Abstract
Docosahexaenoic acid (DHA) is important for central nervous system function during pathological states such as ischemia. DHA reduces neuronal injury in experimental brain ischemia; however, the underlying mechanisms are not well understood. In the present study, we investigated the effects of DHA on acute hippocampal slices subjected to experimental ischemia by transient oxygen and glucose deprivation (OGD) and re-oxygenation and the possible involvement of purinergic receptors as the mechanism underlying DHA-mediated neuroprotection. We observed that cellular viability reduction induced by experimental ischemia as well as cell damage and thiobarbituric acid reactive substances (TBARS) production induced by glutamate (10 mM) were prevented by hippocampal slices pretreated with DHA (5 μM). However, glutamate uptake reduction induced by OGD and re-oxygenation was not prevented by DHA. The beneficial effect of DHA against cellular viability reduction induced by OGD and re-oxygenation was blocked with PPADS (3 μM), a nonselective P2X1–5 receptor antagonist as well as with a combination of TNP-APT (100 nM) plus brilliant blue (100 nM), which blocked P2X1, P2X3, P2X2/3, and P2X7 receptors, respectively. Moreover, adenosine receptors blockade with A1 receptor antagonist DPCPX (100 nM) or with A2B receptor antagonist alloxazine (100 nM) inhibited DHA-mediated neuroprotection. The addition of an A2A receptor antagonist ZM241385 (50 nM), or A3 receptor antagonist VUF5574 (1 μM) was ineffective. Taken together, our results indicated that neuroprotective actions of DHA may depend on P2X, A1, and A2B purinergic receptors activation. Our results reinforce the notion that dietary DHA may act as a local purinergic modulator in order to prevent neurodegenerative diseases.
Keywords: DHA, Adenosine receptors, ATP receptors, Neuroprotection
Introduction
In the USA, stroke represents the third major cause of death in adults, affecting over 800,000 persons per year [73]. Ischemic stroke, the predominant stroke subtype in many populations, has defined risk factors that can, in part, be effectively reduced through preventive regimens [77]. Diet intervention is one example that may impact upon risk factors for stroke [79]. Long-chain n-3 polyunsaturated fatty acids (n-3 FA), especially docosahexaenoic acid (DHA, C22:6 n-3) which is found in high concentration in marine foods, are suggested to be one of these preventive nutrients [8].
DHA is the major n-3 FA in the mammalian central nervous system (CNS) and it can be obtained through diet or to a limited extent via conversion from its precursor, alfa-linolenic acid, in the liver and astrocytes [60]. During brain ischemia and reperfusion, DHA is cleaved from membrane phospholipids to free (unesterified) DHA by phospholipase A2 reaching up micromolar concentrations [58].
Concerning the protective roles of DHA against injuries in animal models, it has been shown that DHA protects rats against excitotoxicity and seizures [44, 71] and reduces neuronal damage in experimental brain ischemia [6, 13]. DHA attenuate brain necrosis after hypoxic ischemic injury, mainly by increasing antioxidant activity for better coping with reactive oxygen species (ROS) [43]. Alternatively, in the onset of brain injury, DHA could be released from the membrane phospholipids and generate the neuroprotectin D1 (NPD1), a docosanoid responsible for some protective effects mediated by DHA [4]. In spite of that, the exact mechanism by which DHA exerts neuroprotective effect against ischemic insult is not fully understood and DHA is believed to affect multiple pathways [3, 24].
Oxygen and glucose deprivation (OGD) in hippocampal brain slices is an established model of ischemia in vitro that can be used to explore potential protective compounds in a cerebral structure particularly susceptible to ischemic insult. Moreover, the hippocampal slice is a fresh tissue preparation that maintains the cellular architecture and connections observed in situ [2, 56]. During brain ischemia, the rapid energy failure causes ATP depletion and ionic imbalance, Na+/K+ electrochemical gradient collapse, resulting in increased glutamate release to the extracellular milieu. After OGD, when oxygen levels are normalized in the re-oxygenation period, additional generation of ROS mostly due to the mitochondrial electron transport chain, may reverse glutamate transporters activity, leading to excessive glutamate release [23] and excitotoxicity [39]. It has been previously demonstrated that the decrease on glutamate uptake evoked by OGD was attenuated in hippocampal slices obtained from rats supplemented with n-3 FA, suggesting DHA may modulate excitotoxicity [49].
It has been shown that DHA release from membranes follows activation of ATP receptors [70] and DHA modulates ATP-induced inward currents [28]. ATP is a pleiotropic cell signaling molecule in the brain that functions through activation of the P2 receptors, named ionotropic P2X or metabotropic P2Y receptors. Noxious brain insults can increase the extracellular levels of ATP, and different P2 receptors are involved in the control of ischemic brain damage [14, 31, 32, 57].
Adenosine extracellular concentration has been shown to be increased during an ischemic situation. It can occur due to the imbalance between ATP degradation and re-synthesis, increasing intracellular adenosine levels, leading to its release to extracellular space by the activity of nucleoside transporters. Additionally, adenosine formation can also take place at the extracellular space, through the hydrolysis of released ATP [81]. Adenosine displays its effects mediated by activation of members of a family of G protein-coupled receptors, A1, A2A, A2B, and A3 receptors. In the CNS, adenosine exerts a wide range of effects [33, 34], but it has a general inhibitory presynaptic activity on glutamatergic transmission [19] and a protective role against excitotoxic/ischemic damage in the brain [40].
In this study, we investigated the neuroprotective role of DHA-enrichment of hippocampal slices against cell damage induced by experimental ischemia. Studies on DHA-mediated neuroprotection have prompted the question regarding the mechanism by which DHA provide neuroprotection. Thus, we have verified if the neuroprotective effect of DHA against OGD-induced cell damage in hippocampal slices involves the modulation of processes that contributes to neuroprotection against brain ischemia, such as suppression of excitotoxicity and modulation of the purinergic system. Here, we demonstrate that OGD produces a substantial reduction on cellular viability in hippocampal slices which can be prevented by preincubation with DHA. The neuroprotection afforded by DHA against OGD and excitotoxicity was not dependent on glutamate transport, but it involves the modulation of P2X as well as A1 and A2B adenosine receptors.
Materials and methods
Drugs
L-[3H]glutamate was purchased from GE Healthcare. All other reagents were obtained from Sigma, St. Louis, MO, USA. DHA was dissolved weekly in ethanol at 10 mM concentration and stored at −20 °C before use. The experimental concentration of DHA was obtained by dilution of the stocks. When DHA stocks were diluted to the concentrations tested (1–50 μM), the concentration of ethanol was 0.16 % (g/100 ml). Control studies revealed that this concentration of ethanol had no effect on hippocampal cell viability (data not shown). Nonselective P2 receptor antagonist pyridoxal phosphate-6-azophenyl-2,4-disulfonic acid (PPADS), P2X1, P2X3, and P2X2/3 receptor antagonist (2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate monolithium trisodium salt) (TNP-ATP); P2X7 receptor antagonist brilliant blue (BBG) were dissolved in water. A1 receptor selective antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), A2A receptor antagonist 4-(−2-[7-amino-2-{2-furyl}{1,2,4}triazolo{2,3-a} {1,3,5}triazin-5-yl-amino]ethyl)phenol (ZM241385) and alloxazine were dissolved in dimethylsufoxide (DMSO). The final concentration of these compounds did not exceed 0.01 % DMSO when added to slices. The selective A3 receptor antagonist N-(2-methoxyphenyl)-N′-[2-(3-pyridinyl)-4-quinazolinyl]-urea (VUF5574, 1 μM) was also prepared in DMSO and added to slices at a final concentration of 1 % DMSO. The antagonists were then diluted in physiological buffer to reach its final concentration: 3 μM PPADS; 100 nM TNP-ATP and 100 nM BBG [11, 38]; 100 nM DPCPX and 50 nM ZM241385 [47]; 100 nM alloxazine and 1 μM VUF5574 [30].
