Abstract
Liquid chromatography-selected reaction monitoring/mass spectrometry-based methodology has evolved to the point where accurate analyses of trace levels of estrogens and androgens in postmenopausal serum and plasma can be accomplished with high precision and accuracy. A suite of derivatization procedures has been developed, which together with modern mass spectrometry instrumentation provide investigators with robust and sensitive methodology. Preionized derivatives are proving to be useful as they are not subject to suppression of the electrospray signal. Postmenopausal women with elevated plasma or serum estrogens are thought to be at increased risk for breast and endometrial cancer. Therefore, significant advances in risk assessment should be possible now that reliable methodology is available. It is also possible to conduct analyses of multiple estrogens in plasma or serum. Laboratories that are currently employing liquid chromatography/mass spectrometry methodology can now readily implement this strategy. This will help conserve important plasma and serum samples available in Biobanks, as it will be possible to conduct high sensitivity analyses using low initial sample volumes. Reported levels of both conjugated and non-conjugated estrogen metabolites are close to the limits of sensitivity of many assays to date, urging caution in the interpretation of these low values. The analysis of serum androgen precursors in postmenopausal women has not been conducted routinely in the past using liquid chromatography/mass spectrometry methodology. Integration of serum androgen levels into the panel of metabolites analyzed could provide additional information for assessing cancer risk and should be included in the future.
Keywords: estrogens, androgens, stable isotope dilution, liquid chromatography/mass spectrometry, pre-ionized derivatives
1. Introduction
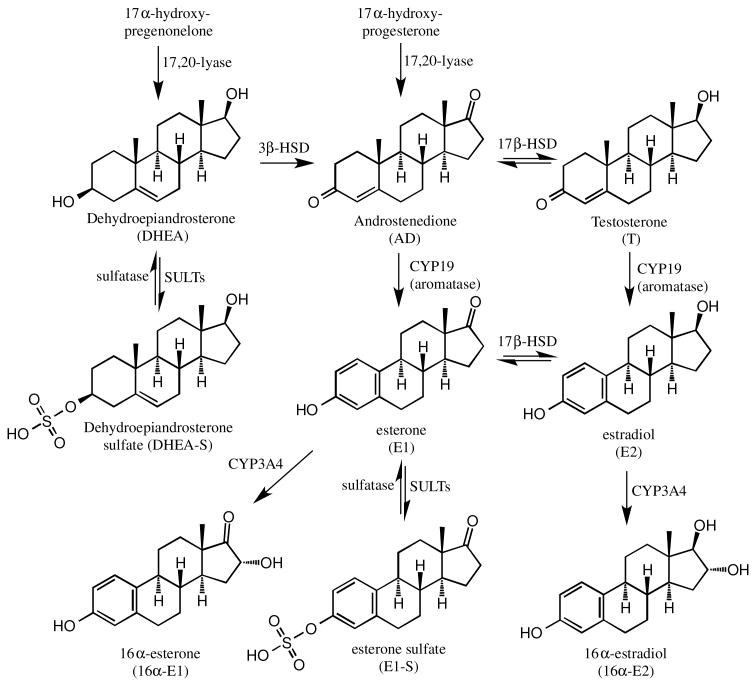
There is a compelling need for reliable methodology capable of quantifying estrogens in the serum of postmenopausal women because increased levels appear to be associated with increased breast cancer risk [1,2]. Estrogen carcinogenesis arises through a dual mechanism in which estradiol can act either as a hormone to stimulate aberrant cell proliferation or as the precursor to the formation of genotoxic catechol metabolites [3]. Estrogen levels in the breast tissues of postmenopausal women are dependent upon the availability of circulating C-19 androgen precursors, which are converted to estrogens in the tissue (Figure 1). Estrogens can then be released into the circulation, providing biomarkers of tissue estrogen biosynthesis if it is assumed that the circulating levels are reflective of tissue concentrations. This assumption has been questioned because tissue levels of estrogens are significantly higher than the corresponding circulating levels and breast tissue-specific metabolism is known to occur. A pharmacokinetic model has been proposed in which there is rapid equilibrium between tissue and plasma estrogens that may might explain this conundrum [4].
Figure 1.
The formation of estrogens in the tissue postmenopausal women from circulating C-19 androgens and sulfate precursors.
The analysis of circulating androgens concentrations can provide insight into availability of relevant androgen precursors, such as androstenedione and testosterone, which can be taken up into tissue (Figure 1). In postmenopausal women, such an analysis could provide useful additional biomarkers of breast cancer risk. Circulating sulfate conjugates have the potential to provide a source of estrogens in breast tissue through the action of sulfatases, which would release the corresponding non-conjugated steroids [5]. This is particularly relevant to circulating estrone-3-sulfate (a precursor to estrone) and dehydroepiandrosterone (DHEA) sulfate, a precursor to DHEA, which is a substrate for 3β-hydroxysteroid dehydrogenase (HSD)-mediated conversion to androstenedione. The androstenedione can in turn be converted to estrone by aromatase (Figure 1). However, there is little evidence that the conversion of circulating sulfate conjugates to tissue androgens and estrogens actually takes place [4]. Furthermore, the polar nature of the sulfate conjugates suggests that they are not good substrates for passive diffusion from the plasma into breast tissue. However, the ability of multiple drug transporter (MRP)-1 (ABCC1) to transport estrone-3-sulfate [6] and MRP-1 and MRP-4 (ABCC4) to transport DHEA sulfate [7] does provide an alternative mechanism for the conjugated steroids to be taken up by breast tissue. Therefore, the analysis of circulating estrone-3-sulfate and DHEA sulfate in postmenopausal women could also be informative.
Aromatase inhibitors have significantly improved the recurrence-free and overall survival rates in breast cancer patients [8]. Unfortunately, only incremental progress has been made over the last decade in preventing breast cancer among postmenopausal women. There is a compelling need to improve this situation in view of the aging world population and the role of aging as an important determinant of breast cancer risk [9,10]. It is clear that implementation of breast cancer prevention programs will require selection of women with high breast cancer risk in order to maximize the benefit/risk ratio [11,12]. It is anticipated that significant advances in risk assessment will be possible if reliable methodology is available to quantify estrogens and androgens in the plasma or serum of postmenopausal women [9]. These measurements can be coupled with other risk factors such as mammographic density [13], bone density [14], body mass index (BMI) [15], and single-nucleotide polymorphisms associated with breast cancer [16] to provide an improved model of breast cancer risk [11]. The present review will focus on the analysis of non-conjugated and conjugated estrogens and androgens using highly specific and sensitive stable isotope dilution liquid chromatography/mass spectrometry methodology that can be used to assess breast cancer risk.
