Abstract
Purinergic receptors activated by extracellular nucleotides (adenosine 5′-triphosphate (ATP) and uridine 5′-triphosphate (UTP)) are well known to exert physiological effects on the cardiovascular system, whether nucleotides participate functionally in embryonic heart development is not clear. The responsiveness of embryonic cardiomyocytes (E) 12 to P2 receptor agonists by measuring Ca2+ influx did not present response to ATP, but responses to P2 agonists were detected in cardiomyocytes taken from E14 and E18 rats. Photometry revealed that the responses to ATP were concentration-dependent with an EC50 of 1.32 μM and 0.18 μM for E14 and E18 cardiomyocytes, respectively. In addition, other P2 agonists were also able to induce Ca2+ mobilization. RT-PCR showed the presence of P2X2 and P2X4 receptor transcripts on E14 cardiomyocytes with a lower expression of P2X3 and P2X7 receptors. P2X1 and a low level of P2X5 receptor messenger RNA (mRNA) were also expressed at E18. Immunofluorescence data indicated that only P2X2 and P2X4 receptor proteins were expressed in E14 cardiomyocytes while protein for all the P2X receptor subtypes was expressed in E18, except for P2X3 and P2X6. Responses mediated by agonists specific for P2Y receptors subtypes showed that P2Y receptors (P2Y1, P2Y2, P2Y4 and P2Y6) were also present in both E14 and E18 cardiomyocytes. Dye transfer experiments showed that ATP induces coupling of cells at E12, but this response is decreased at E14 and lost at E18. Conversely, UTP induced coupling with five or more cells in most cells from E12 to E18. Our results show that specific P2 receptor subtypes are present in embryonic rat cardiomyocytes, including P2X7 and P2Y4 receptors that have not been identified in adult rat cardiomyocytes. The responsiveness to ATP stimulation even before birth, suggests that ATP may be an important messenger in embryonic as well as in adult hearts.
Keywords: ATP, P2X receptors, P2Y receptors, P1 receptors, Rat embryo, Heart, Cardiomyocyte, FLIPR, Calcium mobilization
Introduction
Extracellular purines and pyrimidines (adenosine 5′-triphosphate (ATP), adenosine 5′-diphosphate (ADP) and uridine 5′-triphosphate (UTP)) regulate a variety of physiological functions in many different cell types [1]. In 1978, it was proposed that there were specific receptors for adenine nucleosides (P1) and nucleotides (P2 receptors) [2]. Based initially on pharmacology and later on cloning and second messenger systems, P2 receptors were divided into two receptor families, classified as ionotropic P2X receptors and metabotropic P2Y receptors [3–5]. P2X receptors are ligand-gated ion channels formed from either homomeric or heteromeric association of seven receptor subunits (P2X1–7) [6, 7] whereas P2Y receptors are G protein-coupled receptors, and so far eight mammalian subtypes (P2Y1,2,4,6,11–14) have been identified [8, 9].
ATP is known to mediate various physiological activities in the adult heart of different species [10–12], although only a few studies have focused specifically on P2 receptor expression in cardiomyocytes [13–15]. The messenger RNA (mRNA) transcripts for P2Y1, P2Y2, P2Y4 and P2Y6 receptors are all expressed in rat neonatal cardiomyocytes, whereas the P2Y4 receptor transcript is not detected in the adult [16], suggesting that dynamic changes in expression of P2Y receptors are occurring during heart development [17]. While it was demonstrated that the P2X1 receptor protein was specifically expressed in the intercalated disks of rat cardiac muscle [18], Nori et al. [13] also detected mRNA transcripts for P2X1, P2X2 and P2X4 receptors in the micro-dissected tissues from various areas of the heart, with strong signals in the atria and only the typical band for P2X4 receptor transcripts in ventricles [19, 20]. In addition, Hansen et al. [14] showed an abundance of co-localized P2X2 and P2X5 receptor clusters on the sarcolemma of neonatal rat heart.
The heart is the first organ to function in developing embryos [21], and the tubular heart undergoes sequential morphological changes, such as looping, segmentation, trabeculation and septation [22]. Similar to skeletal or smooth muscle, the arrangement of the myocardial cells is not random, but shows certain patterns which change during ontogenetic development, which is of great importance to ensure the efficiency of the myocardial pump. It is considered that dynamic changes in spatiotemporal patterns of gene expression resulting in complex structural changes during heart ontogeny are involved in the developing embryo [23, 24]; thus, the gene expression may be variable among different developmental stages and in the adult [22]. Nevertheless, identifying the presence of P2 receptor genes may not provide the information as to whether the gene products are in a functional state. Previously, the expression of P2X2 and P2Y1,2,4,6 receptors in the embryonic rat heart has been demonstrated [25–27], but the function of the receptors remains unclear. The expression of P2X receptor subtypes in the chick embryonic heart has been reported, where P2X4 and P2X5 receptor mRNA was identified [28–30]. In the human embryonic heart, mRNA for P2X1, P2X3, P2X4, P2Y2, P2Y4 and P2Y6 receptors were shown to be expressed [31]. However, information on the developmental expression profile together with the functional status of other P2X receptor subunits in rat embryo is lacking. Therefore, the fluorometric imaging plate reader (FLIPR) and fluorescence photometer approach associated with RT-PCR analysis and immunofluorescence were used to study the expression profile of P2 receptors during rat cardiomyocyte development. In addition, dye coupling experiments added a functional characterization of the expressed P2 receptors. Such combinatorial information on the gene expression and pharmacological analysis may clarify the specific receptors responsible for purine- and pyrimidine-mediated activities during cardiac embryogenesis.
Materials and methods
Cell culture
Female time-mated rats were sacrificed using carbon dioxide inhalation and cervical dislocation according to Home Office (UK) regulations covering schedule one procedures. The presence of a vaginal plug was designated as day zero (E0). Embryos were removed and placed into ice-cold phosphate-buffered saline (PBS, pH 7.4). The hearts were dissected out, placed in Ca2+-free PBS at 4 °C and washed, and the heart tissues were digested in a solution containing 1.25 % pancreatin (Gibco-BRL) and 3 mg/ml bovine serum albumin (BSA; Sigma, St. Louis, MO, USA) diluted in (g/100 ml) 8.0 NaCl, 0.2 KCl, 0.05 Na2HPO4, 1.0 NaHCO3 and 2.0 dextrose, pH 7.2–7.4 at 37 °C. The homogenate was then transferred to a 25-ml Erlenmeyer flask containing 5 ml of the above solution and placed in a water bath (37 °C) for 5 min while continuously stirring. The supernatant fraction containing single cells from each digestion period was collected in a conical 15 ml tube and spun at 300×g for 5 min. The pellet was re-suspended in 1 ml of Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % (v/v) foetal bovine serum (FBS; Gibco-BRL) and 1 % penicillin/streptomycin (Gibco-BRL). The dissociated cells were then placed in an incubator at 37 °C and 5 % CO2. This procedure was repeated until the heart tissue was totally dissociated. The cells were pooled and pre-plated in 100-mm plastic culture dishes for 1 h, to allow the non-muscle cells to attach. Then, the remaining unattached cells were plated according to the experiments.
