Abstract
Adenosine (Ado) is a ubiquitous metabolite that plays a prominent role as a paracrine homeostatic signal of metabolic imbalance within tissues. It quickly responds to various stress stimuli by adjusting energy metabolism and influencing cell growth and survival. Ado is also released by dead or dying cells and is present at significant concentrations in solid tumors. Ado signaling is mediated by Ado receptors (AdoR) and proteins modulating its concentration, including nucleoside transporters and Ado deaminases. We examined the impact of genetic manipulations of three Drosophila genes involved in Ado signaling on the incidence of somatic mosaic clones formed by the loss of heterozygosity (LOH) of tumor suppressor and marker genes. We show here that genetic manipulations with the AdoR, equilibrative nucleoside transporter 2 (Ent2), and Ado deaminase growth factor-A (Adgf-A) cause dramatic changes in the frequency of hyperplastic outgrowth clones formed by LOH of the warts (wts) tumor suppressor, while they have almost no effect on control yellow (y) clones. In addition, the effect of AdoR is dose-sensitive and its overexpression leads to the increase in wts hyperplastic epithelial outgrowth rates. Consistently, the frequency of mosaic hyperplastic outgrowth clones generated by the LOH of another tumor suppressor, discs overgrown (dco), belonging to the wts signaling pathway is also dependent on AdoR. Our results provide interesting insight into the maintenance of tissue homeostasis at a cellular level.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-014-9435-2) contains supplementary material, which is available to authorized users.
Keywords: Cell competition, Adgf-A, Ent2, LATS1, Warts, Discs overgrown
Introduction
Adenosine (Ado) is a ubiquitous metabolite that plays a prominent role as a paracrine homeostatic signal of metabolic imbalance within tissues [1]. Ado can be formed extracellularly by the dephosphorylation of adenine nucleotides that are released from stressed or damaged cells [2]. Ado is also formed intracellularly from ATP and transported to the extracellular space via bidirectional nucleoside transporters [2]. The adenosine gradient then acts in neighboring cells, allowing them to deal more effectively with potentially stressful events [1].
While there is a lot of information on the effects of Ado and various metabolically stable adenosine receptor (AdoR) analogs at the organismal and organ levels, our knowledge of adenosine signaling in maintaining tissue homeostasis at a cellular level remains poor. Recent studies have shown that Ado transport, metabolism, and signaling are all parts of the Ado homeostatic signaling, which plays a more important biological role in diverse organisms than previously thought. For example, the local concentration of extracellular Ado was shown to provide a signal for the localization of hematopoietic progenitor cells in the cortical zone of the Drosophila lymph gland [3] or to regulate the regeneration of pancreatic beta cells in zebrafish and mice in vivo [4].
AdoRs, which belong to the G-protein-coupled receptor family [5] mediate most of the physiological effects of extracellular adenosine in vertebrates and invertebrates. Four distinct AdoR isoforms were identified in mammals. Two of them are positively coupled to adenylate cyclase (A2A and A2B) and the other two (A1 and A3) negatively regulate the enzyme. Drosophila has a single AdoR, which is positively coupled to adenylate cyclase [6]. Its sequence shows about 38 % identity with the N-terminal region of 350 amino acids of the A2A human homologue [7]. Drosophila AdoR mutants are viable and show no obvious phenotypic changes [7, 8].
Several other conserved gene products are involved in adenosine signaling and modulate the extracellular concentration of adenosine, including nucleoside transporters and Ado deaminases [9, 10]. Our previous research showed genetic interactions between them including compensatory changes in the expression of the equilibrative nucleoside transporter 2 (Ent2) in the AdoR mutants and adenosine deaminase growth factor A (Adgf-A) in the Ent2 mutants [11]. The Adgf-A mutant is homozygously lethal, causing elevated levels of extracellular Ado [12, 13]. Null mutants in Ent2 are also homozygously lethal, while Ent2 hypomorphs show similar defects in synaptic transmission and associative learning as the AdoR1 mutant [11].
Since Ado exerts a broad range of cytoprotective, growth-promoting, and immunosuppressive effects and was observed at a high concentration in a number of human tumors, it was suggested to be an important factor regulating tumor growth [14–17]. In this report, we examined the effect of three Drosophila genes involved in Ado signaling on the incidence of somatic mosaic clones, including AdoR, Ent2, and Adgf-A. We show that genetic manipulations with these genes cause dramatic changes in the frequency of hyperplastic outgrowth clones by the loss of heterozygosity (LOH) of the warts (wts) tumor suppressor gene, while they have no or very little effect on control clones. The Drosophila wts clones are formed in the epithelium of imaginal discs, neural epithelium, and histoblast nests [18, 19]. The wts homologue in humans is called LATS1 and its downregulation has been reported in a number of tumors [20]. This report is the first to demonstrate how changes in Ado signaling influence the frequency of the wts mosaic hyperplastic epithelial outgrowth clones in Drosophila.
Materials and methods
Fly stocks
The AdoR1 mutant is viable and fertile and was generated earlier by homologous recombination [12]. We prepared an AdoR1 stock isogenized to white1118 flies by serial backcrosses.
Two additional AdoR alleles were received from public stock collections. The AdoR deletion mutant designated AdoRKG0396ex was generated by Wu et al. [8]. The mutation-designated AdoRDf(3R)Exel6214, which contains a larger deletion located in 3R (99D5-99E2), encompasses approximately 17 genes including the AdoR locus and was from the Exelixis collection (Harvard University, USA). The Drosophila RNA interference strains carrying upstream activation sequence (UAS -AdoRRNAi-VDRC, line number 1385 and UAS-Ent2RNAi-VDRC, line number 7618) hairpins were received from the Vienna Drosophila RNA interference (RNAi) Center (VDRC). The UAS-AdoRRNAi-TRIP (TRIP, Bloomington stock BL-27536) flies were received from the Transgenic RNAi Project of Harvard Medical School. The wtsx1 mutant was received from Dr. Tian Xu (Yale University). The UAS-AdoR, UAS-Ent2, and UAS-ADGF-A strains were constructed earlier[7, 11]. All other Drosophila strains, including Act-Gal4, Tub-Gal80, en-Gal4, y, discs overgrown (dco3) were obtained from the Bloomington Drosophila stock center.
Drug treatment
For testing cisplatin and 2,6-diaminopurine (DAP), 10 female and 4 male flies were placed into standard rearing vials for mating and egg laying. After 36–40 h, the parents were transferred to fresh medium. The first instar larvae were treated by adding 0.3 ml of the solutions or suspensions of tested substances onto the surface of the food. Cisplatin, cis-diaminedichloroplatinum (Sigma-Aldrich) was used in a 0.2 mg/ml aqueous (0.67 mM) solution unless otherwise specified. The DAP assays (Sigma-Aldrich) were performed using 12–80 mM (toxicity assay) and 40 mM (Somatic Mutation and Recombination test, SMART) concentrations in 5 % dimethyl sulfoxide, (DMSO, Sigma-Aldrich). Because of its low solubility, DAP was added as a suspension to larval food. The toxicity of paraquat (Sigma-Aldrich, 20 mM solution in 5 % DMSO) was tested on adults for 3 days (males, 24 h after eclosion). Control flies were treated with the equivalent concentration of DMSO. Lethality was expressed in the percentage of surviving flies (with respect to the original number of embryos taken to the experiment).
SMART and clonal analysis
SMART, as described by Stern [21], is based on LOH and mitotic crossing-over. We used a variation of SMART based on flies heterozygous for marker genes (y/+ or wtsx1/+), in which the mosaic clones can be detected over the entire body surface of the adults and more than 70 % of the wts hyperplastic outgrowths are found in larval wing imaginal discs or in adult wings, notum, and sternopleura [22]. We examined either spontaneous or mutagen (cisplatin, diaminopurine, paraquat)-induced somatic recombination and compared the frequencies of clones that appeared in the examined genetic backgrounds (AdoR+, AdoR1 or AdoR+/AdoR1). Cisplatin showed a strong statistically significant recombinogenic effect (P < 0.01) at the doses that had low toxicity (Fig. S1A). We therefore chose cisplatin to induce mitotic recombination in most of our experiments.
The wtsx1 clones were classified into four size categories as follows: (1) “Smallest detectable”—The clones of the minimal detectable size (∼20 cells, based on the wing hair counting), (2) “Small”—Readily detectable, 4–5 times bigger than the smallest (∼100 cells), (3) “Average”—3–10 times bigger than the small (up to one third of the total organ size), and (4) “Large”—2–3 times bigger than the average (up to the total size of the organ).
