Abstract
Eye formation in vertebrates is controlled by a conserved pattern of molecular networks. Homeobox transcription factors are crucially involved in the establishment and maintenance of the retina. A previous study of Massé et al. (Nature, 449: 1058–62, 2007) using morpholino knockdown identified the ectonucleotidase NTPDase2 and the P2Y1 receptor as essential elements for eye formation in embryos of the clawed frog Xenopus laevis. In order to investigate whether a similarly essential mechanism would be active in mammalian eye development, we analyzed mice KO for Entpd2 or P2ry1 as well as double KO for Entpd2/P2ry1. These mice developed normal eyes. In order to identify potential deficits in the molecular identity or in the arrangement of the cellular elements of the retina, we performed an immunohistological analysis using a variety of retinal markers. The analysis of single and double KO mice demonstrated that NTPDase2 and P2Y1 receptors are not required for murine eye formation, as previously shown for eye development in Xenopus laevis.
Keywords: NTPDase2, ATP, ADP, P2Y1 receptor, Purinergic signaling, Eye development
Introduction
Signaling via extracellular nucleotides is involved in the control of neurogenesis both in vitro and in vivo [1]. ATP, ADP, or UTP stimulates proliferation or migration of embryonic or adult neural progenitor cells in vitro via the metabotropic nucleotide receptors P2Y1 and P2Y2 [2–6]. Neural precursors in acute rat and mouse embryonic brain slices release ATP via hemichannels. ATP released from neural precursor cells induces receptor-mediated Ca2+ fluctuations in ventricular zone precursors of the developing brain and facilitates progenitor cell proliferation and migration within the developing cortex [7–9]. P2Y1 receptor-mediated increase in progenitor cell proliferation has been shown in situ for both murine adult neurogenic niches, the subventricular zone (SVZ) of the lateral ventricles [10, 11]), and the hippocampus [12]. The availability of extracellular nucleotides is controlled by ectonucleotidases, nucleotide-hydrolyzing enzymes bound to the cell surface. The ectonucleotidase nucleoside triphosphate diphosphohydrolase 2 (NTPDase2) is highly expressed in the neurogenic niches of the adult mouse brain. By dephosphorylating nucleoside triphosphates, it generates nucleoside diphosphates and eventually the respective nucleoside monophosphates, thus controlling their effect on nearby nucleotide receptors. We recently showed that mice null for Entpd2 reveal increased progenitor cell proliferation in the SVZ and the hippocampal dentate gyrus, suggesting that—by scavenging mitogenic extracellular nucleoside triphosphates—NTPDase2 acts as a homeostatic regulator of nucleotide-mediated neural progenitor cell proliferation and expansion [13].
In a previous study, single knockdown of NTPDase2 or of the P2Y1 receptor with specific antisense morpholino oligonucleotides impaired eye formation in tadpoles of the clawed frog Xenopus laevis. Simultaneous knockdown essentially abolished eye formation, suggesting that these two molecular components of the purinergic signaling pathway are essential for eye development [14]. Whether a similar mechanism also applies to the mammalian eye is unknown. Since the Xenopus NTPDase2 [15] and P2Y1 receptors [16] correspond to their mammalian counterparts, we analyzed the retinas of mice, in which the genes encoding NTPDase2 and P2Y1 were deleted. Our results suggest that eye formation is normal in Entpd2 single, P2ry1 single, or Entpd2/P2ry1 double KO mice.
Materials and methods
Animals
All animal experiments were approved by the local government and conducted under veterinary supervision in accordance with European regulations. Experiments were performed using mice aged 8–12 weeks. Animals were kept under 12 h light and dark cycle with food and water ad libitum. Entpd2 KO mice [13] and the corresponding wild types (litters) were bred in house. The resultant mutant mice were screened by PCR and homozygous Entpd2 mice were created, in which the gene deletion was validated by PCR and immunohistochemistry. Entpd2/P2ry1 double KO mice were generated by the combination of the homozygous deletion of the Entpd2 allele and the P2ry1 allele [17] within one mouse line.