Ca2+ mobilization analysis
Cells were cultured in 96-well plates (black well, clear bottom) (BD Biosciences, Franklin Lakes, NJ, USA). The growth medium was then aspirated and replaced with 100 μl of loading medium (PBS containing 1 mM Fluo-4-AM, 10 % pluronic acid and 2.5 mM probenecid) and incubated for 1 h at room temperature. The cells were subsequently washed three times with PBS, and 100 μl of PBS supplemented with 1 mM CaCl2 were added to each well. The cells were then placed in a FLIPR (Molecular Devices Corp., Sunnyvale, CA, USA), and changes in cellular fluorescence were recorded after the addition of 50 μl control buffer or 50 μl of 100 μM ATP diluted in PBS. Maximum change in fluorescence over baseline was used to determine the response towards ATP. In addition, the P2 receptor agonists 2′,3′-O-(4-benzoylbenzoyl)-ATP (BzATP), α,β-methylene ATP (α,β-meATP), 2-methylthio ADP (2-MeSADP), UTP and uridine 5′-diphosphate (UDP) were also used for examining the Ca2+ mobilizing response of the cardiomyocytes. To investigate if pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), a P2 receptor selective antagonist, blocked the ATP-induced response, cells were treated with PPADS (30 μM) for 30 min before addition of ATP. ATP and related nucleotides were obtained from Sigma except for PPADS, which was from Tocris Cookson (Bristol, UK).
For the dose-response curves, E12, E14 and E18 cardiomyocytes were grown on sterile glass coverslips for 48 h. Cells were pre-incubated or not with Apyrase 4 U/mL for 2 h. Cells were then loaded with 2.5 μM Fura-2-AM (Molecular Probes) for 30 min at room temperature in culture medium. The coverslips were washed three times in warm PBS and mounted in a three-compartment superfusion chamber attached to the stage of a Nikon Diaphot 300 TMD-inverted microscope whose base was formed by a cover slip containing the cells. The central chamber containing the cells had a volume of 200 μl and was perfused with Ca2+-containing saline (PBS supplemented with 1 mM CaCl2) at room temperature at a rate of 1 ml/min. The intracellular calcium concentration of groups of 20–30 cells was monitored continuously with the use of a fluorescence photometer (Photon Technology, Princeton, NJ, USA). Fura-2 was excited alternately at 340 and 380 nm, and the emission at 510 nm was measured. The ratio measurement, which is proportional to the intracellular calcium mobilization, was determined every 50 ms. The nucleotides were injected as a bolus, allowing the drugs to persist in the presence of the cells for 1 min, at room temperature. Alternatively, nucleotides were injected as a bolus every 4 min in a dose-concentration manner.
Dye transfer
Confluent cultures of cardiomyocytes at three different embryonic stages (E12, E14 and E18) plated on 35-mm Petri dishes were treated with 100 μM of ATP or UTP or 4 U/ml of Apyrase. After 24 h, the Lucifer Yellow CH dye (5 % in 150 mM LiCl) (457.2 Da) was injected into cardiomyocytes using glass microelectrodes (resistance between 40 and 70 MΩ) by the application of short hyperpolarizing current pulses (0.1 nA, 100 ms using a WPI amplifier, model 7060; USA), at room temperature. Fluorescence was observed on an Axiovert 100 microscope (Carl Zeiss, Oberkochen, Germany) equipped with appropriate filters (Zeiss BP450-490/FT510/LP520) and photographs were taken using the software Image Pro, 1 min after dye injection. To measure the degree of coupling under different culture conditions, a minimum of 35 cells were injected in at least three independent experiments. Coupling degree was arbitrarily divided into four classes: no coupling (zero); cells that were coupled to one or two cells; three or four cells; or more than five neighbouring cells coupled to the cell injected with Lucifer Yellow. Results are presented as percentage (%) of coupled cells.
Double-labelling immunofluorescence
Cardiomyocytes were grown on gelatin-coated eight-well chambered slides (Fisher Scientific UK Ltd., Loughborough, UK). The cells were fixed in 4 % paraformaldehyde in phosphate buffer (PB, pH 7.4) for 2 h at room temperature. Cells were washed with PBS, blocked with 10 % normal horse serum in 0.1 % Triton, and the cells were stained with the monoclonal antibody against sarcomeric actin (1:500; Sigma), together with the polyclonal antibodies against P2X1–6 (1:200; Roche Palo Alto, CA, USA) and P2X7 receptors (1:500; Alomone Labs, Jerusalem, Israel) followed by an FITC-conjugated donkey anti-mouse IgG antibody and cy3-coupled donkey anti-rabbit IgG antibody (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h. Images of immunohistochemical staining were taken with the Leica DC 200 digital camera (Leica, Heerbrugg, Switzerland) attached to a Zeiss Axioplan microscope (Zeiss, Oberkochen, Germany). Images were imported into a graphics package (Adobe Photoshop 5.0, USA). Control experiments were performed using an excess of the appropriate homologue peptide antigen to absorb the primary antibodies and thus confirm a specific immunoreaction (data not shown).
RT-PCR analysis
Embryonic hearts at E14 and E18 were dissected and total RNA was extracted from the dissected hearts using the SV Total RNA Isolation system (Promega, Madison, WI, USA). Reverse transcription and cDNA amplification for all the P2 receptors was carried out with a thermal cycler (Hybaid, Ashford, UK) in a two-step protocol using Ready-To-Go RT-PCR Beads (Amersham Bioscience, Chalfont St. Giles, UK). Every sample was further treated with amplification grade DNase I (Sigma) to remove any residual DNA contamination that may generate false positive results. Briefly, 1 μg of total RNA from each sample was reverse transcribed using the pd(T)12–18 as the first-strand primer at 42 °C for 30 min and the enzyme was denatured at 95 °C for 5 min. The sequence specific primers (Life Technologies, NY, USA) for P2X receptors [32] were then added to the reaction mixtures and the PCR cycling parameters were 95 °C for 30 s, 58 °C for 1 min (58 °C for P2X1, P2X3, P2X4, P2X5, P2X7; 61 °C for P2X2; and 64 °C for P2X6), 72 °C for 1.5 min for 35 cycles, followed by a further stage of 10-min extension at 72 °C. All primers were based on unique sequences comprising bases in case of: P2X1: 5′CAGAAAGGAAAGCCCAAGGTATTC 3′ (forward), 5′GCGTCAAGTCCGGATCTCGACTAA3′ (backward), product length 452 bp; P2X2: 5′ GAATCAGAGTGCAACCCCAA 3′ (forward), 5′TCACAGGCCATCTACTTGAG3′ (backward) product length 357 bp; P2X3: 5′TGGCGTTCTGGGTATTAAGATCGG3′ (forward), 5′CAGTGGCCTGGTCACTGGCGA3′ (backward) product length 440 bp; P2X4: 5′GAGGCATCATGGGTATCCAGATCAAG3′ (forward), 5′GAGCGGGGTGGAAATGTAACTTTAG3′ (backward) product length 447 bp; P2X5: 5′GCCGAAAGCTTCACCATTTCCATAA3′ (forward), 5′CCTACGGCATCCGCTTTGATGTGATAG3′ (backward) product length 418 bp; P2X6: 5′AAAGACTGGTCAGTGTGTGGCGTTC (forward), 5′TGCCTGCCCAGTGACAAGAATGTCAA3′ (backward) product length 520 bp; P2X7: 5′GTGCCATTCTGACCAGGGTTGTATAAA3′ (forward), 5′GCCACCTCTGTAAAGTTCTCTCCGATT3′ (backward) product length 354 bp.
The resulting PCR products were resolved in a 2 % agarose gel, stained with ethidium bromide and observed under ultraviolet illumination. At least three separate RT-PCR experiments were performed for each P2 receptor on each individual sample.
Statistical analysis
All data are presented as a representative from at least three independent experiments. Statistical data described in text are presented as mean ± S.E.M. Statistical differences between treatments were calculated using t-test.
