Abstract
Pulse dipolar electron-spin resonance in the form of double electron electron resonance was applied to strategically placed, site-specifically attached pairs of nitroxide spin labels to monitor changes in the mini TAR DNA stem-loop structure brought on by the HIV-1 nucleocapsid protein NCp7. The biophysical structural evidence was at Ångstrom-level resolution under solution conditions not amenable to crystallography or NMR. In the absence of complementary TAR RNA, double labels located in both the upper and the lower stem of mini TAR DNA showed in the presence of NCp7 a broadened distance distribution between the points of attachment, and there was evidence for several conformers. Next, when equimolar amounts of mini TAR DNA and complementary mini TAR RNA were present, NCp7 enhanced the annealing of their stem-loop structures to form duplex DNA-RNA. When duplex TAR DNA-TAR RNA formed, double labels initially located 27.5 Å apart at the 3′- and 5′-termini of the 27-base mini TAR DNA relocated to opposite ends of a 27 bp RNA-DNA duplex with 76.5 Å between labels, a distance which was consistent with the distance between the two labels in a thermally annealed 27-bp TAR DNA-TAR RNA duplex. Different sets of double labels initially located 26–27 Å apart in the mini TAR DNA upper stem, appropriately altered their interlabel distance to ∼35 Å when a 27 bp TAR DNA-TAR RNA duplex formed, where the formation was caused either through NCp7-induced annealing or by thermal annealing. In summary, clear structural evidence was obtained for the fraying and destabilization brought on by NCp7 in its biochemical function as an annealing agent and for the detailed structural change from stem-loop to duplex RNA-DNA when complementary RNA was present.
Introduction
This structural study uses pulse dipolar spectroscopy (PDS) implemented at the current state of the art (1–3) to understand the change in physical structure of a stem-loop oligonucleotide from HIV-1. The stem-loop structure is found in the TAR (transactivation response) region of TAR DNA and TAR RNA. The binding of NCp7 inhibits self-priming within such a stem-loop, destabilizes stem-loops, and effectively catalyzes the annealing of complementary oligonucleotide strands so that duplexes form between complementary DNA and RNA. This annealing behavior in the presence NCp7 has been shown by gel techniques to occur both in vivo (4) and in vitro (5,6). The purpose of this work is to determine the nature of the oligonucleotide structural change brought on by NCp7 upon mini TAR DNA and then in conjunction with the complementary mini TAR RNA. The functional biochemical relevance of the annealing and the oligonucleotide structural change is that it aids in rapid HIV genome replication and integration into the host.
The in vitro annealing studies were carried out using the 1–55 form of NCp7, which we also use, that has a basic 1–11 tail (5,6). The mini TAR constructs, which we used for this structural study and for our previous dynamic study (7), are also very similar to those used for in vitro annealing (5,6). For our present structural study three pairs of nitroxide labeling sites on mini TAR DNA, which are the SLAB, SLCD, and SLEF sites shown in Fig. 1, were selected. Structural changes reported by SLAB, SLCD, and SLEF constructs occurred as these stem-loops were destabilized by NCp7 or reacted with equimolar complementary mini TAR RNA in the presence of NCp7.
Figure 1.
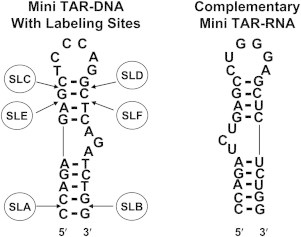
The secondary structure of mini TAR DNA together with positions of spin labels attached via phosphorothioate linkages. The complementary mini TAR RNA is also shown. On the mini TAR DNA the spin labels were attached in pairs, SLA-SLB, SLC-SLD, SLE-SLF, and the constructs were called SLAB, SLCD, and SLEF, respectively.
Over the last decade PDS has been widely used to determine distances within doubly labeled oligonucleotide molecules in the range of 1.5–8 nm (8–23). Further developments in PDS methods (3) enable even greater distances to be measured. For example, Fig. 7 b of (3) shows how long distances are readily obtained for TAR RNA-TAR DNA complexes. Not only the distance, but distance distributions, can be measured by PDS under solution conditions, which do not lend themselves to high resolution NMR or crystallography. Such distributions give insight into naturally occurring, disorder-inducing processes.