Animals
Male Swiss mice (60–90 days of postnatal age and weighted 20–30 g) were maintained on a 12-hour (h) light-12 h dark schedule at 25 °C, with food and water ad libitum. Experiments followed the “Principles of laboratory animal care” (NIH publication No. 85–23, revised 1985) and were approved by the local Ethical Committee of Animal Research (CEP/UnC 08/2012).
Hippocampal slices preparation and incubation
Mice were killed by decapitation, and the hippocampi were rapidly removed and placed in ice-cold HEPES-saline buffer (HS) of the following composition: 124 mM NaCl, 3 mM KCl, 1.0 mM CaCl2, 1.2 mM MgSO4, 1.25 mM KH2PO4, 12 mM D-glucose, 20 mM HEPES. The buffer was bubbled with 95 % O2 up to pH 7.4. Slices (0.4 mm thick) were rapidly prepared using a McIlwain Tissue Chopper, separated in HS at 4 °C and allowed to recover for 30 min in HS at 37 °C to slices stabilization [56].
Experimental ischemia by transient oxygen and glucose deprivation in hippocampal slices
To induce OGD, the slices were incubated in the oxygen and glucose deprived buffer (were D-glucose was replaced by 2-deoxyglucose) under 95 % N2/5 % CO2 for 15 min [68]. After OGD, hippocampal slices were incubated in the presence of oxygen and glucose (HS and bubbled with 95 % O2) for another 2 h (re-oxygenation period) (2). In order to verify the neuroprotective effect of DHA, slices treatment was performed as follows: (a) slices were preincubated with DHA diluted in HS for 15 min, subjected to OGD in the presence of DHA, and then allowed to re-oxygenate for 2 h without DHA; (b) slices were subjected to OGD and then DHA was added in re-oxygenation period [9]. In experiments where adenosine or ATP receptors antagonists were tested, hippocampal slices were treated with the antagonists for 15 min before adding DHA and they were kept together with DHA during preincubation and OGD period. These included the following: nonselective P2X receptors antagonist PPADS (3 μM); P2X1, P2X3, and P2X2/3 selective receptor antagonist TNP-ATP (100 nM); P2X7 selective receptor antagonist BBG (100 nM); A1 selective receptor antagonist (DPCPX, 100 nM); A2A selective receptor antagonist (ZM241385, 50 nM); A2B antagonist (aloxazine, 0.1 μM); and A3R antagonist (VUF5574, 1 μM). In all sets of experiments, control group was incubated for the entire duration of the experiments in the presence of oxygen and glucose (HS and bubbled with 95 % O2) and cellular viability was consider as 100 %.
Excitotoxicity protocol
To verify the neuroprotective effect of DHA against excitotoxicity, hippocampal slices were incubated for 2 h in HF solution containing 10 mM of glutamate [48]. When present, DHA was preincubated for 15 min before adding glutamate. The cellular viability was evaluated at the end of 2 h incubation with glutamate. In all sets of experiments, control group was incubated for the entire duration of the experiments in HS and cellular viability considered as 100 %.
Cellular viability assay
Cell viability was determined through the ability of cells to reduce MTT (3-(4,5-dimethylthiazol-2-yl-diphenyltetrazolium bromide, Sigma) [51]. Hippocampal slices were incubated with MTT (0.5 mg/ml) in HS for 30 min at 37 °C. The tetrazolium ring of MTT can be cleaved by active dehydrogenases in order to produce a precipitated formazan. The formazan produced was solubilized by adding 200 μl DMSO, resulting in a colored compound which optical density was measured in a microplate reader (Labsystems Multiskan MS-550 nm).
Glutamate uptake assay
L-[3H]glutamate uptake was evaluated as previously described [47]. After OGD and re-oxygenation protocol, hippocampal slices were maintained for 15 min at 37 °C in a Hank’s balanced salt solution (HBSS), composition in mM: 1.3 CaCl2, 137 NaCl, 5 KCl, 0.65 MgSO4, 0.3 Na2HPO4, 1.1 KH2PO4, 2 glucose, and 5 HEPES. Uptake was assessed by adding 0.33 μCi/ml L-[3H]glutamate with 100 μM of unlabeled glutamate. Incubation was stopped immediately after 7 min by discarding the incubation medium, and slices were subjected to two ice-cold washes with 1 ml HBSS. Slices were solubilized by adding a solution with 0.1 % NaOH/0.01 % SDS and incubated overnight. Aliquots of slice lysates were taken for determination of the intracellular content of L-[3H]glutamate by scintillation counting. Sodium-independent uptake was determined by using choline chloride, instead of sodium chloride in the HBSS buffer. Unspecific sodium-independent uptake (approximately 30 % of total glutamate uptake) was subtracted from total uptake to obtain the specific sodium-dependent glutamate uptake. Results were expressed in nmol of L [3H]glutamate and taken up per mg of protein per min.
Thiobarbituric acid reactive substances assay
The lipid peroxidation end-products were determined by the thiobarbituric acid reactive substances (TBARS) assay originally described by Ohkawa et al. [54]. At the end of the experiment, samples were incubated with 0.45 M acetic acid/HCl buffer, pH 3.4, 0.28 % thiobarbituric acid, 1.2 % SDS, and thereafter at 95 °C for 60 min to promote color reaction, measured at 532 nm. Malondialdehyde (0 to 3 nmol/mL) was used as a standard. Protein concentration was determined by the method of Lowry [41] using bovine serum albumin as standard.
Statistical analysis
Results are expressed as means ± standard errors of means (SEM). Comparisons among groups were performed by one-way analysis of variance (ANOVA) followed by Tukey’s test with P < 0.05 considered to be statistically significant.
Results
OGD-induced cell damage is prevented by DHA
The aim of the current study was to elucidate the possible involvement of excitotoxicity inhibition and/or purinergic system in the neuroprotective effect of DHA against transient oxygen and glucose deprivation (OGD)-induced damage in mice hippocampal slices.