2. Non-conjugated estrogens
Non-conjugated estradiol and its downstream non-conjugated metabolites are present in plasma and serum in the free form (not bound to steroid binding proteins) in postmenopausal women in the fg/mL range, which puts them below the limit of quantitation (LOQ) of routine assays [17,18]. Therefore, estrogens are quantified as a mixture of non-conjugated free and non-conjugated protein-bound forms. Typical serum concentrations of only 2.7-15.9 pg/mL for estradiol and 11.8-37.4 pg/mL for estrone in postmenopausal women [19-26] (Table 1) are still very challenging for most LC-MS-based procedures. Concentrations of non-conjugated free forms are determined by analyzing the amount of plasma steroid binding protein [27] and subtracting the amount of each individual non-conjugated estrogen calculated to non-covalently bind to this protein [28,29]. Clearly, estrogen assays with high sensitivity, specificity, and reproducibility are required in order for meaningful data to be obtained for postmenopausal women [30]. There are three major bioanalytical methods currently in use: radioimmunoassay coupled with chromatography [31], gas chromatography-selected reaction monitoring/mass spectrometry (GC-SRM/MS) [32], and stable isotope dilution liquid chromatography (LC)-SRM/MS [33]. There is increasing reliance on the use of LC-SRM/MS-based methodology because of the relative simplicity of the triple quadrupole instruments that are employed and the potential for future increased specificity by coupling LC with high-resolution ion-trap-based instruments [34,35].
Table 1. Concentrations of non-conjugated estrogens in postmenopausal serum determined by LC-SRM/MS.
| Reference | Xu 2007 [19] | Caron 2009 [20] | Gao 2010 [21] | Walsh 2011 [22] | Rangiah 2011 [23] | Rothman 2011 [24] | Fuhrman 2012 [25] | Falk 2013 [26] | Overall |
|---|---|---|---|---|---|---|---|---|---|
| Concentration | Mean pg/mL | Mean pg/mL | Mean pg/mL | Median pg/mL | Mean pg/mL | Mean pg/mL | Median pg/mL | Median pg/mL | Mean pg/mL |
| E2 | 15.0 | 6.6 | 15.9 | 3.4 | x | 3.1 | 4.2 | 2.7 | 7.3 |
| E1 | 32.7 | 32.3 | x | 18.4 | 12.3 | 37.4 | 14.6 | 11.8 | 22.8 |
| 16α-OH-E2 | 7.9 | x | x | x | x | x | 13.5 | 7.7 | 9.7 |
| 16α-OH-E1 | BLQ | x | x | x | BLQ | x | 11.2 | 10.7 | 10.9 |
| 2-OH-E2 | BLQ | x | x | x | x | x | BLQ | BLQ | BLQ |
| 2-OH-E1 | BLQ | x | x | x | x | x | BLQ | BLQ | BLQ |
| 4-OH-E2 | x | x | x | x | x | x | x | x | x |
| 4-OH-E1 | BLQ | x | x | x | x | x | BLQ | BLQ | BLQ |
| 2-MeO-E2 | BLQ | x | x | x | x | x | 1.4 | 0.7 | 1.0 |
| 2-MeO-E1 | BLQ | x | x | x | 2.7 | x | 2.3 | 4.6 | 3.2 |
| 4-MeO-E2 | BLQ | x | x | x | x | x | BLQ | BLQ | BLQ |
| 4-MeO-E1 | BLQ | x | x | x | BLQ | BLQ | BLQ | BLQ |
x = not analyzed
Abbreviations: BLQ = below lower limit of quantitation; E2 = estradiol; E1 = estrone; OH = hydroxy; MeO = methoxy.
For reliable measurements of multiple non-conjugated estrogens in plasma or serum, it is necessary to employ stable isotope internal standards, which have identical physical properties to the endogenous metabolites, but differ only in mass. Losses that occur during the extraction and chromatographic analysis are then compensated for because the ratio of each endogenous analyte to its internal standard remains the same. Stable isotope analogs also act as carriers to prevent selective losses of trace analytes through binding to active surfaces during extraction and analysis [36]. Until recently, this ideal condition was not possible for estradiol and its metabolites because only deuterated analogs were available for use as internal standards. Deuterated internal standards are not ideal as they can separate from the corresponding endogenous analyte, with a potential for differential suppression of their ESI signals and inaccurate quantification. The availability of many [13C6]-estrogen analogs from Cambridge Isotope Laboratories (Andover, MA) provides internal standards that do not suffer from this potential problem.
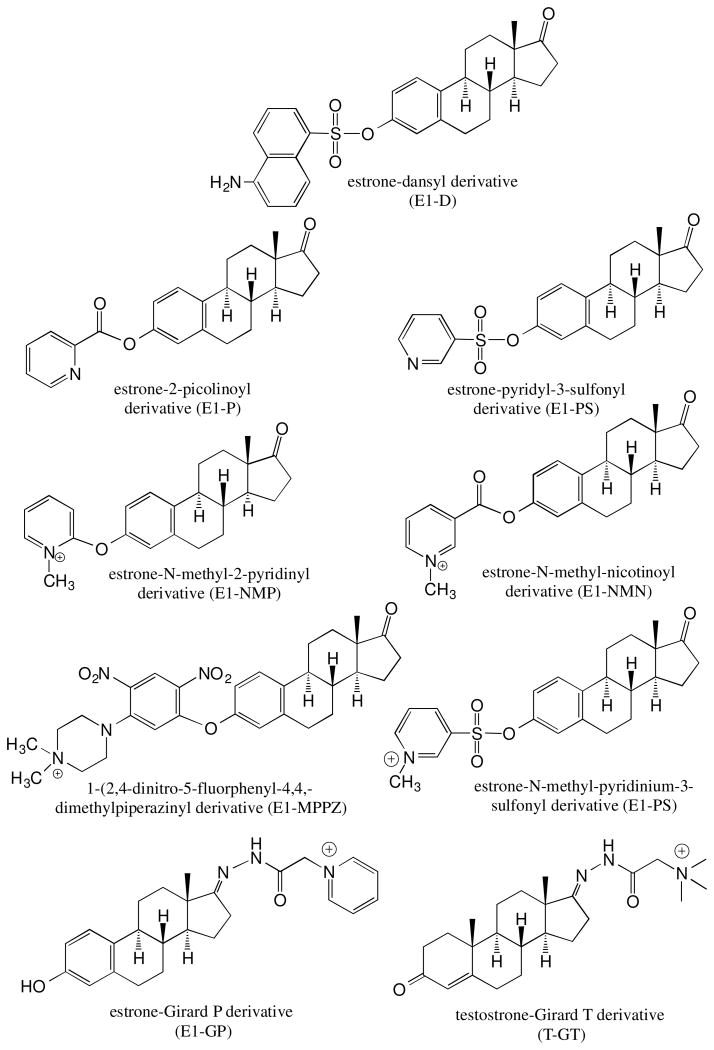
Unfortunately, endogenous non-conjugated estrogens in postmenopausal serum or plasma cannot be quantified using conventional electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI) methodology. Therefore, it is necessary enhance the ionization characteristics of estrogens by first converting them to suitable derivatives. Three approaches to enhancing the sensitivity of LC-ESI/MS-based estrogen analysis through derivatization have been reported. The first approach, which we employed originally involves the preparation of an electron capturing pentafluorobenzyl (PFB) derivative of the estrogen coupled with the use of electron capture atmospheric pressure chemical ionization (ECAPC)/MS [37]. The Higashi group has also explored the utility of ECAPC/MS for estrogen analysis by using different electron capturing derivatives [38]. We showed that it was possible to quantify estrogens in the low pg/mL range in plasma using LC-ECAPCI/MS [39]. The second approach, which has much wider utility, involves the use of derivatives that enhance the ESI signal, a strategy that greatly improves sensitivity during LC-ESI/MS analysis. This approach is exemplified by studies from the Singh [40], Tai [41], Ziegler [42], and Kushnir [33] groups who used the dansyl (D) derivative