In order to label the clones with green fluorescent protein (GFP) and/or to express various transgenes, we used the mosaic analysis with a repressible cell marker (MARCM) system [23], based on the loss of Gal80 expression in clones and subsequent derepression of Gal4-dependent transcription (Figs. 1b–f and S4). For the MARCM expression in wtsx1 or AdoR1 clones, we used the Gal80 transgene localized in the cytological position 84B, in the chromosomal arm 3R. In wts+Tub-Gal80/wtsx1 + heterozygous flies, the mosaic wtsx1 clones lose the repressor and express UAS constructs driven by the Act-Gal4 driver. The following UAS constructs were used: UAS-GFP, UAS-Adgf-A, UAS-Ent2, UAS-Ent2RNAi, UAS-AdoR, and UAS-AdoRRNAi.
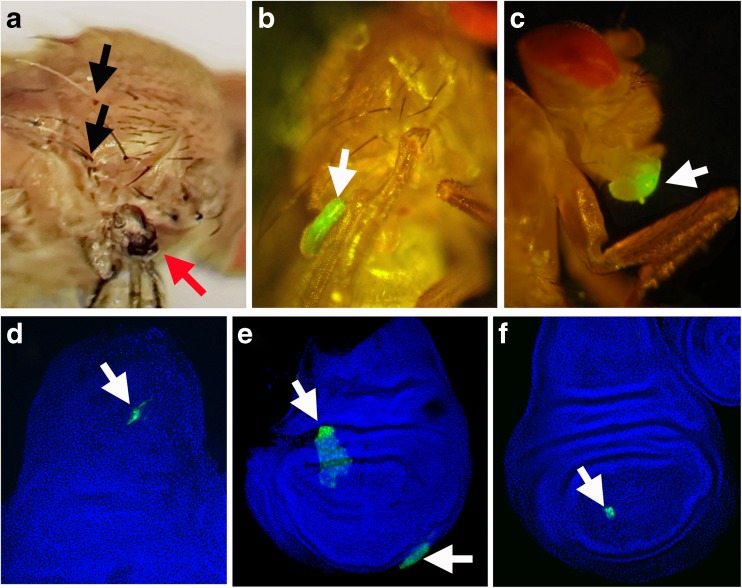
Fig. 1.
Examples of somatic mosaic clones in adult flies and larval imaginal discs; a–c adult flies, d–f imaginal discs. The clones are indicated by arrows. a yellow (black arrows point to yellow bristles) and wts x1 clones (red arrow) on the thorax of a y/+; wts x1 AdoR 1/+ + imago. b A large wts x1 GFP clone (white arrow) in the wing (Act-Gal4 UAS-GFP/+ +; + wts x1/Tub-Gal80 +); c non-hyperplastic GFP clone (white arrow) in proboscis labeled using the MARCM system (Act-Gal4 UAS-GFP/+ +; +/Tub-Gal80). d UAS-AdoR RNAi-VDRC UAS-GFP clone (white arrow) in the imaginal disc of third instar larva Act-Gal4 UAS-GFP/+; AdoR RNAi-VDRC/Tub-Gal80 genotype; e wts x1 clone (white arrow) labeled with GFP using the MARCM system in the imaginal disc of an Act-Gal4 UAS-GFP/+ +; + wts x1/Tub-Gal80 + third instar larva. f GFP-labeled clone (white arrow) using the MARCM system in the imaginal disc of third instar larva (Act-Gal4 UAS-GFP/+ +; +/Tub-Gal80); d–f Nuclei are marked by DAPI stain (blue)
Adult flies were screened for hyperplastic outgrowths or color markers under a binocular microscope. The GFP clones were detected with a fluorescent microscope in dissected imaginal discs from wandering stage third instar larvae.
Quantitative PCR
Total RNA was extracted from 60 Drosophila adults using the RiboZol RNA extraction reagent (AMRESCO) followed by NucleoSpin RNA II kit (Macherey-Nagel) including an on-column rDNase I digestion step. Total RNA (1000 ng) was reverse transcribed using PrimeScript Reverse Transcriptase (Takara) with oligo(dT)17 primer. Quantitative PCR was performed using the HOT FIREPol EvaGreen qPCR Mix Plus (Solis BioDyne). Primer sequences are listed in Table S1. RT-qPCR was performed in triplicates and all results are presented with means and SEM from four independent biological samples. We used rp49 and Rack1 messenger RNA (mRNA) levels for normalization.
Statistical data evaluation
Clone frequency p (%) was calculated as (number of clones)/(total number of flies) × 100. Clone frequencies were compared using Student’s t test and Fisher’s correction ϕ = 2 × arcsin(√p) for frequency. The t test was calculated for confidence intervals (CI) for P < 0.05 and P < 0.01, if appropriate. These intervals are shown as error bars in the figures unless stated otherwise. Low-sample t-criterion correction was applied to samples of fewer than 500 flies, according to Urbakh [24]. We applied the χ2 criterion to assess the lethality of various heteroallelic AdoR mutant combinations.
Results
AdoR mutant flies are homozygous viable
We reported earlier that the homozygous AdoR1 mutation generated by homologous recombination was viable and able to partially rescue the lethality of adenosine deaminase growth factor-A (Adgf-A) mutants [12]. The mutation AdoRKG0396ex lacking the entire AdoR coding region was also reported to be homozygous viable [8]. In contrast, the survival of AdoRRNAi-VDRC flies with long hairpin constructs was dramatically reduced when driven by the Act-Gal4 driver. We conclude that the lethality of the AdoRRNAi-VDRC construct was presumably caused by off-target effects. The larger deletion AdoRDf(3R)Exel6214 was homozygous lethal in our experiments.
To verify that there is no negative effect of AdoR mutations on fly viability, we prepared heteroallelic combinations of various AdoR mutations. As shown in Table S2, the frequencies among the offspring of all examined combinations, including AdoR1/AdoR1, AdoR1/AdoRKG03964ex, AdoRKG03964ex/AdoRDf(3R)Exel6214, and AdoR1/AdoRDf(3R)Exel6214, were in accordance with the expected Mendelian ratios, showing no significant deviation from the expected frequencies. Our results confirmed that the AdoR1 and AdoRKG03964ex mutations have no negative effect on fly viability under standard rearing conditions.
AdoR1 strongly reduces wtsx1 clone frequency in SMART
In order to examine the effect of the loss of AdoR function on the incidence of somatic mosaic clones in flies in vivo, we adapted a robust test, originally designed for assaying the mutagenicity of chemicals, or the effect of genetic background on the frequency of mutations [25, 26]. The mutations are visualized as somatic mosaic clones generated by LOH of the recessive allele of the marker gene. In our experiments, it was advantageous to induce clone formation with mutagens since the spontaneous LOH occurred at a relatively low frequency. In this way, we were able to reduce the number of flies in the experiments and still reach statistical significance.
