Abstract
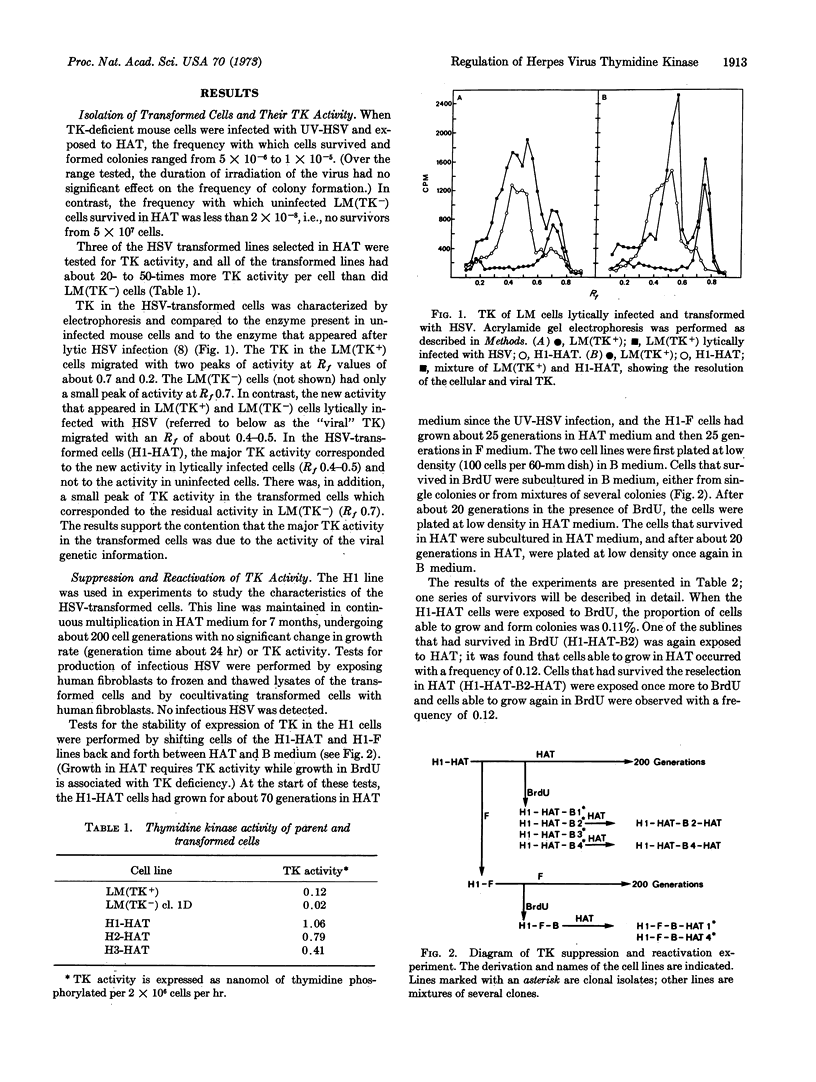
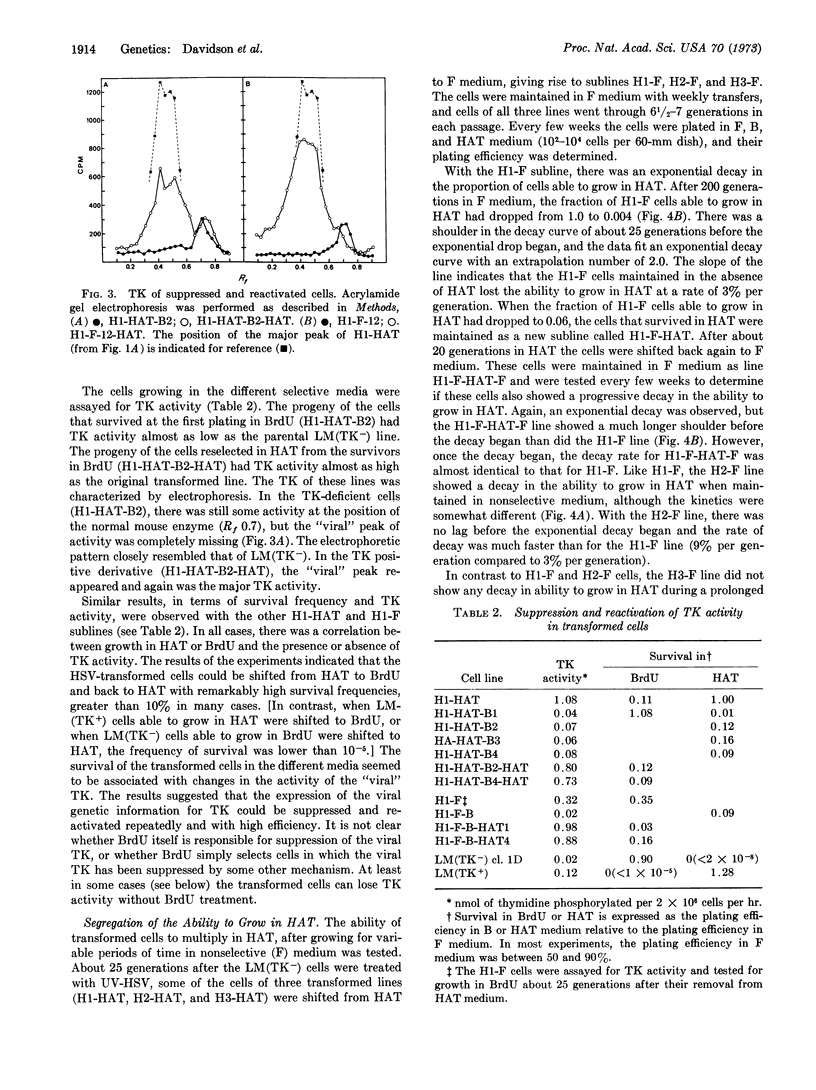
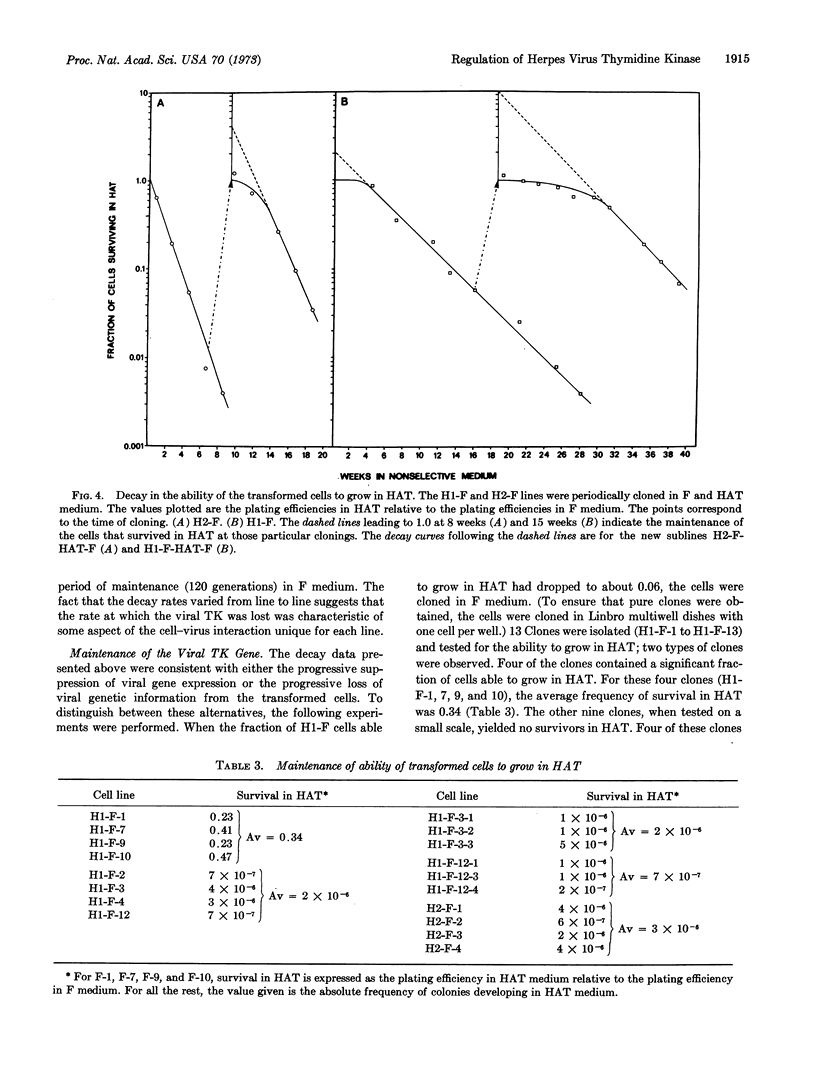
Thymidine kinase (EC 2.7.1.21)-deficient mouse cells were infected with inactivated herpes simplex virus, after which “transformed” cells that produce viral thymidine kinase were isolated. Shortly after transformation, the expression of the viral enzyme could be suppressed and reactivated with high efficiency. On continued multiplication in nonselective medium, the proportion of cells producing the viral enzyme decayed exponentially. This decay seemed to represent a change in the expression of the viral gene for thymidine kinase rather than the loss of the gene from the cells, since the viral enzyme could be apparently reactivated in every cell, albeit at a very low frequency.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein S. J., Baldwin C., Kohn H. I. Thymidine kinase in mouse liver: variations in soluble and mitochondrial-associated activity that are dependent on age, regeneration, starvation, and treatment with actinomycin D and puromycin. Dev Biol. 1971 Dec;26(4):537–546. doi: 10.1016/0012-1606(71)90139-4. [DOI] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. MUTANT STRAINS OF HERPES SIMPLEX DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Apr;22:493–502. doi: 10.1016/0042-6822(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Davidson R., Ephrussi B. Factors influencing the "effective mating rate" of mammalian cells. Exp Cell Res. 1970 Jul;61(1):222–226. doi: 10.1016/0014-4827(70)90282-x. [DOI] [PubMed] [Google Scholar]
- HAM R. G. An improved nutrient solution for diploid Chinese hamster and human cell lines. Exp Cell Res. 1963 Feb;29:515–526. doi: 10.1016/s0014-4827(63)80014-2. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Munyon W., Buchsbaum R., Paoletti E., Mann J., Kraiselburd E., Davis D. Electrophoresis of thymidine kinase activity synthesized by cells transformed by herpes simplex virus. Virology. 1972 Sep;49(3):683–689. doi: 10.1016/0042-6822(72)90525-9. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Lander M. R., Pugh W. E., Teich N. Noninfectious AKR mouse embryo cell lines in which each cell has the capacity to be activated to produce infectious murine leukemia virus. Virology. 1971 Dec;46(3):866–876. doi: 10.1016/0042-6822(71)90087-0. [DOI] [PubMed] [Google Scholar]