to improve sensitivity of detection of non-conjugated estrogens from human biofluid samples (Figure 2). Alternative derivatives that have been employed include picolinoyl (P) by the Yamashita group [43] and pyridyl-3-sulfonyl (PS) by Spink group [44] group (Figure 2). The third approach involves the preparation of pre-ionized (quaternized) derivatives, so that ionization is not required in the ESI source of the mass spectrometer. This approach was reported in studies by the Chen [45], Adamec [46] and Higashi [47] groups in which N-methyl-2-pyridyl (NMP), N-methyl-nicotinyl (NMN) or 1-(2,4-dinitro-5-fluorphenyl-4,4,-dimethylpiperaziny (MPPZ) derivatives, respectively were attached to the 3-hydroxy phenolic moiety of the estrogen (Figure 2). Our group has also used pre-ionized derivatives to improve sensitivity by adding a Girard P (GP) derivative to the 17-oxo moiety of estrone and its metabolites [23] or by adding a Girard T (GT) derivative to the 17-oxo-moiety of androgens [48] (Figure 2). We have also recently developed the pre-ionized N-methyl-3-sulfonyl-pyridinium (NMPS) derivative that can be used for both estradiol and estrone metabolites (Figure 2).
Figure 2. Derivatives used to enhance the ionization efficiency of estrogens in order to improve sensitivity for LC-MS/MS analysis.
The concentrations of non-conjugated estradiol in the serum of postmenopausal women determined by LC-SRM/MS were reported to be the range of 2.7 to 15.9 pg/mL with a mean value of 7.3 pg/mL (Table 1). Concentrations of serum estrone that were also determined by LC-SRM/MS were reported to be significantly higher – in the range 11.8 to 37.4 pg/mL with a mean value of 22.8 pg/mL (Table 1). These values are in reasonable agreement with those obtained for estradiol in the serum of postmenopausal women (mean 5.1 pg/mL, range 2.9 to 7.3 pg/mL) [32,49-51] and serum estrone (mean 15.2 pg/mL, range 12.7 to 17.6 pg/mL) [50,51] using high sensitivity GC-SRM/MS. This suggests that values in excess of 15 pg/mL for estradiol and 30 pg/mL for estrone should treated with extreme caution. LC-SRM/MS studies that analyzed non-conjugated serum 16α-hydroxy-estradiol reported levels that were quite consistent with a mean value of 9.7 pg/mL and a range of 7.7 to 13.5 pg/mL (Table 1). In contrast, two studies have reported 16α-hydroxy-estrone concentrations to be in a similar range (10.7 to 11.2 pg/mL) whereas two additional studies reported 16α-hydroxy-estrone to be below the limit of quantification (Table 1). It is noteworthy that the highly sensitive method based on a GP derivative, which has a limit of quantification of 0.15 pg/mL for 16α-hydroxy-estrone was unable to detect any of this analyte [23]. This suggests that trace amounts of interfering substances could be responsible for the very low concentrations of the non-conjugated hydroxylated estrogens that have been reported and that the results of these studies should be re-evaluated with assays capable of more sensitive detection. The non-conjugated catechol estrogens (2- and 4-hydroxy-estradiol, 2- and 4-hydroxy-estrone) represent very challenging analytical targets because of their inherent instability. Most of the LC-SRM/MS studies have reported very low or undetectable amounts of these unstable analytes in serum (Table 1). Similarly, the non-conjugated methoxy-estrogens all appear to be present at levels that are below the limit of quantification of most LC-SRM/MS assays (0.7 to 4.6 pg/mL; Table 1).
3. Conjugated estrogens
Two approaches have been employed for the analysis of conjugated estrogens. The first approach involves hydrolysis of the β-glucuronide and sulfate conjugates with β-glucuronidase/arylsulfatase (G/S) such as that purified from helix pomatia, followed by derivatization using one of the derivatives in shown Figure 2 and LC-SRM/MS analysis. Analytical data are often reported as total (T) values, which is the sum of the non-conjugated and conjugated estrogens (Table 2). The second approach involves analysis of the intact conjugate without G/S hydrolysis or derivatization using negative ESI-based LC-MS methodology. Each method has drawbacks and for many of the conjugated estrogens rigorous stable isotope dilution methodology cannot be employed because appropriate standards are not available. The use of G/S hydrolysis for all of the conjugates (except estradiol and estrone where appropriate standards are available) is dependent upon the assumption that quantitative conversion of the conjugates occurs. Non-conjugated heavy isotope standards can compensate for any decomposition of the endogenous estrogens that occurs during the hydrolysis procedure. It is more problematic that no authentic standards are available for many of the potential β-glucuronide and sulfate estrogen conjugates. Furthermore, no systematic studies have been conducted to evaluate which conjugates are present in postmenopausal serum and whether they are completely hydrolyzed by the typical β-glucuronidase/sulfatases that are employed. One way to provide assurance that hydrolysis is complete is to conduct a separate methanolysis of the sample with anhydrous hydrogen chloride in methanol [52]. The concentrations of non-conjugated estrogens that are determined can then be compared with those obtained from G/S hydrolysis [53,54].