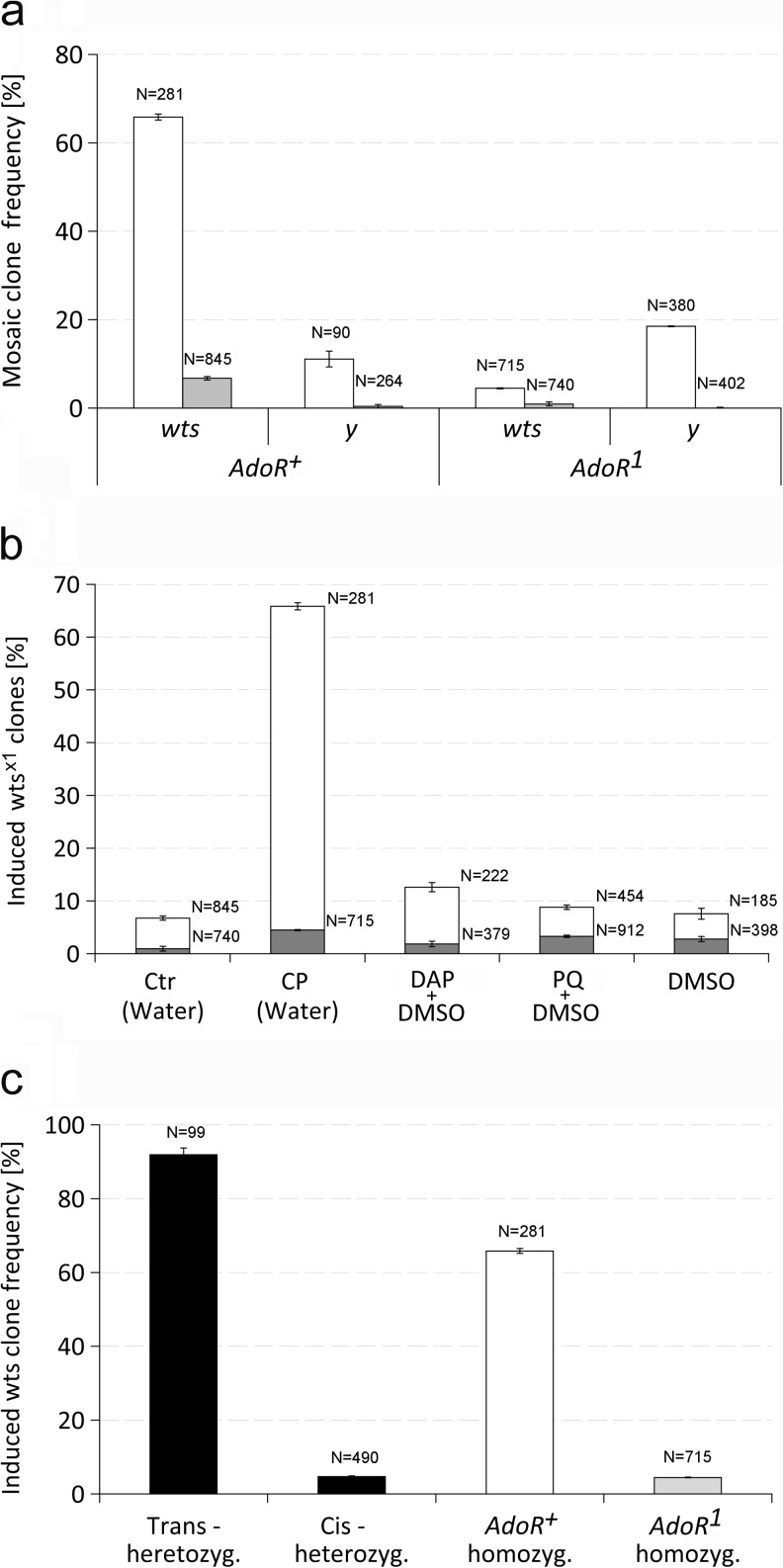
First, we compared the effect of AdoR1 and AdoR+ backgrounds on the rate of somatic mosaic clones labeled with two different types of markers, wtsx1 and y. The wtsx1 is a mutant allele of the wts tumor suppressor causing epidermal tissue overgrowth that is visible as small hyperplastic outgrowths, whereas y causes yellow (y) cuticles and bristles in the adult (Fig. 1a, b). The mosaic clones were induced with cisplatin as described in “Experimental Procedures”. The results are shown in Fig. 2a. The frequency of wtsx1 clones in AdoR1 flies was much lower than in AdoR+ flies; only 4.5 % compared to 65.9 %, respectively. In contrast, y mosaic clones occurred at a frequency of 11.1 % in AdoR+ flies, whereas in AdoR1 flies (y/+; AdoR1wtsx1/AdoR1 +) it reached 18.5 % (similarly for y/+; AdoR1/AdoR1 flies). The higher frequency of y mosaic clones might be connected to the involvement of AdoR in the control of DNA repair or detoxification. The dramatically decreased wtsx1 hyperplastic outgrowth clone frequency in AdoR1 flies was unexpected.
Fig. 2.
The effect of AdoR on the frequency of somatic mosaic clones. a The incidence of wts x1 and y somatic mosaic clones in AdoR + and AdoR 1 flies. The gray and empty bars represent spontaneous and 0.67 mM cisplatin-induced wts x1 and y mosaic clone frequencies, respectively, in homozygous AdoR + (left side, y/+; wts x1/+) and AdoR 1 (right side, y/+; + AdoR 1/+ AdoR 1) flies. Error bars show 95 % confidence interval (CI). The numbers above each bar indicate the number of flies tested. Most wts x1 clones appeared in adult wings, notum, and sternopleura. The yellow clones appear in lower frequency since they must cover a bristle precursor cell for being detected. b The wts x1 clone frequency induced by three chemicals in AdoR + and AdoR 1 flies. The wts x1 clones were induced by 0.67 mM cisplatin (CP), 20 mM paraquat (PQ), and 40 mM diaminopurine (DAP); the solvents (marked in brackets) were water or 5 % aqueous DMSO; CTR control. White and gray bars represent clone frequency in +AdoR +/wts x1 AdoR + and wts x1 AdoR 1/+ AdoR 1 flies, respectively. Error bars show 95 % CI. The numbers above the right of each bar indicate the number of flies tested. c wts x1 clone frequencies in cis- and trans-AdoR 1 wts x1 heterozygous flies. The black bars (left) represent clone frequencies observed in cis- and trans- AdoR 1 wts x1 heterozygous flies. For comparison, wts x1 clone frequencies observed in homozygous AdoR + and AdoR 1 flies are shown on the right (white and gray bars, resp.). The wts x1 clones were induced by 0.67 mM cisplatin. Error bars show 95 % CI. The numbers above each bar indicate the number of flies tested
To show that the observed difference in the incidence of mosaic clones carrying wtsx1 and y markers was not dependent on cisplatin induction, we examined the frequency of spontaneous mosaic clones for both markers. The spontaneous level of wtsx1 clones also dropped by sevenfold in AdoR1 homozygous flies, compared to the AdoR+ controls (from 6.7 to 0.9 %, respectively) showing that the reduction of wtsx1 clone frequency was not caused by cisplatin. The spontaneous frequencies of y/y clones were slightly below the test sensitivity in both fly strains (Fig. 2a).
To further prove that the drop of wtsx1 clone frequency in AdoR1 flies was not caused by cisplatin, we used two other genotoxic compounds for SMART, diaminopurine (DAP), and paraquat. As expected, both DAP and paraquat produced higher clonal rates in both AdoR+ and AdoR1 flies than the spontaneous frequency. Again, the frequencies of wtsx1 mosaic clones induced by DAP and paraquat in the AdoR1 homozygous flies (1.8 and 3.3 %, respectively) were 7–2.7-fold lower compared to those in the corresponding AdoR+ flies (12.6 and 8.8 %, respectively). The results are shown in Fig. 2b. The chemicals were also assayed for toxicity. The results showed that DAP and paraquat have a much higher impact on fly viability than cisplatin (Fig. S1).
These results suggested that the AdoR1 mutation had different effects on the frequency of hyperplastic outgrowth and non-tumor mosaic clones represented by the wtsx1 and y markers, respectively. The effect was independent of the mutagen used and also occurred spontaneously. We did not observe any differences in wtsx1 clone morphology when comparing AdoR+ and AdoR1 flies. The differences in SMART results can be expected because chemicals differ in their genotoxicity, toxicity, and which pathways they activate in different target tissues. The dramatically decreased wtsx1 hyperplastic outgrowth frequency in AdoR1 flies therefore called for further investigation.
Different wtsx1 clone frequencies in cis- and trans-AdoR wts heterozygotes suggest the need of AdoR+ after clone formation
Since both AdoR and wts are localized on the distal tip of the 3R chromosomal arm, mitotic crossing-over break points most frequently occur between the centromere and both loci. To find out whether the AdoR1 mutation affects wtsx1 clone formation by influencing processes such as DNA repair or affects the survival of already established clones, we compared the frequencies of wtsx1 hyperplastic outgrowths in cis- and trans-heterozygotes for AdoR1 and wtsx1, which produce qualitatively different wtsx1 clones. The cells in cis- and trans-heterozygotes have almost the same genetic background and conditions; if the AdoR1 affects wtsx1 clone formation we should observe similar hyperplastic outgrowth frequencies in cis- and trans-heterozygotes. Alternatively, if the AdoR1 influences wtsx1 hyperplastic growth or survival, we should observe different wtsx1 clone frequencies because the mitotic recombination produces different clones: in the cis-heterozygous flies for these genes, the wtsx1 clones lack functional AdoR, whereas in the trans-heterozygous flies, they contain homozygous AdoR+ (Fig. S2). The comparison of wtsx1 clone frequency in cis- and trans-heterozygotes for wtsx1 and AdoR1 therefore may provide important information about whether AdoR-wtsx1 interactions affect clonal formation or survival. The resulting data are shown in Fig. 2c. The wtsx1 mosaic clones containing AdoR+ in trans-heterozygotes occurred at high frequency (91.9 %), whereas their rate in cis-heterozygotes lacking functional AdoR was very low (4.7 %). Such a difference strongly suggests that functional AdoR is needed for wtsx1 hyperplastic clone survival/growth after clone formation.