Results
Effects of agonists on Ca2+ mobilization of cardiomyocytes
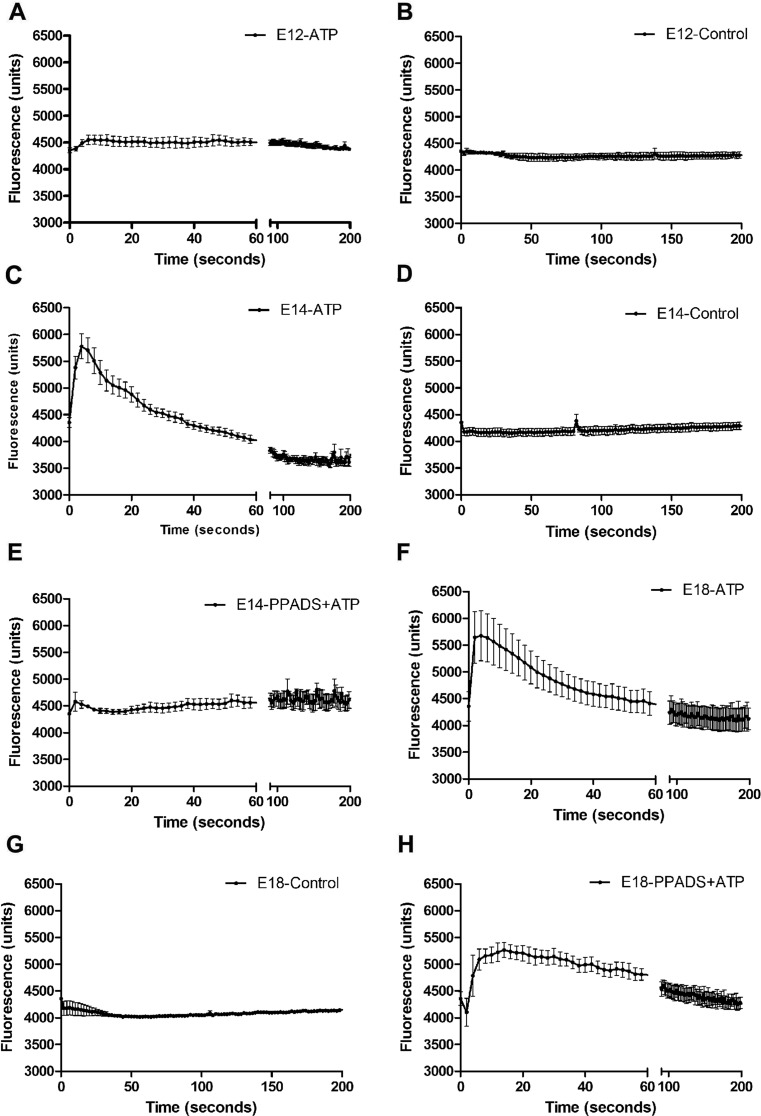
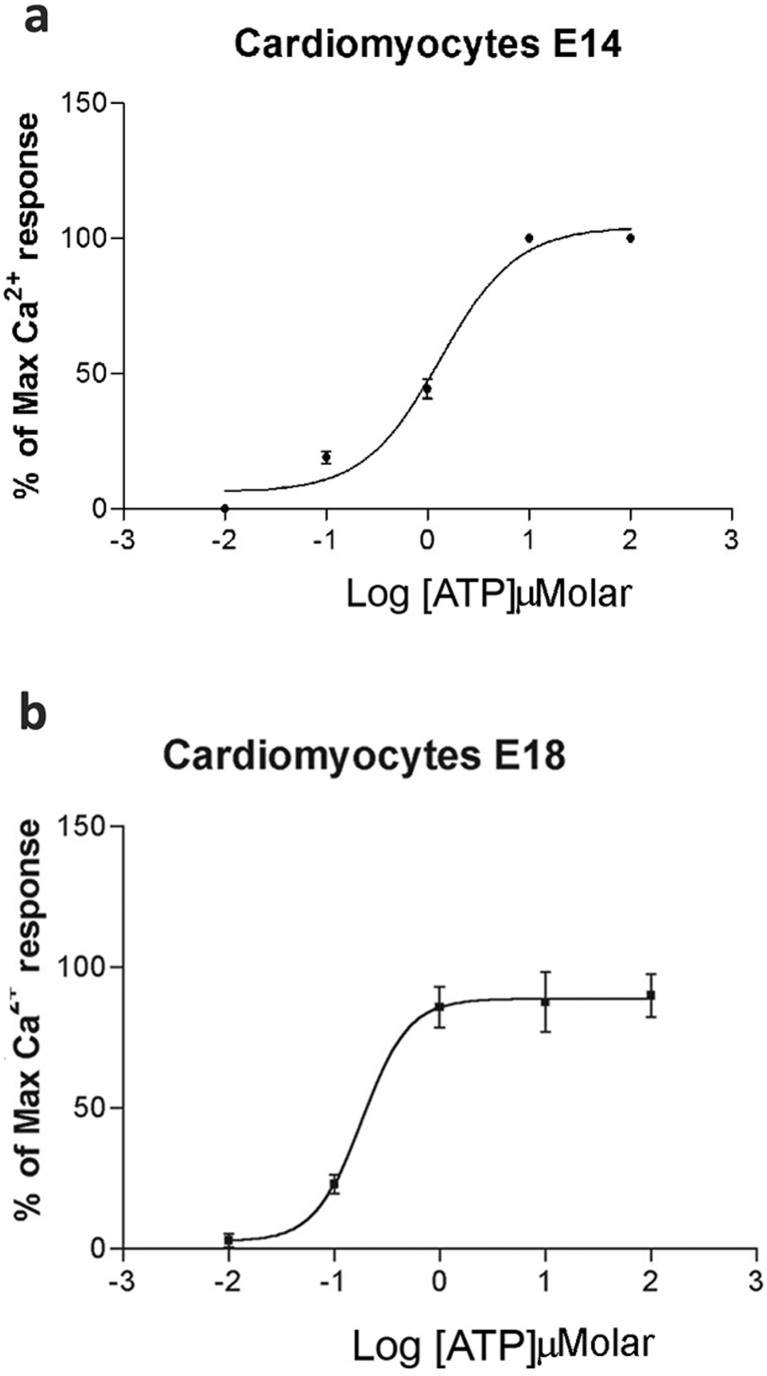
We used FLIPR to study the responses of cultured embryonic cardiomyocytes to exposure to ATP and other P2 receptor agonists. Positive responses were represented by an increase in fluorescence units, indicating Ca2+ mobilization in response to receptor activation. Cardiomyocytes from three embryonic ages (E12, E14 and E18) were taken for analysis. All agonists were used at a concentration of 100 μM. No change was observed in the control group when Ca2+-containing PBS was added to the cells (Fig. 1b, d, g). Cardiomyocytes taken from E12 did not show response in Ca2+ mobilization in response to ATP (Fig. 1a). In contrast, Ca2+ changes were detected with cardiomyocytes taken from E14 1422.4 ± 39.6 (arbitrary units, a.u.) and E18 1317.4 ± 40.2 (a.u.) embryonic hearts when the cells were treated with rapid application of ATP (Fig. 1c, f). A quick increase in fluorescence occurred with few seconds’ exposure to ATP. We then investigated the effect of the P2 receptor antagonist PPADS against ATP responses. The ATP-induced responses of E14 cardiomyocytes were completely blocked by PPADS (30 μM; Fig. 1e), whereas those of E18 cardiomyocytes were only partially blocked (31 %) by PPADS (Fig. 1h). Since we observed transient calcium peaks after 100 μM ATP, ATP dose-response curves were constructed using a more sensitive fluorescence photometer system (Fig. 2a–b). E12 cardiomyocytes exhibited in most experiments an ‘all or nothing’ response to the maximum concentration of ATP tested (100 μM) (data not shown). In contrast, ATP induced concentration-dependent responses in E14 and E18 cardiomyocytes and EC50 concentrations were calculated to be 1.32 ± 0.03 and 0.18 ± 0.02 μM, respectively (Fig. 2a–b). The effect of apyrase on the sensitivity of E12 cardiomyocytes to ATP was examined, and it was found that pretreatment with apyrase did not alter ATP sensitivity (data not shown).