Table 2. Concentrations of conjugated and total estrogens in postmenopausal serum determined by LC-SRM/MS.
| Reference | Xu 2007 [19] |
Labrie 2007 [50] |
Labrie 2008 [51] |
Masi 2009 [57] |
Caron 2009 [20] |
Gao 2010 [21] |
Patel 2011 [58] |
Walsh 2011 [22] |
Fuhrman 2012 [25] |
Falk 2013 [26] |
Dallal 2014 [59] |
Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Technique | G/S | Intact | Intact | G/S | Intact | G/S | G/S | Intact | G/S | G/S | G/S | |
| Concentration | Mean pg/mL |
Mean pg/mL |
Mean pg/mL |
Mean pg/mL |
Mean pg/mL |
Mean pg/mL |
Mean pg/mL |
Median pg/mL |
Median pg/mL |
Median pg/mL |
Median pg/mL |
Mean |
| E2-T | 51.5 | x | x | 31.6 | x | 48.3 | x | x | 9.8 | 6.2 | 10.5 | 26.3 |
| E2-3G | x | x | x | x | 8.4 | x | x | 2.5 | x | x | x | 5.5 |
| E1-T | 442.1 | x | x | 196.7 | x | x | x | x | 105.5 | 61.3 | 77.3 | 176.6 |
| E1-3G | x | x | x | x | 30.9 | x | x | 22.6 | x | x | x | 26.8 |
| E1-S | x | 232.0 | 137.0 | x | 440.0 | x | x | 170.0 | x | x | x | 244.8 |
| 16α-OH-E2-T | 27.9 | x | x | 741.6 | x | x | x | x | 126.0 | 78.4 | 70.5 | 208.9 |
| 16α-OH-E1-T | 8.8 | x | x | 26.4 | x | 11.5 | 19.4 | x | 11.2 | 10.7 | 8.1 | 13.7 |
| 2-OH-E2-T | 11.1 | x | x | 15.9 | x | 12.7 | x | x | 10.4 | 6.6 | 3.5 | 10.0 |
| 2-OH-E1-T | 72.5 | x | x | 136.4 | x | 69.4 | x | x | 19.7 | 21.2 | 17.9 | 56.2 |
| 4-OH-E2-T | x | x | x | x | x | x | x | x | x | x | x | x |
| 4-OH-E1-T | 11.5 | x | x | BLQ | x | x | x | x | 6.3 | 5.0 | 3.0 | 6.5 |
| 2-MeO-E2-T | BLQ | x | x | 19.0 | x | x | x | x | 2.3 | 4.7 | 4.0 | 7.5 |
| 2-MeO-E2-3G | x | x | x | x | 6.9 | x | x | 2.5 | x | x | x | 4.7 |
| 2-MeO-E1-T | BLQ | x | x | 32.8 | x | x | x | x | 3.7 | 12.4 | 8.4 | 14.4 |
| 2-MeO-E1-3G | x | x | x | x | 6.2 | x | x | 2.5 | x | x | x | 4.4 |
| 4-MeO-E2-T | BLQ | x | x | 9.2 | x | x | x | x | 0.8 | 0.6 | 0.8 | 2.9 |
| 4-MeO-E1-T | BLQ | x | x | 16.6 | x | x | x | x | 1.1 | 1.1 | 1.1 | 5.0 |
x = not analyzed
Abbreviations: BLQ = below lower limit of quantitation; E2 = estradiol; E1 = estrone; OH = hydroxy; MeO = methoxy; T= total (non-conjugated + conjugated); G/S= β-glucuronidase/arylsulfatase; -S = sulfate; -G = glucuronide
In general, very few heavy isotope internal standards are available for rigorous quantification of estrogen conjugates. A heavy isotope internal standard for estrone-3-sulfate (2,4,16,16-[2H4]-estrone-3-sulfate) is commercially available from C/D/N isotopes (Pointe-Claire, CA) so that reliable quantitative assays can be conducted for this important analyte [22,55]. It is surprising that this standard has not generally been used for quantitative determinations of estrone-3-sulfate in the serum of postmenopausal women. When conducting quantitative determinations, care has to be taken with calibration standards because both unlabeled and labeled estrone-3-sulfate contain Tris as a stabilizer as well as significant amounts of water [55]. Quantifying the actual amount of estrone-3-sulfate in solutions requires UV spectrophotometry, in which the λmax of estrone at 270 nM (ε 2000) is measured [56]. Three regioisomeric heavy isotope labeled estradiol β-glucuronides ([2H4]-estradiol-3β-glucuronide, [2H4]-estradiol-17-β-glucuronide, [2H4]-estradiol-3,17-bis-β-glucuronide) have been synthesized using rat liver microsomes and used as internal standards in LC-SRM/MS assays for the corresponding endogenous β-glucuronides [20,22]. No heavy isotope internal standards are available for any of the other estrogen conjugates that have been analyzed (Table 2).
The very low concentrations of non-conjugated estradiol in the serum of postmenopausal women are reflected in the low level of total (non-conjugated + conjugated) serum estradiol with a mean value of 26.3 pg/mL and a range of 6.2 to 51.5 pg/mL [19-22,25,26,50,51,57-59] (Table 2). The reported mean levels of estradiol-3β-glucuronide of 5.5 pg/mL suggest that the sulfate conjugate is probably also present in the serum. Recent studies have found total mean estrone concentrations to be 176.6 pg/mL with a range of 61.3 to 442.1 pg/mL (Table 2). However, specific analyses of estrone-sulfate (137 to 440 pg/mL) and estrone-3β-glucuronide (22.6 to 33.9 pg/mL) suggest that some of the total estrone conjugate values obtained after G/S hydrolysis might be an underestimate of the true values (Table 2). Therefore, future studies should focus on the analysis of intact estrone-sulfate using stable isotope LC-SRM/MS methodology [22,55]. This approach would be particularly useful for monitoring the effect of aromatase inhibitors [60].
Mean levels of total 16α-hydroxy-estradiol have been reported to be 208.9 pg/mL with a wide range of 27.9 to 741.6 pg/mL. However, more recent reports suggest that the actual range may be closer to 70.5 to 126.0 pg/mL (Table 2). Levels of total 16α-hydroxy-estrone were reported as being much lower (mean 13.7 pg/mL, range 8.1 to 26.4 pg/mL) (Table 2). These values are similar to the non-conjugated levels (Table 1) adding further concern that these low levels values might simply arise from quantification of trace amounts of interfering substances. Levels of the other total estrogen conjugates except for 2-hydroxy-estrone were all very close to the LOQs (8 pg/mL) that have been reported for most of the assays (Table 2). Therefore, care should be exercised in interpreting these values.