The low frequency of wtsx1/wtsx1 clones formed in cis-heterozygotes also means there was no rescue of wtsx1 hyperplastic outgrowth clones by the surrounding AdoR1/AdoR+ cells. Taking this result into account, the effect of AdoR1 appeared to be cell autonomous. This provided the opportunity for further experiments, which could use the overexpression of the studied genes within somatic clones only.
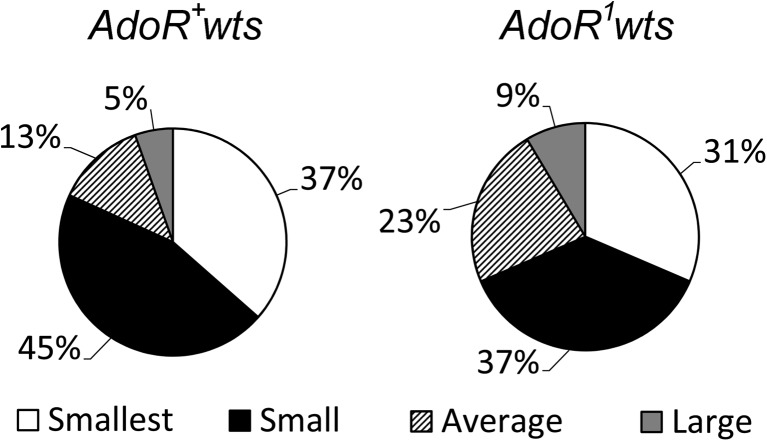
To better understand the impact of AdoR1 on the wtsx1 cells, we also assessed clone sizes. As shown in Fig. 3, we observed significantly higher proportion of larger wtsx1 hyperplastic outgrowths, among the AdoR1wtsx1/++ flies compared to wtsx1/+ flies.
Fig. 3.
The size distribution of wts x1 hyperplastic outgrowth size classes in two Drosophila genotypes. We analyzed 148 spontaneous wts x1 hyperplastic outgrowths detected among 1574 wts x1/+ flies and 35 spontaneous wts x1 AdoR 1 hyperplastic outgrowths found among 2267 wts x1 AdoR 1/++ flies. The differences between the average and large clones in wts x1/+ and wts x1 AdoR 1/++ flies are statistically significant
Confirmation of the specificity of AdoR effect on the frequency of wtsx1 hyperplastic outgrowth clones
To confirm that the observed decrease of the wtsx1 hyperplastic outgrowth clone rate was specific for the loss of the function of AdoR, we used AdoR silencing through the expression of UAS-AdoRRNAi hairpin RNA.
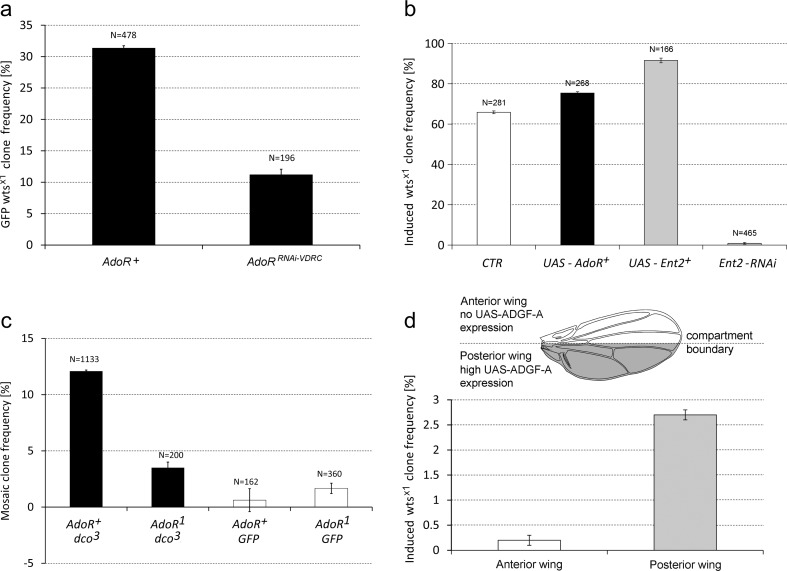
Expression of the UAS-AdoRRNAi-TRIP hairpin in the flies using Act-Gal4 led to the decrease of AdoR mRNA level to 33 % of its wild-type level, as shown by qPCR (Fig. S3). These flies had a significantly decreased frequency of wtsx1 clones by 40.0 % (95 % confidence interval, CI ±0.4 %) compared to control flies without AdoR silencing. This result supports the hypothesis stipulating the requirement of functional AdoR in the wtsx1 clones for their high frequency. The weaker effect of RNAi-silencing as compared to the AdoR1 mutation can be explained by the incomplete downregulation of AdoR.
The AdoR knockdown experiments were also performed with flies expressing the UAS-AdoRRNAi-VDRC construct together with UAS-GFP. Since the global expression of UAS-AdoRRNAi-VDRC caused lethality, we used the MARCM system (see “Materials and methods” and Fig. S4) and targeted its expression just to wtsx1 clones. The frequency of GFP AdoRRNAi-VDRCwtsx1 clones in adults was about threefold lower than in the control flies without UAS-AdoRRNAi-VDRC (Fig. 4a). Consistently, the frequency of wtsx1AdoRRNAi-VDRC clones assayed in larval imaginal discs also showed similar decrease (but these small samples lack statistical power) (Fig. S5). The rate of control MARCM-labeled GFP clones in imaginal discs of AdoR+ larvae with or without UAS-AdoRRNAi-VDRC was statistically indistinguishable (27.3, 95 % CI ±8.5 %, compared to 20.0, 95 % CI ±9.3 %) (Fig. S5). These results support the notion that the loss of AdoR function decreases the frequency of wtsx1 clones and exerts no or very little influence on the frequency of control GFP clones.
Fig. 4.
The frequencies of mosaic clones in various genetic backgrounds. a The incidence of wts x1 GFP clones in adult AdoR RNAi and control flies. The UAS transgenes were expressed by MARCM system. The left bar (AdoR+) y w; act-Gal4 UAS-GFP/+ +; wts x1/tub-Gal80; the right bar (AdoR RNAi) y w; act-Gal4 UAS-GFP/+ +; UAS-AdoR RNAi-VDRC wts x1/tub-Gal80. Error bars show 95 % CI. The numbers above each bar indicate the number of flies tested. b AdoR and Ent2 overexpression and Ent2 silencing affect the frequency of wts x1 clones. The wts x1 clone frequencies, in flies with UAS-AdoR + (black bar, left), UAS-Ent2 + (left gray bar) and UAS-Ent2 RNAi-VDRC (right gray bar) constructs expressed using MARCM system (see the text). For comparison, wts x1 clone frequency observed in homozygous AdoR + flies is shown on the left (white bar). Error bars show 95 % CI. The numbers above each bar indicate the number of flies tested. c The incidence of dco 3 and control mosaic clones in AdoR + and AdoR 1 flies. Black bars (left) the frequency of dco 3 clones in heterozygous dco +/dco 3 flies (Act-Gal4 UAS-GFP/+ +; + AdoR 1 dco 3/Tub-Gal80 + + and Act-Gal4 UAS-GFP/+ +; + dco 3/Tub-Gal80 +); gray bars (right) control GFP clones labeled by MARCM system in AdoR 1 and AdoR + flies (Act-Gal4 UAS-GFP/+ +; + AdoR 1/Tub-Gal80+ and Act-Gal4 UAS-GFP/+ +; +/Tub-Gal80). Error bars show 95 % CI. The numbers above each bar indicate the number of flies tested. d The ectopic expression of adenosine deaminase ADGF-A rescues wts x1 AdoR 1 clones in UAS-ADGF-A/en-Gal4; wts x1 AdoR 1/++ flies. The en-Gal4 was used to drive UAS-ADGF-A + expression in the posterior wing (gray shading in the schematic wing drawing above the graph indicates the en expression pattern). The hyperplastic outgrowth clone rates in the anterior and posterior compartments of 1518 wings examined were 0.2 ± 0.1 (left empty bar) and 2.7 ± 0.1 (right gray bar), respectively
To further test the specificity of the effect of AdoR level on the rate of wtsx1 clones, we used AdoR overexpression. Because the ubiquitous overexpression of UAS-AdoR in the entire body was lethal [9], we expressed it locally in the wtsx1 mosaic hyperplastic clones using the MARCM system (Fig. S4). We constructed UAS-AdoR/Act-Gal4 UAS-GFP;+ Tub-Gal80/wtsx1+ flies carrying the UAS-AdoR and Act-Gal4 transgenes and compared their wtsx1 clone frequency with that of the control flies lacking UAS-AdoR (Fig. 4b). Such AdoR overexpression increased the wtsx1 hyperplastic outgrowth clone frequency to 75.4 compared to 66.0 % in AdoR+ flies without UAS-AdoR (significant at P < 0.05).