Characterization of genotypes by PCR
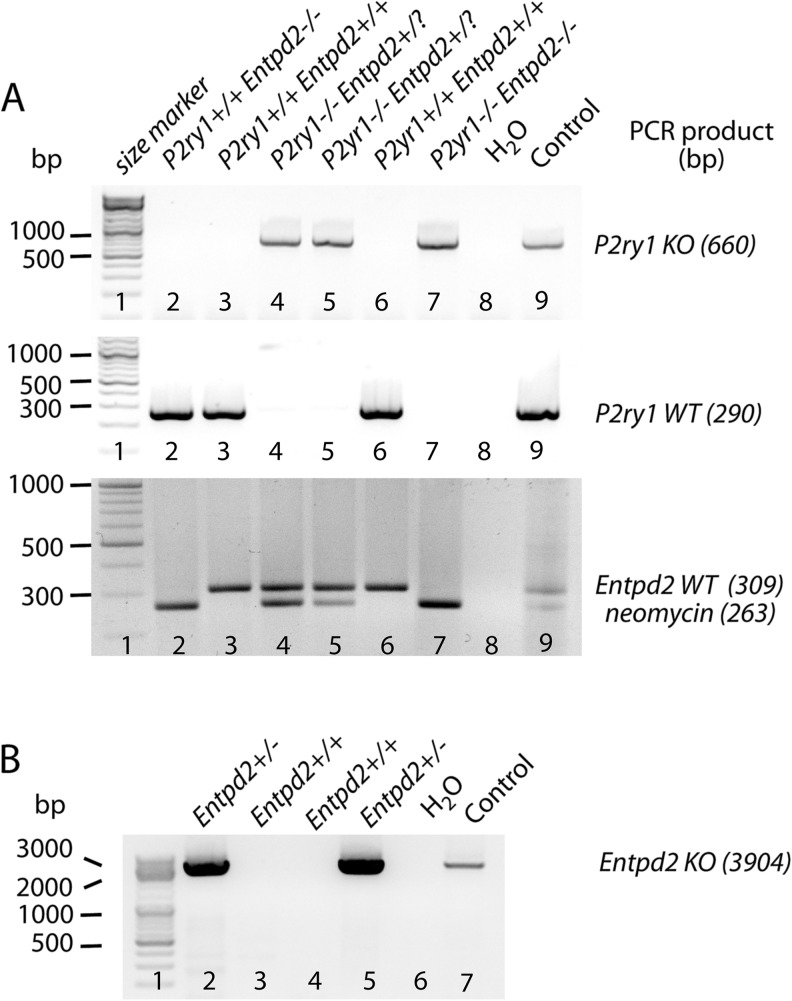
Gene deletion was confirmed by a two-step genotyping procedure using oligonucleotides given in Table 1 (Fig. 1). Genomic DNA was obtained from tail biopsies of 3–4 weeks old pups. One tail biopsy per animal was digested with lysis buffer (1.7 μM sodium dodecyl sulfate, 100 μg/ml proteinase K and 1× gitschier buffer, 16.6 mM ammonium sulfate, 67 mM Tris/HCl, 6.7 mM MgCl2, 5 mM β-mercapto ethanol, and 6.7 μM EDTA) at 55 °C over night. Lysates were heat-inactivated for 30 min at 95 °C. PCR was performed applying oligonucleotides discriminating between P2ry1 mutated and P2ry1 WT alleles. PCRs resulted in fragment sizes of 660 and 290 bp for P2ry1 mutated and WT loci, respectively (Fig. 1a) [17, 18]. For detection of the Entpd2 genotype, PCR was performed using primers selectively identifying the Entpd2 WT allele (fragment size 309 bp) as well as primers, which amplify the neomycin selection cassette (fragment size 263 bp) (Fig. 1a) [13]. Since the neomycin selection cassette is also contained in the P2ry1-mutated allele, tail biopsies of animals with the genotype P2ry1−/− Entpd2+/? (? = unknown genotype of second allele) were further analyzed for the presence of the Entpd2 KO allele (Fig. 1b). Genomic DNA from second tail biopsies was purified using the DNeasy Blood and Tissue Kit (Qiagen), and PCR was performed using a long template polymerase (Expand Long Template PCR System, Roche) including one primer (Entpd2 KO, Table 1) hybridizing within the neomycin selection cassette and one primer annealing in the downstream Entpd2 WT sequence. This only results in the expected 3904 bp fragment if the Entpd2 KO allele is present [13].