4. Non-conjugated and conjugated androgens
The importance of analyzing androgens stems from their potential conversion to estrogens in breast and endometrial tissue (Figure 1). Consequently, a number of studies have reported the analysis of non-conjugated testosterone, DHEA, and androstenedione in serum samples from postmenopausal women (Table 3) [50,51,61]. Levels of non-conjugated testosterone determined by LC-SRM/MS have been reported as a mean level of 173 pg/mL with a range of 109 to 248 pg/mL [22,24,50,51,61-65] (Table 3). This is slightly higher than the mean value of 107 pg/mL (range 90-130 pg/mL) that was reported using GC-SRM/MS [22,24,50,51,62,63,65]. It is noteworthy that when modern very high sensitivity triple quadrupole instrumentation was employed, the concentration of serum testosterone of 187 pg/mL was still higher than the values reported by GC-SRM/MS [64]. The higher values for serum non-conjugated testosterone reported by the LC-SRM/MS methods could be overestimating the actual serum concentrations, so these values should be re-evaluated. Doing so could involve use of derivatization procedures to improve sensitivity and specificity [48] and/or use of highresolution MS [34,35]. The mean level of serum androstenedione was reported to be 380 pg/mL with a range of 354 to 440 pg/mL (Table 3). These consistent values suggest that serum androstenedione concentrations in postmenopausal women are truly in the range of 380 pg/mL. The mean level of non-conjugated DHEA was reported to be 1790 pg/mL with a range 1670 pg/mL to 1910 pg/mL (Table 3). In contrast to non-conjugated serum testosterone, this value agrees well with the mean level of 1203 pg/mL obtained by GC-SRM/MS (range 720 to 1800 pg/mL) [50,51,65]. The mean level of DHEA sulfate was reported to be 499 ng/mL (range from 355 to 600 pg/mL), which is approximately five orders of magnitude higher than the mean value of 1790 pg/mL reported for the non-conjugated form of DHEA (Table 3). If the sulfate conjugate can serve as a precursor to the formation of estrogens (Figure 1) this represents an enormous pool that could potentially be eliminated by the use of sulfatase inhibitors [5]. Surprisingly, no studies have been reported on the use of LC-SRM/MS for the analysis of conjugated testosterone in the serum of postmenopausal women. This would be a worthwhile endeavor for the future if indeed the sulfate conjugate can be transported into tissues and undergo hydrolysis to provide an additional source of non-conjugated testosterone for conversion to estrone (Figure 1).
Table 3. Concentrations of non-conjugated and conjugated androgens in postmenopausal serum determined by LC-SRM/MS.
| Reference | Labrie 2007 [50] | Labrie 2008 [51] | Kushnir 2010 [61] | Bui 2010 [62] | Walsh 2011 [22] | Rothman 2011 [24] | Haring 2012 [63] | Methlie 2013 [64] | Labrie 2013 [65] | Overall |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | Mean | Mean | Median | Mean | Mean | Mean | Mean | Median | Mean | Mean |
| T (pg/mL) | x | x | 180 | 248 | 140 | 109 | x | 187 | x | 173 |
| AD (pg/mL) | x | x | 360 | x | 440 | x | 354 | 367 | x | 380 |
| DHEA (pg/mL) | x | x | 1670 | x | 1910 | x | x | x | x | 1790 |
| DHEA-S (ng/mL) | 502 | 355 | x | x | 600 | x | x | x | 540 | 499 |
x = not analyzed
Abbreviations: AD, androstenedione; T, non-conjugated testosterone, DHEAS, DHEA-sulfate.
Summary and Future Directions
The availability of a suite of derivatization procedures makes it possible to quantify nonconjugated estrogens by LC-SRM/MS (Table 1) with sensitivity comparable to that which can be obtained by GC-SRM/MS [32]. Pre-ionized derivatives are also proving to be useful for the quantification of androgens [48], although this methodology has not yet been applied to postmenopausal serum samples. This suggests that in the future it will be possible to conduct LC-MS/MS assays on multiple estrogen and androgen metabolites in serum and plasma at an order of magnitude lower than current methodology (Table 1). The availability of high sensitivity high-resolution ion trap instrumentation such as the Thermo Q-Exactive (San Jose, CA) should make it possible to conduct analyses with further increases in sensitivity and specificity. Preliminary results are very encouraging with high-resolution instruments [34,35]. Improved specificity could also arise from the use of improved chromatographic separations such as that which can be obtained with supercritical fluid chromatography (SFC) [66]. The availability of modern triple quadrupole mass spectrometers such as the Waters Xevo (Milford, MA), which are integrated with SFC (Acquity UPC2) could prove to be very useful for routine nonconjugated estrogen analyses. The high sensitivity that can be obtained with modern LCSRM/MS will also permit the use of smaller volumes of biofluids to help conserve important plasma and serum samples. This will make it possible to use plasma and serum samples available from existing Biobanks without significantly depleting the total volume available. This could permit additional studies to be conducted on the same samples in order to help understand the factors that cause an increase in breast cancer risk.
Recent LC-SRM/MS assays have revealed that true serum levels of 16α-hydroxy-estrone are likely to be lower than previously reported. This should lead to re-evaluation of the importance of this metabolite, as it has been proposed to be involved in breast cancer progression [67-69]. Several studies were unable to detect non-conjugated 16α-hydroxy-estrone, while other studies found levels of the metabolite to be very close to the reported limits of quantification (Table 1). Reported levels of non-conjugated 16α-hydroxy-estradiol are similarly very close to the limits of quantification of the assays employed. This suggests that when these analytes are analyzed with greater sensitivity and specificity, serum concentration will actually be closer to 1 pg/mL. Intriguingly, very low levels of the conjugated forms of both 16α-hydroxy-estrone and 16α-hydroxy-estradiol have also been reported (Table 2). No methods have been developed to detect the intact β-glucuronide and sulfate conjugates of the 16α-hydroxy estrogens and so it is conceivable that the lack of detection could be due to incomplete hydrolysis by the G/S-based procedures normally employed. Therefore, there is a compelling need to confirm these findings using alternative methodology such as hydrolysis of conjugates with anhydrous hydrogen chloride [52] rather than by G/S.
The quantification of estrone-sulfate is particularly important as it is the majorcirculating form of estrone [60] and can potentially serve as a precursor to estrone in tissues through the action of sulfatases [5]. Furthermore, estrone-sulfate could potentially serve as a biomarker for the effectiveness of aromatase inhibitors [60]. It should be possible to detect low pg/mL levels by stable isotope dilution LC-SRM/MS, which would be approximately 1 % of the original circulating form, confirming complete inhibition of estrogen biosynthesis. Stable isotope dilution LC-SRM/MS assay methodology should be as specific as possible. Unfortunately, the only heavy isotope internal standard available for estrone-sulfate is the tetradeuterated form. Therefore, there is a critical need to synthesize the corresponding [13C]-analog of estrone-sulfate in order to overcome the problems inherent to use of deuterated internal standards. The [13C]-analog would additionally be stable to acid hydrolysis, overcoming any additional concerns that deuterium could exchange for protium during the analytical procedure.
Significant advances have been made in the development of LC-SRM/MS assays over the lastdecade, allowing increasingly sensitive and reliable quantification of serum estrogens and androgens. These advances in analytical methodology will facilitate the development of improved breast cancer risk models that incorporate serum concentrations of a comprehensive panel of estrogen and androgen metabolites. [30]. Previous studies have shown that such models have the potential to significantly improve breast cancer prevention [11,12]. The LCSRM/MS assays have potential utility for discovering biomarkers for the treatment and early detection of endometrial cancer as exemplified in the study of Audet-Walsh et al. [22]. The ability to routinely analyze serum and plasma estrogens and androgens with very high sensitivity and specificity by stable isotope dilution LC-SRM/MS is a promising avenue towards saving a large number of women from these devastating diseases [30,70].
