Abstract
When exposed to nutrient or nonnutrient germinants, individual Bacillus spores can return to life through germination followed by outgrowth. Laser tweezers, Raman spectroscopy, and either differential interference contrast or phase-contrast microscopy were used to analyze the slow dipicolinic acid (DPA) leakage (normally ∼20% of spore DPA) from individual spores that takes place prior to the lag time, Tlag, when spores begin rapid release of remaining DPA. Major conclusions from this work with Bacillus subtilis spores were as follows: (i) slow DPA leakage from wild-type spores germinating with nutrients did not begin immediately after nutrient exposure but only at a later heterogeneous time T1; (ii) the period of slow DPA leakage (ΔTleakage = Tlag − T1) was heterogeneous among individual spores, although the amount of DPA released in this period was relatively constant; (iii) increases in germination temperature significantly decreased T1 times but increased values of ΔTleakage; (iv) upon germination with l-valine for 10 min followed by addition of d-alanine to block further germination, all germinated spores had T1 times of less than 10 min, suggesting that T1 is the time when spores become committed to germinate; (v) elevated levels of SpoVA proteins involved in DPA movement in spore germination decreased T1 and Tlag times but not the amount of DPA released in ΔTleakage; (vi) lack of the cortex-lytic enzyme CwlJ increased DPA leakage during germination due to longer ΔTleakage times in which more DPA was released; and (vii) there was slow DPA leakage early in germination of B. subtilis spores by the nonnutrients CaDPA and dodecylamine and in nutrient germination of Bacillus cereus and Bacillus megaterium spores. Overall, these findings have identified and characterized a new early event in Bacillus spore germination.
INTRODUCTION
Spores of Bacillus species can remain dormant for long periods and are extremely resistant to a variety of environmental stresses. However, under appropriate conditions, normally upon the binding of specific nutrients to spores' nutrient germinant receptors (GRs), spores can rapidly return to life in the process of germination followed by outgrowth (1–3). Germination is important not only to spores but also to the food and medical product industries, since spores of a number of species are major agents of food spoilage and foodborne disease and must germinate to cause their deleterious effects (3). In contrast to the resistant dormant spores that are hard to kill, germinated spores have lost most dormant spore resistance properties and are killed relatively easily (2, 3). Consequently, there is much interest in the mechanism of spore germination, since preventing this process or promoting it efficiently could have significant applied microbiology applications.
Germination of Bacillus subtilis spores can be triggered by l-alanine or l-valine or a combination of l-asparagine, d-glucose, d-fructose, and K+ (AGFK). These nutrient germinants trigger germination by binding to and interacting with GRs present in the spore's inner membrane (IM) (2, 3). A number of events occur in a defined order during spore germination. Initially, exposure of spores to nutrient germinants causes a reaction that commits spores to germinate, even if the germinant is removed or displaced from its cognate GR (3–7). This commitment step is followed by release of monovalent cations, as well as the spore core's large pool (∼25% of core dry weight) of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) along with divalent cations, predominantly Ca2+, that are chelated with DPA (CaDPA). CaDPA release completes stage I of germination and triggers entry into stage II when cortex-lytic enzymes (CLEs) degrade spores' peptidoglycan (PG) cortex. Spores of Bacillus species generally contain two major CLEs, CwlJ and SleB, either of which alone is sufficient to allow completion of spore germination. CwlJ and SleB are likely to be lytic transglycosylases, although this has been shown directly only for SleB (3). Completion of cortex PG degradation allows the spore's germ cell wall to expand and the spore core to expand and take up water. Once the core water content has risen to ∼80% of wet weight, equal to that in the growing cell, metabolism in the core begins, followed by macromolecular synthesis, ultimately converting the germinated spore into a growing cell in the process of outgrowth (2, 8).
Normally, the process of individual spore germination can be divided into three phases according to a spore's image intensity in differential interference contrast (DIC) or phase-contrast (PC) microscopy with the phases ending at Tlag, Trelease, and Tlysis (9). Tlag is the time between germinant addition and the initiation of rapid CaDPA release, Trelease is the time for completion of rapid CaDPA release, and following Trelease, there is a further small decline in spore refractility due to the hydrolysis of the spore cortex and spore core swelling, and the time when spore refractility becomes constant is Tlysis. Previous work also occasionally noted that there were slow decreases in spores' DIC image intensity and some CaDPA release prior to Tlag during spore germination (4, 9–11). However, how these slow changes occur and what factors affect them were not clear. In the current study, we monitored the germination of multiple individual spores of Bacillus species using Raman spectroscopy and DIC and PC microscopy to determine (i) if there is slow CaDPA release early in the initiation of germination of spores of different Bacillus species, (ii) the magnitude and rate of this slow CaDPA release at different germination temperatures, (iii) other factors that affect this slow CaDPA release, and (iv) whether this slow CaDPA release is observed in both GR-dependent and GR-independent germination.
MATERIALS AND METHODS
Bacillus strains and species and spore preparation.
The B. subtilis strains used in this study were PS832 (wild type), a prototrophic 168 strain, and its isogenic derivatives, including (i) PS533 (also a wild-type strain), strain PS832 carrying plasmid pUB110 providing resistance to kanamycin (10 μg/ml) (12); (ii) PS3411, termed ↑SpoVA, which overexpresses SpoVA proteins ∼4-fold in spores (13) (the SpoVA proteins are almost certainly components of the channel in spores' IM through which CaDPA is released in stage I of spore germination [3, 13–15]); and (iii) FB111, which lacks the CLE CwlJ (16). Bacillus cereus T was originally obtained from H. O. Halvorson, and Bacillus megaterium QM B1551 was originally obtained from H. S. Levinson. Spores of B. subtilis strains were prepared at 37°C on 2× Schaeffer's glucose medium agar plates and were harvested, purified, and stored as described previously (17). Spores of B. cereus and B. megaterium were prepared at 30°C in either defined liquid medium (B. cereus) or liquid-supplemented nutrient broth (B. megaterium) and purified as described previously (18, 19). All spores used in this study were >98% free of sporulating cells, germinated spores, and debris, as observed by phase-contrast microscopy.
Spore germination.