Taken together, these data confirms the specific requirement of AdoR for wtsx1 hyperplastic outgrowth occurrence and also revealed a dose effect: AdoR overexpression further increased the frequency of wtsx1 clones while, conversely, incomplete RNAi-silencing of AdoR was not as effective as the null mutation.
AdoR1 mutation also affects the frequency of dco3 hyperplastic outgrowth clones
To further confirm the effect of AdoR1 on hyperplastic outgrowth clone frequency we tested a mutation in another Drosophila tumor suppressor gene discs overgrown (dco3) under the same conditions. Since the morphological outgrowths of dco3 clones are more difficult to detect, we also labeled the clones with GFP using the MARCM system (Act-Gal4 UAS-GFP/+ +; + dco3/Tub-Gal80 +).
As shown in Fig. 4c, the frequency of dco3 mosaic hyperplastic outgrowths in heterozygous dco3/+ flies with AdoR+ and UAS-GFP was about 12.1 % and this rate dropped by approximately a factor of four to 3.5 % in the AdoR1dco3/+ + cis-heterozygotes (Act-Gal4 UAS-GFP/+ +; + AdoR1dco3/Tub-Gal80 + +). This difference was statistically highly significant (P < 0.01). In contrast, in the control experiment, the frequency of GFP clones was 1.72 % in the AdoR1 mutant flies (Act-Gal4 UAS-GFP/+ +; + AdoR1 /Tub-Gal80 +), which is comparable to the 1.1 % value observed in flies that were wild type for AdoR (Act-Gal4 UAS-GFP/+ +; +/Tub-Gal80); the difference was statistically insignificant.
Our results showed that the AdoR1 mutation dramatically decreased the frequency of hyperplastic outgrowth clones induced by LOH of another tumor suppressor gene dco, suggesting that more types of hyperplastic outgrowth clones may require functional AdoR for their growth. The extent of this effect was, however, lower than that observed in the previous experiments with wtsx1 clones, probably reflecting the different growth properties of these hyperplastic outgrowth types. The frequency of the control GFP-labeled non-tumor clones did not decrease in AdoR1 flies in any of the experiments.
The effect of Ent2 and Adgf-A on wtsx1 clone frequency
To find out whether other members of the Ado signaling pathway influence wtsx1 hyperplastic outgrowth frequency, we overexpressed Ent2 and Adgf-A. In addition, we silenced the transporter expression by using UAS-Ent2RNAi.
MARCM expression of UAS-Ent2 in the wtsx1 clones increased the induced mosaic hyperplastic outgrowth rate from 65.8 to 91.6 % (P < 0.01) (Fig. 4b). Conversely, the knockdown of Ent2 expression by RNAi in the UAS- Ent2RNAi-VDRC ++/+ Act-Gal4 UAS-GFP; wtsx1+/+Tub-Gal80 flies decreased the frequency of wtsx1 hyperplastic outgrowths to 0.9 %. These results showed that both ectopic expression and silencing of the Ent2 transporter exerted an even stronger effect on wtsx1 hyperplastic outgrowth frequency than the changes in the level of the Ado receptor.
We also expressed UAS-Adgf-A in AdoR1wtsx1/+ + mutants using an engrailed-Gal4 driver and took advantage of the distinct ectopic expression of the UAS construct in wtsx1 hyperplastic outgrowths in the anterior and posterior compartments of the wing (Fig. 4d). There was a statistically significant increase (13.5 times) of the wtsx1 hyperplastic outgrowth rate in the posterior compartment compared to the intact anterior one—from 0.2 to 2.7 % (P < 0.01). The ratio is quite comparable to the 14-fold difference observed in the wtsx1 hyperplastic outgrowth rates between flies with AdoR+ and AdoR1 homozygous backgrounds (Fig. 2a) suggesting that the overexpression of Adgf-A completely rescues the effect of the AdoR1 mutation in wtsx1 clones. Taken together, our results suggest that the wtsx1 hyperplastic outgrowth clone frequency is regulated by adenosine signaling.
Discussion
Fly genetic methods allow the study of interactions between cells in the mosaic clones and their microenvironment. Our results show that AdoR and extracellular adenosine play key roles in the growth control of mosaic hyperplastic outgrowth clones. This conclusion follows from the observations that the frequency of wtsx1 and dco3 hyperplastic outgrowth clones in the AdoR1 mutant background is dramatically reduced compared to that in the AdoR+ flies. In contrast, the rate of non-hyperplastic clones (yellow or GFP) did not show any reduction, and even showed a significant increase for y. To exclude the possible effect of different recombination frequency on different chromosomes we used yellow on chromosome X and Gal 80 on chromosome 3 (segregation of Gal 80 permits the UAS-GFP expression on chromosome 2).
AdoR seems to influence the SMART results by more than one mechanism. The slightly higher proportion of y mosaic clones in AdoR1 flies compared to that in the AdoR+ ones suggests that the loss of functional AdoR may slightly increase the frequency of LOH. It was reported earlier that adenosine receptor agonists reduced the level of mutations in mouse and human cells treated with alkylating agents or H2O2 [27]. The elevated level of y clones in AdoR1 flies might suggest that the loss of such protective AdoR function slightly increases the rate of somatic mutations.
We found that the lack of functional AdoR causes a dramatic reduction of wtsx1 mosaic hyperplastic outgrowth clone frequency. Our data strongly suggest that AdoR is not needed for wtsx1 clone formation, but rather for their growth/survival. The reduction of wtsx1 clonal rate in AdoR1 or AdoRRNAi flies was consistent for clone established by spontaneous mosaicism, as well as for all chemical treatments, but its extent was different for different SMART conditions and also depended on the expression level of AdoR.
Moreover, AdoR interacts with dco, another tumor suppressor gene, which is also a member of the wts signaling pathway. Both the wtsx1 and dco3 mosaic clones form fast- growing hyperplastic outgrowths. The dco is Drosophila casein kinase Iε/δ and affects the upstream regulator of wts called fat [28, 29]. Wts is a serine/threonine protein kinase belonging to the WTS/LATS tumor suppressor family, which suppresses growth by antagonizing the function of the transcriptional co-activator protein Yorkie (Yki) [30]. Mutations in wts causing elevated levels of Yki activity can convert wild type (WT) cells into faster growing supercompetitors that are able to eliminate adjacent WT cells [31, 32]. Our results suggest that the wtsx1 and dco3 mosaic hyperplastic outgrowths lose their growth advantage in the absence of functional AdoR.
Interestingly, changes in the expression of Ent2 equilibrative nucleoside transporter in wtsx1 clones have a similar cell autonomous effect as changes in AdoR expression. The overexpression of Ent2 in wtsx1 clones leads to their higher frequency, whereas Ent2 silencing dramatically decreases wtsx1 clonal rate (Fig. 4a). The normal frequency of wtsx1 clones therefore requires WT function of both AdoR and Ent2, suggesting some form of interaction between them. We previously observed that AdoR1 and Ent23 mutant flies have similar defects in associative learning and synaptic function [11]. We even observed some compensatory changes including a dramatic increase in Ent2 mRNA in AdoR1 mutants or a reduction of adenosine deaminase transcript levels in Ent2 mutants [11]. Although we cannot directly measure local extracellular Ado concentrations, we know that Ado is critical for clone survival since the clonal rate can be rescued by overexpressing the extracellular adenosine deaminase Adgf-A in AdoR1wtsx1/++ mutants (Fig. 4d). The growth restrictions probably appear early in the clonal development. Significant differences in the distribution of wtsx1 hyperplastic outgrowth sizes between wtsx1AdoR1/++ and wtsx1/+ flies suggest that larger wtsx1AdoR1 clones have lower probability for being eliminated than the smaller ones. Alternative explanation that the fewer clones in wtsx1AdoR1/++ flies exist because the clones have fused is not likely since the overall frequency of wtsx1 clones was too low as well as the proportion of flies having more than one wtsx1 hyperplastic outgrowth.
Earlier, microarray analyses of gene expression in tissue culture cells showed that most of the common Drosophila cell lines, including imaginal disc cells Cl.8+, neuroblasts Bg2-c2, or hematopoietic Mbn2 cells seem to have the Hippo/Warts pathway inactivated [33]. Interestingly, the Cl.8+ imaginal disc cell line also has a very low level of functional AdoR [6]. Our previous tissue culture experiments showed that there is an intriguing connection between the AdoR and Ent2 functions with cell survival. The exposure of Cl.8+ cells to extracellular Ado causes a concentration-dependent block of cell division and apoptosis associated with a loss of mitochondrial membrane potential and excessive ATP production [34]. In contrast, other cell types containing functional AdoR, including Bg2-c2 and Mbn2 cells, are able to decrease Ado uptake upon Ado treatment and avoid apoptosis following adenosine stimulation [34, 35]. Cl.8+ cell death could be prevented by blocking adenosine transport or increasing extracellular adenosine deaminase activity but not by blocking AdoR [34]. These results showed that Ado can induce receptor-independent cell death or block of cell growth.