In our previous dynamic study with 9.5 and 236.6 GHz room temperature EPR (electron paramagnetic resonance), single nitroxide labeling sites in the lower stem, loop, and bulge were used to monitor the change in rotational dynamics of mini TAR DNA in the presence of increasing NCp7 concentrations (7). As a function of the ratio of NCp7 to mini TAR DNA, the tumbling time was found to increase with increasing NCp7, implying that more NCp7 was bound as the concentration of NCp7 increased. The tumbling time increased sharply when the ratio of TAR DNA/NCp7 became 1:4, implying the formation of condensates where there were 6–7 TAR bases per NCp7 (7). Evidence for condensates at that coverage of oligonucleotides by NCp7 has previously been reported through application of light scattering techniques (24,25). The condensing function is thought to be important in the annealing process, but the light scattering from condensates is not conducive to fluorescence resonance energy transfer (FRET), and the disorder that results is not conducive to high resolution NMR. The potential for condensing and disorder is, however, not an impediment to PDS. In this work, as a measure of the unwinding and destabilization of the TAR DNA stem structure by NCp7, PDS was used to probe the distance distributions between bilabels at a coverage of ∼6–7 bases per NCp7 in the absence of TAR RNA. Because of the small size of the nitroxide probes, their short tethers, and the accurate method for extracting pair distributions (26,27), the pulsed EPR technique provided not only precise interprobe distances (whereas FRET yields estimates of distances if the proper donor-acceptor pair is chosen), but also quantitative data on the distribution of end-to-end distances in NCp7-destabilized mini TAR DNA. We provide, with the power of PDS, clear resolution of explicit distance changes and distance distributions at Ångstrom (i.e., 0.1 nm) resolution and additional structural evidence of multiple conformers.
Distance changes from several pairs of double labels (SLAB, SLCD, SLEF) within mini TAR DNA were next followed in the presence of NCp7 and complementary mini TAR RNA. Where both NCp7 and complementary RNA were present, these measured distances showed marked changes both from the initial stem-loop structures in the absence of NCp7 to the case of duplex DNA-RNA. These distance changes were explicit evidence that NCp7 had catalyzed the annealing of complementary oligonucleotide strands so that duplexes had formed between complementary DNA and RNA. To compare the results of annealing by NCp7, control experiments were also performed on TAR DNA-TAR RNA samples that were thermally annealed in the absence of NCp7. This work thus provides a demonstration of the power of pulse dipolar electron-spin resonance (ESR) for resolving distances and distance distributions of oligonucleotide systems when these systems exhibit multiple conformations.
Materials and Methods
Preparation and characterization of spin-labeled mini TAR DNA
Spin labels were attached at the positions SLA, SLB, SLC, SLD, SLE, and SLF of mini TAR DNA (Fig. 1) by the method shown in Fig. 2, using protocols previously described (7). Doubly labeled derivatives at diametrically opposite duplex locations were created as follows: 1) SLAB at the penultimate 3′ and 5′ locations in the lower stem; 2) SLCD in the upper stem near the loop; 3) SLEF in the middle of the upper stem. A thio-methyl-phosphorothioate method of label attachment, indicated in Fig. 2, was used for making SLA, SLB, SLC, SLD, SLE, and SLF (7,14). For SLA and SLB, the thio-amido phosphorothioate method of label attachment was also used for some initial experiments (7). Mini TAR DNA with phosphorothioate modifications was purchased from TriLink (TriLink Bio Technologies, San Diego, CA) or IDT (Integrated DNA Technologies, Skokie, IL). 3-(2-iodoacetamide) proxyl (IPSL) for thio-amido attachment and 3-iodomethyl-(1-oxy-2,2,5,5-tetramethylpyrroline) for thio-methyl attachment were purchased from Toronto Research Chemicals, North York, Ontario, Canada. The detailed preparative protocols for label attachment are provided in the Supporting Material of Sun et al. (7). Purification and characterization of the spin-labeled derivatives were done by high-performance liquid chromatography (HPLC), as shown in Fig. S1 of our Supporting Material. Analytical denaturing gel electrophoresis was used to monitor the formation of the doubly labeled product, as shown in Fig. S2.
Figure 2.
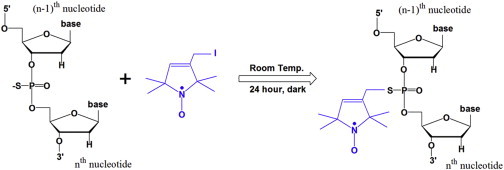
The reaction of a phosphorothioate sulfur with 3-iodomethyl-(1-oxy-2,2,5,5-tetramethylpyrroline) in blue to form a phosphorothioate linkage (14).