Table 1.
Oligonucleotides used for genotyping mice
| Amplified sequence | Forward primer | Reverse primer | Product size (bp) |
|---|---|---|---|
| Entpd2 WT | gttagggggtgtctgtctgg | aaatgctgcctggaaatgg | 309 |
| neomycin cassette | ccgtaaagcacgaggaagc | ctggacgaagagcatcagg | 263 |
| Entpd2 KO | tcctgtcatctcaccttgctcctgc | gtttaagtgttgctctctgaccaactggc | 3904 |
| P2ry1 WT | acagtactgtcgcctcaactgcagcagttt | ccgaagatccagtcagtcttgttgaagtag | 290 |
| P2ry1 KO | gccttctatcgccttcttgacgagttcttc | ccgaagatccagtcagtcttgttgaagtag | 660 |
Fig. 1.
Identification of P2ry1 and Entpd2 genotypes by PCR using a two-step genotyping procedure. a Mice were genotyped for P2ry1 KO alleles (660 bp, upper image), P2ry1 WT alleles (290 bp, middle image), Entpd2 WT alleles (309 bp), and the neomycin selection cassette (263 bp, bottom image). b Tail biopsies of animals revealing the genotype P2ry1−/− Entpd2+/? [e.g., lanes 4, 5 in (a)] were further analyzed for the presence of the Entpd2 KO allele (3904 bp). Water and lysates from heterozygous animal served as controls [lanes 8 and 9 in (a) and lanes 6 and 7 in (b)]. ? Unknown genotype of second allele; bp base pairs
Immunohistochemistry
For analysis of retinas, mice were deeply anesthetized with isoflurane and decapitated. The eyes were enucleated, the anterior segments were removed, and the posterior eyecups were immersion fixed for 15–30 min in 4 % paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. After fixation, retinas were dissected from the eye cup, cryoprotected in graded sucrose solutions (10, 20, and 30 %), and sectioned vertically at 14 μm on a cryostat. Sections were collected on Super-Frost glass slides and stored at −20 °C until use.
Immunolabeling was carried out using the indirect fluorescence method. Retinal sections were blocked for 1 h in a solution containing 10 % normal donkey serum (NDS) and 0.5 % Triton-X-100 in PB. Primary antibodies were diluted in 3 % NDS and 0.5 % Triton-X-100 in PB applied overnight at room temperature. These included rabbit anti-calbindin (1:2000; Swant), mouse anti-CtBP2 (1:5000; BD Transduction), rabbit anti-PKCα (1:10,000; Sigma-Aldrich), goat anti-choline acetyltransferase (1:200, Millipore), goat anti-glycine transporter 1 (1:1000; Millipore), mouse anti-glutamine synthetase (1:500, BD Transduction), and mouse anti-synaptotagmin 2 (1:1000; ZIRC, Eugene). After overnight incubation and washing in PB, secondary antibodies conjugated to Alexa 488 (Invitrogen) or Cy3 (Dianova) were applied for 1 h. Confocal images were taken using a Zeiss LSM 5 Pa confocal microscope, equipped with an argon and a HeNe laser. Optical sections were acquired with a 40×/1.3 Plan-Neofluar objective, and projections of stacks of 3–6 μm were used for figures. Brightness and contrast of the final images were adjusted using Adobe Photoshop. The imaging conditions (laser intensity, pinhole diameter, and detector gain) and digital processing were identical for all images within a series/experiment.