Our earlier experiments showed that global AdoR overexpression in flies causes lethality [9]. Microinjection of Act-Gal4 and UAS-AdoR flies with the AdoR antagonist SCH58261 was able to partially rescue the flies [6]. Similarly, the overexpression of AdoR in Drosophila Cl.8+ and S2 cells in vitro was cytotoxic [6]. These results suggest that the AdoR-mediated signaling can also cause death of Drosophila cells. Strong effects of both the Ado transport and AdoR signaling on cell viability were recently also described in the experiments involving the regeneration of pancreatic β-cells in zebrafish in vivo [4].
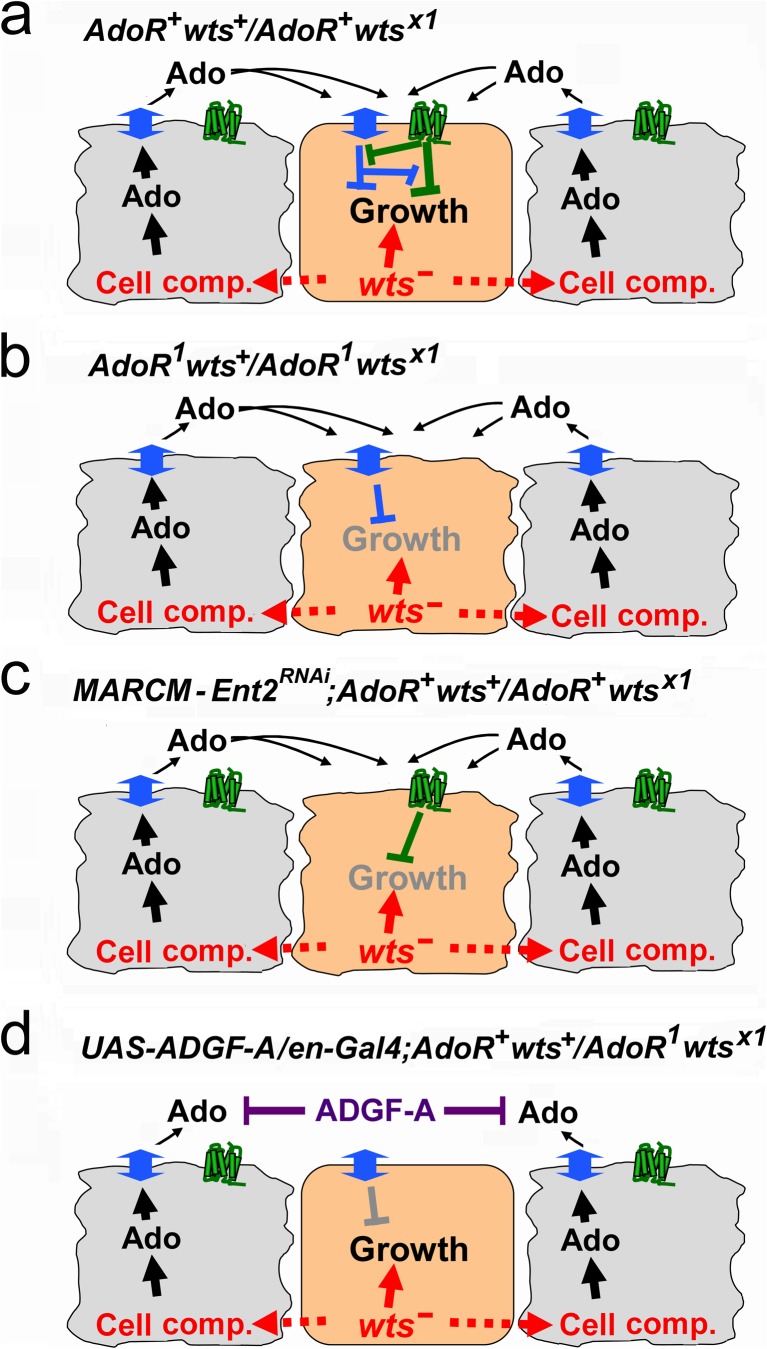
The simplest model that would explain our data on the occurrence of wtsx1 clones is shown in Fig. 5. We postulate that the extracellular Ado represents two opposing signals, which need to be balanced in fast-growing cells. Such hypothesis is supported by earlier experiments in mice brain showing that Ado uptake induces ATP synthesis and stimulates catabolic reactions, while the signal mediated by the AdoR signaling leads to the downregulation of a number of transcripts encoding metabolic enzymes [1]. It was also shown earlier that wts−/wts− cells behave as supercompetitors and eliminate adjacent wts+ cells at clonal boundaries [36–38]. In our experiments, AdoR+wtsx1/AdoR+wts+ flies are characterized by a relatively high frequency of wtsx1 hyperplastic outgrowth clones in which the wtsx1/wtsx1 cells may behave as supercompetitors. The dying wts+ cells become rich sources of extracellular Ado (Fig. 5a, black arrows). Ado is taken up by the wtsx1 hyperplastic outgrowth cells and this uptake is compensated by high AdoR stimulation (Fig. 5a). This extracellular Ado effect is indirectly supported by the experiments with the ADGF-A overexpression (Figs. 5d and 4d). In flies carrying AdoR1 mutation or Ent2RNAi, and therefore lacking such balance, wtsx1 hyperplastic outgrowths cannot grow in most cases (Fig. 5b, c). However, there are some escaper AdoR1wtsx1 or Ent2RNAiwtsx1 clones which probably grow if they reach the critical threshold while providing their own microenvironment. If we overexpress Adgf-A in the posterior wing compartment of AdoR+wts+/AdoR1wtsx1 flies using en-Gal4 and UAS-Adgf-A, the wtsx1 hyperplastic outgrowths in this area occur at the high rate suggesting that the depletion of extracellular Ado eliminates the effect of AdoR mutation and rescues the wtsx1 clones (Fig. 5d).
Fig. 5.
Model of adenosine effect on wts x1 hyperplastic outgrowth survival. Hyperplastic outgrowth cells are shown in orange color, cells with WT phenotype in light gray, green shapes represent AdoR, and blue two-headed arrows denote Ent2. a In AdoR + wts +/AdoR + wts x1 flies, the frequency of wts x1 mosaic hyperplastic outgrowths is high. The wts x1 hyperplastic outgrowth cells may behave as supercompetitors and eliminate adjacent wts + cells. High level of extracellular Ado released by the outcompeted cells causes elevated AdoR stimulation as well as high Ado uptake in hyperplastic outgrowth cells. b Mutation in AdoR leads to dramatic decrease of wts x1 clone frequency, because Ado uptake in those cells is no longer balanced by AdoR signaling. c Silencing of Ent2 in wts x1 hyperplastic outgrowth clones using RNAi also leads to dramatic decrease of wts x1 clonal rate (unbalanced AdoR signal). d Overexpression of Adgf-A in AdoR 1 wts +/AdoR 1 wts x1 flies eliminates the effect of AdoR mutation and rescues the wts x1 clones (example of this effect in the posterior wing compartment is shown in Fig. 4d)
The wts downregulation was shown to play a key role in the stem cell-mediated regenerative response to tissue damage in the Drosophila intestine [39, 40]. The fast-growing supercompetitive cells with blocked Wts function and activated Yki may cause secondary tissue damage. The observed effects of AdoR and Ent2 on the frequency of cells which lost their wts tumor suppressor might be part of a normal tissue protection mechanism which limits the extent of regenerative growth.
A number of recent studies have examined the involvement of adenosine in tumor-protective mechanisms in mice, namely triggering immunosuppression via the A2A adenosine receptor (A2AR) on the surface of activated immune cells [16, 41]. While this mechanism probably evolved for the protection of normal tissues from collateral damage caused by the overactive immune cells, the cancer tissue would also be protected. The best evidence for this was provided by experiments using WT and A2A receptor knockout (A2AR−) mice inoculated with established melanomas. These hyperplastic outgrowths were completely rejected in 60 % of A2AR− hyperplastic outgrowth-bearing mice while there was no rejection in WT mice [42]. The effects of the A2A receptor were T cell autonomous. In contrast, our results in Drosophila showed that the effects of AdoR1 on wtsx1 and dco3 mosaic hyperplastic outgrowth are cell autonomous. Results in both experimental systems suggest the involvement of Ado in the interactions between hyperplastic outgrowths and their neighboring cells in tissues.
