly more frequent development of hepatic decompensation (HR=7.36, p<0.001), overall death (HR=3.57, p=0.002), liver-related death (HR=6.49, p<0.001), and all liver-related adverse events (HR=4.98, p<0.001) (Table 2, Figure 4). When competing risk was adjusted for non-liver-related death, association of the high risk group with liver-related death remained significant (HR= 3.65, 95% confidence interval=1.75–7.61, p<0.001). The probabilities of overall death at 5- and 10-years were 18% and 57% in the high risk group, 11% and 42% in the intermediate risk group, and 4% and 18% in the low risk group, respectively. The prognostic association of the prognostic index remained significant in a subgroup of Child-Turcotte–Pugh class A patients (Figure 5).
Figure 3.
Expression pattern of the 186-gene signature nCounter assay in the validation cohort. Red and blue colors indicate high and low gene expression, respectively.
Table 2.
Association of the prognostic index with clinical outcome (Cox regression, validation cohort).
| Variable | Hazard ratio (95% confidence interval) |
p-value |
|---|---|---|
| Hepatic decompensation (n=45, 31%) | ||
| Intermediate risk group | 3.43 (1.45–8.13) | 0.005 |
| High risk group | 7.36 (2.96–18.27) | <0.001 |
| Overall death (n=50, 34%) | ||
| Intermediate risk group | 2.05 (0.99–4.23) | 0.05 |
| High risk group | 3.57 (1.62–7.88) | 0.002 |
| Liver-related death (n=32, 22%) | ||
| Intermediate risk group | 2.73 (0.97–7.67) | 0.06 |
| High risk group | 6.49 (2.25–18.71) | <0.001 |
| All liver-related adverse events (n=59, 41%) | ||
| Intermediate risk group | 3.46 (1.68–7.12) | <0.001 |
| High risk group | 4.98 (2.21–11.22) | <0.001 |
Hazard ratios were computed by comparing to low risk group. The prognostic index could not be calculated in 10 patients due to missing laboratory data. Hepatic decompensation is the composite of variceal bleeding, ascites, and hepatic encephalopathy. The composite outcome incorporates hepatic decompensation, HCC, and liver-related death. Bonferroni-corrected p-values for the high risk group were <0.001, 0.01, 0.003, and 0.001 for hepatic decompensation, overall death, liver-related death, and all liver-related adverse events, respectively. When the competing risk was adjusted for non-liver-related death by using proportional subdistribution hazards regression modeling,26 the association of the high risk group with liver-related death remained significant (HR= 3.65, 95% confidence interval=1.75–7.61, p<0.001).
Figure 4.
Probability of overall death according to the prognostic index in training cohort (A) and validation cohort (B). P-values were calculated by log-rank test.
Figure 5.
Prognostic association of the prognostic index with clinical outcomes in all patients (A) and Child-Turcotte-Pugh class A patients (B). Hazard ratio and 95% confidence interval in log scale (base 10) for high risk group in comparison to low risk group is shown.
The full 186-gene signature captures markers of molecular pathway deregulation, including up-regulation of epidermal growth factor, nuclear factor kappa-B, interleukin-6, and interferon pathways, hepatic stellate cell activation, and down-regulation of metabolic and protein synthesis pathways and DNA damage repair machinery.18,19 Although some of the information may be lost, a reduction of the number of signature genes could decrease complexity of the signature, and enable its adaptation to a lower throughput assay platform and thus enable more flexible utilization of the signature in clinical practice. By using two different approaches, we found that the number of signature genes could be reduced to 32 or 11 (Supplementary figure 3, Supplementary tables 4 and 5), which were still significantly associated with overall death (Supplementary figure 4). The prognostic index based on the reduced signatures showed relatively inferior, but still statistically significant associations with all of the analyzed clinical outcomes (Supplementary tables 6 and 7), warranting further evaluation in future studies.