Except for CaDPA and dodecylamine germination, spores were heat activated in water before germination by incubation of spores at 70°C for 30 min and then cooling on ice for at least 15 min. Spores of B. subtilis strains were germinated routinely at 37°C in 25 mM K-HEPES buffer (pH 7.4) with various concentrations of l-valine or the AGFK mixture and at other temperatures as noted for individual experiments. To examine slow CaDPA leakage during nonnutrient germination, spores were germinated at 37°C with 50 mM CaDPA in 25 mM K-HEPES buffer (pH 7.4) or at 45°C with 0.5 mM dodecylamine in 25 mM K-HEPES buffer (pH 7.4). B. cereus and B. megaterium spores were germinated at 37°C in either 25 mM Tris-HCl buffer (pH 8.3) with 2 mM l-alanine or 25 mM KPO4 buffer (pH 7.4) with 10 mM glucose, respectively.
In experiments measuring spores' commitment to germinate, spores were germinated with 10 mM l-valine for 10 min at various temperatures, and then the l-valine was replaced with 10 mM d-alanine in 25 mM K-HEPES buffer (pH 7.4) as described previously (20) and incubation was continued at 37°C. Previous work has shown that d-alanine addition to spores germinating with l-valine blocks further commitment but allows completion of the germination of spores that had become committed prior to d-alanine addition (7).
Monitoring the germination of multiple individual spores.
In most experiments, germination of multiple individual spores was monitored by PC microscopy as described previously (11, 20–22). Changes in wild-type spores' PC image intensities at least through Trelease of germination are almost certainly due to changes in spores' CaDPA content, and this is also true for analyses of spore germination by DIC microscopy (see below) (9–11). In brief, spores were spread on the surface of a coverslip that was dried in a vacuum desiccator for ∼10 min, and the coverslips were mounted on and sealed to a microscope sample holder kept at a constant temperature. The PC images of multiple spores adhered on coverslips were recorded at a rate of 1 frame per 15 s for 60 to 120 min by a digital charge-coupled device camera (16 bits; 1,600 by 1,200 pixels) following the addition of preheated germinant solution to the spores on the coverslips. The averaged pixel intensity of an area of 21 by 21 pixels that covered each individual spore's PC image was calculated, and the PC image intensity of each individual spore was plotted as a function of the incubation time with a resolution of 15 s, and with image initial intensity at the first time of measurement, T0, normalized to 1 and the intensity at the end of the measurement period set at zero. Invariably, the PC image intensity had been constant for ≥10 min at the end of measurements. The degree of germination of spore populations was measured by simultaneously monitoring the germination of >200 individual spores by PC microscopy, and at various times, the percentage of these spores that had released their CaDPA was determined as described below.
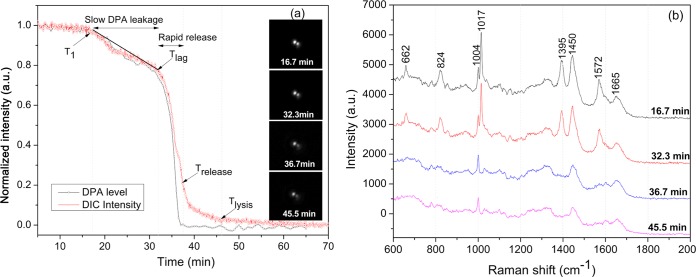
In order to confirm that decreases in a spore's PC image intensity early in spore germination were due to CaDPA release, the kinetics of CaDPA release during germination of individual spores optically trapped by laser tweezers were also measured simultaneously by DIC microscopy and Raman spectroscopy as described previously (9, 11), and CaDPA levels in individual spores were determined from the intensities of the CaDPA-specific Raman band at 1,017 cm−1. As found previously (9, 11), the end of the rapid fall in DIC image intensity during wild-type spore germination with nutrients corresponded to the point at which release of CaDPA was complete, and this time point was defined as Trelease. At this time, the DIC image intensity (I) was 30 to 35% of that at T0, when image intensity at T0 was set at 1 and the intensity at the end of measurements was set at zero. Consequently, the CaDPA content of wild-type spores at any time relative to T0 could be estimated from the DIC image intensity It as 100% × It, since the DIC intensity was found to nearly coincide with CaDPA level prior to Trelease (11) (see Fig. 1). In addition to Trelease, a number of other spore germination parameters have been previously described (9, 11), as well as a new one introduced in this work, including T1, the time when CaDPA leakage starts; Ilag, the DIC image intensity at Tlag; Tlag, the time between T1 and Trelease when CaDPA release becomes much faster; and ΔTleakage and ΔTrelease, which are Tlag − T1 and Trelease − Tlag, respectively.
FIG 1.
Changes in Raman spectra and DIC images of a single optically trapped B. subtilis wild-type spore (wild type) germinating with l-valine. A single B. subtilis PS533 spore (wild type) was optically trapped and germinated with 10 mM l-valine at 37°C, and DIC images and Raman spectra were recorded as described in Materials and Methods. (a) Intensities of the CaDPA-specific Raman band at 1,017 cm−1 and DIC image intensity as a function of germination time. The arrows indicate the times of T1, Tlag, Trelease, and Tlysis. Raman band intensities were normalized to the initial values at the first time of measurement. Spore refractility was calculated by normalizing a spore's DIC image intensity to its initial value at the first time of measurement (corresponding to that of the dormant spore) after normalization to the last unchanged image intensity value corresponding to that of the fully germinated spore that was set at 0. All intensities are given in arbitrary units (a.u.). (b) Sequential Raman spectra during germination of the single trapped spore.
RESULTS
Slow CaDPA leakage occurs during spore germination and is affected by germination temperature.
In previous studies (9, 22), the release of CaDPA during germination of individual spores has been defined by the following parameters: Tlag, the time for initiation of rapid CaDPA release; Trelease, the time of completion of CaDPA release; and ΔTrelease, which is Trelease − Tlag. However, in these previous studies there were indications that significant amounts of CaDPA were released prior to Tlag (9–11). Indeed, careful analysis of individual germinating B. subtilis spores using both DIC microscopy and Raman spectroscopy indicated that CaDPA is released not at a constant rate during germination but rather in two phases, a slower one and a faster one (Fig. 1a; also see below). In this study, the period of slower CaDPA release is defined as CaDPA leakage and begins at a time defined as T1 when a spore's DIC image intensity and its CaDPA content begin to drop after germinant addition, with CaDPA leakage ending at Tlag (Fig. 1a and b; also see below). For the spore shown in Fig. 1a, ∼ 20% of total CaDPA was released in the period between T1 and Tlag, and this was also found with multiple individual wild-type spores germinating with l-valine at 37°C (see below). Additional parameters to describe the germination of the spore in Fig. 1a are thus the following: (i) T1, which is ∼15 min; (ii) ΔTleakage (Tlag − T1), the slow CaDPA leakage time that was ∼17 min in which ∼20% of total CaDPA was released; and (iii) Ilag, the relative DIC image intensity at Tlag that was ∼0.80, with the value at T0 of germination set at 1.0. This spore also lost a further ∼60% of its DIC image intensity and all remaining CaDPA by Trelease, and the time for the faster CaDPA release (ΔTrelease) was 5.5 min.