Fig. 1.
Ca2+ mobilization in embryonic cardiomyocytes using FLIPR. The response of Ca2+ mobilization is represented by an increase in fluorescence. Responses of ATP (100 μM) in cultured cardiomyocytes taken from E12 (a), E14 (c) and E18 (f) and the corresponding controls (b, d, g, respectively). e, h The ATP-induced response after pre-incubation with PPADS (30 μM). Representative tracings from three independent experiments are shown performed with three different embryos each day of experiment
Fig. 2.
Dose-response curves to ATP of embryonic cardiomyocytes. Ca2+ mobilization responses to increasing ATP concentrations using fluorescence photometry. ATP-induced responses in cultured cardiomyocytes taken from E14 (a) and E18 (b). Mean of at least three independent experiments, with at least three different embryos each day of experiment, with 20–30 cells/field
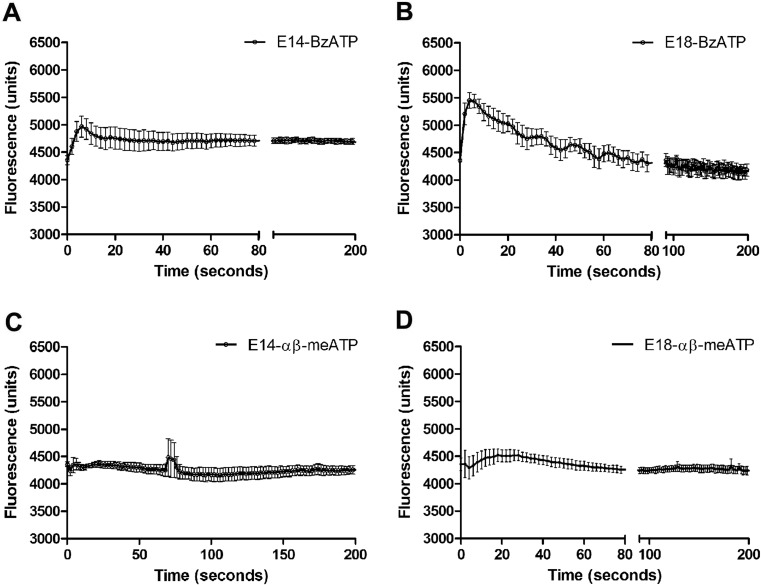
The response to other P2X receptors agonists was recorded. The prototypic P2X7 receptor agonist Bz-ATP did not induce any response in E12 cardiomyocytes (data not shown), but induced an augment of calcium responses 612 (a.u.) in E14 and 1098 (a.u.) in E18 cells, indicating that E18 cardiomyocytes are more responsive to P2X7 agonist than E14 (44 % increase) (Fig. 3a, b). The P2X1/3 agonist α,β-meATP did not induce a very noticeable response in E14 and E18 cardiomyocytes (Fig. 3c, d).
Fig. 3.
Ca2+ mobilization of embryonic cardiomyocytes using FLIPR. The Ca2+ mobilization response is represented by an increase in fluorescence. a, b show responses induced by BzATP (100 μM) in E14 and E18 cardiomyocytes, respectively. c, d Responses induced by α,β-meATP (100 μM) in E14 and E18 cardiomyocytes, respectively (the control data for Fig. 3 are shown in Fig. 1). Representative tracings from three independent experiments are shown performed with three different embryos each day of experiment
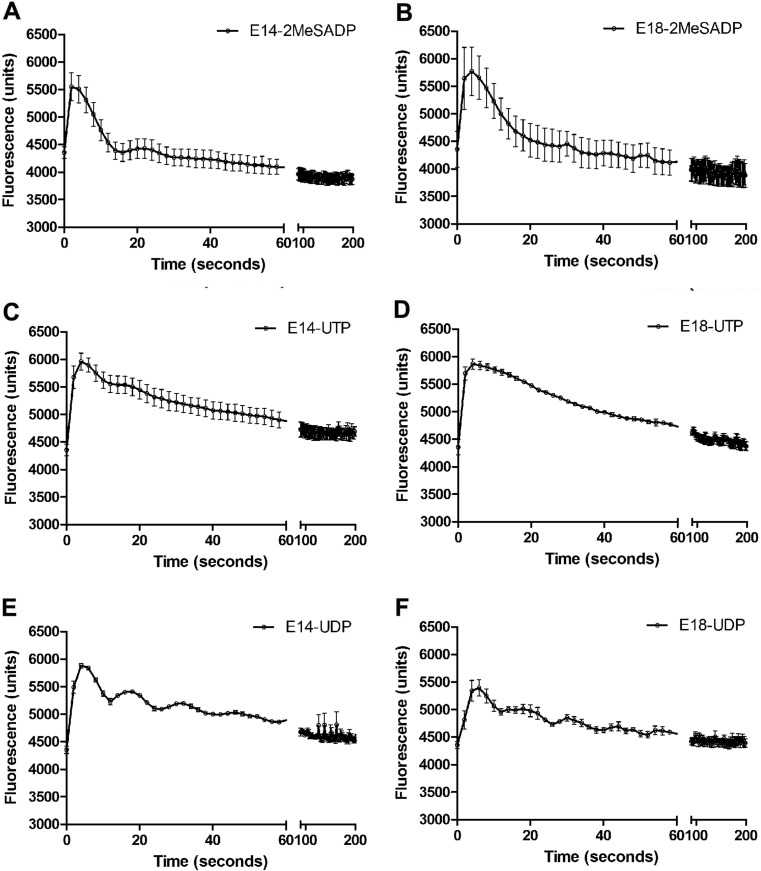
The P2Y receptor agonist’s 2-MeSADP, UTP and UDP induced significant Ca2+ mobilization in cardiomyocytes from embryonic ages E14 and E18 (Fig. 4a–f).
Fig. 4.
Ca2+ mobilization of embryonic cardiomyocytes using FLIPR. The Ca2+ mobilization responses are represented by an increase in fluorescence. a, c, e Responses of E14 cardiomyocytes induced by 2-MeSADP, UTP and UDP (100 μM), respectively; b, d, f Responses of E18 cardiomyocytes induced by the same agonists. Representative tracings from three independent experiments are shown performed with three different embryos each day of experiment
The P2Y1 agonist 2-MeSADP induced an augment of 1193 (a.u.) of E14 cells response and 1416 (a.u.) in E18 cardiomyocytes (Fig. 4a, b). In addition, the P2Y2/4 agonist UTP induced calcium changes of 1605 (a.u.) and 1322 (a.u.) in E14 and E18, respectively (Fig. 4e). A similar reduction of calcium responses were observed when E14 and E18 cardiomyocytes were treated with P2Y6 agonist UDP being 1529 (a.u.) and 1038 (a.u.) for E14 and E18 cells, respectively (Fig. 4f). All changes were significantly different.