DISCUSSION
We have demonstrated that a prognostic index combining genomic and clinical information successfully predicts prognosis of HCV-related compensated cirrhosis patients, for which clinical prognostic information is limited. Several clinical prognostic indicators have been proposed to discriminate late-stage cirrhosis from early-stage cirrhosis28 or to discriminate progressive cirrhosis from less advanced or no fibrosis with hazard ratios smaller than 2.0.29–31 However, prognostic prediction within established but early-stage cirrhosis is more critical because this specific disease stage comprises the majority of the target patient population indicated for regular follow-up and HCC surveillance as recommended in the clinical practice guidelines. Recently emerging non-invasive blood test- or elastography-based methods of liver fibrosis detection are not sensitive enough to classify early-stage cirrhosis patients into prognostic subgroups.15,32 In addition, the increasing use of these non-invasive methods promises to increase the number of newly identified early-stage cirrhosis patients, a prospect that may tax current medical resources.14 Therefore, highly sensitive and accurate molecular prognostic biomarkers are sorely needed. Clinical deployment of a gene-expression-based molecular biomarker has been challenging because of less reproducible measurements.33 Recent development of assay platforms, which are capable of inexpensively analyzing archived FFPE tissues with minimal experimental variation, is a breakthrough that will facilitate clinical implementation of gene signature tests.34–36
Risk prediction, early detection, and prevention of lethal cirrhosis complications including hepatic decompensation and HCC has been recognized as the most effective strategy to substantially impact patient prognosis.16,37 Recent large-scale HCC chemoprevention trials have demonstrated the proof of concept for this strategy, although none of the therapies tested in the trials have been established as a standard of care due to the modest effect and/or unacceptable toxicities.38–40 These studies and others also highlight the challenge in conducting chemoprevention trials, i.e., requirement of larger sample size and longer follow-up time compared to therapeutic trials enrolling patients with advanced diseases such as end-stage cancer.41 Enrichment of high-risk populations through use of prognostic indices like ours could overcome this handicap and facilitate the implementation of chemoprevention trials.42 In a pre-clinical animal study, the 186-gene signature was correlated with anti-fibrotic and anti-carcinogenic effect of an FDA-approved kinase inhibitor, erlotinib.43 Interestingly, the study showed that the gene signature was strikingly induced before histologically recognizable accumulation of fibrosis, suggesting that the signature sensitively detected activated fibrogenic pathways in the liver. Based on the study, a clinical trial of the drug has been planned with assessment of the 186-gene signature assay as a companion diagnostic (cancerpreventionnetwork.org). The significant association of the signature with long-term clinical outcomes confirmed in multiple patient cohorts may support the use of the gene signature as a surrogate endpoint in other chemoprevention trials.
Recently emerging highly effective and less toxic direct-acting anti-HCV drugs will eventually halt the development of HCV-induced advanced fibrosis and cirrhosis and prevent the lethal complications in newly infected patients.9 However, recent epidemiological studies have suggested that patients with advanced fibrosis are still at risk of progressive disease even after viral eradication.7 Also, given the high worldwide prevalence of HCV infection already affecting 170 million individuals and anticipated obstacles in disseminating the expensive direct-acting anti-HCV drugs, it is expected that risk prediction and chemopreventive interventions will remain relevant in the coming decade and beyond.
Recent emergence of selective molecular targeted agents has highlighted the need to obtain tissue specimens for more precise characterization of the molecular targets and subsequent personalized therapeutic decisions.13,44 Circulating cells or biomolecules such as microRNAs may be alternative sources to obtain similar molecular information less invasively, although more studies are needed.45 The current validation cohort may have been limited for assessment of HCC development due to an insufficient number of clinical events. Further follow-up data from this cohort will require evaluation. We have also made the assumption that the variables in the prognostic index, the 186-gene signature, bilirubin, and platelet count, capture molecular and clinical information for progressive cirrhosis in general, and speculate that the index will also be prognostic in cirrhosis caused by other etiologies, namely chronic hepatitis B, alcohol, and non-alcoholic fatty liver diseases.46 This prospect also should be evaluated in future studies. The risk index validation was conducted in a retrospective cohort enrolled from a tertiary referral center. Therefore, the possibility of the presence of selection biases and information biases caused by variation in the quality and reliability in the outcome definition and measurement exists.
In conclusion, our novel genomic and clinical prognostic index was successfully validated for prognostic capability in HCV-related early-stage cirrhosis patients, in whom clinical prognostic indicators are limited. One of the components of the prognostic index, the gene signature, was implemented in an assay platform readily available for clinical implementation. The prognostic index has the potential to refine the intensity of follow-up and enable more cost-effective clinical trials of chemoprevention and/or anti-cirrhosis therapy by enriching for high-risk populations, which could contribute to substantial improvements in predicting a patient’s prognosis and improving outcomes, a vital need in view of the projected growth of the cirrhotic patient population and its associated medical care costs.
Supplementary Material
Summary box.
What is already known about this subject?
Hepatitis C virus (HCV)-related early-stage cirrhosis is increasing and overtaxing the medical resources for regular follow-up and hepatocellular carcinoma (HCC) surveillance as evidenced by the low application rate (only 12% of new HCC patients were diagnosed through the surveillance in the U.S.).