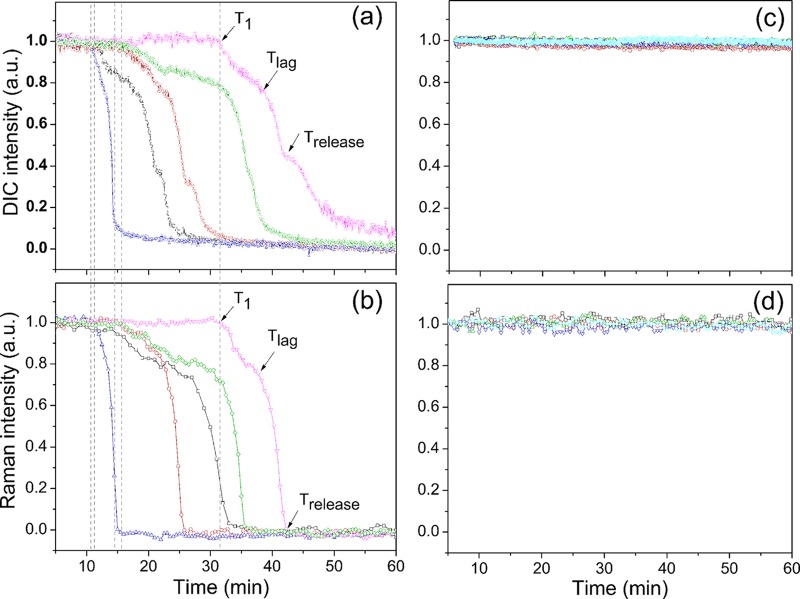
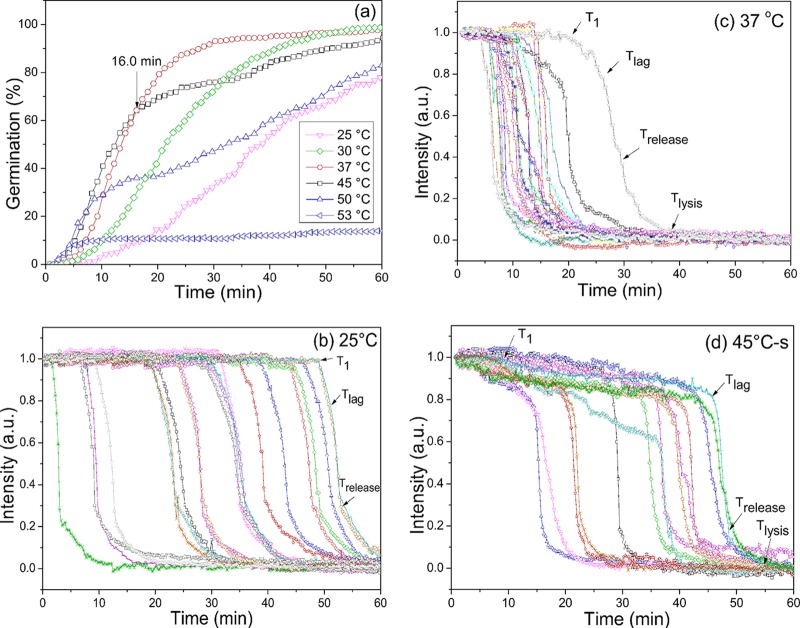
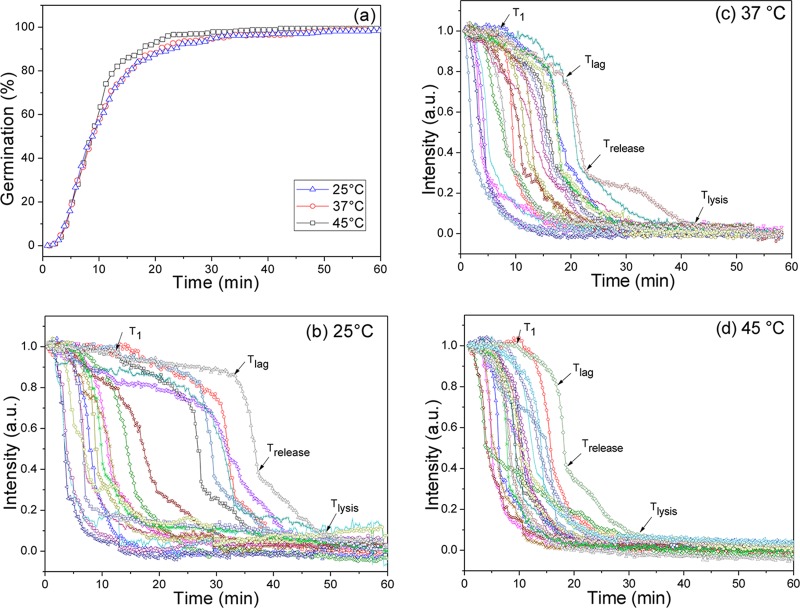
Monitoring the l-valine or AGFK germination at various temperatures of multiple individual spores that were laser trapped or adhered on a coverslip and using either PC microscopy or both Raman spectroscopy and DIC microscopy demonstrated that all spores exhibited slow decreases in DIC image intensity paralleled by slow CaDPA leakage prior to Tlag (Fig. 2 and 3; see also Fig. S1 in the supplemental material). In contrast, spores incubated at temperatures of 37 or 50°C in buffer alone exhibited no decreases in DIC image intensity or CaDPA level (Fig. 2c and d and data not shown). Notable findings from determination of the l-valine and AGFK germination parameters for hundreds of individual spores (Table 1; also see Table S1 in the supplemental material) included the following. (i) Values of Ilag were always ∼0.85 for spores germinating with either l-valine or AGFK at different germination temperatures, but values of T1, Tlag, and ΔTleakage varied considerably between individual spores. (ii) As expected (9–11, 23), spores germinating with l-valine or AGFK and at different temperatures had essentially identical ΔTrelease times. (iii) T1 and Tlag times for l-valine and AGFK germination were highest when germination was at 25°C and were lower at higher germination temperatures; one exception was with 50°C l-valine germination, for which Tlag values increased considerably. (iv) ΔTleakage times were lower than ΔTrelease times for lower-temperature germinations but increased 4- to 10-fold as germination temperature increased. Since only ∼20% of CaDPA was released in ΔTleakage with 80% in ΔTrelease, CaDPA release rates in these two periods were 2.5- to 3-fold lower in ΔTleakage at 25°C and 12- to 20-fold lower at 45°C. (v) At temperatures of 45 and 50°C, a fraction of spores germinated rapidly and exhibited germination kinetics similar to those at 37°C (Fig. 3a; see also Fig. S1a in the supplemental material; also data not shown). However, a smaller fraction of the spores incubated at these higher temperatures germinated very slowly and had much larger T1, Tlag, Trelease, and ΔTleakage times (Fig. 3d; see also Fig. S1d). These results are consistent with significant heterogeneity in the germination of individual spores in this spore population.