Acknowledgments
We acknowledge the support of NIH grants R01CA158328, P30ES013508, and R25CA101871. We are grateful for helpful discussions with Dr. Richard Santen of the University of Virginia and Dr. Trevor Penning of the University of Pennsylvania.
Abbreviations used
- 2-hydroxy-estradiol
2,3-dihydroxy-17β-estradiol
- 2-methoxy-estradiol
2-methoxy-3-hydroxy-17β-estradiol
- 4-methoxy-estradiol
3-hydroxy-4-methoxy-17β-estradiol
- 4-hydroxy-estradiol
3,4-dihydroxy-17β-estradiol
- 16α-hydroxy-estradiol
3,16α-dihydroxy-17β-estradiol
- estradiol
17β-estradiol
- APCI
atmospheric pressure chemical ionization
- BMI
body mass index
- CYP
cytochrome P-450
- D
dansyl
- DHEA
dehydroepiandrosterone
- ECAPCI
electron capture atmospheric pressure chemical ionization
- ESI
electrospray ionization
- GP
Girard P
- GT
Girard T
- G/S
β-glucuronidase/arylsulfatase
- LC
liquid chromatography
- HSD
hydroxysteroid dehydrogenase
- MPPZ
1-(2,4-dinitro-5-fluorphenyl-4,4,-dimethylpiperazinyl
- MS
mass spectrometry
- NMN
N-methyl-nicotinyl
- NMP
N-methyl-2-pyridyl
- NMPS
N-methylpyridynium-3-sulfonyl
- P
picolinoyl
- PFB
pentafluorobenzyl
- PS
pyridyl-3-sulfonyl
- SRM
selected reaction monitoring
- SULT
sulfotransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000;21:40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- 2.Blair IA. Analysis of estrogens in serum and plasma from postmenopausal women: past, present, and future. Steroids. 2010;75:297–306. doi: 10.1016/j.steroids.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liehr JG. Genotoxicity of the steroidal oestrogens oestrone and oestradiol: possible mechanism of uterine and mammary cancer development. Hum Reprod Update. 2001;7:273–281. doi: 10.1093/humupd/7.3.273. [DOI] [PubMed] [Google Scholar]
- 4.Lonning PE, Haynes BP, Straume AH, Dunbier A, Helle H, Knappskog S, et al. Exploring breast cancer estrogen disposition: the basis for endocrine manipulation. Clin Cancer Res. 2011;17:4948–4958. doi: 10.1158/1078-0432.CCR-11-0043. [DOI] [PubMed] [Google Scholar]
- 5.Purohit A, Foster PA. Steroid sulfatase inhibitors for estrogen- and androgen-dependent cancers. J Endocrinol. 2012;212:99–110. doi: 10.1530/JOE-11-0266. [DOI] [PubMed] [Google Scholar]
- 6.Qian YM, Song WC, Cui H, Cole SP, Deeley RG. Glutathione stimulates sulfated estrogen transport by multidrug resistance protein 1. J Biol Chem. 2001;276:6404–6411. doi: 10.1074/jbc.M008251200. [DOI] [PubMed] [Google Scholar]
- 7.Gustafson AM, Soldi R, Anderlind C, Scholand MB, Qian J, Zhang X, et al. Airway PI3K pathway activation is an early and reversible event in lung cancer development. Sci Transl Med. 2010;2:26ra25. doi: 10.1126/scitranslmed.3000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falandry C, Canney PA, Freyer G, Dirix LY. Role of combination therapy with aromatase and cyclooxygenase-2 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2009;20:615–620. doi: 10.1093/annonc/mdn693. [DOI] [PubMed] [Google Scholar]
- 9.Santen RJ. Assessing individual risk for breast cancer: role of oestrogens and androgens. Breast Cancer Res. 2008;10:S10. doi: 10.1186/bcr2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ESHRE Capri Workshop Group. Hormones and breast cancer. Hum Reprod Update. 2004;10:281–293. doi: 10.1093/humupd/dmh025. [DOI] [PubMed] [Google Scholar]
- 11.Santen RJ, Boyd NF, Chlebowski RT, Cummings S, Cuzick J, Dowsett M, et al. Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. Endocr Relat Cancer. 2007;14:169–187. doi: 10.1677/ERC-06-0045. [DOI] [PubMed] [Google Scholar]
- 12.Chan K, Morris GJ. Chemoprevention of breast cancer for women at high risk. Semin Oncol. 2006;33:642–646. doi: 10.1053/j.seminoncol.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Boyd N, Martin L, Gunasekara A, Melnichouk O, Maudsley G, Peressotti C, et al. Mammographic density and breast cancer risk: evaluation of a novel method of measuring breast tissue volumes. Cancer Epidemiol Biomarkers Prevent. 2009;18:1754–1762. doi: 10.1158/1055-9965.EPI-09-0107. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Arendell L, Aickin M, Cauley J, Lewis CE, Chlebowski R. Hip bone density predicts breast cancer risk independently of Gail score: results from the Women's Health Initiative. Cancer. 2008;113:907–915. doi: 10.1002/cncr.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gail MH. Value of adding single-nucleotide polymorphism genotypes to a breast cancer risk model. J Natl Cancer Inst. 2009;101:959–963. doi: 10.1093/jnci/djp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McShane LM, Dorgan JF, Greenhut S, Damato JJ. Reliability and validity of serum sex hormone measurements. Cancer Epidemiol Biomarkers Prevent. 1996;5:923–928. [PubMed] [Google Scholar]
- 18.Stanczyk FZ, Cho MM, Endres DB, Morrison JL, Patel S, Paulson RJ. Limitations of direct estradiol and testosterone immunoassay kits. Steroids. 2003;68:1173–1178. doi: 10.1016/j.steroids.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 20.Caron P, Audet-Walsh E, Lepine J, Belanger A, Guillemette C. Profiling endogenous serum estrogen and estrogen-glucuronides by liquid chromatography-tandem mass spectrometry. Anal Chem. 2009;81:10143–10148. doi: 10.1021/ac9019126. [DOI] [PubMed] [Google Scholar]
- 21.Gao W, Zeng C, Cai D, Liu B, Li Y, Wen X, et al. Serum concentrations of selected endogenous estrogen and estrogen metabolites in pre- and post-menopausal Chinese women with osteoarthritis. J Endocrinol Invest. 2010;33:644–649. doi: 10.1007/BF03346664. [DOI] [PubMed] [Google Scholar]
- 22.Audet-Walsh E, Lepine J, Gregoire J, Plante M, Caron P, Tetu B, et al. Profiling of endogenous estrogens, their precursors, and metabolites in endometrial cancer patients: association with risk and relationship to clinical characteristics. J Clin Endocrinol Metab. 2011;96:E330–E339. doi: 10.1210/jc.2010-2050. [DOI] [PubMed] [Google Scholar]