Eradication of HCV reduces, but does not eliminate the risk of disease progression including HCC development especially when cirrhosis is present. Therefore, prognostic prediction is critical in clinical management of this sizable patient population.
Clinical prognostic variables have demonstrated limited capability with hazard ratios (HRs) less than 2 in identifying a subset of patients who most need close follow-up for long-term clinical deterioration and mortality.
What are the new findings?
A prognostic index comprised of a gene-expression signature, serum bilirubin, and platelet count showed significant association with disease progression and death with substantially higher HRs up to 6.70 in a cohort of HCV cirrhosis patients prospectively followed for a median of 10 years.
The gene signature test was implemented in an assay platform specifically designed for clinical use.
The prognostic index based on the clinically applicable gene signature assay was successfully validated in an independent cohort of early-stage HCV cirrhosis patients for disease progression and death with HRs as high as 7.36.
How might it impact on clinical practice in the forseeable future?
The prognostic index will help prioritize a subset of early-stage HCV cirrhosis patients for regular follow-up and HCC surveillance.
The prognostic index can be used for clinical trial stratification and enrichment for preventive intervention with anti-cirrhosis and/or anti-carcinogenic therapies.
ACKNOWLEDGEMENTS
The nCounter assay was performed at Mount Sinai qPCR Shared Resource Facility. Bioinformatics analysis was performed by using High Power Computing facility at Mount Sinai Genomics Core and Department of Scientific Computing.
FINANCIAL SUPPORT
This research was supported by the National Institute of Health (DK099558 to Y.H.; DA033541, DK098079, DK078772 to R.T.C.; DK56621 and AA020709 to S.L.F.; DK076986 to J.M.L.; DK007191 to L.K.Y. and K.B.J.); the European Commission Framework Programme 7 (Heptromic, proposal number 259744 to Y.H., J.M.L.); the Samuel Waxman Cancer Research Foundation, the Spanish National Health Institute (SAF-2010-16055), and the Asociación Española para el Estudio del Cáncer (AECC) to J.M.L.
Abbreviations
- HCV
hepatitis C virus
- HCC
hepatocellular carcinoma
- FFPE
formalin-fixed paraffin-embedded
- DEALE
Declining Exponential Approximation of Life Expectancy
- IQR
inter-quartile range
- HR
hazard ratio
Footnotes
COMPETING INTEREST
Y.H., A.V., and J.M.L. are named investigators on a pending patent application entitled “Compositions, kits, and methods for detecting, characterizing, preventing, and treating hepatic disorders (USPTO application #: #20110263441)". NanoString, Inc. has secured the option to an exclusive worldwide license. NanoString has no role in conduction of the current study. There is no other relevant declaration relating to employment, consultancy, patents, products in development or modified products etc.
REFERENCES
- 1.Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7(8):425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson IM, Davis GL, El-Serag H, et al. Prevalence and challenges of liver diseases in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2010;8(11):924–933. doi: 10.1016/j.cgh.2010.06.032. quiz e117. [DOI] [PubMed] [Google Scholar]
- 4.Zalesak M, Francis K, Gedeon A, et al. Current and future disease progression of the chronic HCV population in the United States. PLoS One. 2013;8(5):e63959. doi: 10.1371/journal.pone.0063959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ly KN, Xing J, Klevens RM, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 6.Myers RP, Krajden M, Bilodeau M, et al. Burden of disease and cost of chronic hepatitis C infection in Canada. Canadian journal of gastroenterology & hepatology. 2014;28(5):243–250. doi: 10.1155/2014/317623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. Jama. 2012;308(24):2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Singh PP, Roberts LR, et al. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014;11:45–54. doi: 10.1038/nrgastro.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung RT, Baumert TF. Curing chronic hepatitis C--the arc of a medical triumph. N Engl J Med. 2014;370(17):1576–1578. doi: 10.1056/NEJMp1400986. [DOI] [PubMed] [Google Scholar]
- 10.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 11.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EASL-EORTC. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 15.Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther. 2009;30(6):557–576. doi: 10.1111/j.1365-2036.2009.04062.x. [DOI] [PubMed] [Google Scholar]
- 16.Hoshida Y, Fuchs BC, Tanabe KK. Prevention of hepatocellular carcinoma: potential targets, experimental models, and clinical challenges. Curr Cancer Drug Targets. 2012;12(9):1129–1159. [PMC free article] [PubMed] [Google Scholar]