Nondenaturing gel-shift annealing assays
These assays were used to probe the annealing process of mini TAR DNA with complementary mini TAR RNA (5,6), where this process is enhanced by increasing NCp7. The primary purpose of these assays was to show the similarity in annealing behavior between unlabeled mini TAR DNA and doubly labeled mini TAR DNA. Separate bands for mini TAR RNA, mini TAR DNA, and the annealed RNA-DNA duplex were observed, where the presence of the latter duplex was greatly enhanced by increasing amounts of NCp7. The bands of unlabeled mini TAR DNA were similar in their location to the bands of the doubly labeled mini TAR DNA. Detailed gel shift annealing assays are presented in Fig. S3. A low intensity annealed mini TAR DNA-mini TAR RNA duplex band appeared even in the absence of NCp7 both for labeled and unlabeled TAR derivatives, consistent with the findings of Vo et al. (6).
Thermal melting of doubly labeled mini TAR DNA
The temperature dependence of the UV-260 absorbance was monitored as previously (7) to detect the hyperchromic increase in absorbance at 260 nm associated with the loss of basepairing and thermal melting of the TAR stem-loops. Absorption spectra were obtained by a Cary 3 UV-Vis Spectrophotometer equipped with a thermostated Peltier temperature controller. The controller was ramped at a rate of 0.3°C/min from 20 to 95°C, and the thermal melting profile, as shown below in Fig. 3, was obtained from the first derivative of the absorbance with respect to the temperature. The melting profiles provided melting comparisons of the unlabeled mini TAR DNA with the doubly labeled SLAB, SLCD, and SLEF forms of mini TAR DNA. From these profiles estimates of Tm (melting temperature), ΔH (van’t Hoff enthalpy), and ΔS (van’t Hoff entropy) were obtained by use of a previously developed nonlinear least-squares fitting routine under the assumption of two-state sequential unfolding (28). The melting profiles of unlabeled mini TAR DNA and the doubly labeled SLAB, SLCD, and SLEF derivatives were acquired under the same buffer conditions, 50 mM HEPES buffer, pH 7.5.
Figure 3.
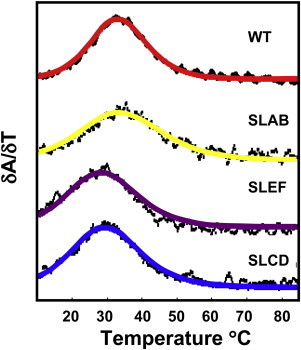
This figure provides a comparison of the melting behaviors of unlabeled mini TAR DNA and the SLAB, SLCD, and SLEF doubly labeled derivatives as shown by the first derivative of absorbance at 260 nm.
Nucleocapsid protein NCp7
NCp7 was prepared by solid phase peptide synthesis and with analysis methods described previously (29–32). The final NCp7 concentration was determined by using an extinction coefficient of ε280 = 6050 M−1 cm−1 (33).
Pulse dipolar spectroscopy: double electron-electron resonance
DEER measurements were performed in the 50 to 60 K temperature range at a 17.35 GHz working frequency using a home-built Ku-band pulse EPR spectrometer (2,34,35) optimized for double electron-electron resonance (DEER) and double-quantum coherence measurements. The 4-pulse DEER sequence (35–37) was applied with respective π/2-π-π detection pulse widths of 16 ns, 32 ns, and 32 ns, and a 32 ns π pump pulse was used (7,38). The detection pulses were positioned at the low-field edge of the nitroxide spin-label spectrum and the pump pulse was positioned at the center of the spectrum, so that the frequency separation between detected and pumped pulses was 70 MHz. Interlabel distances were measured in the range of 2–10 nm (20–100 Å) well suited for the DEER, and the DEER evolution time period (τ2) of 2.5–20 μs was chosen to provide at least two periods of dipolar oscillations for the largest distances encountered in this work. The homogeneous background was removed from the raw time-domain DEER signal, V(t), in a standard way (36,37) by fitting its latter (typically 50%) points of log[V(t)] to a first or second degree polynomial, extrapolating to early points, and subtracting out. The remainder was then normalized as (V(t) – 1)/V(0) to give the intramolecular dipole-dipole interaction-induced modulation of the time-domain signal. The distances were reconstructed from such baseline corrected and normalized signals (termed dipolar signals in the following) by using the Tikhonov regularization (L-curve) method (27) and refined by the maximum entropy method (26). The distance probability distributions, P(r), in units of nm−1, are thus model free, and not assumed to have a certain profile (e.g., to be Gaussian). Examples of the detailed procedure of DEER signal processing and distance reconstruction are given in Figs. S4–S6 and in Georgieva et al. (38).