FIG 2.
Germination of multiple individual optically trapped wild-type B. subtilis spores monitored by Raman spectroscopy (b and d) and DIC microscopy (a and c). Optically trapped B. subtilis PS533 spores (wild type) were germinated at 37°C with 10 mM l-valine (a and b) or only 25 mM K-HEPES (pH 7.4) (c and d), and spore germination was monitored as described in Materials and Methods. Germination curves were determined from the loss in spore DIC image intensity or from the intensities of the CaDPA-specific Raman band at 1,017 cm−1. The DIC image and Raman band intensities in arbitrary units (a.u.) were normalized to 1 based on the respective values at the first time of measurement, and DIC image intensities at the end of the experiment were set at 0. The arrows indicate the times of T1, Tlag, and Trelease for a single spore. The dashed lines indicate T1 times of each individual spore.
FIG 3.
Germination extents and PC image intensities of individual wild-type B. subtilis spores germinating with l-valine at various temperatures. B. subtilis PS533 (wild-type) spores were germinated with 10 mM l-valine at various temperatures, and spore germination was monitored by PC image intensity changes as described in Materials and Methods. The PC image intensities (a.u.) were normalized to 1 based on the respective values at the first time of measurement, and PC image intensities at the end of the experiment were set at 0. The arrows indicate the times of T1, Tlag, Trelease, and Tlysis for a single spore. In panel a, the germination curves at the different temperatures are from data with >262 spores each, and a germinated spore was defined as one that had reached Trelease. In panel d, “s” means spores germinating slowly at 45°C (Trelease of >16.0 min in panel a).
TABLE 1.
Mean values and standard deviations of germination parameters of multiple individual B. subtilis wild-type spores germinating with l-valine at different temperaturesa
| Germination temp (°C) | Mean ± SD |
No. of germinated spores (% germination) | |||||
|---|---|---|---|---|---|---|---|
| T1 (min) | Tlag (min) | ΔTleakage (min) | Trelease (min) | ΔTrelease (min) | Ilag | ||
| 25 | 33.1 ± 17.3 | 34.6 ± 17.5 | 1.6 ± 1.0 | 37.3 ± 17.8 | 2.6 ± 1.2 | 0.87 ± 0.06 | 206 (78.6) |
| 30 | 18.5 ± 10.1 | 20.2 ± 10.5 | 1.7 ± 1.2 | 22.6 ± 10.6 | 2.4 ± 0.8 | 0.85 ± 0.08 | 459 (98.9) |
| 37 | 8.9 ± 4.8 | 12.3 ± 7.4 | 3.4 ± 4.6 | 15.3 ± 7.8 | 2.9 ± 1.2 | 0.85 ± 0.10 | 552 (97.7) |
| 45 | 7.8 ± 7.2 | 14.2 ± 12.2 | 6.4 ± 8.2 | 16.8 ± 12.6 | 2.5 ± 1.0 | 0.84 ± 0.11 | 467 (98.1) |
| 50 | 9.8 ± 7.7 | 25.9 ± 17.3 | 16.0 ± 13.8 | 29.0 ± 18.1 | 3.1 ± 1.3 | 0.87 ± 0.08 | 295 (82.8) |
As described in the legend to Fig. 3, PS533 spores (wild type) were germinated with 10 mM l-valine at various temperatures, and spore germination parameters were determined as described in Materials and Methods.
Spores that initiate slow CaDPA leakage are committed to germinate.
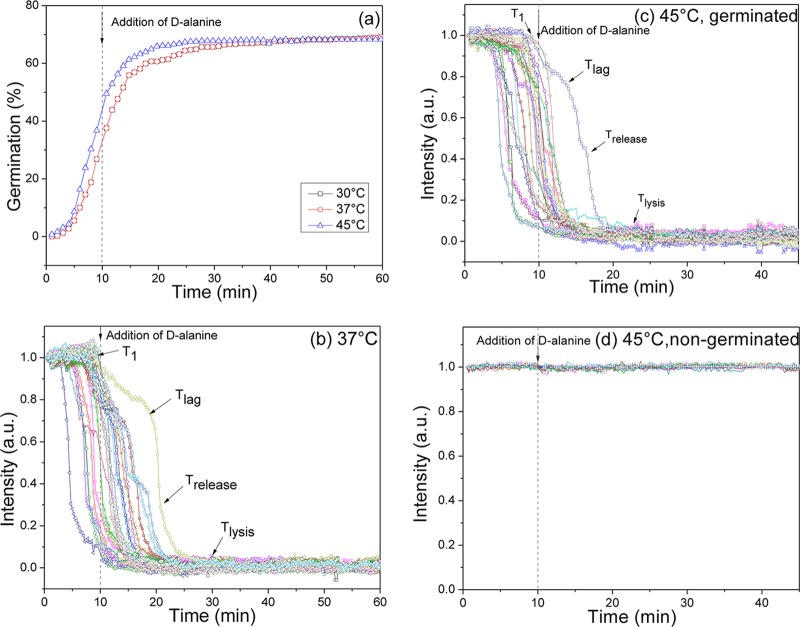
Since the initiation of slow CaDPA leakage begins soon after initiation of germination, an obvious question is what other germination events are associated with initiation of CaDPA leakage at T1? Since commitment to germinate is a major event prior to Tlag (3, 7), it seemed reasonable that T1 is at or near the time of commitment (Tc) for individual spores. To test this idea, B. subtilis spores were germinated with l-valine at various temperatures, the l-valine was removed after 10 min and replaced with d-alanine, and the germination of multiple individual spores was monitored for 45 to 60 min (Fig. 4a to c; Table 2). Previous work showed that d-alanine blocks spore commitment and yet allows committed spores to complete germination (7). The results of this experiment showed that at 30 to 45°C T1 values from germinated spores were less than 10 min, indicating that all germinated spores had committed before T1. In other words, if spores had reached T1 and initiated slow CaDPA leakage, they were committed to germinate. In contrast, spores incubated without l-valine exhibited no germination events before or after d-alanine addition, even at 45°C (Fig. 4d). Therefore, it seems likely that T1 is identical to Tc, and all spores that initiate slow CaDPA leakage are committed to germinate.