Results
In order to investigate whether NTPDase2 and the P2Y1 receptor are essential for the development of the mammalian eye, we analyzed the eyes of single knockouts for NTPDase2 and P2Y1. Both Entpd2 and P2ry1 KO mice developed apparently normal eyes. We therefore generated in addition Entpd2/P2ry1 double KO mice. These mice also developed normal eyes. We could not exclude, however, that there might be deficits in the molecular identity or the arrangement of the cellular elements of the retinas. We therefore performed a detailed immunohistological analysis using a variety of retinal markers. These included protein kinase C alpha (PKCα; marker for rod bipolar cells [19]), C-terminal binding protein 2 (CtBP2; marker for synaptic ribbons in photoreceptor and bipolar cell terminals [20]), calbindin (marker for horizontal cells in the outer retina, amacrine, displaced amacrine, and ganglion cells in the inner retina [19]), choline acetyltransferase (marker for starburst amacrine cells [19]), glycine transporter 1 (marker for glycinergic amacrine cells [19]), glutamine synthetase (marker for Müller glia cells [19]), and synaptotagmin 2 (marker for bipolar cell terminals [21]). All knockout animals, single or double, revealed the normal retinal structure and cellular distribution. This is depicted in Fig. 2 for calbindin and in Fig. 3 for CtBP2 and PKCα. Figure 4 further shows the retinal distribution of synaptotagmin 2, glycine transporter 1, choline acetyltransferase, and glutamine synthase in wild type and Entpd2/P2ry1 double KO mice. Accordingly, KO mice reveal a normal retinal pattern of horizontal cells in the outer retina, amacrine, displaced amacrine, and ganglion cells in the inner retina, photoreceptor and bipolar cell terminals, rod bipolar cells, glycinergic amacrine cells, starbust amacrine cells, and Müller glia cells. These data suggest that deletion of Entpd2, P2ry1, or both does not prevent formation of structurally normal eyes in mice.
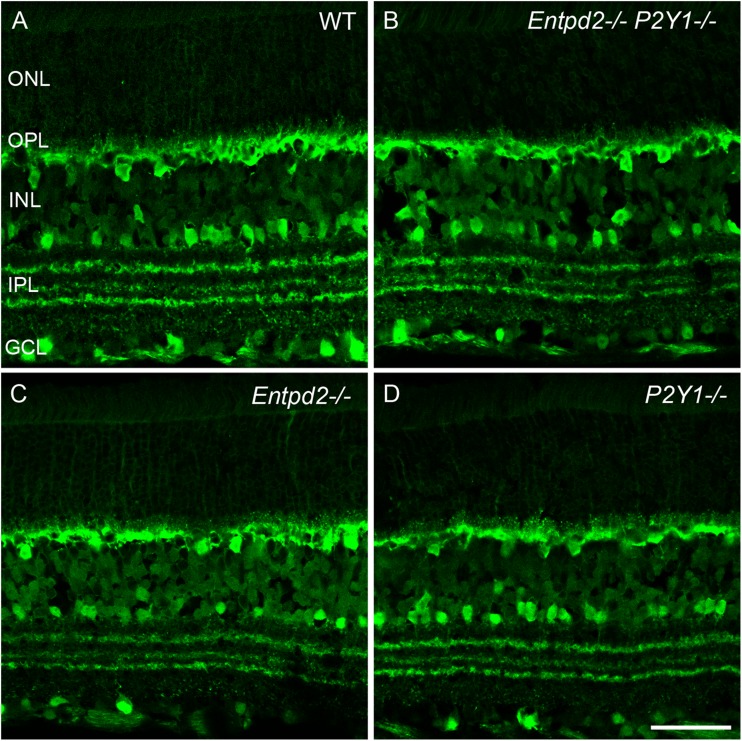
Fig. 2.