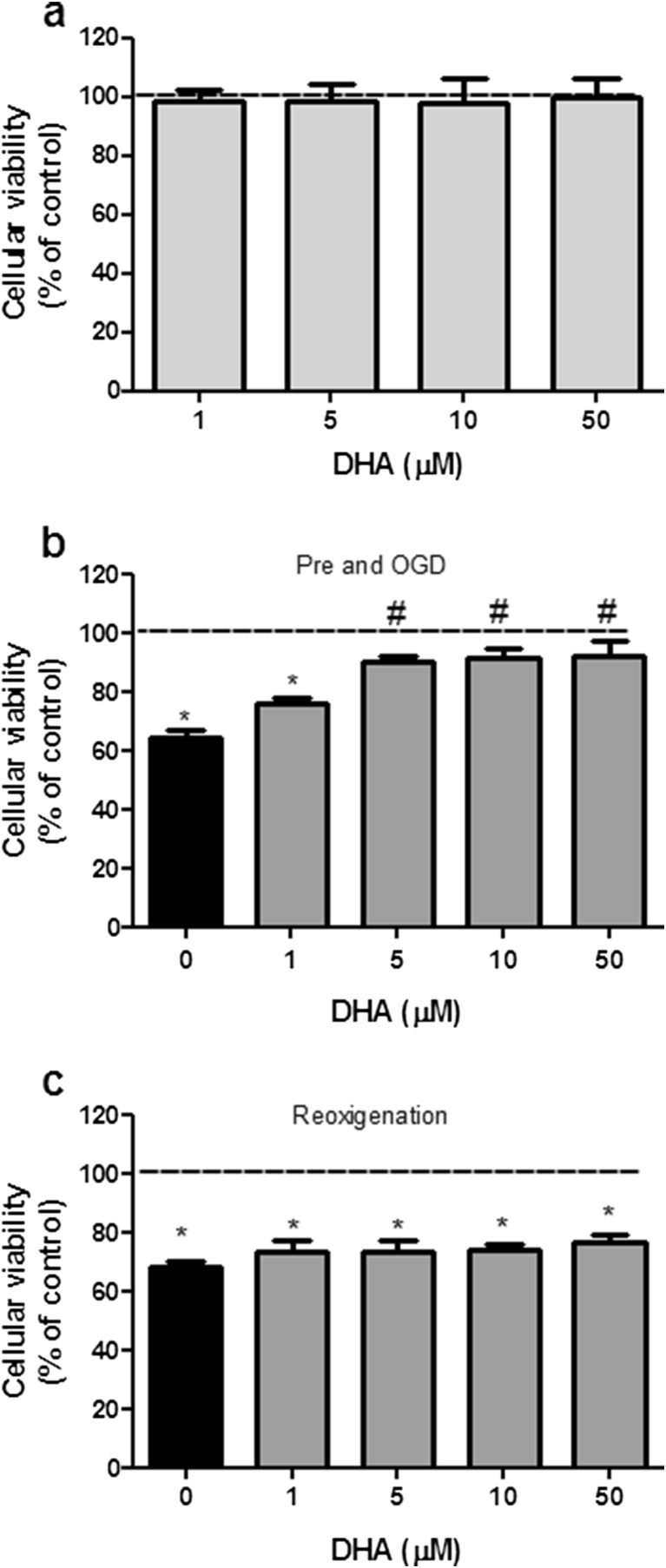
Firstly, we tested the concentration of DHA necessary to afford neuroprotection in our model and whether DHA-mediated neuroprotection was achieved during or after OGD (re-oxygenation) period. Under physiological conditions, hippocampal slices incubation with increasing concentrations of DHA (1–50 μM) alone did not affect cellular viability (Fig. 1a). When subjected to OGD, cellular viability of hippocampal slices was significantly reduced by 33 % (Fig. 1b and c). However, when DHA was preincubated for 15 min and maintained during OGD period, it was able to prevent cell viability reduction in a concentration-dependent manner (Fig. 1b), being the lower effective concentration 5 μM. In contrast, DHA did not show any protective effect when it is added after OGD, during the re-oxygenation period (Fig. 1c). Based on these results, in the following experiments, DHA was used at 5 μM, preincubated and maintained during OGD, in order to study DHA-mediated neuroprotective mechanism.
Fig. 1.
Neuroprotective properties of DHA against oxygen/glucose deprivation and re-oxygenation (OGD)-induced toxicity in brain hippocampal slices. a Effect of increasing concentrations of DHA on cellular viability of hippocampal slices incubated in control conditions (1–50 μM). b Effect of DHA (1–50 μM) when added to hippocampal slices 15 min before OGD and maintained through OGD. c Effect of DHA (1–50 μM) incubation during the re-oxygenation period. Control group (dashed lines) was considered as 100 %. These results represent means ± SEM of six experiments carried out in triplicates. *P < 0.05 when compared to control group (100 % of cellular viability); # P < 0.05 when compared to OGD treated group (one-way ANOVA followed by Tukey’s test)
DHA prevents excitotoxicity-induced cell damage, but it does not alter glutamate uptake
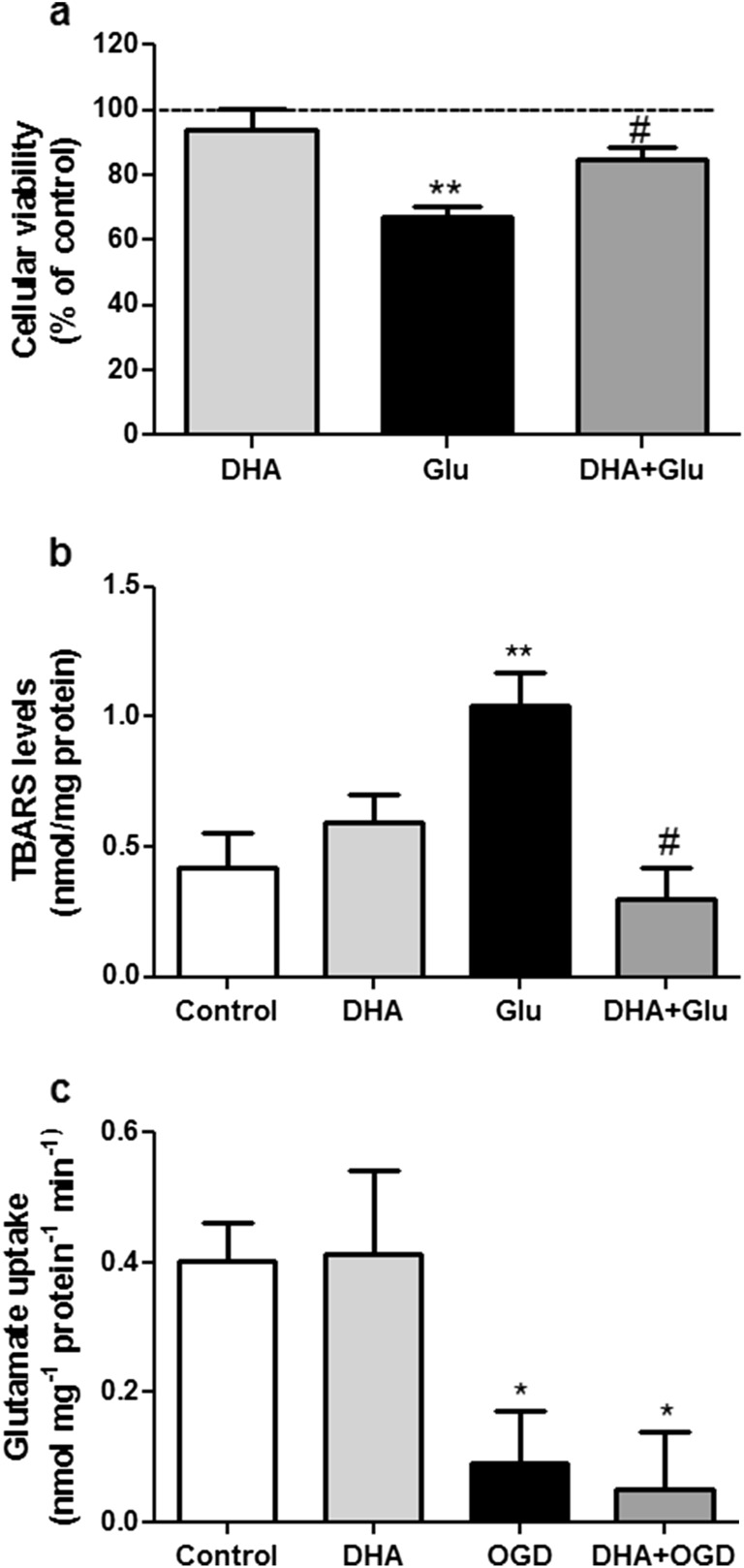
Studies of excitotoxicity mimic one consequence (high extracellular glutamate) of energy failure associated with cerebral ischemia [17]. Thus, we also verified if DHA was able to prevent excitotoxicity-induced cell damage in hippocampal brain slices [48]. As shown in Fig. 2a and b, 5 μM DHA was effective in preventing cellular viability reduction as well as lipid peroxidation as an index of ROS production induced by 10 mM glutamate.