Our report is the first to show the interaction between the adenosine and Hippo/Warts signaling pathways in Drosophila. We believe that further investigation of adenosine signaling and its impact on competitive cell interactions may clarify important aspects regarding the maintenance of tissue homeostasis at a cellular level.
Electronic supplementary material
Toxicity of DAP, paraquat and cisplatin. a Cisplatin was used for treatment of Drosophila larvae as a 0.67 mM water solution. b The toxicity of 2,6-diaminopurine (DAP) was tested on larvae as 12-80 mM aqueous solutions in 5 % dimethyl sulfoxide. c The toxicity of paraquat was tested on adults for 3 days (males, 24 h after eclosion) as a 20 mM solution containing 5% DMSO and 1% sucrose. Lethality is expressed in the percentage of surviving flies. Black bars AdoR 1 flies, empty bars AdoR + flies, ctr control. Error bars show standard deviations. (GIF 12 kb)
Mitotic recombination can convert heterozygous cells into homozygous. In cis- heterozygote for AdoR 1 and wts x1 mutations, the recombination results in AdoR 1 homozygous wts x1 hyperplastic outgrowth progeny cells, whereas in trans-heterozygote, the recombination produces AdoR + wts x1 tumors and AdoR 1 wts + clones. The wts x1 hyperplastic outgrowth cells are shown in orange. AR 1 = AdoR 1, AR + = AdoR +. (GIF 38 kb)
Real-time RT-PCR validation of RNAi tools used for AdoR gene silencing. (Ctr) The expression level of AdoR mRNA in control w 1118 flies is shown as a white bar. AdoR mRNA levels in (Ctr Bal), (Ctr GAL4), and (Ctr TRIP) flies carrying CyO balancer (yw/w; CyO/+; Tb/+), Gal4 driver (yw/w; Act-Gal4/+; Tb/+) and RNAi construct– (yw/w; CyO/+; UAS-AdoR RNAi-TRIP,wts x1/+), respectively, are represented by gray bars. (RNAi) AdoR mRNA level in flies with ubiquitous overexpression of AdoR RNAi-TRIP (yw/w; Act-Gal4/+; UAS-AdoR RNAi-TRIP, wts x1/+) is shown as a black bar. The AdoR expression levels were normalized to Rack1 and rp49 mRNAs. Ordinate: The levels of AdoR mRNA are expressed as percentages of values for the Ctr (error bars show SEM from four independent biological samples). (GIF 59 kb)
The expression of UAS constructs in mosaic clones using MARCM. The mosaic analysis with a repressible cell marker (MARCM) expression system is based on the generation of homozygous daughter cells lacking GAL80 expression in otherwise heterozygous tissues, therefore allowing GAL4-dependent activation of UAS-reporter or other UAS-transgenes. We generated MARCM clones by cisplatin-induced recombination; upon division, recombined chromosomes segregate so that one daughter cell receives both copies of GAL80 and the other receives both copies of wts x1 (thus creating a hyperplastic cell which lacks the inhibition of the GAL4 system). The wts x1 hyperplastic cell lacking GAL80 expression is shown in orange. (GIF 14 kb)
The incidence of wts x1 GFP and control GFP clones in larval imaginal discs of AdoR + and AdoR RNAi flies. The UAS transgenes were expressed by MARCM system. The black bars left (AdoR+) - y w; act-Gal4 UAS-GFP/+ +; wts x1/tub-Gal80; the black right bar (AdoR-RNAi) y w; act-Gal4 UAS-GFP/+ +; UAS-AdoR RNAi-VDRC wts x1/tub-Gal80. White bars left (AdoR+) - y w; act-Gal4 UAS-GFP/+ +; + /tub-Gal80; the white right bar (AdoR-RNAi) - y w; act-Gal4 UAS-GFP/+ +; UAS-AdoR RNAi-VDRC + /tub-Gal8. Error bars show 95 % CI. Numbers above each bar indicate the number of flies tested. (GIF 41 kb)
(DOC 25 kb)
(DOC 35 kb)
Acknowledgments
We thank Ruzenka Kuklova for the technical assistance. We also thank Dr. Dana Carroll, Dr. Gennady Belitsky, Dr. Elizabeth Khovanova and Dr. Tamas Szlanka for critical reading of the manuscript and Dr. Tian Xu for the wts x1 Drosophila strain. We acknowledge the use of research infrastructure that has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 316304. This research was supported by the Czech Science Foundation, grant no. GA14-27816S.
References
- 1.Cunha RA. Adenosine neuromodulation and neuroprotection. In: Lajtha A, editor. Handbook of neurochemistry and molecular neurobiology. New York: Springer; 2008. pp. 256–273. [Google Scholar]
- 2.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14(7):1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 3.Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, Banerjee U. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147(7):1589–1600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson O, Adams BA, Yoo D, Ellis GC, Gut P, Anderson RM, German MS, Stainier DY. Adenosine signaling promotes regeneration of pancreatic beta cells in vivo. Cell Metab. 2012;15(6):885–894. doi: 10.1016/j.cmet.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf) 2009;195(4):415–447. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- 6.Kucerova L, Broz V, Fleischmannova J, Santruckova E, Sidorov R, Dolezal V, Zurovec M. Characterization of the Drosophila adenosine receptor: the effect of adenosine analogs on cAMP signaling in Drosophila cells and their utility for in vivo experiments. J Neurochem. 2012;121(3):383–395. doi: 10.1111/j.1471-4159.2012.07701.x. [DOI] [PubMed] [Google Scholar]
- 7.Dolezelova E, Nothacker HP, Civelli O, Bryant PJ, Zurovec M. A Drosophila adenosine receptor activates cAMP and calcium signaling. Insect Biochem Mol Biol. 2007;37(4):318–329. doi: 10.1016/j.ibmb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Wu MN, Ho K, Crocker A, Yue Z, Koh K, Sehgal A. The effects of caffeine on sleep in Drosophila require PKA activity, but not the adenosine receptor. J Neurosci. 2009;29(35):11029–11037. doi: 10.1523/JNEUROSCI.1653-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolezelova E, Zurovec M, Dolezal T, Simek P, Bryant PJ. The emerging role of adenosine deaminases in insects. Insect Biochem Mol Biol. 2005;35(5):381–389. doi: 10.1016/j.ibmb.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Hirsh AJ, Stonebraker JR, van Heusden CA, Lazarowski ER, Boucher RC, Picher M. Adenosine deaminase 1 and concentrative nucleoside transporters 2 and 3 regulate adenosine on the apical surface of human airway epithelia: implications for inflammatory lung diseases. Biochemistry. 2007;46(36):10373–10383. doi: 10.1021/bi7009647. [DOI] [PubMed] [Google Scholar]
- 11.Knight D, Harvey PJ, Iliadi KG, Klose MK, Iliadi N, Dolezelova E, Charlton MP, Zurovec M, Boulianne GL. Equilibrative nucleoside transporter 2 regulates associative learning and synaptic function in Drosophila. J Neurosci. 2010;30(14):5047–5057. doi: 10.1523/JNEUROSCI.6241-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolezal T, Dolezelova E, Zurovec M, Bryant PJ. A role for adenosine deaminase in Drosophila larval development. Plos Biology. 2005;3(7):e201. doi: 10.1371/journal.pbio.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolezal T, Gazi M, Zurovec M, Bryant PJ. Genetic analysis of the ADGF multigene family by homologous recombination and gene conversion in Drosophila. Genetics. 2003;165(2):653–666. doi: 10.1093/genetics/165.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fishman P, Bar-Yehuda S, Synowitz M, Powell JD, Klotz KN, Gessi S, Borea PA. Adenosine receptors and cancer. Handb Exp Pharmacol. 2009;193:399–441. doi: 10.1007/978-3-540-89615-9_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA. Adenosine receptors and cancer. Biochim Biophys Acta. 2011;1808(5):1400–1412. doi: 10.1016/j.bbamem.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Sitkovsky M, Ohta A. Targeting the hypoxia-adenosinergic signaling pathway to improve the adoptive immunotherapy of cancer. J Mol Med. 2013;91(2):147–155. doi: 10.1007/s00109-013-1001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spychala J. Tumor-promoting functions of adenosine. Pharmacol Ther. 2000;87(2–3):161–173. doi: 10.1016/S0163-7258(00)00053-X. [DOI] [PubMed] [Google Scholar]
