Abstract
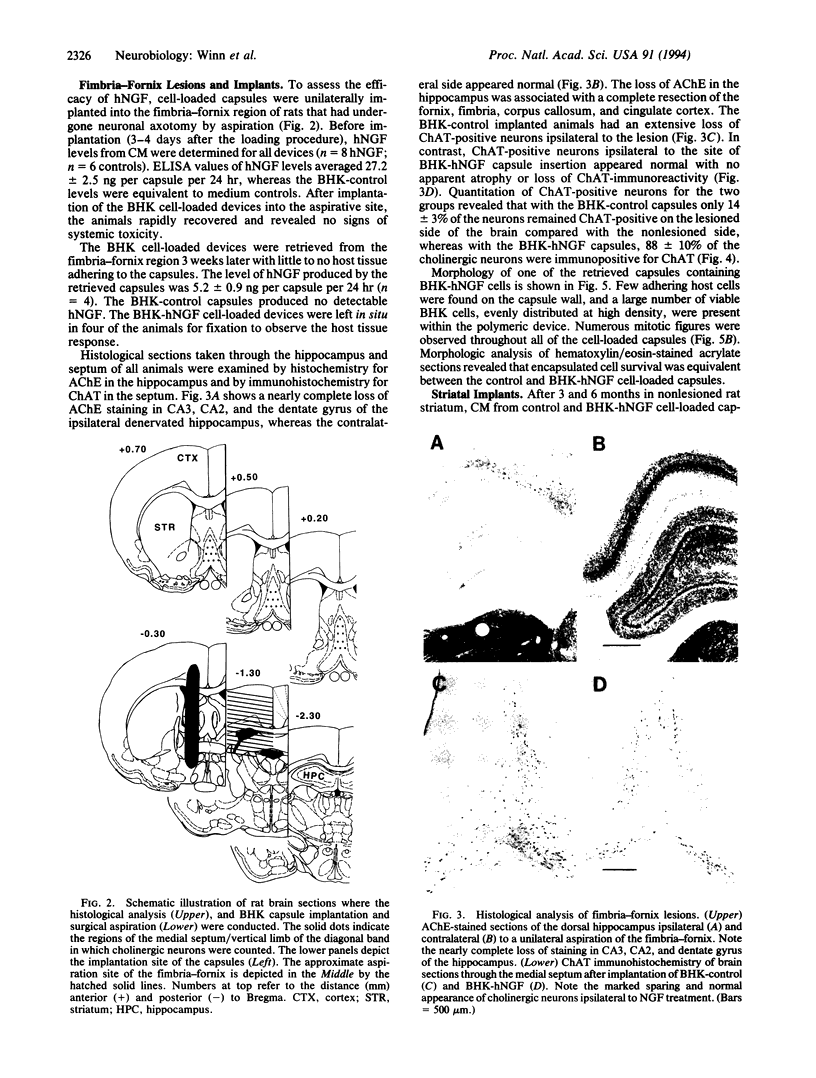
Effective treatments for neurodegenerative disorders are limited by our inability to alter the progression of the diseases. A number of proteins have specific neuroprotective activities in vitro; however, the delivery of these factors into the central nervous system over the long term at therapeutic levels has been difficult to achieve. BHK cells engineered to express and release human nerve growth factor were encapsulated in an immunoisolation polymeric device and transplanted into both fimbria-fornix-lesioned rat brains and naive controls. In the lesioned rat brain, chronic delivery of human nerve growth factor by the encapsulated BHK cells provided nearly complete protection of axotomized medial septal cholinergic neurons. Human nerve growth factor continued to be released by encapsulated cells upon removal from the aspirative site after 3 weeks or from normal rat striatum after 3 and 6 months in vivo. Long-term encapsulated cell survival was confirmed by histologic analysis. This encapsulated xenogeneic system may provide therapeutically effective amounts of a number of neurotrophic factors, alone or in combination, to virtually any site within the body.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebischer P., Tresco P. A., Sagen J., Winn S. R. Transplantation of microencapsulated bovine chromaffin cells reduces lesion-induced rotational asymmetry in rats. Brain Res. 1991 Sep 27;560(1-2):43–49. doi: 10.1016/0006-8993(91)91212-j. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Terry R. D., Deteresa R. M., Bruce G., Hersh L. B., Gage F. H. Response of septal cholinergic neurons to axotomy. J Comp Neurol. 1987 Oct 15;264(3):421–436. doi: 10.1002/cne.902640309. [DOI] [PubMed] [Google Scholar]
- Ayer-LeLievre C., Olson L., Ebendal T., Seiger A., Persson H. Expression of the beta-nerve growth factor gene in hippocampal neurons. Science. 1988 Jun 3;240(4857):1339–1341. doi: 10.1126/science.2897715. [DOI] [PubMed] [Google Scholar]
- Baetge E. E., Suh Y. H., Joh T. H. Complete nucleotide and deduced amino acid sequence of bovine phenylethanolamine N-methyltransferase: partial amino acid homology with rat tyrosine hydroxylase. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5454–5458. doi: 10.1073/pnas.83.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavicchioli L., Flanigan T. P., Dickson J. G., Vantini G., Dal Toso R., Fusco M., Walsh F. S., Leon A. Choline acetyltransferase messenger RNA expression in developing and adult rat brain: regulation by nerve growth factor. Brain Res Mol Brain Res. 1991 Mar;9(4):319–325. doi: 10.1016/0169-328x(91)90079-d. [DOI] [PubMed] [Google Scholar]
- Freed W. J., Poltorak M., Becker J. B. Intracerebral adrenal medulla grafts: a review. Exp Neurol. 1990 Nov;110(2):139–166. doi: 10.1016/0014-4886(90)90026-o. [DOI] [PubMed] [Google Scholar]
- Gage F. H., Armstrong D. M., Williams L. R., Varon S. Morphological response of axotomized septal neurons to nerve growth factor. J Comp Neurol. 1988 Mar 1;269(1):147–155. doi: 10.1002/cne.902690112. [DOI] [PubMed] [Google Scholar]