Fig. 2.
Effect of DHA on glutamate-induced excitotoxicity and on glutamate uptake into hippocampal slices subjected to oxygen/glucose deprivation and re-oxygenation (OGD). Effect of DHA (5 μM) on a cellular viability and b TBARS levels in hippocampal slices incubated with 10 mM glutamate (Glu) for 2 h. Control group (dashed lines) was considered as 100 %. These results represent means ± SEM of six experiments carried out in triplicates. Two asterisk indicate means significantly different from control group (P < 0.01) and number sign indicates mean significantly different from glutamate (Glu) group (P < 0.05) (one-way ANOVA followed by Tukey’s test). c Effect of DHA (5 μM) on glutamate uptake impairment induced by oxygen/glucose deprivation and re-oxygenation (OGD). The values are expressed as nmol of L-[3H]glutamate taken up/mg protein/min and represent means ± SEM of five experiments carried out in triplicates. Asterisk indicates means significantly different from control group (P < 0.05) (one-way ANOVA followed by Tukey’s test)
The cascade of events in response to cerebral ischemia is still being debated. However, it is generally accepted that a large amount of glutamate released can lead to excitotoxicity, so, we decided to investigate if DHA was able to modulate glutamate transport in OGD-stimulated slices. Figure 2c demonstrates that OGD significantly reduced glutamate uptake which was not prevented by DHA, indicating that modulation of glutamate transporters activity seems not to be the mechanism involved in DHA-mediated neuroprotection against OGD. When only DHA was applied to hippocampal slices, glutamate uptake was unchanged in comparison to control.
DHA prevents OGD-induced cell damage via ATP receptors activation
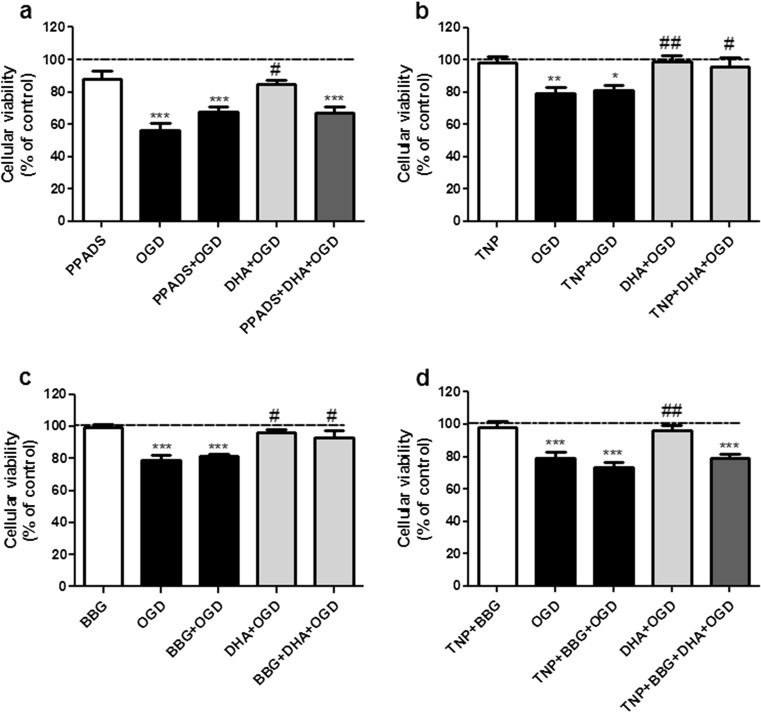
ATP is released during ischemia [22] and induces DHA release from membrane [70], so, we evaluated if the nonselective P2 receptor antagonist PPADS (3 μM) which blocks P2X1–5 could inhibit the neuroprotective effect of DHA. Our results showed that neuroprotection afforded by DHA was fully inhibited by PPADS (Fig. 3a) indicating that P2X receptors are involved in DHA-mediated neuroprotection.
Fig. 3.
Blockade of purinergic P2 receptors prevents DHA-mediated protective effect against oxygen/glucose deprivation and re-oxygenation (OGD). The P2 receptors selective antagonists (PPADS, TNP-ATP, or BBG) were incubated for 15 min prior to DHA exposure and maintained throughout OGD in the presence or absence of DHA. Control group (dashed lines) was considered as 100 %. The values are expressed as percentage of control group (considered as 100 %) and represent means ± SEM of 5–7 experiments carried out in triplicates. a Blockade of P2 receptor with the nonselective antagonist PPADS. In this figure, three asterisks (P < 0.001) indicate means significantly different from control group; number sign represents mean different from OGD and PPADS + DHA + OGD group (P < 0.05). b Blockade of P2X1, P2X3, and P2X2/3 receptors with the selective antagonist TNP-ATP. In this figure, two asterisks (P < 0.01) and asterisk (P < 0.05) represent means significantly different from control group; two number signs (P < 0.01) and number sign (P < 0.05) represent means different from OGD. c Blockade of P2X7 receptors with the selective antagonist BBG. In this figure, three asterisks (P < 0.001) indicate means significantly different from control group and number sign (P < 0.05) represents mean different from OGD group. d Blockade of P2X1, P2X3, P2X2/3, and P2X7 receptors with the combination of selective antagonists TNP-ATP and BBG. In this figure, three asterisks (P < 0.001) represent means significantly different from control group and two number signs (P < 0.01) represent means significantly different from OGD and TNP + BBG + DHA + OGD groups. Statistical analysis was performed by ANOVA followed by Tukey’s test
The P2X1–7 receptors are composed of homomeric or heteromeric assemblies of three or six subunits [11]. All seven isoforms of P2X receptors identified are expressed in the CNS and assembled to different homomeric and heteromeric channels, e.g., P2X2/3. The P2Y receptor family is activated by extracellular adenine and/or uracil nucleotides or in the case of the P2Y14 receptor, by sugar nucleotides. Because we are interested only in adenine nucleotide effects, we decided to evaluate the involvement of some selective P2X receptors in the neuroprotective mechanism of DHA. To achieve this, hippocampal slices were preincubated with TNP-ATP (100 nM), which inhibits ATP-induced currents in cells expressing P2X1, P2X3, and heteromeric P2X2/3 receptors [62], or with BBG (100 nM), a selective P2X7 receptor antagonist [37]. Figure 3b and c show that neither TNP-ATP nor BBG could inhibit DHA-mediated neuroprotection against OGD. However, when both antagonists were co-incubated, a complete inhibition of DHA-induced neuroprotection was observed (Fig. 3d). These results corroborate the effect of PPADS and indicate that at least P2X1–5, heteromeric P2X2/3, and P2X7 receptor activation are required for the neuroprotective effect of DHA against OGD-induced cell injury.