Distribution of calbindin in the retinas of WT mice (a) and mice KO for Entpd2 (c) or P2ry1 (d), and Entpd2/P2ry1 double KO (b). All KO mice reveal a normal retinal distribution of the calcium-binding protein. ONL outer nuclear layer; OPL outer plexiform layer; INL inner nuclear layer; IPL inner plexiform layer; GCL ganglion cell layer. (Scale bar, 50 μm for A-D)
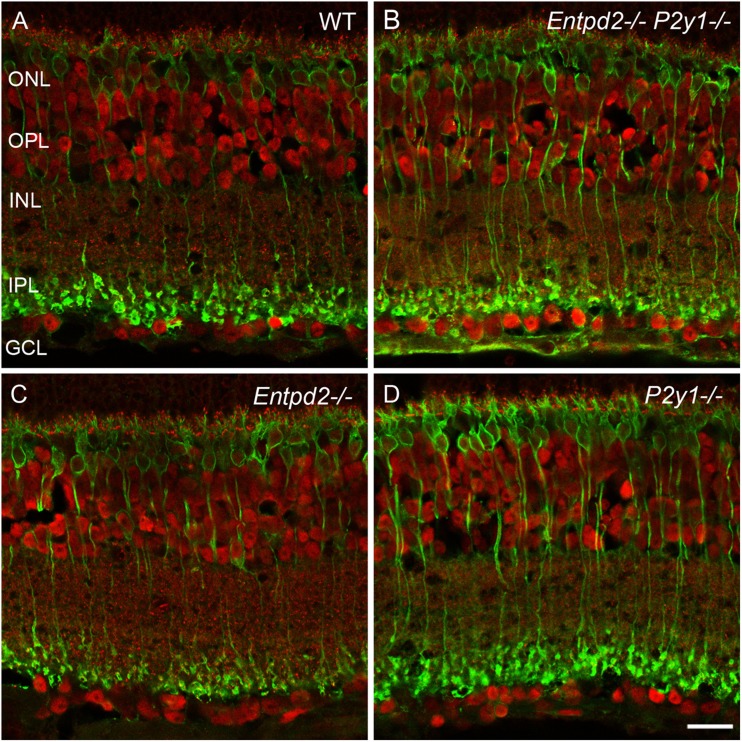
Fig. 3.
Distribution of CtBP2 (C-terminal binding protein 2) (red) and protein kinase C α (green) in the retinas of WT mice (a) and mice KO for Entpd2 (c) or P2ry1 (d), and Entpd2/P2ry1 double KO (b). All KO mice reveal a normal retinal pattern of ribbon synapses and rod bipolar cells, respectively. ONL outer nuclear layer; OPL outer plexiform layer; INL inner nuclear layer; IPL inner plexiform layer; GCL ganglion cell layer. (Scale bar, 20 μm for a–d)
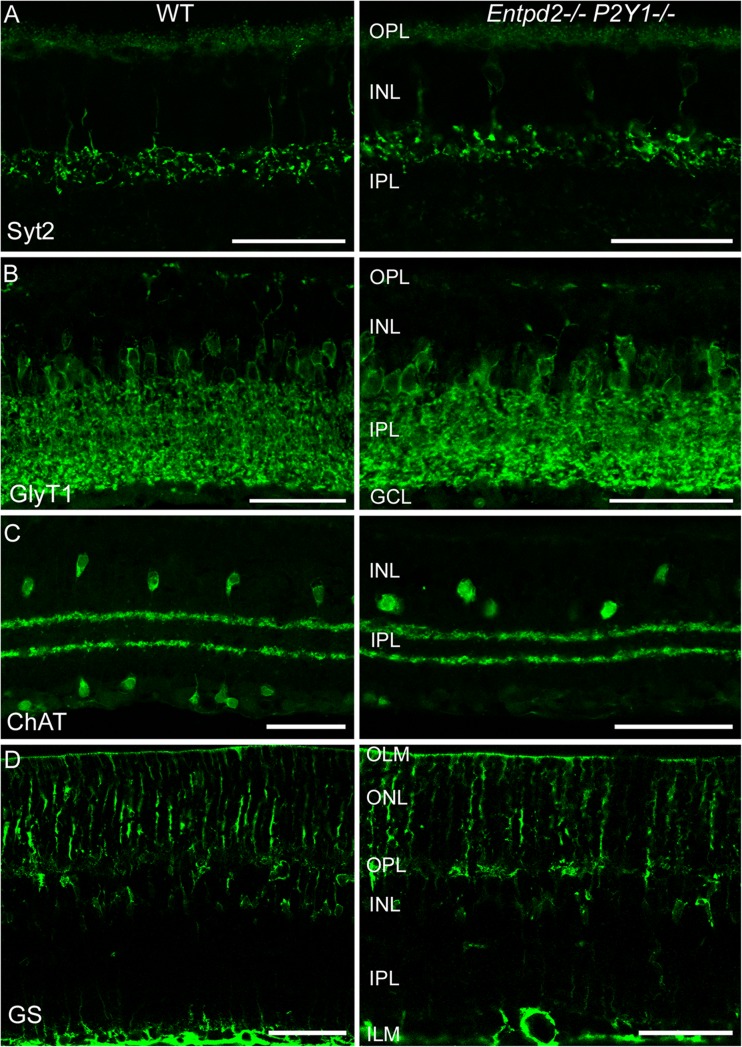
Fig. 4.