Samples of doubly labeled oligonucleotide were typically frozen in 10% glycerol with an oligonucleotide concentration of 50–100 μM. For better resolution of longer distances out to ∼100 Ǻ, deuterated solvent was preferable because proton spin-spin interactions diminish the phase memory time for the transient EPR signal and hinder resolution of long-period oscillations and distances beyond ∼50 Å. Water that was 98% deuterated and glycerol-d8 that was 99% perdeuterated were used to prepare deuterated samples. Samples that contained mixtures of double-labeled mini TAR DNA, mini TAR RNA, and NCp7 were allowed to react at room temperature for 5 min before freezing.
Prediction of interlabel distances and distance distributions by the algorithm NASNOX
To independently estimate some distances experimentally measured by DEER, we applied the NASNOX conformational search algorithm, which was developed by Qin and co-workers (11–13,23). Using labels site-specifically attached in silico to known oligonucleotide Protein Data Bank (PDB) structures, NASNOX introduces a systematic series of spin label rotamer conformations, eliminates those that clash with nearby oligonucleotide moieties, and from the allowed ensemble of rotamers computes inter-NO double-label distances, uncertainty in the distances, and interlabel distance histogram distributions.
Results
Thermal melting of doubly labeled mini TAR DNA
Thermal melting profiles, obtained as the first derivative of the UV-260 absorbance with respect to temperature in the 20–95°C range, are shown in Fig. 3 to provide a melting comparison of the unlabeled and the doubly labeled SLAB, SLCD, and SLEF forms of mini TAR DNA. As described in the Materials and Methods section, values of Tm, ΔH, and ΔS were estimated, and their values are tabulated in Table 1. The melting temperature for unlabeled mini TAR DNA in low salt concentration was lowered by ∼3.5°C for SLCD and ∼4.5°C for SLED and slightly increased by ∼1°C for SLAB. The enthalpy of melting was 37 kcal/mol for unlabeled mini TAR DNA, and this number was diminished by 11, 8, and 8 kcal/mol for SLAB, SLCD, and SLED, respectively. At 20°C the ΔG 20◦C values (ΔG 20◦C = ΔH - 293ΔS), which are the overall free energies of melting at 20°C, were estimated. The values of ΔG 20◦C indicated that in their stem-loop forms unlabeled mini TAR DNA, SLAB, SLCD, and SLEF, were respectively stabilized by 2.7, 2.0, 1.9, and 1.8 kcal/mol with respect to their melted forms.
Table 1.
Melting temperatures, Tm, and the thermodynamic melting parameters ΔH, ΔS, ΔG20°C for unlabeled mini TAR DNA and doubly labeled SLAB, SLCD, and SLEF constructs
| DNA | UNLABELED | SLAB | SLCD | SLEF | |
|---|---|---|---|---|---|
| Buffer 50 mM Hepes, pH 7.5 | Tm (°C) | 43.4 ± 1.4 | 44.4 ± 4.2 | 39.9 ± 1.8 | 38.9 ± 2.9 |
| ΔH (Kcal/mol) | 37.0 ± 3.0 | 25.8 ± 3.4 | 29.4 ± 3.8 | 28.9 ± 3.8 | |
| ΔS (cal/mol/K) | 117 ± 9 | 81 ± 11 | 94 ± 12 | 93 ± 12 | |
| ΔG20°C (Kcal/mol) | 2.7 ± 0.3 | 2.0 ± 0.4 | 1.9 ± 0.3 | 1.8 ± 0.3 | |
| ΔTm (°C) | – | +1.0 ± 4.4 | −3.5 ± 2.2 | −4.5 ± 3.2 | |
| ΔΔG20°C (Kcal/mol) | – | 0.7 ± 0.5 | 0.8 ± 0.4 | 0.9 ± 0.4 | |
Destabilizing doubly labeled mini TAR DNA in the presence of NCp7
SLAB, labeled at the 5′ (SLA) and 3′ (SLB) positions shown in Fig. 1, previously was reported by DEER measurement to be partially unfolded at a molar ratio of 1 mini TAR DNA/4 NCp7 (7). Such unfolding and destabilization were interpreted as part of the NCp7-induced annealing process. In this work SLCD mini TAR DNA with double labels in the upper stem showed evidence for partial unfolding in the presence of a fourfold excess of NCp7, which corresponded to 6–7 TAR bases per NCp7. This partial unfolding, shown by broadening of the initial feature occurring near 26 Å and the appearance of new features in the 30–50 Å range, is indicated in Fig. 4. The pulse dipolar time domain traces relevant to SLCD by itself and with a fourfold excess of NCp7 in Fig. 4 are shown as Fig. S4.