Nonetheless, direct application of ATP to brain primary neuronal and organotypic cultures is per se toxic [1] and in these situations, antagonists of P2 receptors have been often neuroprotective [12, 25, 31], so, we also tested the effect of the various ATP antagonists in OGD-induced cell damage. As we can also see in Figs. 3a–d, preincubation and maintenance of PPADS, TNP-ATP, BBG, or TNP-ATP plus BBG during OGD did not interfere with cell damage induced by OGD, which indicates that in our experimental model, ATP itself had no neuroprotective or detrimental effect on OGD-induced cell damage, but it can mediate neuroprotection evoked by DHA.
DHA prevents OGD-induced cell damage via adenosine receptors activation
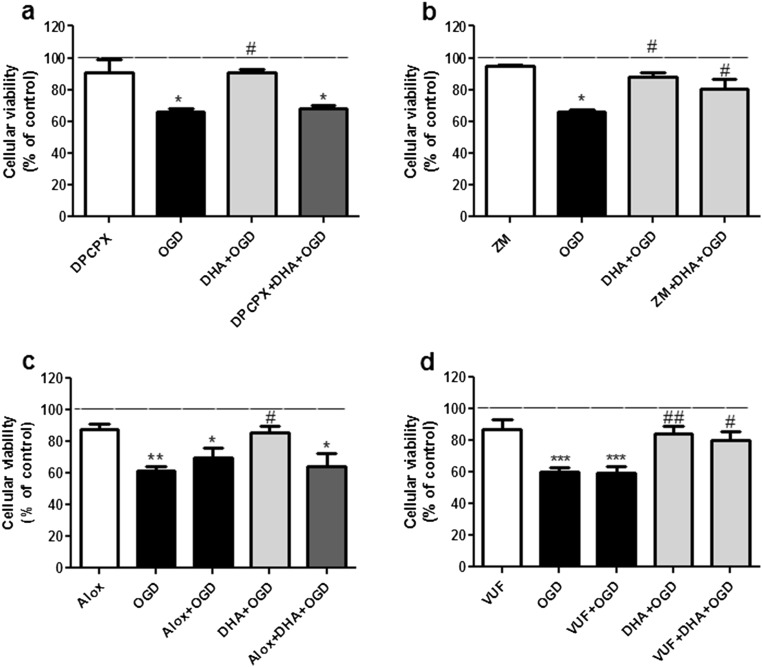
As previously mentioned, adenosine can derive from ATP through the enzymatic activity of ecto-nucleotidases which are ubiquitously distributed in the nervous system. Thus, we also investigated the effect of DHA in OGD-stimulated hippocampal slices upon blockade of adenosine receptors. Hippocampal slices preincubation with A1 (DPCPX) or A2B (alloxazine), but not with A2A (ZM 241385) or A3 (VUF 5574) receptor antagonists abolished the neuroprotective effect of DHA (Fig. 4a–d). These results indicate that A1 and A2B receptor activation are required for the neuroprotective effect of DHA against OGD-induced cell viability reduction. In the presented study, we also showed that blockade of A2B or A3 receptors did not influence the extent of cell damage caused by OGD, and a previous study conducted by our group [55] already demonstrated that DPCPX and ZM 241385 did not alter cellular viability reduction induced by OGD, which indicates that A1 and A2A receptors are not involved in cell damage induced by OGD. Altogether, these results indicate that in our experimental model, adenosine alone has no neuroprotective effect against OGD-induced cell damage, but it can mediate neuroprotection in the presence of DHA.
Fig. 4.
Blockade of adenosine receptors prevents DHA-mediated protective effect against oxygen/glucose deprivation and re-oxygenation (OGD). Adenosine receptors selective antagonists (DPCPX, ZM241385, alloxazine, and VUF5574) were incubated for 15 min prior to DHA exposure and maintained throughout OGD. Control group (dashed lines) was considered as 100 %. The values are expressed as percentage of control and represent means ± SEM of 5–7 experiments carried out in triplicates. a Blockade of A1 receptor with the selective antagonist DPCPX. In this figure, asterisk (P < 0.05) represents means significantly different from control group and number sign (P < 0.05) represents mean different from OGD and DPCPX + DHA + OGD group. b Blockade of A2A receptor with the selective antagonist ZM241385. In this figure, asterisk (P < 0.05) represents mean significantly different from control group and number sign (P < 0.05) represents means different from OGD group. c Blockade of A2B receptors with the selective antagonist alloxazine. In this figure, two asterisks and asterisk represent means significantly different from control group (P < 0.01 and 0.05, respectively); number sign (P < 0.05) represents mean different from OGD and Alox + DHA + OGD group. d Blockade of A3 receptors with VUF5574. In this figure, three asterisks represent means significantly different from control (P < 0.001); two asterisks and asterisks indicate means different from OGD group (P < 0.01 and 0.05, respectively). Statistical analysis was performed by ANOVA followed by Tukey’s test
Discussion
Despite evidences of DHA as an important neuroprotective agent in the CNS, the exact mechanism by which DHA exerts its effect is not fully understood. In the present study, the involvement of purinergic receptors on the neuroprotective effect of DHA against OGD-induced cell damage to hippocampal slices was tested. Our results demonstrate that DHA blocks OGD-induced hippocampal degeneration through activation of P2X, A1, and A2B receptors.
In order to assess the effect of DHA on cellular viability, hippocampal slices from adult mice were subjected to OGD and re-oxygenation, an in vitro model of brain ischemia. DHA preincubation, in a concentration-dependent manner, abolished hippocampal cell viability reduction evoked by OGD and re-oxygenation. These results are in good agreement with previous reports in the literature that used hippocampal slices [69], or neuronal [80] and astrocytes cultures [5] where DHA protects against in vitro ischemia at low micromolar range. Besides evidence that acute treatment with DHA reduces the injury after a permanent focal cerebral ischemia in rats [27], in our study, the presence of DHA only in the re-oxygenation period did not protect hippocampal slices from OGD-induced cell damage. Epidemiological studies demonstrated that n-3 FA consumption provides benefits in stroke outcomes [42, 45, 50, 63]. Since DHA is widely available at low cost and has an excellent safety profile, our data support these data and suggests that diet-induced accumulation of DHA in the brain may prevent post-ischemic injury.
During OGD, there is a lack of glucose and oxygen with subsequent depletion of ATP which leads to collapse of Na+/K+ electrochemical gradient, leading to reversal of glutamate transport. After OGD, additional generation of ROS might result in oxidation of glutamate transporters [74] with consequent excessive glutamate release [23]. Our results demonstrated that DHA is able to prevent cellular viability reduction and lipid peroxidation probably due to ROS production evoked by excitotoxicity, but it failed to prevent OGD-induced decrease of glutamate uptake. Newly taken up DHA incorporates predominantly into phospholipids, thereby increasing the ROS-scavenging capacity of the cells [29, 52] and prevention of excitotoxicity [44, 76]. Regarding the effect of DHA on glutamate uptake, it has been shown that DHA reduces D-[3H]aspartate uptake in astrocytes cultured under basal conditions but not in reactive astrocytes [36]. So, our results reinforce the idea that DHA does not regulate glutamate transport under pathological situations and suggest that neuroprotection afforded by DHA may involve direct interaction with glutamate receptors, as previously suggested [53, 78].