- 23.Rangiah K, Shah SJ, Vachani A, Ciccimaro E, Blair IA. Liquid chromatography-mass spectrometry of pre-ionized Girard P derivatives for quantifying estrone and its metabolites in serum from postmenopausal women. Rapid Commun Mass Spectrom. 2011;25:1297–1307. doi: 10.1002/rcm.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman MS, Carlson NE, Xu M, Wang C, Swerdloff R, Lee P, et al. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry. Steroids. 2011;76:177–182. doi: 10.1016/j.steroids.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuhrman BJ, Schairer C, Gail MH, Boyd-Morin J, Xu X, Sue LY, et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2012;104:326–339. doi: 10.1093/jnci/djr531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falk RT, Brinton LA, Dorgan JF, Fuhrman BJ, Veenstra TD, Xu X, et al. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res. 2013;15:R34. doi: 10.1186/bcr3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longcope C, Hui SL, Johnston CC., Jr Free estradiol, free testosterone, and sex hormonebinding globulin in perimenopausal women. J Clin Endocrinol Metab. 1987;64:513–518. doi: 10.1210/jcem-64-3-513. [DOI] [PubMed] [Google Scholar]
- 28.Rinaldi S, Geay A, Dechaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prevent. 2002;11:1065–1071. [PubMed] [Google Scholar]
- 29.Endogenous Hormones and Breast Cancer Collaborative Group. Free estradiol and breast cancer risk in postmenopausal women: comparison of measured and calculated values. Cancer Epidemiol Biomarkers Prevent. 2003;12:1457–1461. [PubMed] [Google Scholar]
- 30.Santen RJ, Lee JS, Wang S, Demers LM, Mauras N, Wang H, et al. Potential role of ultrasensitive estradiol assays in estimating the risk of breast cancer and fractures. Steroids. 2008;73:1318–1321. doi: 10.1016/j.steroids.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Geisler J, Ekse D, Helle H, Duong NK, Lonning PE. An optimised, highly sensitive radioimmunoassay for the simultaneous measurement of estrone, estradiol and estrone sulfate in the ultra-low range in human plasma samples. J Steroid Biochem Mol Biol. 2008;109:90–95. doi: 10.1016/j.jsbmb.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, et al. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Kushnir MM, Rockwood AL, Bergquist J, Varshavsky M, Roberts WL, Yue B, et al. Highsensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am J Clin Pathol. 2008;129:530–539. doi: 10.1309/LC03BHQ5XJPJYEKG. [DOI] [PubMed] [Google Scholar]
- 34.Franke AA, Custer LJ, Morimoto Y, Nordt FJ, Maskarinec G. Analysis of urinary estrogens, their oxidized metabolites, and other endogenous steroids by benchtop orbitrap LCMS versus traditional quadrupole GCMS. Anal Bioanal Chem. 2011;401:1319–1330. doi: 10.1007/s00216-011-5164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bloomston M, Zhou JX, Rosemurgy AS, Frankel W, Muro-Cacho CA, Yeatman TJ. Fibrinogen gamma overexpression in pancreatic cancer identified by large-scale proteomic analysis of serum samples. Cancer Res. 2006;66:2592–2599. doi: 10.1158/0008-5472.CAN-05-3659. [DOI] [PubMed] [Google Scholar]
- 36.Oe T, Ackermann BL, Inoue K, Berna MJ, Garner CO, Gelfanova V, et al. Quantitative analysis of amyloid beta peptides in cerebrospinal fluid of Alzheimer's disease patients by immunoaffinity purification and stable isotope dilution liquid chromatography/negative electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:3723–3735. doi: 10.1002/rcm.2787. [DOI] [PubMed] [Google Scholar]
- 37.Singh G, Gutierrez A, Xu K, Blair IA. Liquid chromatography/electron capture atmospheric pressure chemical ionization/mass spectrometry: analysis of pentafluorobenzyl derivatives of biomolecules and drugs in the attomole range. Anal Chem. 2000;72:3007–3013. doi: 10.1021/ac000374a. [DOI] [PubMed] [Google Scholar]
- 38.Higashi T, Takayama N, Nishio T, Taniguchi E, Shimada K. Procedure for increasing the detection responses of estrogens in LC-MS based on introduction of a nitrobenzene moiety followed by electron capture atmospheric pressure chemical ionization. Anal Bioanal Chem. 2006;386:658–665. doi: 10.1007/s00216-006-0371-z. [DOI] [PubMed] [Google Scholar]
- 39.Penning TM, Lee SH, Jin Y, Gutierrez A, Blair IA. Liquid-chromatography mass spectrometry (LC-MS) of steroid hormone metabolites and its applications. J Steroid Biochem Mol Biol. 2010;121:546–555. doi: 10.1016/j.jsbmb.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 41.Tai SS, Welch MJ. Development and evaluation of a reference measurement procedure for the determination of estradiol-17beta in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2005;77:6359–6363. doi: 10.1021/ac050837i. [DOI] [PubMed] [Google Scholar]
- 42.Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, et al. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77:6646–6654. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita K, Okuyama M, Watanabe Y, Honma S, Kobayashi S, Numazawa M. Highly sensitive determination of estrone and estradiol in human serum by liquid chromatography-electrospray ionization tandem mass spectrometry. Steroids. 2007;72:819–827. doi: 10.1016/j.steroids.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Xu L, Spink DC. Analysis of steroidal estrogens as pyridine-3-sulfonyl derivatives by liquid chromatography electrospray tandem mass spectrometry. Anal Biochem. 2008;375:105–114. doi: 10.1016/j.ab.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin YH, Chen CY, Wang GS. Analysis of steroid estrogens in water using liquid chromatography/tandem mass spectrometry with chemical derivatizations. Rapid Commun Mass Spectrom. 2007;21:1973–1983. doi: 10.1002/rcm.3050. [DOI] [PubMed] [Google Scholar]
- 46.Yang WC, Regnier FE, Sliva D, Adamec J. Stable isotope-coded quaternization for comparative quantification of estrogen metabolites by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B. 2008;870:233–240. doi: 10.1016/j.jchromb.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishio T, Higashi T, Funaishi A, Tanaka J, Shimada K. Development and application of electrospray-active derivatization reagents for hydroxysteroids. J Pharm Biomed Anal. 2007;44:786–795. doi: 10.1016/j.jpba.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Tamae D, Byrns MC, Brettt M, Mostaghel E, Nelson PS, Lange P, et al. Validation and application of a atable isotope dilution Liquid Chromatography electrospray ionization/selected reaction monitoring/mass spectrometry (SID-LC/ESI/SRM/MS) method for quantification of keto-androgens in human serum. J Steroid Biochem Mol Biol. 2013;128:281–289. doi: 10.1016/j.jsbmb.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JS, Ettinger B, Stanczyk FZ, Vittinghoff E, Hanes V, Cauley JA, et al. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab. 2006;91:3791–3797. doi: 10.1210/jc.2005-2378. [DOI] [PubMed] [Google Scholar]