Figure 4.
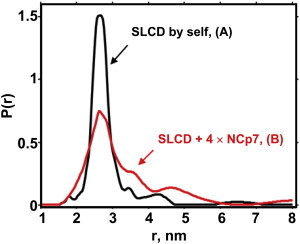
This figure provides evidence for the destabilization of the upper stem of doubly labeled SLCD mini TAR DNA. (A) Black shows the DEER-derived interprobe SLC-SLD distance distribution, P(r), in units of nm−1, from SLCD mini TAR DNA in the absence of NCp7. (B) Red provides the interprobe SLC-SLD distance distribution in the presence of a 1:4 ratio of the SLCD mini TAR DNA construct/NCp7. Sample conditions: 20 mM HEPES, 20 mM NaCl, 0.2 mM MgCl2, pH 7.5. Samples were frozen in 10% glycerol to prevent tube breakage.
Annealing doubly labeled mini TAR DNA in the presence of complementary mini TAR RNA and NCp7
The annealing process in the presence of complementary mini TAR RNA was monitored to provide evidence for conversion of the destabilized mini TAR DNA to duplex mini TAR DNA-mini TAR RNA. In Fig. 5 we provide the interprobe distance distributions from DEER measurements on the doubly labeled SLAB mini TAR DNA. The interprobe distance distribution of SLAB TAR DNA was unchanged in the presence of an equimolar concentration of TAR RNA. The implication is that little formation (<5%) of duplex TAR DNA-TAR RNA occurred without NCp7 present. For SLAB with no NCp7 present, the separation of labels at the peak of the distribution was 27.5 ± 0.5 Å, and the peak width at half height was 7.0 ± 0.5 Å. The 1:1:4 mixture of the SLAB mini TAR DNA/unlabeled mini TAR RNA/NCp7 provided striking evidence, shown in the red trace of Fig. 5, for a much longer interlabel SLA-SLB distance, consistent with duplex formation. For the red trace in Fig. 5, the separation of SLA and SLB labels at the peak of the distribution was 76.5 ± 2.0 Å, and the peak width of the interprobe distance distribution was 19.5 ± 2.0 Å. Fig. 5 was obtained with the deuterated solvent system that provides a longer phase memory time for better resolution of distance distributions beyond 60 Å. The pulse dipolar time domain traces relevant to SLAB in Fig. 5 are shown as Fig. S5. We provide in Fig. S7, evidence of the similarity of DEER distance distributions from a NCp7-annealed and from a thermally annealed 1:1 mixture of SLAB mini TAR DNA and mini TAR RNA. (These annealed distributions in Fig. S7 were the result of early measurements, where deuteration was not in use, where the peak positions and the peak widths beyond 60 Å were less accurate, and where double labeling was done with the iodoacetamido spin label that has a longer, flexible tether.)
Figure 5.
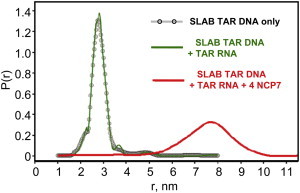
DEER distance distributions for doubly labeled mini TAR DNA showing the annealing function of NCp7 when complementary mini TAR RNA was present. The black line is SLAB by itself, the green trace is from a 1:1 mixture of SLAB + mini TAR RNA, and the red trace is a 1:1:4 mixture of SLAB mini TAR DNA/mini TAR RNA/NCp7. Sample conditions were 20 mM HEPES, 20 mM NaCl, 0.2 mM MgCl2, pH 7.5 with 10% glycerol. The samples were deuterated as described in Materials and Methods.