- 18.Reddy BV, Rauskolb C, Irvine KD. Influence of fat-hippo and notch signaling on the proliferation and differentiation of Drosophila optic neuroepithelia. Development. 2010;137(14):2397–2408. doi: 10.1242/dev.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121(4):1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 20.Katoh M. Function and cancer genomics of FAT family genes (review) Int J Oncol. 2012;41(6):1913–1918. doi: 10.3892/ijo.2012.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stern C. Somatic crossing over and segregation in Drosophila melanogaster. Genetics. 1936;21(6):625–730. doi: 10.1093/genetics/21.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidorov RA, Ugnivenko EG, Khovanova EM, Belitsky GA. Induction of tumor clones in D. melanogaster wts/+ heterozygotes with chemical carcinogens. Mutat Res. 2001;498(1–2):181–191. doi: 10.1016/S1383-5718(01)00277-7. [DOI] [PubMed] [Google Scholar]
- 23.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22(3):451–461. doi: 10.1016/S0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 24.Urbakh VY. Mathematical statistics for biologists and medicine specialists. Moscow: USSR Academy of Science Publishers; 1963. [Google Scholar]
- 25.Busygina V, Suphapeetiporn K, Marek LR, Stowers RS, Xu T, Bale AE. Hypermutability in a Drosophila model for multiple endocrine neoplasia type 1. Hum Mol Genet. 2004;13(20):2399–2408. doi: 10.1093/hmg/ddh271. [DOI] [PubMed] [Google Scholar]
- 26.Szakmary A, Huang SM, Chang DT, Beachy PA, Sander M. Overexpression of a Rrp1 transgene reduces the somatic mutation and recombination frequency induced by oxidative DNA damage in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1996;93(4):1607–1612. doi: 10.1073/pnas.93.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Zheng J, Linden J, Holoshitz J. Genoprotective pathways. Part I. Extracellular signaling through G(s) protein-coupled adenosine receptors prevents oxidative DNA damage. Mutat Res. 2004;546(1–2):93–102. doi: 10.1016/j.mrfmmm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Feng YQ, Irvine KD. Processing and phosphorylation of the Fat receptor. Proc Natl Acad Sci U S A. 2009;106(29):11989–11994. doi: 10.1073/pnas.0811540106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sopko R, Silva E, Clayton L, Gardano L, Barrios-Rodiles M, Wrana J, Varelas X, Arbouzova NI, Shaw S, Saburi S, Matakatsu H, Blair S, McNeill H. Phosphorylation of the tumor suppressor fat is regulated by its ligand Dachsous and the kinase discs overgrown. Curr Biol. 2009;19(13):1112–1117. doi: 10.1016/j.cub.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138(1):9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neto-Silva RM, de Beco S, Johnston LA. Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev Cell. 2010;19(4):507–520. doi: 10.1016/j.devcel.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyler DM, Baker NE. Expanded and fat regulate growth and differentiation in the Drosophila eye through multiple signaling pathways. Dev Biol. 2007;305(1):187–201. doi: 10.1016/j.ydbio.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cherbas L, Willingham A, Zhang D, Yang L, Zou Y, Eads BD, Carlson JW, Landolin JM, Kapranov P, Dumais J, Samsonova A, Choi JH, Roberts J, Davis CA, Tang H, van Baren MJ, Ghosh S, Dobin A, Bell K, Lin W, Langton L, Duff MO, Tenney AE, Zaleski C, Brent MR, Hoskins RA, Kaufman TC, Andrews J, Graveley BR, Perrimon N, Celniker SE, Gingeras TR, Cherbas P. The transcriptional diversity of 25 Drosophila cell lines. Genome Res. 2011;21(2):301–314. doi: 10.1101/gr.112961.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleischmannova J, Kucerova L, Sandova K, Steinbauerova V, Broz V, Simek P, Zurovec M. Differential response of Drosophila cell lines to extracellular adenosine. Insect Biochem Mol Biol. 2012;42(5):321–331. doi: 10.1016/j.ibmb.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Zurovec M, Dolezal T, Gazi M, Pavlova E, Bryant PJ. Adenosine deaminase-related growth factors stimulate cell proliferation in Drosophila by depleting extracellular adenosine. Proc Natl Acad Sci U S A. 2002;99(7):4403–4408. doi: 10.1073/pnas.062059699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyler DM, Li W, Zhuo N, Pellock B, Baker NE. Genes affecting cell competition in Drosophila. Genetics. 2007;175(2):643–657. doi: 10.1534/genetics.106.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117(1):107–116. doi: 10.1016/S0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- 38.Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117(1):117–129. doi: 10.1016/S0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- 39.Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137(24):4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137(24):4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnstock G, Di Virgilio F. Purinergic signalling and cancer. Purinergic Signal. 2013;9(4):491–540. doi: 10.1007/s11302-013-9372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MKK, Huang XJ, Caldwell S, Liu KB, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M (2006) A2A adenosine receptor protects tumors from antitumor T cells. P Natl Acad Sci USA 103 (35):13132–13137 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Toxicity of DAP, paraquat and cisplatin. a Cisplatin was used for treatment of Drosophila larvae as a 0.67 mM water solution. b The toxicity of 2,6-diaminopurine (DAP) was tested on larvae as 12-80 mM aqueous solutions in 5 % dimethyl sulfoxide. c The toxicity of paraquat was tested on adults for 3 days (males, 24 h after eclosion) as a 20 mM solution containing 5% DMSO and 1% sucrose. Lethality is expressed in the percentage of surviving flies. Black bars AdoR 1 flies, empty bars AdoR + flies, ctr control. Error bars show standard deviations. (GIF 12 kb)
Mitotic recombination can convert heterozygous cells into homozygous. In cis- heterozygote for AdoR 1 and wts x1 mutations, the recombination results in AdoR 1 homozygous wts x1 hyperplastic outgrowth progeny cells, whereas in trans-heterozygote, the recombination produces AdoR + wts x1 tumors and AdoR 1 wts + clones. The wts x1 hyperplastic outgrowth cells are shown in orange. AR 1 = AdoR 1, AR + = AdoR +. (GIF 38 kb)
Real-time RT-PCR validation of RNAi tools used for AdoR gene silencing. (Ctr) The expression level of AdoR mRNA in control w 1118 flies is shown as a white bar. AdoR mRNA levels in (Ctr Bal), (Ctr GAL4), and (Ctr TRIP) flies carrying CyO balancer (yw/w; CyO/+; Tb/+), Gal4 driver (yw/w; Act-Gal4/+; Tb/+) and RNAi construct– (yw/w; CyO/+; UAS-AdoR RNAi-TRIP,wts x1/+), respectively, are represented by gray bars. (RNAi) AdoR mRNA level in flies with ubiquitous overexpression of AdoR RNAi-TRIP (yw/w; Act-Gal4/+; UAS-AdoR RNAi-TRIP, wts x1/+) is shown as a black bar. The AdoR expression levels were normalized to Rack1 and rp49 mRNAs. Ordinate: The levels of AdoR mRNA are expressed as percentages of values for the Ctr (error bars show SEM from four independent biological samples). (GIF 59 kb)
The expression of UAS constructs in mosaic clones using MARCM. The mosaic analysis with a repressible cell marker (MARCM) expression system is based on the generation of homozygous daughter cells lacking GAL80 expression in otherwise heterozygous tissues, therefore allowing GAL4-dependent activation of UAS-reporter or other UAS-transgenes. We generated MARCM clones by cisplatin-induced recombination; upon division, recombined chromosomes segregate so that one daughter cell receives both copies of GAL80 and the other receives both copies of wts x1 (thus creating a hyperplastic cell which lacks the inhibition of the GAL4 system). The wts x1 hyperplastic cell lacking GAL80 expression is shown in orange. (GIF 14 kb)
The incidence of wts x1 GFP and control GFP clones in larval imaginal discs of AdoR + and AdoR RNAi flies. The UAS transgenes were expressed by MARCM system. The black bars left (AdoR+) - y w; act-Gal4 UAS-GFP/+ +; wts x1/tub-Gal80; the black right bar (AdoR-RNAi) y w; act-Gal4 UAS-GFP/+ +; UAS-AdoR RNAi-VDRC wts x1/tub-Gal80. White bars left (AdoR+) - y w; act-Gal4 UAS-GFP/+ +; + /tub-Gal80; the white right bar (AdoR-RNAi) - y w; act-Gal4 UAS-GFP/+ +; UAS-AdoR RNAi-VDRC + /tub-Gal8. Error bars show 95 % CI. Numbers above each bar indicate the number of flies tested. (GIF 41 kb)
(DOC 25 kb)
(DOC 35 kb)