- Gage F. H., Batchelor P., Chen K. S., Chin D., Higgins G. A., Koh S., Deputy S., Rosenberg M. B., Fischer W., Bjorklund A. NGF receptor reexpression and NGF-mediated cholinergic neuronal hypertrophy in the damaged adult neostriatum. Neuron. 1989 Feb;2(2):1177–1184. doi: 10.1016/0896-6273(89)90184-0. [DOI] [PubMed] [Google Scholar]
- Gage F. H., Wictorin K., Fischer W., Williams L. R., Varon S., Bjorklund A. Retrograde cell changes in medial septum and diagonal band following fimbria-fornix transection: quantitative temporal analysis. Neuroscience. 1986 Sep;19(1):241–255. doi: 10.1016/0306-4522(86)90018-7. [DOI] [PubMed] [Google Scholar]
- Hefti F., Dravid A., Hartikka J. Chronic intraventricular injections of nerve growth factor elevate hippocampal choline acetyltransferase activity in adult rats with partial septo-hippocampal lesions. Brain Res. 1984 Feb 20;293(2):305–311. doi: 10.1016/0006-8993(84)91237-x. [DOI] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986 Aug;6(8):2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman D., Breakefield X. O., Short M. P., Aebischer P. Transplantation of a polymer-encapsulated cell line genetically engineered to release NGF. Exp Neurol. 1993 Jul;122(1):100–106. doi: 10.1006/exnr.1993.1111. [DOI] [PubMed] [Google Scholar]
- Hoffman D., Wahlberg L., Aebischer P. NGF released from a polymer matrix prevents loss of ChAT expression in basal forebrain neurons following a fimbria-fornix lesion. Exp Neurol. 1990 Oct;110(1):39–44. doi: 10.1016/0014-4886(90)90049-x. [DOI] [PubMed] [Google Scholar]
- Horellou P., Brundin P., Kalén P., Mallet J., Björklund A. In vivo release of dopa and dopamine from genetically engineered cells grafted to the denervated rat striatum. Neuron. 1990 Oct;5(4):393–402. doi: 10.1016/0896-6273(90)90078-t. [DOI] [PubMed] [Google Scholar]
- Hoyle G. W., Mercer E. H., Palmiter R. D., Brinster R. L. Expression of NGF in sympathetic neurons leads to excessive axon outgrowth from ganglia but decreased terminal innervation within tissues. Neuron. 1993 Jun;10(6):1019–1034. doi: 10.1016/0896-6273(93)90051-r. [DOI] [PubMed] [Google Scholar]
- Kawaja M. D., Fagan A. M., Firestein B. L., Gage F. H. Intracerebral grafting of cultured autologous skin fibroblasts into the rat striatum: an assessment of graft size and ultrastructure. J Comp Neurol. 1991 May 22;307(4):695–706. doi: 10.1002/cne.903070414. [DOI] [PubMed] [Google Scholar]
- Kawaja M. D., Gage F. H. Morphological and neurochemical features of cultured primary skin fibroblasts of Fischer 344 rats following striatal implantation. J Comp Neurol. 1992 Mar 1;317(1):102–116. doi: 10.1002/cne.903170108. [DOI] [PubMed] [Google Scholar]
- Kawaja M. D., Rosenberg M. B., Yoshida K., Gage F. H. Somatic gene transfer of nerve growth factor promotes the survival of axotomized septal neurons and the regeneration of their axons in adult rats. J Neurosci. 1992 Jul;12(7):2849–2864. doi: 10.1523/JNEUROSCI.12-07-02849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos V. E., Applegate M. D., Knüsel B., Junard E. O., Burton L. E., Mobley W. C., Hefti F. F., Price D. L. Recombinant human nerve growth factor prevents retrograde degeneration of axotomized basal forebrain cholinergic neurons in the rat. Exp Neurol. 1991 May;112(2):161–173. doi: 10.1016/0014-4886(91)90066-l. [DOI] [PubMed] [Google Scholar]