Distribution of bipolar cell terminals (synaptotagmin 2, Syt2) (a), glycinergic amacrine cells (glycine transporter 1, GlyT1) (b), starburst amacrine cells (choline acetyltransferase, ChAT) (c), and Müller glia cells (glutamine synthetase, GS) (d) in the retinas of WT (left) and Entpd2/P2ry1 double KO (right) mice. OLM outer limiting membrane; ONL outer nuclear layer; OPL outer plexiform layer; INL inner nuclear layer; IPL inner plexiform layer; GCL ganglion cell layer; ILM inner limiting membrane. (Scale bars, 50 μm for a–d)
Discussion
The observation that in the frog Xenopus laevis eye development depends on nucleotide signaling involving NTPDase2 and P2Y1 receptors [14] raised the question whether this would also apply to other vertebrates, including mammals. Notably, in this study, the signaling pathways involving the P2Y1 receptor and NTPDase2 were found to lie upstream of eye field transcription factors such as Pax6, Rx1, and Six3, and thus apparently control the development of the entire organ. NTPDase2 knockdown greatly reduced Pax6 expression. Purine-mediated signaling would thus trigger both eye field transcription factors and eye development. This is of considerable interest since in vertebrates key factors involved in early eye field specification and retinal-specific transcription factors are highly conserved [22, 23]. Nucleotide signaling involving the ectonucleotidase NTPDase2 and the P2Y1 receptor might therefore represent an additional essential pathway involved in early eye development.
In adult mammals, purinergic signaling is involved in the modulation of the physiological and patho-physiological responses of ocular tissue, involving both P2X and P2Y receptors [24]. P2Y1 receptors are the principal receptors on Müller glial cells [25], but they are also expressed by cholinergic amacrine cells [26]. It has previously been shown that ATP stimulates proliferation of neural retinal progenitor cells in the embryonic chicken retina [27, 28]. ATP also controls the cell cycle and induces proliferation in retinal explants of newborn mice, presumably via the P2Y1 receptor [29]. NTPDase2 is mainly associated with Müller glial cells [30, 31].
Our finding that eye formation and retinal functional topography was not affected in Entpd2 KO, P2ry1 KO, or Entpd2/P2ry1 double KO mice does not exclude a contribution of P2Y1 and NTPDase2 to early murine eye induction or development in wild-type animals. But it suggests that these molecular components are not essential. Analysis of mature eyes cannot exclude the compensatory up-regulation of other purine receptors or ectonucleotidases at early stages of eye development. However, deletion of Entpd2 does not induce up-regulation of any other ectonucleotidase in mouse brain [13]. Furthermore, visual external inspection of the eyes of mice deficient of the P2Y2 or the P2Y13 receptor revealed no apparent abnormalities suggesting that these animals have developed normal eyes (Gampe and Zimmermann, unpublished observations) and for none of the other ectonucleotidases or P2 receptor KO animals’ aberrant eye development has been reported. In summary, our data reveal that NTPDase2 and the P2Y1 receptors are not required for murine eye formation, as previously shown for eye development in Xenopus laevis [14].