Another way to counteract excitotoxicity-induced cell damage during ischemia is through modulation of purinergic system [57]. Either adenosine or ATP seems to become particularly important signaling molecules under ischemia, when extracellular concentrations of either compound drastically rises [18, 22]. Usually, brain damage results in ATP release and upregulation of P2 receptors in both neuronal and glial membranes, which contribute to the enhancement of purinergic transmission and sometimes to the aggravation of toxicity [15, 31]. However, the presence of ATP receptors antagonists does not influence the amount of OGD and re-oxygenation-induced cell damage to hippocampal slices, indicating that the release of ATP is not sufficient to reduce cellular viability in our experimental model. In contrast, blockade of P2X receptors, particularly P2X1–5, P2X2/3, and P2X7 receptors, completely abolished DHA-mediated neuroprotection against OGD, suggesting an inhibition of DHA release from astrocyte membrane [69] or direct interaction between DHA and ATP receptors [5, 28, 64].
In the SNC, the modulatory effects of ATP are difficult to clarify due to its rapid degradation by the ecto-enzymes and the consequent formation of adenosine [19]. It is well known that adenosine exerts important neuroprotective effects during brain ischemic insults by activating A1 receptor, which profoundly inhibits synaptic transmission and, in particular, the release of glutamate [20]. However, despite that adenosine is released during ischemia, A1 receptor does not participate in the modulation of excitotoxic glutamate release, which is nonsynaptic and is due to the reverse operation of transporters [66]. Instead, extrasynaptic A1 receptor might be responsible for the neuroprotection afforded by A1 receptor activation. In addition, in hippocampal neurons, A1 receptor is mainly present in nerve terminals and enriched in the postsynaptic density [61, 72] where it modulates glutamate receptors [26]. Since DHA was unable to prevent OGD-induced decrease of glutamate uptake, but its neuroprotective effect is blocked by A1 receptor antagonist, we may speculate that DHA-evoked neuroprotection involves extracellular adenosine formation from ATP and/or direct adenosine release. Accordingly, it was demonstrated that arachidonic acid, another polyunsaturated fatty acid, facilitated adenosine release from hippocampal nerve terminals [21]. Moreover, it has been shown in malignant cells that the protective effect of DHA is achieved due to increased expression of adenosine receptor [65]. Taken together, these data suggest that DHA neuroprotective mechanism of action may involve bolstering of purinergic receptors.
There are three other adenosine receptors (A2A, A2B, and A3) in the hippocampus, but their density and adenosine affinity are lower than A1 receptor [35]. Evidence point out for A2B involvement in the survival of cortical neurons against excitotoxicity [46] and in the presented study, blockade of A2B receptors with alloxazine prevented DHA-mediated neuroprotection against OGD. However, one may consider that the alloxazine concentration used in our study is not selective to A2B receptor and may also block A1 receptor [10], reinforcing the above-mentioned effects. A3 receptor activation has been shown to promote the recovery of synaptic responses following OGD in the CA1 region [59]; however, reports are unclear and contrasting on the neuroprotective effects of A3 receptor modulation during ischemia [75]. In our study A3 receptor activation is not related to DHA-mediated neuroprotection against OGD. Regarding A2A receptor, it has been shown that in certain situations, adenosine can exacerbate neurotoxicity via A2A receptor-mediated effects [67] and A2A receptor antagonists are confirmed to have neuroprotective role in different models of ischemia [16]. In accordance, in our study, DHA-promoted neuroprotection is not mediated by A2A receptors activation.
Purines, especially adenosine, and their receptors have been viewed as potential therapeutic agents for the treatment of stroke. In a translational point of view, however, adenosine-based therapies for the treatment of stroke are complex in their implementation [7]. Difficulties stem from the widespread distribution of adenosine receptors within CNS and throughout the body. For this reason, direct targeting of specific adenosine receptors leads to widespread side effects. So, this study hypothesizes that an optimal brain DHA status, conferred by an adequate n-3 FA intake, would limit ischemic-related brain damage by optimizing endogenous brain repair mechanisms such as local modulation of purinergic signaling.
Acknowledgments
This study and FKL were supported by grants from Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC). GO was supported by Fundo de Apoio à Manutenção e ao Desenvolvimento da Educação Superior (FUMDES). C.I.T. is recipient of CNPq productivity fellowship. The authors thank the Universidade do Contestado for the animal house facility.
Conflict of interest
The authors state that there is no conflict of interest.
References
- 1.Amadio S, D’Ambrosi N, Cavaliere F, Murra B, Sancesario G, Bernardi G, Burnstock G, Volonte C. P2 receptor modulation and cytotoxic function in cultured CNS neurons. Neuropharmacology. 2002;42:489–501. doi: 10.1016/S0028-3908(01)00197-6. [DOI] [PubMed] [Google Scholar]
- 2.Arias RL, Tasse JR, Bowlby MR. Neuroprotective interaction effects of NMDA and AMPA receptor antagonists in an in vitro model of cerebral ischemia. Brain Res. 1999;816:299–308. doi: 10.1016/S0006-8993(98)01051-8. [DOI] [PubMed] [Google Scholar]
- 3.Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu Rev Nutr. 2011;31:321–351. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazan NG, Musto AE, Knott EJ. Endogenous signaling by omega-3 docosahexaenoic acid-derived mediators sustains homeostatic synaptic and circuitry integrity. Mol Neurobiol. 2011;44:216–222. doi: 10.1007/s12035-011-8200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begum G, Kintner D, Liu Y, Cramer SW, Sun D. DHA inhibits ER Ca2+ release and ER stress in astrocytes following in vitro ischemia. J Neurochem. 2012;120:622–630. doi: 10.1111/j.1471-4159.2011.07606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belayev L, Khoutorova L, Atkins KD, Eady TN, Hong S, Lu Y, Obenaus A, Bazan NG. Docosahexaenoic acid therapy of experimental ischemic stroke. Transl Stroke Res. 2011;2:33–41. doi: 10.1007/s12975-010-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boison D. Adenosine augmentation therapies (AATs) for epilepsy: prospect of cell and gene therapies. Epilepsy Res. 2009;85:131–141. doi: 10.1016/j.eplepsyres.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC. Alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fat Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Brongholi K, Souza DG, Bainy AC, Dafre AL, Tasca CI. Oxygen-glucose deprivation decreases glutathione levels and glutamate uptake in rat hippocampal slices. Brain Res. 2006;1083:211–218. doi: 10.1016/j.brainres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Brown P, Dale N. Adenosine A1 receptors modulate high voltage-activated Ca2+ currents and motor pattern generation in the Xenopus embryo. J Physiol. 2000;525(Pt 3):655–667. doi: 10.1111/j.1469-7793.2000.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnstock G, Krugel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Calon F, Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot Essent Fat Acids. 2007;77:287–293. doi: 10.1016/j.plefa.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Carmo MR, Simoes AP, Fonteles AA, Souza CM, Cunha RA, Andrade GM. ATP P2Y1 receptors control cognitive deficits and neurotoxicity but not glial modifications induced by brain ischemia in mice. Eur J Neurosci. 2014;39:614–622. doi: 10.1111/ejn.12435. [DOI] [PubMed] [Google Scholar]