FIG 4.
Germination and PC image intensities of individual B. subtilis spores germinating with l-valine and after l-valine was replaced by d-alanine. PS533 spores (wild type) were germinated with 10 mM l-valine (a to c) at various temperatures or with buffer alone at 45°C (d), and spore germination was monitored by PC image intensity changes as described in Materials and Methods. In panels a to c, after 10 min the germinant was removed and replaced with 10 mM d-alanine, and incubations were continued at initial incubation temperatures. PC image intensities (a.u.) were normalized to 1 based on the respective values at the first time of measurement and with PC image intensities at the end of the experiment set at 0. The arrows indicate the times of T1, Tlag, Trelease, and Tlysis for a single spore. In panel a, the germination curves at the different temperatures are from data with >217 spores each, and a germinated spore was defined as one that had reached Trelease.
TABLE 2.
Mean values and standard deviations of germination parameters of multiple individual wild-type B. subtilis spores germinating with l-valine and then with its replacement, d-alaninea
| Germination temp (°C) | Mean ± SD |
No. of germinated spores (% germination) | |||||
|---|---|---|---|---|---|---|---|
| T1 (min) | Tlag (min) | ΔTleakage (min) | Trelease (min) | ΔTrelease (min) | Ilag | ||
| 30 | 7.1 ± 1.9 | 8.9 ± 2.6 | 1.9 ± 1.1 | 11.4 ± 2.9 | 2.5 ± 0.7 | 0.87 ± 0.09 | 78 (35.9) |
| 37 | 6.3 ± 2.3 | 9.9 ± 7.3 | 3.6 ± 6.0 | 12.4 ± 7.6 | 2.5 ± 0.9 | 0.86 ± 0.12 | 202 (69.2) |
| 45 | 4.6 ± 2.2 | 8.2 ± 5.9 | 3.6 ± 5.1 | 10.8 ± 6.3 | 2.6 ± 1.2 | 0.86 ± 0.10 | 191 (68.5) |
As described in the legend to Fig. 4, PS533 spores were germinated with 10 mM l-valine at various temperatures; after 10 min, the germinant was removed and replaced with 10 mM d-alanine, incubations were continued at initial incubation temperatures, and spore germination parameters were determined as described in Materials and Methods.
High levels of SpoVA proteins increase CaDPA leakage during spore germination.
SpoVA proteins are present in spores' IM and are involved in CaDPA release during germination with nutrients, most likely directly (3, 13–15). Previous work showed that ↑SpoVA B. subtilis (PS3411) spores with ∼4-fold-higher levels of SpoVA proteins have smaller Tlag values in nutrient germination than do wild-type spores (10). The l-valine germination of multiple individual ↑SpoVA spores at various temperatures (Fig. 5; Table 3) exhibited Ilag values similar to those of germinating wild-type spores, indicating that elevated SpoVA levels did not result in more CaDPA leakage prior to Tlag. However, not only were Tlag values of germinating spores lower than those for wild-type spores at all temperatures tested, as expected (10), but T1 values for ↑SpoVA spores were also lower than those for wild-type spores (Tables 1 and 3). In addition, the CaDPA leakage from ↑SpoVA spores was more obvious than that from wild-type spores, in particular at 25°C (Fig. 3b and 5b). There were also some small differences in the ΔTleakage and ΔTrelease times for ↑SpoVA and wild-type spores, although these are not especially significant (Tables 1 and 3). Consequently, compared with wild-type spores, elevated SpoVA protein levels result in earlier-than-normal initiation of CaDPA leakage. In addition, this leakage from ↑SpoVA spores is faster but over a shorter time period than that from wild-type spores.
FIG 5.
Germination extents (a) and PC image intensities of multiple individual B. subtilis PS3411 (↑SpoVA) spores germinating with l-valine at various temperatures. PS3411 spores were germinated with 2 mM l-valine at various temperatures, and spore germination was monitored by PC image intensity changes as described in Materials and Methods. The phase-contrast image intensities (a.u.) were normalized to 1 based on the respective values at the first time of measurement, and PC image intensities at the end of the experiment were set at 0. The arrows indicate the times of T1, Tlag, Trelease, and Tlysis for a single spore. In panel a, the germination curves at the different temperatures are from data with >323 spores each, and a germinated spore was defined as one that had reached Trelease.
TABLE 3.
Mean values and standard deviations of germination parameters of multiple individual ↑SpoVA and cwlJ B. subtilis spores germinating with l-valine at different temperaturesa
| Spore strain | Germination temp (°C) | Mean ± SD |
No. of germinated spores (% germination) | |||||
|---|---|---|---|---|---|---|---|---|
| T1 (min) | Tlag (min) | ΔTleakage (min) | Trelease (min) | ΔTrelease (min) | Ilag | |||
| ↑SpoVA | 25 | 3.9 ± 3.8 | 7.9 ± 7.7 | 4.0 ± 5.5 | 11.8 ± 8.4 | 3.9 ± 1.8 | 0.89 ± 0.11 | 354 (98.3) |
| 37 | 3.8 ± 3.0 | 8.0 ± 7.9 | 4.2 ± 6.5 | 11.2 ± 8.6 | 3.1 ± 1.3 | 0.88 ± 0.09 | 335 (98.9) | |
| 45 | 3.8 ± 3.0 | 7.4 ± 7.7 | 3.6 ± 6.3 | 10.6 ± 8.3 | 3.1 ± 1.3 | 0.87 ± 0.10 | 321 (99.3) | |
| FB111 (cwlJ) | 25 | 20.0 ± 11.1 | 72.2 ± 25.6 | 52.2 ± 19.9 | 81.1 ± 27.1 | 8.9 ± 3.5 | 0.48 ± 0.07 | 360 (93.5)b |
| 37 | 8.2 ± 4.7 | 34.1 ± 12.3 | 25.9 ± 10.4 | 38.9 ± 12.8 | 4.8 ± 1.3 | 0.57 ± 0.07 | 432 (94.5)c | |
| 45 | 8.1 ± 5.7 | 24.7 ± 9.1 | 16.6 ± 6.9 | 28.4 ± 9.4 | 3.7 ± 0.84 | 0.61 ± 0.07 | 316 (97.8)c | |
As described in the legend to Fig. 5, PS3411 (↑SpoVA) spores were germinated with 2 mM l-valine at various temperatures, and spore germination parameters were determined as described in Materials and Methods. As described in the legend to Fig. S2 in the supplemental material, FB111 (cwlJ) spores were germinated with 10 mM l-valine at various temperatures, and spore germination parameters were determined as described in Materials and Methods.