Coupling measurements of cardiomyocytes
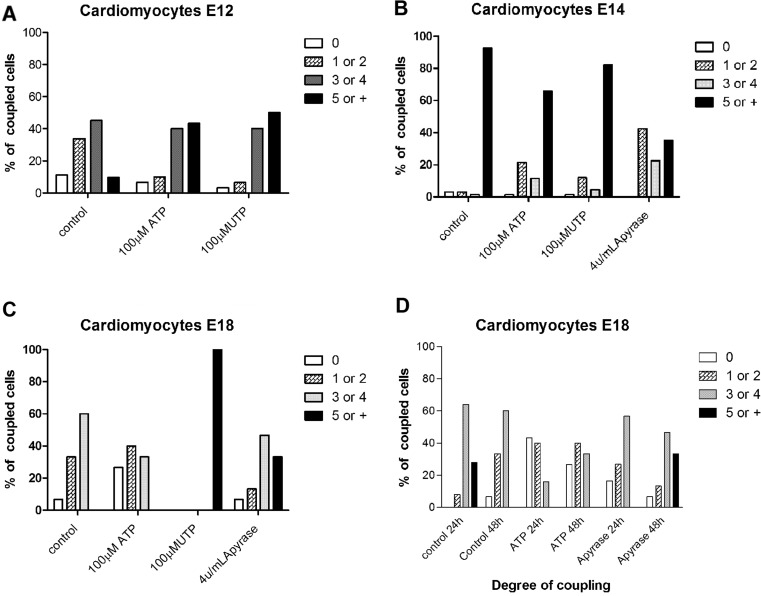
At E12, microinjection data showed that 0 coupling and 5 or more coupling represent about 10 % of the observed cells in control situations, and about 34 and 45 % of the cells were couple to one or two and three or four, respectively (Fig. 5a). After the application of ATP or UTP, the number of 1 or two coupled cells diminished and more than five couplings got higher (approximately 43 %) (Fig. 5a). At E14, approximately 90 % of the cardiomyocytes were coupled to more than five cells in control conditions (Fig. 5b). In contrast, when ATP or UTP were added, the number of coupled cells was reduced to 65.71 and 82.08 %, respectively (Fig. 5b). At E18, the majority of cardiomyocytes (60 %) was coupled to up to four cells in control conditions, while addition of ATP resulted in 27 % of no coupled cells, 40.3 % coupled to one or two cells and 33.3 % three or four cells coupled being observed. However, in the presence of UTP 100 % of the cells were coupled to more than five cells (Fig. 5c). In terms of apyrase effects, we observed that in E14 the number of one or two and three or four coupled cardiomyocytes increased, while five or more coupling was diminished in about 57.4 % (Fig. 5b). Data concerning E18 apyrase-treated cells showed a 33 % augment of five or more coupled cells (Fig. 5c). In view of this, E18 cardiomyocytes were treated for 24 or 48 h with nucleotides before microinjection (Fig. 5d). At E18 (control conditions—24 and 48 h), the majority of cardiomyocytes were coupled to three or four cells (Fig. 5d). Furthermore, both 24- and 48-h ATP treatments reduced the number of coupled cells; the coupling was only slightly changed in 24 h as well as in 48 h after apyrase addition in comparison to control (Fig. 5d).
Fig. 5.
Coupling measurements of cardiomyocytes. Percentages of coupled cells after microinjection in E12 cardiomyocytes (a) after ATP and UTP treatments and in E14 cardiomyocytes (b) after ATP and UTP application. c E18 cardiomyocytes. d The number of coupled cells after 24 and 48 h ATP or apyrase treatment. Bars show degree of coupling divided into four classes: no coupling (zero); one or two; three or four; or more than five neighboring cells coupled to the cell injected with Lucifer Yellow. Representative of n ≥ 2 independent experiments, with at least two different embryos each day of experiment, 20–30 cells/field
RT-PCR analysis
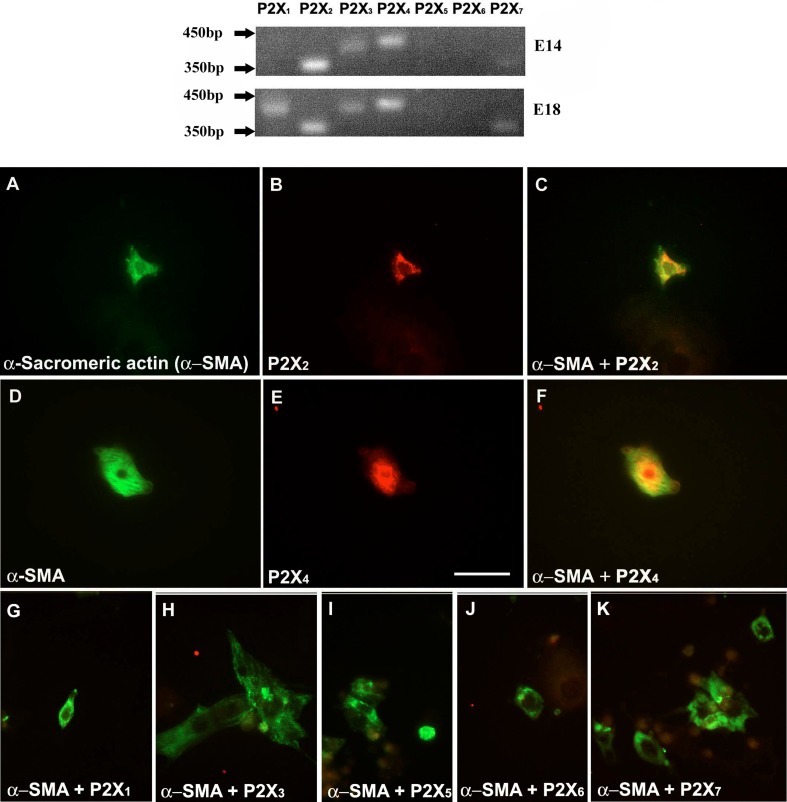
Two developmental ages of embryonic heart, E14 and E18, were studied for the expression of mRNA for the seven P2X receptor subtypes (P2X1–7). Results of the mRNA expression are summarized in Fig. 6. P2X2, P2X3, P2X4 and P2X7 receptor mRNA was expressed in the heart at both E14 and E18. P2X1 and P2X5 receptor mRNA was expressed at E18, but not at E14, although the expression of P2X5 receptor mRNA in the heart was barely detectable at E18. P2X6 receptor mRNA, however, was not expressed in cardiomyocytes at either E14 or E18.
Fig. 6.
P2X receptor subtype expression on E14 and E18 heart tissues. Primers for P2X6 receptors that gave negative results at both stages gave positive signals using rat brain tissue extract (data not shown). Cardiomyocytes were identified by a monoclonal antibody against α-sarcomeric actin (α-SMA, green) (a, d). P2X receptor-immunostained cells are shown in red (b, e). Cardiomyocytes showing co-expression (yellow) of P2X receptors (P2X2 and P2X4) with the α-SMA (c, f). Cardiomyocytes at this stage did not express P2X1, P2X3, P2X5, P2X6 and P2X7 receptors (g–k, respectively). Scale bar = 25 μm. Images representative of n = 2 independent experiments, with at least two different embryos
Double-labelling immunocytochemistry
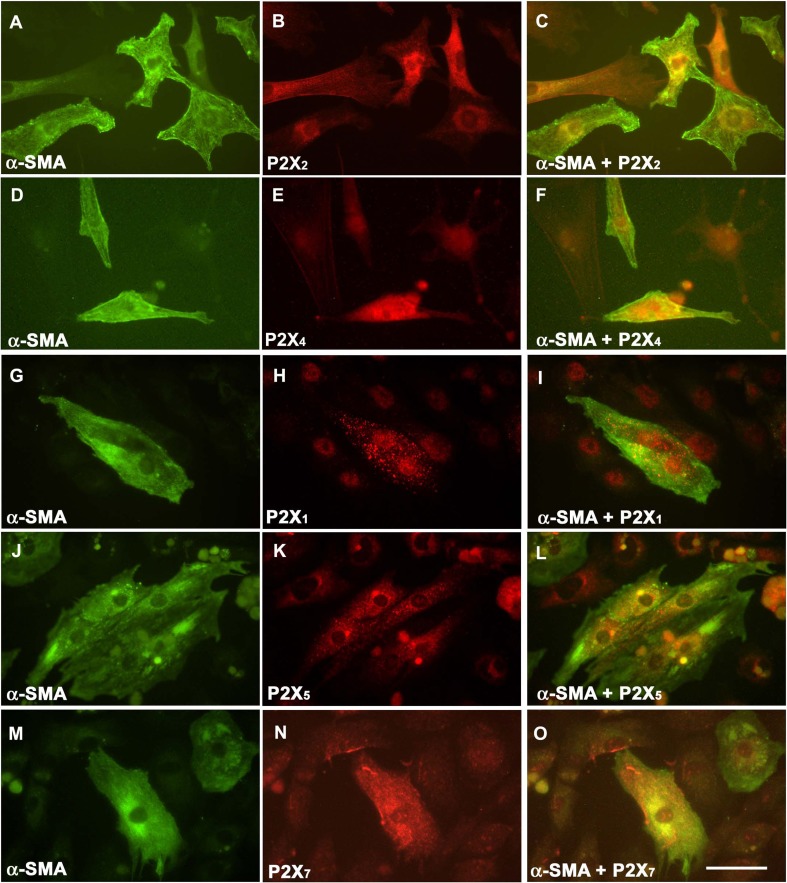
Fluorescence immunocytochemical staining has shown that of the P2X receptors examined, only P2X2 and P2X4 receptors were expressed in cardiomyocytes at E14 (Fig. 6a–f), as shown by double-labelling immunocytochemical staining with α-sarcomeric actin. None of the other P2X receptor subunits were co-expressed with α-sarcomeric actin (Fig. 6g–k). P2X2 and P2X4 receptors were detected in E18 cardiomyocytes labelled with α-sarcomeric actin (Fig. 7a–f). P2X1, P2X5 and P2X7 receptors were also expressed in E18 cultured cardiomyocytes (Fig. 7g–o). However, immunoreactivity for P2X3 and P2X6 receptors was not detected at any of the stages examined.