- 17.Nault JC, De Reynies A, Villanueva A, et al. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology. 2013;145(1):176–187. doi: 10.1053/j.gastro.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 18.Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359(19):1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshida Y, Villanueva A, Sangiovanni A, et al. Prognostic Gene-Expression Signature for Patients with Hepatitis C-Related Early-Stage Cirrhosis. Gastroenterology. 2013;144(5):1024–1030. doi: 10.1053/j.gastro.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villanueva A, Hoshida Y, Battiston C, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology. 2011;140(5):1501–1512. doi: 10.1053/j.gastro.2011.02.006. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colombo M, de Franchis R, Del Ninno E, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325(10):675–680. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 22.Sangiovanni A, Del Ninno E, Fasani P, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126(4):1005–1014. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 23.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 24.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20(1 Pt 1):15–20. [PubMed] [Google Scholar]
- 25.Hoshida Y. Nearest Template Prediction: A Single-Sample-Based Flexible Class Prediction with Confidence Assessment. PLoS One. 2010;5(11):e15543. doi: 10.1371/journal.pone.0015543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 27.Beck JR, Pauker SG, Gottlieb JE, et al. A convenient approximation of life expectancy (the "DEALE"). II. Use in medical decision-making. Am J Med. 1982;73(6):889–897. doi: 10.1016/0002-9343(82)90787-2. [DOI] [PubMed] [Google Scholar]
- 28.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 29.Singal AG, Mukherjee A, Elmunzer BJ, et al. Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am J Gastroenterol. 2013;108(11):1723–1730. doi: 10.1038/ajg.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Serag HB, Kanwal F, Davila JA, et al. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology. 2014;146(5):1249–1255. doi: 10.1053/j.gastro.2014.01.045. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Meer AJ, Hansen BE, Fattovich G, et al. Reliable prediction of clinical outcome in patients with chronic HCV infection and compensated advanced hepatic fibrosis: a validated model using objective and readily available clinical parameters. Gut. 2014 doi: 10.1136/gutjnl-2013-305357. [DOI] [PubMed] [Google Scholar]
- 32.Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158(11):807–820. doi: 10.7326/0003-4819-158-11-201306040-00005. [DOI] [PubMed] [Google Scholar]
- 33.Koscielny S. Why most gene expression signatures of tumors have not been useful in the clinic. Sci Transl Med. 2010;2(14) doi: 10.1126/scitranslmed.3000313. 14ps2. [DOI] [PubMed] [Google Scholar]
- 34.Reis PP, Waldron L, Goswami RS, et al. mRNA transcript quantification in archival samples using multiplexed, color-coded probes. BMC Biotechnol. 2011;11:46. doi: 10.1186/1472-6750-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Northcott PA, Shih DJ, Remke M, et al. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta neuropathologica. 2012;123(4):615–626. doi: 10.1007/s00401-011-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kojima K, April C, Canasto-Chibuque C, et al. Transcriptome profiling of archived sectioned formalin-fixed paraffin-embedded (AS-FFPE) tissue for disease classification. PLoS One. 2014;9(1):e86961. doi: 10.1371/journal.pone.0086961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science. 2013;339(6127):1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lok AS, Everhart JE, Wright EC, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology. 2011;140(3):840–849. doi: 10.1053/j.gastro.2010.11.050. quiz e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruix J, Poynard T, Colombo M, et al. Maintenance therapy with peginterferon alfa-2b does not prevent hepatocellular carcinoma in cirrhotic patients with chronic hepatitis C. Gastroenterology. 2011;140(7):1990–1999. doi: 10.1053/j.gastro.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Okita K, Izumi N, Matsui O, et al. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J Gastroenterol. 2014 doi: 10.1007/s00535-014-0956-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simon R. The use of genomics in clinical trial design. Clin Cancer Res. 2008;14(19):5984–5993. doi: 10.1158/1078-0432.CCR-07-4531. [DOI] [PubMed] [Google Scholar]
- 43.Fuchs BC, Hoshida Y, Fujii T, et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59(4):1577–1590. doi: 10.1002/hep.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garraway LA. Genomics-driven oncology: framework for an emerging paradigm. J Clin Oncol. 2013;31(15):1806–1814. doi: 10.1200/JCO.2012.46.8934. [DOI] [PubMed] [Google Scholar]
- 45.Wong KF, Xu Z, Chen J, et al. Circulating markers for prognosis of hepatocellular carcinoma. Expert opinion on medical diagnostics. 2013;7(4):319–329. doi: 10.1517/17530059.2013.795146. [DOI] [PubMed] [Google Scholar]
- 46.Dufour JF. Modern hepatomancy. Gastroenterology. 2013;144(5):876–878. doi: 10.1053/j.gastro.2013.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.