Germination was for 2 h.
Germination was for 1 h.
The absence of the CLE CwlJ affects CaDPA leakage during spore germination.
CwlJ is one of two redundant CLEs that play partially redundant roles in cortex PG degradation during stage II of B. subtilis spore germination (3). Previous work has shown that individual cwlJ spores have different germination curves from those with wild-type spores, in particular because ΔTrelease times are ∼15-fold higher than those for wild-type spores, making assignment of Tlag difficult (3, 23, 24). In view of the new evidence for slow CaDPA leakage prior to Tlag, the germination of multiple individual B. subtilis cwlJ spores was reexamined, focusing on changes early in l-valine germination (see Fig. S2 in the supplemental material; also Table 3). The results of this reanalysis indicated that (i) at all temperatures tested, cwlJ spores had higher Tlag, ΔTleakage, Trelease, and ΔTrelease values than wild-type spores (Tables 1 and 3); (ii) Ilag values of cwlJ spores were much smaller than those for wild-type spores, indicating that more CaDPA was released prior to Tlag during cwlJ spore germination; and (iii) values of ΔTrelease for cwlJ spores germinating at 37°C were only 1.5-fold higher than those for wild-type spores, much lower than determined previously; this large difference is primarily because much of the CaDPA now assigned as released in ΔTleakage was previously assigned as released in ΔTrelease.
CaDPA leakage during nonnutrient germination of B. subtilis spores and nutrient germination of B. cereus and B. megaterium spores.
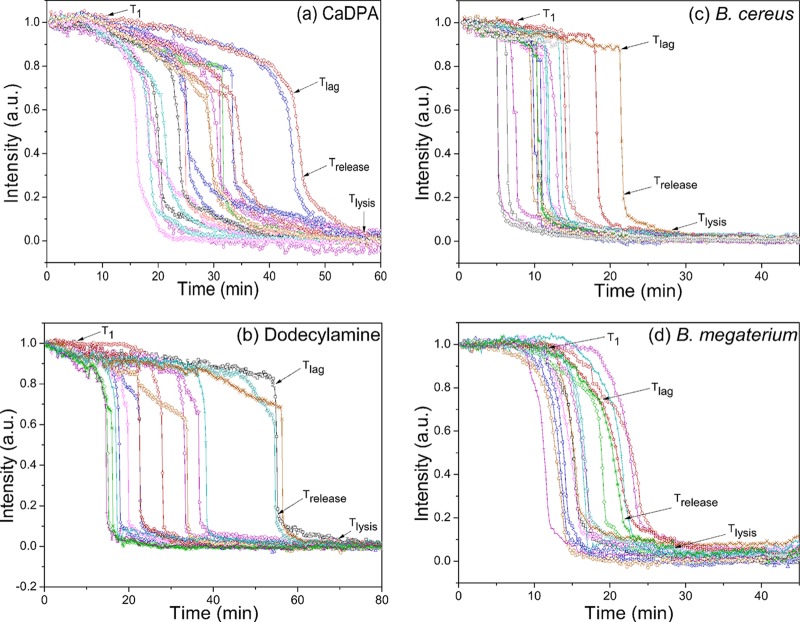
While CaDPA leakage was very evident in B. subtilis spores germinating with l-valine or AGFK, these are both GR-dependent germinants. In order to examine if slow CaDPA leakage also occurs during germination with GR-independent germinants, wild-type B. subtilis spores were germinated with CaDPA and dodecylamine (Fig. 6a and b; see Table S2 in the supplemental material), since these two germinants act in a GR-independent manner (3). The results of this experiment indicated that there was obvious slow CaDPA leakage in CaDPA and dodecylamine germination and that the amount of CaDPA released in this slow leakage was even slightly higher than in nutrient germination, as determined from Ilag values, although ΔTleakage values for CaDPA and dodecylamine germination were significantly larger than for nutrient germination at the same temperatures.
FIG 6.
(a to d) Germination curves of multiple individual spores of various species germinating with different germinants. (a) PS533 spores germinating with CaDPA; (b) PS832 spores germinating with dodecylamine; (c) B. cereus spores germinating with l-alanine; (d) B. megaterium spores germinating with glucose. Spores were germinated, and phase-contrast image intensities were determined as described in Materials and Methods. The image intensities (a.u.) were normalized to 1 based on the respective values at the first time of measurement, and image intensities at the end of the experiment were set at 0. The arrows indicate the times of T1, Tlag, Trelease, and Tlysis for a single spore.
Although slow CaDPA leakage prior to Tlag seems to be a general feature of B. subtilis spore germination with both GR-dependent and GR-independent germinants, an important question is whether this phenomenon is a general feature of the germination of Bacillus spores. To examine this question, the nutrient germination of spores of two other Bacillus species, B. cereus and B. megaterium, was also examined. Just as seen with B. subtilis spores, B. cereus and B. megaterium spore germination with nutrients also exhibited slow leakage of ∼8% and ∼19% of CaDPA, respectively, prior to fast CaDPA release beginning at Tlag (Fig. 6c and d; see Table S2 in the supplemental material). Thus, slow CaDPA leakage appears to be a general feature of the germination of spores of Bacillus species.