- 15.Cavaliere F, Florenzano F, Amadio S, Fusco FR, Viscomi MT, D’Ambrosi N, Vacca F, Sancesario G, Bernardi G, Molinari M, Volontà C. Up-regulation of P2X2, P2X4 receptor and ischemic cell death: prevention by P2 antagonists. Neuroscience. 2003;120:85–98. doi: 10.1016/S0306-4522(03)00228-8. [DOI] [PubMed] [Google Scholar]
- 16.Chen JF, Pedata F. Modulation of ischemic brain injury and neuroinflammation by adenosine A2A receptors. Curr Pharm Des. 2008;14:1490–1499. doi: 10.2174/138161208784480126. [DOI] [PubMed] [Google Scholar]
- 17.Choi D. Antagonizing excitotoxicity: a therapeutic strategy for stroke? Mt Sinai J Med. 1998;65:133–138. [PubMed] [Google Scholar]
- 18.Chu S, Xiong W, Zhang D, Soylu H, Sun C, Albensi BC, Parkinson FE. Regulation of adenosine levels during cerebral ischemia. Acta Pharmacol Sin. 2013;34:60–66. doi: 10.1038/aps.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38:107–125. doi: 10.1016/S0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 20.Cunha RA. Neuroprotection by adenosine in the brain: from A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunha RA, Almeida T, Ribeiro JA. Modification by arachidonic acid of extracellular adenosine metabolism and neuromodulatory action in the rat hippocampus. J Biol Chem. 2000;275:37572–37581. doi: 10.1074/jbc.M003011200. [DOI] [PubMed] [Google Scholar]
- 22.Dale N, Frenguelli BG. Release of adenosine and ATP during ischemia and epilepsy. Curr Neuropharmacol. 2009;7:160–179. doi: 10.2174/157015909789152146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/S0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 24.Denis I, Potier B, Vancassel S, Heberden C, Lavialle M. Omega-3 fatty acids and brain resistance to ageing and stress: body of evidence and possible mechanisms. Ageing Res Rev. 2013;12:579–594. doi: 10.1016/j.arr.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32:79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 27.Eady TN, Khoutorova L, Anzola DV, Hong SH, Obenaus A, Mohd-Yusof A, Bazan NG, Belayev L. Acute treatment with docosahexaenoic acid complexed to albumin reduces injury after a permanent focal cerebral ischemia in rats. PLoS ONE. 2013;8:e77237. doi: 10.1371/journal.pone.0077237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eto K, Arimura Y, Mizuguchi H, Nishikawa M, Noda M, Ishibashi H. Modulation of ATP-induced inward currents by docosahexaenoic acid and other fatty acids in rat nodose ganglion neurons. J Pharmacol Sci. 2006;102:343–346. doi: 10.1254/jphs.SC0060053. [DOI] [PubMed] [Google Scholar]
- 29.Farooqui AA, Horrocks LA. Plasmalogens, phospholipase A2, and docosahexaenoic acid turnover in brain tissue. J Mol Neurosci. 2001;16:263–272. doi: 10.1385/JMN:16:2-3:263. [DOI] [PubMed] [Google Scholar]
- 30.Fedalto ML, Ludka FK, Tasca CI, Molz S. Neuroprotection of Persea major extract against oxygen and glucose deprivation in hippocampal slices involves increased glutamate uptake and modulation of A1 and A2A adenosine receptors. Rev Bras Farmacognosia. 2013;23:789–795. doi: 10.1590/S0102-695X2013000500011. [DOI] [Google Scholar]
- 31.Franke H, Krugel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006;452:622–644. doi: 10.1007/s00424-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 32.Franke H, Verkhratsky A, Burnstock G, Illes P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012;8:629–657. doi: 10.1007/s11302-012-9300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 34.Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- 35.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/S0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 36.Grintal B, Champeil-Potokar G, Lavialle M, Vancassel S, Breton S, Denis I. Inhibition of astroglial glutamate transport by polyunsaturated fatty acids: evidence for a signalling role of docosahexaenoic acid. Neurochem Int. 2009;54:535–543. doi: 10.1016/j.neuint.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- 38.Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci. 2001;2:165–174. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- 39.Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, Dasgupta S, Barral PM, Hedvat M, Diaz P, Reed JC, Stebbins JL, Pellecchia M, Sarkar D, Fisher PB. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol. 2011;226:2484–2493. doi: 10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopes LV, Sebastiao AM, Ribeiro JA. Adenosine and related drugs in brain diseases: present and future in clinical trials. Curr Top Med Chem. 2011;11:1087–1101. doi: 10.2174/156802611795347591. [DOI] [PubMed] [Google Scholar]
- 41.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 42.Mahe G, Ronziere T, Laviolle B, Golfier V, Cochery T, De Bray JM, Paillard F. An unfavorable dietary pattern is associated with symptomatic ischemic stroke and carotid atherosclerosis. J Vasc Surg. 2010;52:62–68. doi: 10.1016/j.jvs.2010.02.258. [DOI] [PubMed] [Google Scholar]
- 43.Mayurasakorn K, Williams JJ, Ten VS, Deckelbaum RJ. Docosahexaenoic acid: brain accretion and roles in neuroprotection after brain hypoxia and ischemia. Curr Opin Clin Nutr Metab Care. 2011;14:158–167. doi: 10.1097/MCO.0b013e328342cba5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menard C, Patenaude C, Gagne AM, Massicotte G. AMPA receptor-mediated cell death is reduced by docosahexaenoic acid but not by eicosapentaenoic acid in area CA1 of hippocampal slice cultures. J Neurosci Res. 2009;87:876–886. doi: 10.1002/jnr.21916. [DOI] [PubMed] [Google Scholar]
- 45.Miyagawa N, Miura K, Okuda N, Kadowaki T, Takashima N, Nagasawa SY, Nakamura Y, Matsumura Y, Hozawa A, Fujiyoshi A, Hisamatsu T, Yoshita K, Sekikawa A, Ohkubo T, Abbott RD, Okamura T, Okayama A, Ueshima H. Long-chain n-3 polyunsaturated fatty acids intake and cardiovascular disease mortality risk in Japanese: a 24-year follow-up of NIPPON DATA80. Atherosclerosis. 2014;232:384–389. doi: 10.1016/j.atherosclerosis.2013.11.073. [DOI] [PubMed] [Google Scholar]
- 46.Moidunny S, Vinet J, Wesseling E, Bijzet J, Shieh CH, van Ijzendoorn SC, Bezzi P, Boddeke HW, Biber K. Adenosine A2B receptor-mediated leukemia inhibitory factor release from astrocytes protects cortical neurons against excitotoxicity. J Neuroinflammation. 2012;9:198. doi: 10.1186/1742-2094-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molz S, Dal-Cim T, Tasca CI. Guanosine-5′-monophosphate induces cell death in rat hippocampal slices via ionotropic glutamate receptors activation and glutamate uptake inhibition. Neurochem Int. 2009;55:703–709. doi: 10.1016/j.neuint.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 48.Molz S, Decker H, Dal-Cim T, Cremonez C, Cordova FM, Leal RB, Tasca CI. Glutamate-induced toxicity in hippocampal slices involves apoptotic features and p38 MAPK signaling. Neurochem Res. 2008;33:27–36. doi: 10.1007/s11064-007-9402-1. [DOI] [PubMed] [Google Scholar]