DEER was next used to follow interlabel distance changes between positions in the upper stem. The double-label positions were at SLC and SLD just below the upper loop, with interlabel distributions shown in Fig. 6 a, and SLE and SLF one basepair below C and D, with interlabel distributions shown in Fig. 6 b. For these doubly labeled constructs a compendium of traces is presented to show the evolution of the interlabel distance distribution, P(r), due to progressive duplex formation. The duplex formation proceeded in the following order: 1) the initial doubly labeled mini TAR DNA by itself; 2) a 1:1 mixture of doubly labeled mini TAR DNA and unlabeled mini TAR RNA; 3) a 1:1:1 mixture of doubly labeled mini TAR DNA/unlabeled mini TAR RNA/NCp7; 4) a 1:1:4 mixture of doubly labeled mini TAR DNA/mini TAR RNA/NCp7; and 5) a 1:1 mixture of doubly labeled mini TAR DNA/unlabeled mini TAR RNA that had been thermally annealed at 80°C in the absence of NCp7 and then allowed to cool in air to room temperature before freezing. When either SLCD or SLEF were present with equimolar mini TAR RNA, there was a slight increase in a species having a larger interlabel distance of ∼35 Å, implying that a small amount of annealing was occurring in the absence of NCp7. The presence of NCp7 catalyzed duplex DNA-RNA formation shown by the feature with the interlabel 35 Å distance. The pulse dipolar time domain traces relevant to SLCD and SLEF in Fig. 6 are shown as Fig. S6.
Figure 6.
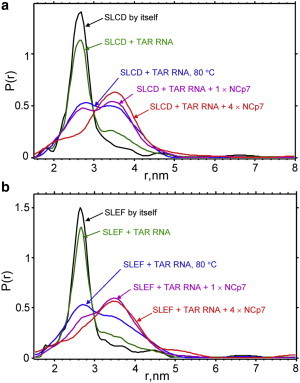
The DEER-derived interprobe distance distributions for mini TAR DNA doubly labeled at diametrically opposite phosphorothioate positions CD or EF in the TAR upper stem. The DNA was annealed to complementary unlabeled mini TAR RNA in the presence of NCp7 to form hybrid DNA-RNA duplexes. In panel a, the black line is SLCD by itself, the green trace is from a 1:1 mixture of SLCD + mini TAR RNA, the magenta trace is a 1:1:1 mixture of SLCD mini TAR DNA/mini TAR RNA/NCp7, the red trace is from a 1:1:4 mixture of SLCD mini TAR DNA/mini TAR RNA/NCp7, and finally, the blue traces is a thermally annealed mixture of 1:1 mixture of doubly labeled SLCD and mini TAR RNA. Panel b shows the same combinations as panel a, but with SLEF substituted for SLCD. Sample conditions were 20 mM HEPES, 20 mM NaCl, 0.2 mM MgCl2, pH 7.5 with 10% glycerol. The samples were deuterated as described in Materials and Methods.
The separation of double labels for both SLCD by itself in Fig. 6 a and SLEF by itself in Fig. 6 b was 26–27 Å and the separation of labels increased to ∼35 Å when the doubly labeled mini TAR DNA was annealed with complementary mini TAR RNA. Distances between labels within SLAB, SLCD, or SLEF in duplex mini TAR RNA-DNA are shown schematically in Fig. 7, and the details of interlabel distances and the interlabel distribution widths are provided in Table 2. Although the distributions showing conversion from stem-loop to apparent duplex superficially seem to show an isosbestic point near 30 Å, there appear to be more species, especially in the presence of fourfold excess NCp7, than just the original stem-loop with the peak near 27 Å and the RNA-DNA duplex with the peak near 35 Å. There is a broad shoulder with amplitude extending above 50 Å, in particular for SLED in 1:1:4 DNA/RNA/NCp7, implying the presence of other possibly more disordered species. The presence of these species may diminish the amount of available doubly labeled TAR DNA for duplex formation.
Figure 7.
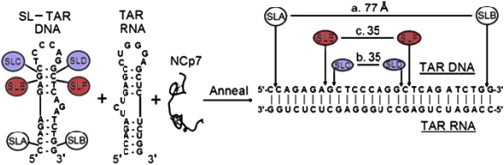
Summary of most probable interspin label distances from DEER measurements for the annealed TAR DNA-TAR RNA species of three different samples SLAB, SLCD, and SLEF.