DISCUSSION
Previous work strongly indicates that the time Tc at which individual spores of Bacillus species commit to germinate is at or very near the time at which the permeability of the spores' IM increases in some fashion such that (i) the monovalent cations Na+, K+, and H+ are rapidly released and (ii) CaDPA is also rapidly released by heat treatments such as that at 70°C that have no effects on dormant spores (25, 26). The new results from the current work provide further support for a drastic change in Bacillus spores' IM permeability associated with Tc, as CaDPA also begins to slowly leak from spores of several Bacillus species at the time designated T1 (Fig. 7), which is equivalent or very close to Tc, and well before Tlag, with this leakage much more evident at high than at low germination temperatures. While the mechanism for monovalent cation release in spore germination is not known, the current work indicates that CaDPA leakage is most likely via the SpoVA CaDPA channel, since T1 values at which CaDPA leakage began during nutrient germination were much lower in ↑SpoVA spores and presumably Tc values are also much lower in ↑SpoVA spores. Slow CaDPA leakage was observed not only in GR-dependent germination of spores of three Bacillus species but also in germination of B. subtilis spores by the GR-independent germinants CaDPA and dodecylamine, suggesting that slow CaDPA leakage beginning at ∼Tc is a general feature of germination of spores of all Bacillus species. There is also evidence that dodecylamine triggers spore germination by directly opening the SpoVA protein channel for CaDPA in spores' IM (15, 27), and B. subtilis spore germination by a high pressure (HP) of 140 megapascals (MPa) that acts via GR activation also exhibits obvious CaDPA leakage prior to Tlag (28).
FIG 7.
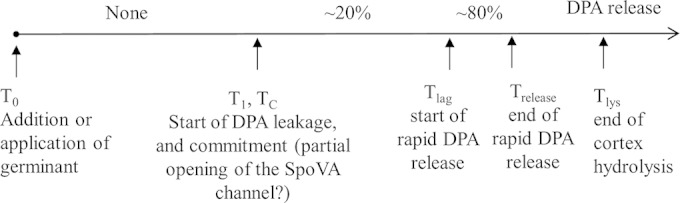
Temporal sequence of events in germination of a single Bacillus spore exposed to a germinant. The germinant (nutrient, dodecylamine, CaDPA, or a high pressure of 150 MPa [28]) is added or applied to spores at T0; the spore begins slow DPA leakage at T1, commits to germinate at Tc, and T1 and Tc may be identical; the spore starts rapid DPA release at Tlag; rapid DPA release ends at Trelease; and the spore completes cortex hydrolysis at Tlys, thus completing spore germination.
A possible model to fit the observations noted above into a conceptual framework is as follows. (i) Nutrient germinants and HPs of ∼140 MPa through action on GRs, dodecylamine through action on the SpoVA channel for CaDPA, and exogenous CaDPA via its activation of the CLE CwlJ that cleaves some bonds in cortex PG all lead to a partial opening or loosening of the SpoVA channel at ∼Tc, such that slow CaDPA leakage begins, and it is through this partially opened channel that CaDPA is rapidly released when committed spores are exposed to temperatures of 60 to 70°C (25). This initial partial opening of the SpoVA channel generally takes longer at low rather than higher temperatures and takes less time if spores have more SpoVA channels, as in ↑SpoVA spores. (ii) The initial slow CaDPA leakage via SpoVA channels then either slowly activates endogenous CwlJ (3, 16), leading to completion of spore germination, or can indirectly activate the other redundant CLE, SleB, perhaps by changes in the overall cortex structure due to CwlJ action or core water uptake accompanying CaDPA leakage, or both of these changes. However, more total CaDPA leakage appears to be required for SleB activation than for activation of CwlJ, and presumably, this is why there is much more CaDPA leakage from germinating CwlJ spores. Indeed, spores with no or low CaDPA levels germinate spontaneously through SleB (29).
While the simple model described above has some attractive features, there are a number of unanswered questions and uncertainties as follows. (i) What is the change that triggers a spore to actually commit to germinate and initiate slow CaDPA leakage? In nutrient germination, Tc values are decreased by elevated GR levels or increases in nutrient germinant concentration at least up to GR saturation, and almost certainly by increased SpoVA levels (7, 27). Since interaction of germinants with GRs leads to germination by opening the SpoVA channel for CaDPA, which can also be opened by at least dodecylamine, perhaps directly (15, 27), it seems reasonable that it is some partial opening or loosening of the SpoVA channel that is the crucial event that commits a spore to germinate. The dramatic effects of elevated SpoVA levels on the T1 times for initiation of CaDPA leakage during nutrient germination also suggest the possibility that partial opening of the SpoVA channel is a cooperative process requiring the partial opening of many individual SpoVA channels. At present, we know very little about the details of the composition, structure, or regulation of the SpoVA channel. However, in contrast to GRs, which are localized in a small focus in spores' IM termed the germinosome, at least one of the 7 SpoVA proteins, each of which is present at much higher levels per spore than are GRs, is dispersed throughout the IM (30). (ii) Spores germinated at 25°C had much higher T1 values than spores germinating at higher temperatures, although elevation of SpoVA levels largely reversed this effect of low-temperature germination. This suggests that at low temperatures, the SpoVA channel is more difficult to partially activate, something that seems reasonable if SpoVA activation requires a conformational change in one or more of the channel proteins, but the molecular mechanism for this phenomenon is far from known. (iii) Why do ΔTleakage values differ so much in germination triggered by nutrients, dodecylamine, and CaDPA, if all CaDPA release is via partial activation of SpoVA channels? Clearly, these different germinants are acting differently on SpoVA channels, but is this sufficient to result in different timing of CaDPA leakage and different rates of the leakage process, and if so, why? Clearly, more information is needed on the composition, structure, and regulation of the function of SpoVA channels for CaDPA in order to completely understand the early events in the germination of spores of Bacillus species.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a Department of Defense Multi-disciplinary University Research Initiative through the U.S. Army Research Laboratory and the U.S. Army Research Office under contract number W911F-09-1-0286 (P.S. and Y.-Q.L.) and by a grant from the Army Research Office under contract number W911NF-12-1-0325.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02490-14.