Acknowledgments
This work was supported by grants from the Cluster of Excellence EXC 115 and Gutenberg Research College (GCR) Mainz University (to A A-P) and from NIH (R21 CA164970/NCI and HL R01 094400/NHLBI) (to SC R).
Conflict of interest
The authors indicate no potential conflicts of interest.
References
- 1.Zimmermann H. Purinergic signaling in neural development. Semin Cell Dev Biol. 2011;22:194–204. doi: 10.1016/j.semcdb.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Scemes E, Duval N, Meda P. Reduced expression of P2Y1 receptors in connexin43-null mice alters calcium signaling and migration of neural progenitor cells. J Neurosci. 2003;23:11444–11452. doi: 10.1523/JNEUROSCI.23-36-11444.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra SK, Braun N, Shukla V, Füllgrabe M, Schomerus C, Korf H-W, Gachet C, Ikehara Y, Sévigny J, Robson SC, Zimmermann H. Extracellular nucleotide signaling in adult neural stem cells: Synergism with growth factor-mediated cellular proliferation. Development. 2006;133:675–684. doi: 10.1242/dev.02233. [DOI] [PubMed] [Google Scholar]
- 4.Lin JHC, Takano T, Arcuino G, Wang XH, Hu FR, Darzynkiewicz Z, Nunes M, Goldman SA, Nedergaard M. Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev Biol. 2007;302:356–366. doi: 10.1016/j.ydbio.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Striedinger K, Meda P, Scemes E. Exocytosis of ATP from astrocyte progenitors modulates spontaneous Ca2+ oscillations and cell migration. Glia. 2007;55:652–662. doi: 10.1002/glia.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimm I, Ullsperger SN, Zimmermann H. Nucleotides and epidermal growth factor induce parallel cytoskeletal rearrangements and migration in cultured adult murine neural stem cells. Acta Physiol. 2010;199:181–189. doi: 10.1111/j.1748-1716.2010.02092.x. [DOI] [PubMed] [Google Scholar]
- 7.Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Liu XX, Hashimoto-Torii K, Torii M, Haydar TF, Rakic P. The role of ATP signaling in the migration of intermediate neuronal progenitors to the neocortical subventricular zone. Proc Natl Acad Sci U S A. 2008;105:11802–11807. doi: 10.1073/pnas.0805180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Hashimoto-Torii K, Torii M, Ding C, Rakic P. Gap junctions/hemichannels modulate interkinetic nuclear migration in the forebrain precursors. J Neurosci. 2010;30:4197–4209. doi: 10.1523/JNEUROSCI.4187-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suyama S, Sunabori T, Kanki H, Sawamoto K, Gachet C, Koizumi S, Okano H. Purinergic signaling promotes proliferation of adult mouse subventricular zone cells. J Neurosci. 2012;32:9238–9247. doi: 10.1523/JNEUROSCI.4001-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boccazzi M, Rolando C, Abbracchio MP, Buffo A, Ceruti S. Purines regulate adult brain subventricular zone cell functions: Contribution of reactive astrocytes. Glia. 2014;62:428–439. doi: 10.1002/glia.22614. [DOI] [PubMed] [Google Scholar]
- 12.Cao X, Li LP, Qin XH, Li SJ, Zhang M, Wang Q, Hu HH, Fang YY, Gao YB, Li XW, Sun LR, Xiong WC, Gao TM, Zhu XH. Astrocytic ATP release regulates the proliferation of neural stem cells in the adult hippocampus. Stem Cells. 2013;31:1633–1643. doi: 10.1002/stem.1408. [DOI] [PubMed] [Google Scholar]
- 13.Gampe K, Stefani J, Hammer K, Brendel P, Pötzsch A, Enikolopov G, Enjyoji K, Acker-Palmer A, Robson SC, Zimmermann H. NTPDase2 and purinergic signaling control progenitor cells proliferation in neurogenic niches of the adult mouse brain. Stem Cells. 2014 doi: 10.1002/stem.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massé K, Bhamra S, Eason R, Dale N, Jones EA. Purine-mediated signalling triggers eye development. Nature. 2007;449:1058–1062. doi: 10.1038/nature06189. [DOI] [PubMed] [Google Scholar]