- Koliatsos V. E., Clatterbuck R. E., Nauta H. J., Knüsel B., Burton L. E., Hefti F. F., Mobley W. C., Price D. L. Human nerve growth factor prevents degeneration of basal forebrain cholinergic neurons in primates. Ann Neurol. 1991 Dec;30(6):831–840. doi: 10.1002/ana.410300613. [DOI] [PubMed] [Google Scholar]
- Koliatsos V. E., Nauta H. J., Clatterbuck R. E., Holtzman D. M., Mobley W. C., Price D. L. Mouse nerve growth factor prevents degeneration of axotomized basal forebrain cholinergic neurons in the monkey. J Neurosci. 1990 Dec;10(12):3801–3813. doi: 10.1523/JNEUROSCI.10-12-03801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer L. F. Nerve growth factor treatment after brain injury prevents neuronal death. Science. 1987 Jan 9;235(4785):214–216. doi: 10.1126/science.3798108. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. B., Friedmann T., Robertson R. C., Tuszynski M., Wolff J. A., Breakefield X. O., Gage F. H. Grafting genetically modified cells to the damaged brain: restorative effects of NGF expression. Science. 1988 Dec 16;242(4885):1575–1578. doi: 10.1126/science.3201248. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Otten U., Agid Y., Thoenen H. Nerve growth factor (NGF) in the rat CNS: absence of specific retrograde axonal transport and tyrosine hydroxylase induction in locus coeruleus and substantia nigra. Brain Res. 1979 Jun 8;168(3):473–483. doi: 10.1016/0006-8993(79)90303-2. [DOI] [PubMed] [Google Scholar]
- Schweitzer E. S., Kelly R. B. Selective packaging of human growth hormone into synaptic vesicles in a rat neuronal (PC12) cell line. J Cell Biol. 1985 Aug;101(2):667–676. doi: 10.1083/jcb.101.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg I., Wetmore C. J., Ebendal T., Ernfors P., Persson H., Olson L. Rescue of basal forebrain cholinergic neurons after implantation of genetically modified cells producing recombinant NGF. J Neurosci Res. 1990 Mar;25(3):405–411. doi: 10.1002/jnr.490250318. [DOI] [PubMed] [Google Scholar]
- Tuszynski M. H., Buzsaki G., Gage F. H. Nerve growth factor infusions combined with fetal hippocampal grafts enhance reconstruction of the lesioned septohippocampal projection. Neuroscience. 1990;36(1):33–44. doi: 10.1016/0306-4522(90)90349-9. [DOI] [PubMed] [Google Scholar]
- Tuszynski M. H., Sang H., Yoshida K., Gage F. H. Recombinant human nerve growth factor infusions prevent cholinergic neuronal degeneration in the adult primate brain. Ann Neurol. 1991 Nov;30(5):625–636. doi: 10.1002/ana.410300502. [DOI] [PubMed] [Google Scholar]
- Tuszynski M. H., U H. S., Amaral D. G., Gage F. H. Nerve growth factor infusion in the primate brain reduces lesion-induced cholinergic neuronal degeneration. J Neurosci. 1990 Nov;10(11):3604–3614. doi: 10.1523/JNEUROSCI.10-11-03604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. R., Varon S., Peterson G. M., Wictorin K., Fischer W., Bjorklund A., Gage F. H. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9231–9235. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooteghem S. A., Shipley M. T. Factors affecting the sensitivity and consistency of the Koelle-Friedenwald histochemical method for localization of acetylcholinesterase. Brain Res Bull. 1984 May;12(5):543–553. doi: 10.1016/0361-9230(84)90170-9. [DOI] [PubMed] [Google Scholar]