Fig. 7.
P2X receptor expression in cultured E18 cardiomyocytes. Cardiomyocytes were identified by a monoclonal antibody against α-sarcomeric actin (α-SMA, green) (a, d, g, j, m). P2X receptor-expressing cells are shown in red (P2X2 (b), P2X4 (e), P2X1 (h), P2X5 (k) and P2X7 (n)). P2X receptor subtypes co-expressing α-SMA on cardiomyocytes are shown in yellow (c, f, i, l, o). P2X3 and P2X6 receptors were not expressed in E18 cardiomyocytes (data not shown). Scale bar = 50 μm. Images representative of n = 2 independent experiments, with at least three different embryos
Discussion
It is well known that extracellular ATP exerts a wide range of activities on the adult myocardium (for reviews see [10, 33–35]). However, combined functional, molecular and immunocytochemical information about P2 receptor expression in the embryonic heart is lacking and would aid our understanding of purinergic signalling during embryonic development. The present study focused on the expression pattern of P2 receptors in the rat heart during embryonic development.
With the use of FLIPR, the responsiveness of cardiomyocytes taken at different embryonic ages to ATP was analysed, where it was found that cells at different embryonic ages responded differently to ATP. Previous reports have demonstrated the presence of functional P1 adenosine receptors in mammalian embryonic cardiomyocytes [36–38], and the results of the present study have clearly indicated the presence of functional P2X and P2Y receptors in rat cardiomyocytes from E12 onwards.
The results of the RT-PCR study and immunocytochemical staining showed that P2X2 and P2X4 receptors were the most likely P2X receptor subtypes responsive ATP at E14. The discriminating feature between the P2X2 and the P2X4 receptor is the blockade of the latter, but not the former, by PPADS [39]. The ATP-induced response at E14 was almost completely blocked by PPADS, which suggested that it was probably the P2X2 receptor that accounted for the ATP-mediated response in E14 cardiomyocytes; this was supported by the RT-PCR studies with the P2X2 receptor mRNA band being dominant. In addition, the P2X2 receptor was also expressed in the embryonic rat heart as early as E12 [25], the stage at which none of the other P2X receptors were detected. In contrast, the ATP-induced response at E18 cardiomyocytes was partially sustained after treating with PPADS, suggesting the involvement of P2X4, P2X6 and/or P2X7 receptors (see [39]). However, the absence of P2X6 receptor mRNA transcript could indicate a low possibility of P2X6 receptor involvement. Dose-response experiments showed that E18 cardiomyocytes are more sensitive to ATP than E14 and E12, suggesting that during embryonic cardiac development, there are changes in P2 receptor sensitivity. The microinjection data revealed that ATP inhibits the coupling of cardiomyocytes more intensively at E18 than at previous developmental stages. These results indicate that endogenous nucleotides may control couplings, with the progression of tissue development; more couplings can be observed, as well as P2 receptor subtype expression. Gap junction channels provide a critical component of the cardiac conduction system. Intercellular couplings in embryonic rat hearts are sufficient for the entire ventricular myocardium to contract synchronously at E10 [40]. It has been shown that mRNA for connexins are expressed as early as E9, but in terms of connexin 43 protein expression, it was shown that this occurred about 11 days after the mRNA expression [41]. Others connexins, such as connexin 32, were found to be expressed by E6 in the developing heart [42]. Gap junctions are formed early in the embryonic heart and are maintained. P2Y receptor subtype expression has overlapping or complementary expression and functional patterns that are similar to gap junction expression. Conversely, ATP induces a decrease in the couplings accompanying the embrionary development, indicating that ATP responsive receptors are downregulated as trabeculations occur and are established. The developmental increase in the density of gap junctions in embryonic hearts correlates well with the reported developmental increase in conduction velocity. It was shown that connexin 40 is highly expressed at E11 and maintains a stable expression pattern until E14, after which expression decreases [43]. A parallel connection with our results indicates a possible alignment of P2X receptor expression and functionality can be made with connexin expression, indicating a possible importance of P2X receptor expression together with connexins for the development of the electrical conduction system. Our immunostaining data show that P2X receptors are steadily expressed between E12 and E18, and the coupling data suggest that ATP-activated P2X receptors may possess a downregulatory role in the coupling at E18, while UTP-activated receptors present a positive effect after E12. Such responses might be controlled by endogenous apyrase, the balance of P2X receptor expression and endogenous apyrase throughout embryonic development must control the function of the cardiac tissue. Studies using RT-PCR and immunocytochemistry indicated the presence of P2X1, P2X3, P2X4 and P2X7 receptors in E18 hearts. In addition, BzATP, a P2X1 and P2X7 receptor agonist, triggered Ca2+ mobilization in E18 cardiomyocytes. Although P2X1 receptors are also sensitive to BzATP, α,β-meATP, a P2X1 and P2X3 receptor specific agonist, did not induce significant responses in either E14 or E18 cardiomyocytes. It is therefore suggested that the BzATP-induced response is probably via the activation of functional P2X7 rather than P2X1 receptors; this has not been reported previously. In contrast, P2X4 receptor expression has been reported in the human foetal and chick embryonic heart [19, 28, 30, 31]. ATP is known to stimulate a large increase in cytosolic calcium transient and myocyte contractile amplitude (see [10]). Transgenic mice overexpressing human P2X4 receptors demonstrated an increase in contractility in cardiomyocytes subject to ATP stimulation [19]. In contrast to P2X4 receptors, little is known about the function of P2X2 and P2X7 receptors in the myocardium, although it has been shown previously that activation of P2X7 receptors induce contraction of the human saphenous vein smooth muscle [44]. It is still not clear what factors underlie early embryonic changes in contractility in rat myocardium, as both changes in Ca2+ sensitivity and troponin isoforms occur after 7 days postnatal [45, 46]. However, the possibility of an increase in contractility in the embryonic rat heart as a result of P2X4 and P2X7 receptor activation (and probably P2X2 receptors) mediated by ATP is worth investigating.