- 49.Moreira JD, Knorr L, Thomazi AP, Simao F, Battu C, Oses JP, Gottfried C, Wofchuk S, Salbego C, Souza DO, Perry ML, Vinade L. Dietary omega-3 fatty acids attenuate cellular damage after a hippocampal ischemic insult in adult rats. J Nutr Biochem. 2009;21:351–356. doi: 10.1016/j.jnutbio.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 50.Mori TA. Omega-3 fatty acids and cardiovascular disease: epidemiology and effects on cardiometabolic risk factors. Food Funct. 2014;5:2004–2019. doi: 10.1039/C4FO00393D. [DOI] [PubMed] [Google Scholar]
- 51.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 52.Nagan N, Zoeller RA. Plasmalogens: biosynthesis and functions. Prog Lipid Res. 2001;40:199–229. doi: 10.1016/S0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 53.Nishikawa M, Kimura S, Akaike N. Facilitatory effect of docosahexaenoic acid on N-methyl-D-aspartate response in pyramidal neurones of rat cerebral cortex. J Physiol. 1994;475:83–93. doi: 10.1113/jphysiol.1994.sp020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 55.Oleskovicz SP, Martins WC, Leal RB, Tasca CI. Mechanism of guanosine-induced neuroprotection in rat hippocampal slices submitted to oxygen-glucose deprivation. Neurochem Int. 2008;52:411–418. doi: 10.1016/j.neuint.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 56.Oliveira IJ, Molz S, Souza DO, Tasca CI. Neuroprotective effect of GMP in hippocampal slices submitted to an in vitro model of ischemia. Cell Mol Neurobiol. 2002;22:335–344. doi: 10.1023/A:1020724102773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pedata F, Melani A, Pugliese AM, Coppi E, Cipriani S, Traini C. The role of ATP and adenosine in the brain under normoxic and ischemic conditions. Purinergic Signal. 2007;3:299–310. doi: 10.1007/s11302-007-9085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phillis JW, O’Regan MH. A potentially critical role of phospholipases in central nervous system ischemic, traumatic, and neurodegenerative disorders. Brain Res Brain Res Rev. 2004;44:13–47. doi: 10.1016/j.brainresrev.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Pugliese AM, Coppi E, Spalluto G, Corradetti R, Pedata F. A3 adenosine receptor antagonists delay irreversible synaptic failure caused by oxygen and glucose deprivation in the rat CA1 hippocampus in vitro. Br J Pharmacol. 2006;147:524–532. doi: 10.1038/sj.bjp.0706646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fat Acids. 2007;77:251–261. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rebola N, Pinheiro PC, Oliveira CR, Malva JO, Cunha RA. Subcellular localization of adenosine A(1) receptors in nerve terminals and synapses of the rat hippocampus. Brain Res. 2003;987:49–58. doi: 10.1016/S0006-8993(03)03247-5. [DOI] [PubMed] [Google Scholar]
- 62.Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci. 2005;25:6286–6295. doi: 10.1523/JNEUROSCI.0628-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saremi A, Arora R. The utility of omega-3 fatty acids in cardiovascular disease. Am J Ther. 2009;16:421–436. doi: 10.1097/MJT.0b013e3180a5f0bb. [DOI] [PubMed] [Google Scholar]
- 64.Sergeeva M, Strokin M, Reiser G. Regulation of intracellular calcium levels by polyunsaturated fatty acids, arachidonic acid and docosahexaenoic acid, in astrocytes: possible involvement of phospholipase A2. Reprod Nutr Dev. 2005;45:633–646. doi: 10.1051/rnd:2005050. [DOI] [PubMed] [Google Scholar]
- 65.Sheng H, Li P, Chen X, Liu B, Zhu Z, Cao W. Omega-3 PUFAs induce apoptosis of gastric cancer cells via ADORA1. Front Biosci (Landmark Ed) 2014;19:854–861. doi: 10.2741/4252. [DOI] [PubMed] [Google Scholar]
- 66.Sperlagh B, Vizi ES. The role of extracellular adenosine in chemical neurotransmission in the hippocampus and Basal Ganglia: pharmacological and clinical aspects. Curr Top Med Chem. 2011;11:1034–1046. doi: 10.2174/156802611795347564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stone TW, Ceruti S,Abbracchio MP (2009) Adenosine receptors and neurological disease: neuroprotection and neurodegeneration. Handb Exp Pharmacol 535–87 [DOI] [PubMed]
- 68.Strasser U, Fischer G. Protection from neuronal damage induced by combined oxygen and glucose deprivation in organotypic hippocampal cultures by glutamate receptor antagonists. Brain Res. 1995;687:167–174. doi: 10.1016/0006-8993(95)00519-V. [DOI] [PubMed] [Google Scholar]
- 69.Strokin M, Chechneva O, Reymann KG, Reiser G. Neuroprotection of rat hippocampal slices exposed to oxygen-glucose deprivation by enrichment with docosahexaenoic acid and by inhibition of hydrolysis of docosahexaenoic acid-containing phospholipids by calcium independent phospholipase A2. Neuroscience. 2006;140:547–553. doi: 10.1016/j.neuroscience.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 70.Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ Br J Pharmacol. 2003;139:1014–1022. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taha AY, Jeffrey MA, Taha NM, Bala S, Burnham WM. Acute administration of docosahexaenoic acid increases resistance to pentylenetetrazol-induced seizures in rats. Epilepsy Behav. 2010;17:336–343. doi: 10.1016/j.yebeh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 72.Tetzlaff W, Schubert P, Kreutzberg GW. Synaptic and extrasynaptic localization of adenosine binding sites in the rat hippocampus. Neuroscience. 1987;21:869–875. doi: 10.1016/0306-4522(87)90043-1. [DOI] [PubMed] [Google Scholar]
- 73.Thrift AG, Cadilhac DA, Thayabaranathan T, Howard G, Howard VJ, Rothwell PM, Donnan GA. Global stroke statistics. Int J Stroke. 2014;9:6–18. doi: 10.1111/ijs.12245. [DOI] [PubMed] [Google Scholar]
- 74.Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci. 1998;19:328–334. doi: 10.1016/S0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- 75.von Lubitz DK, Ye W, McClellan J, Lin RC. Stimulation of adenosine A3 receptors in cerebral ischemia. Neuronal death, recovery, or both? Ann N Y Acad Sci. 1999;890:93–106. doi: 10.1111/j.1749-6632.1999.tb07984.x. [DOI] [PubMed] [Google Scholar]
- 76.Wang X, Zhao X, Mao ZY, Wang XM, Liu ZL. Neuroprotective effect of docosahexaenoic acid on glutamate-induced cytotoxicity in rat hippocampal cultures. Neuroreport. 2003;14:2457–2461. doi: 10.1097/00001756-200312190-00033. [DOI] [PubMed] [Google Scholar]
- 77.Wei JW, Wang JG, Huang Y, Liu M, Wu Y, Wong LK, Cheng Y, Xu E, Yang Q, Arima H, Heeley EL, Anderson CS. Secondary prevention of ischemic stroke in urban China. Stroke. 2010;41:967–974. doi: 10.1161/STROKEAHA.109.571463. [DOI] [PubMed] [Google Scholar]
- 78.Wilding TJ, Chai YH, Huettner JE. Inhibition of rat neuronal kainate receptors by cis-unsaturated fatty acids. J Physiol. 1998;513(Pt 2):331–339. doi: 10.1111/j.1469-7793.1998.331bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 80.Zhang M, Wang S, Mao L, Leak RK, Shi Y, Zhang W, Hu X, Sun B, Cao G, Gao Y, Xu Y, Chen J, Zhang F. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J Neurosci. 2014;34:1903–1915. doi: 10.1523/JNEUROSCI.4043-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedeberg’s Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]