- 50.Labrie F, Belanger A, Belanger P, Berube R, Martel C, Cusan L, et al. Metabolism of DHEA in postmenopausal women following percutaneous administration. J Steroid Biochem Mol Biol. 2007;103:178–188. doi: 10.1016/j.jsbmb.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 51.Labrie F, Cusan L, Gomez JL, Cote I, Berube R, Belanger P, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol. 2008;111:178–194. doi: 10.1016/j.jsbmb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Tang PW, Crone DL. A new method for hydrolyzing sulfate and glucuronyl conjugates of steroids. Anal Biochem. 1989;182:289–294. doi: 10.1016/0003-2697(89)90596-4. [DOI] [PubMed] [Google Scholar]
- 53.Hobe G, Schon R, Goncharov N, Katsiya G, Koryakin M, Gesson-Cholat I, et al. Some new aspects of 17alpha-estradiol metabolism in man. Steroids. 2002;67:883–893. doi: 10.1016/s0039-128x(02)00058-2. [DOI] [PubMed] [Google Scholar]
- 54.Wong CH, Leung DK, Tang FP, Wong JK, Yu NH, Wan TS. Rapid screening of anabolic steroids in horse urine with ultra-high-performance liquid chromatography/tandem mass spectrometry after chemical derivatisation. J Chromatogr A. 2012;1232:257–265. doi: 10.1016/j.chroma.2011.12.095. [DOI] [PubMed] [Google Scholar]
- 55.Giton F, Caron P, Berube R, Belanger A, Barbier O, Fiet J. Plasma estrone sulfate assay in men: Comparison of radioimmunoassay, mass spectrometry coupled to gas chromatography (GC-MS), and liquid chromatography-tandem mass spectrometry (LCMS/MS) Clin Chim Acta. 2010;411:1208–1213. doi: 10.1016/j.cca.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 56.Slaunwhite WR, Jr, Engel LL, Olmsted PC, Carter P. The fluorescence and extinction and partition coefficients of estrogens. J Biol Chem. 1951;191:627–631. [PubMed] [Google Scholar]
- 57.Masi CM, Hawkley LC, Xu X, Veenstra TD, Cacioppo JT. Serum estrogen metabolites and systolic blood pressure among middle-aged and older women and men. Am J Hypertens. 2009;22:1148–1153. doi: 10.1038/ajh.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel S, Hawkley LC, Cacioppo JT, Masi CM. Dietary fiber and serum 16alphahydroxyestrone, an estrogen metabolite associated with lower systolic blood pressure. Nutrition. 2011;27:778–781. doi: 10.1016/j.nut.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dallal CM, Tice JA, Buist DS, Bauer DC, Lacey JV, Jr, Cauley JA, et al. Estrogen metabolism and breast cancer risk among postmenopausal women: a case-cohort study within B∼FIT. Carcinogenesis. 2014;35:346–355. doi: 10.1093/carcin/bgt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lonning PE, Ekse D. A sensitive assay for measurement of plasma estrone sulphate in patients on treatment with aromatase inhibitors. J Steroid Biochem Mol Biol. 1995;55:409–412. doi: 10.1016/0960-0760(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 61.Kushnir MM, Blamires T, Rockwood AL, Roberts WL, Yue B, Erdogan E, et al. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56:1138–1147. doi: 10.1373/clinchem.2010.143222. [DOI] [PubMed] [Google Scholar]
- 62.Bui HN, Struys EA, Martens F, de RW, Thienpont LM, Kenemans P, et al. Serum testosterone levels measured by isotope dilution-liquid chromatography-tandem mass spectrometry in postmenopausal women versus those in women who underwent bilateral oophorectomy. Ann Clin Biochem. 2010;47:248–252. doi: 10.1258/acb.2010.009171. [DOI] [PubMed] [Google Scholar]
- 63.Haring R, Hannemann A, John U, Radke D, Nauck M, Wallaschofski H, et al. Age-specific reference ranges for serum testosterone and androstenedione concentrations in women measured by liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2012;97:408–415. doi: 10.1210/jc.2011-2134. [DOI] [PubMed] [Google Scholar]
- 64.Methlie P, Hustad SS, Kellmann R, Almas B, Erichsen MM, Husebye E, et al. Multisteroid LC-MS/MS assay for glucocorticoids and androgens, and its application in Addison's disease. Endocr Connect. 2013 doi: 10.1530/EC-13-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Labrie F, Martel C, Berube R, Cote I, Labrie C, Cusan L, et al. Intravaginal prasterone (DHEA) provides local action without clinically significant changes in serum concentrations of estrogens or androgens. J Steroid Biochem Mol Biol. 2013;138:359–367. doi: 10.1016/j.jsbmb.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Xu X, Roman JM, Veenstra TD, Van AJ, Ziegler RG, Issaq HJ. Analysis of fifteen estrogen metabolites using packed column supercritical fluid chromatography-mass spectrometry. Anal Chem. 2006;78:1553–1558. doi: 10.1021/ac051425c. [DOI] [PubMed] [Google Scholar]
- 67.Cauley JA, Zmuda JM, Danielson ME, Ljung BM, Bauer DC, Cummings SR, et al. Estrogen metabolites and the risk of breast cancer in older women. Epidemiology. 2003;14:740–744. doi: 10.1097/01.ede.0000091607.77374.74. [DOI] [PubMed] [Google Scholar]
- 68.Kabat GC, O'Leary ES, Gammon MD, Sepkovic DW, Teitelbaum SL, Britton JA, et al. Estrogen metabolism and breast cancer. Epidemiology. 2006;17:80–88. doi: 10.1097/01.ede.0000190543.40801.75. [DOI] [PubMed] [Google Scholar]
- 69.Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Circulating 2-hydroxy- and 16alpha-hydroxy estrone levels and risk of breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2029–2035. doi: 10.1158/1055-9965.EPI-08-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitra R, Guo Z, Milani M, Mesaros C, Rodriguez M, Nguyen J, et al. CYP3A4 mediates growth of estrogen receptor-positive breast cancer cells in part by inducing nuclear translocation of phospho-Stat3 through biosynthesis of (+/-)-14,15-epoxyeicosatrienoic acid (EET) J Biol Chem. 2011;286:17543–17559. doi: 10.1074/jbc.M110.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]