The presence of receptor transcripts for P2Y1, P2Y2, P2Y4 and P2Y6 receptors in the embryonic rat heart has been demonstrated previously [26], as well as P2Y2, P2Y4 and P2Y6 receptors in the human foetal heart [31]. The present study helps to uncover the functional status of the receptors on cardiomyocytes during heart development. The P2Y receptor agonists all induced Ca2+ mobilization on cardiomyocytes at E14. 2-MeSADP, which is a stable analogue of ADP, induced a P2Y1-specific response on Ca2+ mobilization in cardiomyocytes. The uracil nucleotides that preferentially activate P2Y2 and P2Y4 receptors (UTP) and P2Y6 receptor (UDP) also induced Ca2+ mobilization in cardiomyocytes. The receptor proteins were expressed as early as E12 [26], and were shown to be responsive to the agonists in cardiomyocytes from E12. We have previously shown that P2Y receptor proteins were initially expressed all over the heart, but were soon restricted to the trabeculated layers; the expression was absent in compact myocardium after E14 [26]. Trabeculation differs from compact myocardium in terms of the extent of differentiation, rate of contractions and impulse conduction [22]. P2Y receptor expression in the trabeculated layer, and the responsiveness towards agonists from E14 onwards, suggested that the receptor may be involved in either differentiation or contraction.
Small spontaneous responses were occasionally observed in E14 and E18 cardiomyocytes. Such responses appeared to be artefacts, as they remained in the presence of PPADS, and were probably due to the contractile property of the cardiomyocytes that generate the responses. The spontaneous responses were particularly noticeable in UDP-treated cardiomyocytes, raising the possibility that UDP may have some influence on cardiomyocyte contraction.
Bogdanov et al. [31] have shown the presence of P2X1 and P2X3 receptor mRNA transcripts in the human foetal heart. In the present study of rat myocytes, we were also able to amplify the mRNA transcripts of P2X1 and P2X3 receptors in E18 hearts. However, α,β-meATP did not triggered response in E18 cardiomyocytes compared to ATP, BzATP and the P2Y receptor agonists. Therefore, P2X1 receptors (which also respond to BzATP) are also likely to be involved in the development of cardiomyocytes in rats.
Ruppelt et al. [29] demonstrated the mRNA expression of the P2X5 receptor subtype in the embryonic chick heart. Although the results of RT-PCR from the present study showed very weak P2X5 mRNA transcript expression in E18 rat heart, double-labelling experiments confirmed the presence of P2X5 receptor protein in cardiomyocytes.
Although the expression profile of P2 receptors on rat heart development has been examined previously, expression patterns, together with functional analysis of embryonic cardiomyocytes, have not been studied previously. Our observations support the hypothesis that ATP acts on particular P2 receptor subtypes during different stages of development, suggesting that complex mechanisms are involved in governing sequential P2 receptor subtype expression.
In summary, we have identified P2X receptor subtypes (P2X1, P2X2, P2X4, P2X5 and P2X7) and P2Y receptor subtypes (P2Y1, P2Y2, P2Y4 and P2Y6) in rat embryonic cardiomyocytes. All these receptor subtypes, except for P2X7 and P2Y4 were shown to be present in adult cardiomyocytes [13, 14, 27]. It therefore appears that purinoceptors play important roles early in embryos and that P2Y4 and P2X7 have additional roles in the embryo that are not continued into adults. As complex remodelling occurs during the embryonic heart development, the question of whether these embryonic ATP receptor subtypes play comparable functional roles in the adult awaits further investigation.
Acknowledgments
The authors would like to thank Dr. Chrystalla Orphanides and Dr. Gillian E. Knight for excellent editorial assistance. We also thank Sam Ranasinghe for technical assistance on FLIPR. Dr. Coutinho-Silva was supported by a fellowship from Wellcome Trust. This work was supported by funds from the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico do Brasil (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Programa de Núcleos de Excelência (PRONEX) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Conflict of interest
The authors claim no conflict of interest.
Abbreviations
- ATP
Adenosine 5′-triphosphate
- ADP
Adenosine 5′-diphosphate
- UTP
Uridine 5′-triphosphate
- FLIPR
Fluorometric imaging plate reader
- BzATP
2′,3′-O-(4-benzoylbenzoyl)-ATP
- α,β-meATP
α,β-Methylene ATP
- 2-MeSADP
2-Methylthio ATP
- UDP
Uridine 5′-diphosphate
- PPADS
Pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid
Footnotes
Kwok-Kuen Cheung and Camila Marques-da-Silva Both contributed equally to this work.
References
- 1.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis L, editors. Cell membrane receptors for drugs and hormones: a multidisciplinary approach. New York: Raven; 1978. pp. 107–118. [Google Scholar]
- 3.Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 4.Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G. Introduction and perspective, historical note. Front Cell Neurosci. 2013;7:227. doi: 10.3389/fncel.2013.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Köles L, Fürst S, Illes P. Purine ionotropic (P2X) receptors. Curr Pharm Des. 2007;13:2368–2384. doi: 10.2174/138161207781368747. [DOI] [PubMed] [Google Scholar]
- 7.Fountain SJ. Primitive ATP-activated P2X receptors: discovery, function and pharmacology. Front Cell Neurosci. 2013;7:247. doi: 10.3389/fncel.2013.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G. Introduction: P2 receptors. Curr Top Med Chem. 2004;4:793–803. doi: 10.2174/1568026043451014. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson KA, Balasubramanian R, Deflorian F, Gao ZG. G protein-coupled adenosine (P1) and P2Y receptors: ligand design and receptor interactions. Purinergic Signal. 2012;8:419–436. doi: 10.1007/s11302-012-9294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vassort G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- 11.Blayney L, Beck K, MacDonald E, D'Cruz L, Nomikos M, Griffiths J, Thanassoulas A, Nounesis G, Lai FA. ATP interacts with the CPVT mutation-associated central domain of the cardiac ryanodine receptor. Biochim Biophys Acta. 2013;1830:4426–4432. doi: 10.1016/j.bbagen.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 12.Burley DS, Cox CD, Zhang J, Wann KT, Baxter GF. Natriuretic peptides modulate ATP-sensitive K+ channels in rat ventricular cardiomyocytes. Basic Res Cardiol. 2014;109:402. doi: 10.1007/s00395-014-0402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nori S, Fumagalli L, Bo X, Bogdanov Y, Burnstock G. Coexpression of mRNAs for P2X1, P2X2 and P2X4 receptors in rat vascular smooth muscle: an in situ hybridization and RT-PCR study. J Vasc Res. 1998;35:179–185. doi: 10.1159/000025582. [DOI] [PubMed] [Google Scholar]