- 15.Massé K, Eason R, Bhamra S, Dale N, Jones EA. Comparative genomic and expression analysis of the conserved NTPDase gene family in Xenopus. Genomics. 2006;87:366–381. doi: 10.1016/j.ygeno.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Cheng AWM, Kong LW, Tung EKK, Siow NL, Choi RCY, Zhu SQ, Peng BH, Tsim KWK. cDNA encodes Xenopus P2Y1 nucleotide receptor: Expression at the neuromuscular junctions. Neuroreport. 2003;14:351–357. doi: 10.1097/00001756-200303030-00011. [DOI] [PubMed] [Google Scholar]
- 17.Léon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, Dierich A, LeMeur M, Cazenave JP, Gachet C. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y1 receptor-null mice. J Clin Invest. 1999;104:1731–1737. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laplante MA, Monassier L, Freund M, Bousquet P, Gachet C. The purinergic P2Y1 receptor supports leptin secretion in adipose tissue. Endocrinology. 2010;151:2060–2070. doi: 10.1210/en.2009-1134. [DOI] [PubMed] [Google Scholar]
- 19.Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. doi: 10.1002/1096-9861(20000814)424:1<1::AID-CNE1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 20.tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, Fejtová A, Bracko O, Gundelfinger ED, Brandstätter JH. Molecular dissection of the photoreceptor ribbon synapse: Physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J Cell Biol. 2005;168:825–836. doi: 10.1083/jcb.200408157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wässle H, Puller C, Müller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- 23.Sinn R, Wittbrodt J. An eye on eye development. Mech Dev. 2013;130:347–358. doi: 10.1016/j.mod.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Sanderson J, Dartt DA, Trinkaus-Randall V, Pintor J, Civan MM, Delamere NA, Fletcher EL, Salt TE, Grosche A, Mitchell CH. Purines in the eye: Recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Müller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp Eye Res. 2014;127:270–279. doi: 10.1016/j.exer.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wurm A, Erdmann I, Bringmann A, Reichenbach A, Pannicke T. Expression and function of P2Y receptors on Müller cells of the postnatal rat retina. Glia. 2009;57:1680–1690. doi: 10.1002/glia.20883. [DOI] [PubMed] [Google Scholar]
- 26.Dilip R, Ishii T, Imada H, Wada-Kiyama Y, Kiyama R, Miyachi E, Kaneda M. Distribution and development of P2Y1-purinoceptors in the mouse retina. J Mol Histol. 2013;44:639–644. doi: 10.1007/s10735-013-9525-4. [DOI] [PubMed] [Google Scholar]
- 27.Sugioka M, Zhou WL, Hofmann HD, Yamashita M. Involvement of P2 purinoceptors in the regulation of DNA synthesis in the neural retina of chick embryo. Int J Dev Neurosci. 1999;17:135–144. doi: 10.1016/S0736-5748(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 28.Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Sholl-Franco A, Fragel-Madeira L, Macama AC, Linden R. Ventura AL (2010) ATP controls cell cycle and induces proliferation in the mouse developing retina. Int J Dev Neurosci. 2010;28:63–73. doi: 10.1016/j.ijdevneu.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Iandiev I, Wurm A, Pannicke T, Wiedemann P, Reichenbach A, Robson SC, Zimmermann H, Bringmann A. Ectonucleotidases in Müller glial cells of the rodent retina: Involvement in inhibition of osmotic cell swelling. Purinergic Signal. 2007;3:423–433. doi: 10.1007/s11302-007-9061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricatti MJ, Alfie LD, Lavoie EG, Sévigny J, Schwarzbaum PJ, Faillace MP. Immunocytochemical localization of NTPDases1 and 2 in the neural retina of mouse and zebrafish. Synapse. 2009;63:291–307. doi: 10.1002/syn.20605. [DOI] [PubMed] [Google Scholar]