REFERENCES
- 1.Paredes-Sabja D, Setlow P, Sarker MR. 2011. Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol 19:85–94. doi: 10.1016/j.tim.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Setlow P, Johnson EA. 2012. Spores and their significance, p 45–79. In Doyle MP, Beuchat LR (ed), Food microbiology: fundamentals and frontiers, 3rd ed ASM Press, Washington, DC. [Google Scholar]
- 3.Setlow P. 2013. When the sleepers wake: the germination of spores of Bacillus species. J Appl Microbiol 115:1251–1268. doi: 10.1111/jam.12343. [DOI] [PubMed] [Google Scholar]
- 4.Stewart GSAB, Johnstone K, Hagelberg E, Ellar DJ. 1981. Commitment of bacterial spores to germinate. A measure of the trigger reaction. Biochem J 198:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster SJ, Johnstone K. 1986. The use of inhibitors to identify early events during Bacillus megaterium KM spore germination. Biochem J 237:865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatasubramanian P, Johnstone K. 1989. Biochemical analysis of the Bacillus subtilis 1604 spore germination response. J Gen Microbiol 135:2723–2733. [DOI] [PubMed] [Google Scholar]
- 7.Yi X, Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J Bacteriol 192:3424–3433. doi: 10.1128/JB.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paidhungat M, Setlow P. 2002. Spore germination and outgrowth, p 537–548. In Sonenshein AL, Hoch JA, Losick R (ed), Bacillus subtilis and its relatives: from genes to cells. ASM Press, Washington, DC. [Google Scholar]
- 9.Kong L, Zhang P, Wang G, Yu J, Setlow P, Li Y-Q. 2011. Characterization of bacterial spore germination using phase-contrast and fluorescence microscopy, Raman spectroscopy and optical tweezers. Nat Protoc 6:625–639. doi: 10.1038/nprot.2011.307. [DOI] [PubMed] [Google Scholar]
- 10.Wang G, Yi X, Li Y-Q, Setlow P. 2011. Germination of individual Bacillus subtilis spores with alterations in the GerD and SpoVA proteins, which are important in spore germination. J Bacteriol 193:2301–2311. doi: 10.1128/JB.00122-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Kong L, Wang G, Setlow P, Li Y-Q. 2010. Combination of Raman tweezers and quantitative differential interference contrast microscopy for measurement of dynamics and heterogeneity during the germination of individual bacterial spores. J Biomed Opt 15:056010. doi: 10.1117/1.3494567. [DOI] [PubMed] [Google Scholar]
- 12.Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol 178:3486–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vepachedu VR, Setlow P. 2005. Localization of SpoVAD to the inner membrane of spores of Bacillus subtilis. J Bacteriol 187:5677–5682. doi: 10.1128/JB.187.16.5677-5682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Valdespino A, Li Y, Setlow B, Ghosh S, Pan D, Korza G, Feeherry FF, Doona CJ, Li YQ, Hao B, Setlow P. 2014. Properties and function of the SpoVAEa and SpoVAF proteins in Bacillus subtilis spores. J Bacteriol 196:2077–2088. doi: 10.1128/JB.01546-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velásquez J, Schuurman-Wolters G, Birkner JP, Abee T, Poolman B. 2014. Bacillus subtilis protein SpoVAC functions as a mechanosensitive channel. Mol Microbiol 92:813–823. doi: 10.1111/mmi.12591. [DOI] [PubMed] [Google Scholar]
- 16.Paidhungat M, Ragkousi K, Setlow P. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J Bacteriol 183:4886–4893. doi: 10.1128/JB.183.16.4886-4893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450. In Harwood CR, Cutting SM (ed), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 18.Ghosh S, Setlow P. 2010. The preparation, germination properties and stability of superdormant spores of Bacillus cereus. J Appl Microbiol 108:582–590. doi: 10.1111/j.1365-2672.2009.04442.x. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh S, Setlow P. 2009. Isolation and characterization of superdormant spores of Bacillus species. J Bacteriol 191:1787–1797. doi: 10.1128/JB.01668-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang P, Liang J, Yi X, Setlow P, Li YQ. 2014. Monitoring of commitment, blocking and continuation of nutrient germination of individual Bacillus subtilis spores. J Bacteriol 196:2443–2454. doi: 10.1128/JB.01687-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang P, Garner W, Yi X, Yu J, Li Y-Q, Setlow P. 2010. Factors affecting variability in time between addition of nutrient germinants and rapid dipicolinic acid release during germination of spores of Bacillus species. J Bacteriol 192:3608–3619. doi: 10.1128/JB.00345-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang G, Zhang P, Setlow P, Li Y. 2011. Kinetics of germination of wet-heat-treated individual spores of Bacillus. Appl Environ Microbiol 77:3368–3379. doi: 10.1128/AEM.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Thomas SS, Li YQ, Setlow P. 2012. Effects of cortex peptidoglycan structure and cortex hydrolysis on the kinetics of Ca2+-dipicolinic acid release during Bacillus subtilis spore germination. J Bacteriol 194:646–652. doi: 10.1128/JB.06452-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng L, Chen D, Setlow P, Li YQ. 2009. Elastic and inelastic light scattering from single bacterial spores in an optical trap allows monitoring of spore germination dynamics. Anal Chem 81:4035–4042. doi: 10.1021/ac900250x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luu S, Setlow P. 2014. Analysis of the loss in heat and acid resistance during germination of spores of Bacillus species. J Bacteriol 196:1733–1740. doi: 10.1128/JB.01555-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swerdlow BM, Setlow B, Setlow P. 1981. Levels of H+ and other monovalent cations in dormant and germinated spores of Bacillus megaterium. J Bacteriol 148:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vepachedu VR, Setlow P. 2007. Role of SpoVA proteins in release of dipicolinic acid during the germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J Bacteriol 189:1565–1572. doi: 10.1128/JB.01613-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong L, Doona CJ, Setlow P, Li YQ. 2014. Monitoring rates and heterogeneity of high-pressure germination of Bacillus spores by phase-contrast microscopy of individual spores. Appl Environ Microbiol 80:345–353. doi: 10.1128/AEM.03043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magge A, Granger AC, Wahome P, Setlow B, Vepachedu VR, Loshon CA, Peng L, Chen D, Li YQ, Setlow P. 2008. Role of dipicolinic acid in the germination, stability and viability of spores of Bacillus subtilis. J Bacteriol 190:4798–4807. doi: 10.1128/JB.00477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffiths KK, Zhang J, Cowan AE, Yu J, Setlow P. 2011. Germination proteins in the inner membrane of dormant Bacillus subtilis spores colocalize in a discrete cluster. Mol Microbiol 81:1061–1077. doi: 10.1111/j.1365-2958.2011.07753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.