- 14.Hansen MA, Bennett MR, Barden JA. Distribution of purinergic P2X receptors in the rat heart. J Auton Nerv Syst. 1999;78:1–9. doi: 10.1016/S0165-1838(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 15.Horckmans M, Robaye B, Léon-Gómez E, Lantz N, Unger P, Dol-Gleizes F, Clouet S, Cammarata D, Schaeffer P, Savi P, Gachet C, Balligand JL, Dessy C, Boeynaems JM, Communi D. P2Y4 nucleotide receptor: a novel actor in post-natal cardiac development. Angiogenesis. 2012;15:349–360. doi: 10.1007/s10456-012-9265-1. [DOI] [PubMed] [Google Scholar]
- 16.Webb TE, Boluyt MO, Barnard EA. Molecular biology of P2Y purinoceptors: expression in rat heart. J Auton Pharmacol. 1996;16:303–307. doi: 10.1111/j.1474-8673.1996.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 17.Hochhauser E, Cohen R, Waldman M, Maksin A, Isak A, Aravot D, Jayasekara PS, Müller CE, Jacobson KA, Shainberg A. P2Y2 receptor agonist with enhanced stability protects the heart from ischemic damage in vitro and in vivo. Purinergic Signal. 2013;9:633–642. doi: 10.1007/s11302-013-9374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci U S A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu B, Mei QB, Yao XJ, Smith E, Barry WH, Liang BT. A novel contractile phenotype with cardiac transgenic expression of the human P2X4 receptor. FASEB J. 2001;15:2739–2741. doi: 10.1096/fj.01-0102com. [DOI] [PubMed] [Google Scholar]
- 20.Shen JB, Pappano AJ, Liang BT. Extracellular ATP-stimulated current in wild-type and P2X4 receptor transgenic mouse ventricular myocytes: implications for a cardiac physiologic role of P2X4 receptors. FASEB J. 2006;20:277–284. doi: 10.1096/fj.05-4749com. [DOI] [PubMed] [Google Scholar]
- 21.Fishman MC, Chien KR. Fashioning the vertebrate heart: earliest embryonic decisions. Development. 1997;124:2099–2117. doi: 10.1242/dev.124.11.2099. [DOI] [PubMed] [Google Scholar]
- 22.Franco D, Lamers WH, Moorman AF. Patterns of expression in the developing myocardium: towards a morphologically integrated transcriptional model. Cardiovasc Res. 1998;38:25–53. doi: 10.1016/S0008-6363(97)00321-0. [DOI] [PubMed] [Google Scholar]
- 23.Olson EN, Srivastava D. Molecular pathways controlling heart development. Science. 1996;272:671–676. doi: 10.1126/science.272.5262.671. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Z, Rivkees SA. Programmed cell death in the developing heart: regulation by BMP4 and FGF2. Dev Dyn. 2000;217:388–400. doi: 10.1002/(SICI)1097-0177(200004)217:4<388::AID-DVDY6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 25.Cheung K-K, Burnstock G. Localisation of P2X3 and co-expression with P2X2 receptors during rat embryonic neurogenesis. J Comp Neurol. 2002;443:368–382. doi: 10.1002/cne.10123. [DOI] [PubMed] [Google Scholar]
- 26.Cheung K-K, Ryten M, Burnstock G. Abundant and dynamic expression of G protein-coupled P2Y receptors in mammalian development. Dev Dyn. 2003;228:254–266. doi: 10.1002/dvdy.10378. [DOI] [PubMed] [Google Scholar]
- 27.Musa H, Tellez JO, Chandler NJ, Greener ID, Maczewski M, Mackiewicz U, Beresewicz A, Molenaar P, Boyett MR, Dobrzynski H. P2 purinergic receptor mRNA in rat and human sinoatrial node and other heart regions. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:541–549. doi: 10.1007/s00210-009-0403-2. [DOI] [PubMed] [Google Scholar]
- 28.Ruppelt A, Liang BT, Soto F. Cloning, functional characterization and developmental expression of a P2X receptor from chick embryo. Prog Brain Res. 1999;120:81–90. doi: 10.1016/S0079-6123(08)63547-5. [DOI] [PubMed] [Google Scholar]
- 29.Ruppelt A, Ma W, Borchardt K, Silberberg SD, Soto F. Genomic structure, developmental distribution and functional properties of the chicken P2X5 receptor. J Neurochem. 2001;77:1256–1265. doi: 10.1046/j.1471-4159.2001.00348.x. [DOI] [PubMed] [Google Scholar]
- 30.Hu B, Senkler C, Yang A, Soto F, Liang BT. P2X 4 receptor is a glycosylated cardiac receptor mediating a positive inotropic response to ATP. J Biol Chem. 2002;277:15752–15757. doi: 10.1074/jbc.M112097200. [DOI] [PubMed] [Google Scholar]
- 31.Bogdanov Y, Rubino A, Burnstock G. Characterisation of subtypes of the P2X and P2Y families of receptors in the foetal human heart. Life Sci. 1998;62:697–703. doi: 10.1016/S0024-3205(97)01168-5. [DOI] [PubMed] [Google Scholar]
- 32.Shibuya I, Tanaka K, Hattori Y, Uezono Y, Harayama N, Noguchi J, Ueta Y, Izumi F, Yamashita H. Evidence that multiple P2X purinoceptors are functionally expressed in rat supraoptic neurones. J Physiol. 1999;514:351–367. doi: 10.1111/j.1469-7793.1999.351ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsson RA, Pearson JD. Cardiovascular purinoceptors. Physiol Rev. 1990;70:761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- 34.Ralevic V, Burnstock G. Roles of P2-purinoceptors in the cardiovascular system. Circulation. 1991;84:1–14. doi: 10.1161/01.CIR.84.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Pelleg A, Belardinelli L. Effects of extracellular adenosine and ATP on cardiomyocytes. Austin: R.G. Landes Company; 1998. pp. 1–225. [Google Scholar]
- 36.Zhao Z, Rivkees SA. Inhibition of cell proliferation in the embryonic myocardium by A1 adenosine receptor activation. Dev Dyn. 2001;221:194–200. doi: 10.1002/dvdy.1130. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Z, Yaar R, Ladd D, Cataldo LM, Ravid K. Overexpression of A3 adenosine receptors in smooth, cardiac, and skeletal muscle is lethal to embryos. Microvasc Res. 2002;63:61–69. doi: 10.1006/mvre.2001.2366. [DOI] [PubMed] [Google Scholar]
- 38.Mustafa SJ, Morrison RR, Teng B, Pelleg A (2009) Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol 161–188 [DOI] [PMC free article] [PubMed]
- 39.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 40.Guthrie SC, Gilula NB. Gap junctional communication and development. Trends Neurosci. 1989;12:12–16. doi: 10.1016/0166-2236(89)90150-1. [DOI] [PubMed] [Google Scholar]
- 41.Fishman GI, Hertzberg EL, Spray DC, Leinwand LA. Expression of connexin43 in the developing rat heart. Circ Res. 1991;68:782–787. doi: 10.1161/01.RES.68.3.782. [DOI] [PubMed] [Google Scholar]
- 42.Becker DL, Cook JE, Davies CS, Evans WH, Gourdie RG. Expression of major gap junction connexin types in the working myocardium of eight chordates. Cell Biol Int. 1998;22:527–543. doi: 10.1006/cbir.1998.0295. [DOI] [PubMed] [Google Scholar]
- 43.Delorme B, Dahl E, Jarry-Guichard T, Marics I, Briand JP, Willecke K, Gros D, Theveniau-Ruissy M. Developmental regulation of connexin 40 gene expression in mouse heart correlates with the differentiation of the conduction system. Dev Dyn. 1995;204:358–371. doi: 10.1002/aja.1002040403. [DOI] [PubMed] [Google Scholar]
- 44.Cario-Toumaniantz C, Loirand G, Ladoux A, Pacaud P. P2X7 receptor activation-induced contraction and lysis in human saphenous vein smooth muscle. Circ Res. 1998;83:196–203. doi: 10.1161/01.RES.83.2.196. [DOI] [PubMed] [Google Scholar]
- 45.Reiser PJ, Westfall MV, Schiaffino S, Solaro RJ. Tension production and thin-filament protein isoforms in developing rat myocardium. Am J Physiol. 1994;267:H1589–H1596. doi: 10.1152/ajpheart.1994.267.4.H1589. [DOI] [PubMed] [Google Scholar]
- 46.Jin JP. Alternative RNA splicing-generated cardiac troponin T isoform switching: a non-heart-restricted genetic programming synchronized in developing cardiac and skeletal muscles. Biochem Biophys Res Commun. 1996;225:883–889. doi: 10.1006/bbrc.1996.1267. [DOI] [PubMed] [Google Scholar]