Table 2.
Interlabel distances and distribution widths of doubly labeled mini TAR DNA
| Sample | 〈rDEER〉 (Å)a | W1/2 (Å)a |
|---|---|---|
| SLAB by itself | 27.5 ± 0.5 | 7.0 ± 0.5 |
| SLCD by itself | 26.8 ± 0.4 | 5.2 ± 0.4 |
| SLEF by itself | 26.3 ± 0.4 | 4.3 ± 0.4 |
| 1:1:4 SLCD/mini TAR RNA/NCp7 | 35.1 ± 0.6 | 10.8 ± 1.0 |
| 1:1:4 SLEF/mini TAR RNA/NCp7b | 34.8 ± 0.6 | 14.0 ± 1.0 |
| 1:1:4 SLAB/mini TAR RNA/NCp7 | 76.5 ± 2.0 | 19.5 ± 2.0 |
〈rDEER〉 and W1/2 are the respective experimental interlabel distance and the full peak width at half height of the interlabel distance distribution, as determined experimentally by DEER.
In the presence of a fourfold excess of NCp7 with respect to mini TAR DNA and mini TAR RNA, there is evidence for a broad shoulder in the interlabel distribution that extends above 50 Å separation; it is most notable in the SLEF sample.
Predicted inter-NO distances compared to DEER findings
The NASNOX routine was developed (13,14) to estimate interlabel distances between double labels attached via phosphorothioate linkages. NASNOX is an efficient conformational search algorithm to accurately predict the internitroxide distances and distributions from rotamer ensembles of phosphorothioate-attached spin labels; the predictions are obtained within minutes on a PC, without the substantial computational resources required for molecular dynamics computations (13,14). Application of NASNOX requires that spin labels be attached in silico to known oligonucleotide PDB model structures, which are not themselves varied during the application of the NASNOX algorithm. For estimating distances between the labels SLA and SLB, SLC and SLD, or SLE and SLF when they were attached diametrically opposite one another in duplex regions of the TAR DNA stem-loop, a duplex DNA structure (PDB 1CS2 (11,39)) was initially chosen. Labels were then attached in silico to this structure and interlabel distances, distribution widths, and interlabel distance histograms were computed. For DNA-RNA hybrid duplexes, relevant to the DNA-RNA duplexes in which SLC and SLD or SLE and SLF reside after annealing, a duplex DNA-RNA model structure (PDB 1EFS (40)) was chosen and labels then attached to it in silico. Detailed NASNOX results are tabulated (Table S1) and compared to DEER results. Interlabel histograms for SLCD and SLEF are compared to DEER spectra in Figs. S8 and S9. The distances and distance distributions obtained from NASNOX were in excellent agreement with those obtained by DEER.
In the TAR DNA-TAR RNA 27 bp duplex, SLA and SLB were separated by 25 phosphates, as indicated in Fig. 7. PDB structures of such lengthy duplex forms are not available, and NASNOX, which requires preexisting PDB structures, is therefore not immediately useful. To approximate the distance between phosphorothioate-attached SLA and SLB spin labels near the opposite ends of a 27-mer DNA-RNA duplex, we worked from literature estimates of the axial rise and the angular rotation per residue (41,42). The calculation of the interlabel distance is explained in the Supporting Material and given in Table S1. For a 25 phosphate separation, the prediction was for a SLA-SLB separation of 74.5 ± 2.8 Å, in excellent agreement with the separation of 76.5 ± 2.0 Å obtained by DEER.
Discussion
Structural and thermodynamic differences between the SLAB, SLCD, and SLEF spin-labeled constructs
The thermal melting temperatures of SLAB, SLCD, and SLEF constructs were within 1.5% (in °K) of native mini TAR DNA. However, the enthalpies (ΔH) and entropies (ΔS) of melting for doubly labeled SLAB, SLCD, and SLEF were smaller by ∼25% than those of native mini TAR DNA, reflecting the perturbation to lower and upper stem formation from phosphorothioate label attachment. If the entropy of all melted forms is about the same, then the smaller value of ΔS for SLAB (81 cal/mol/K), compared to ΔS for SLCD and SLEF (93–94 cal/mol/K), implies the greater disorder of SLAB in its folded form. The SLA and SLB labels are immediately adjacent to the 3′–5′ termini of the lower duplex region, a region which has exhibited evidence by NMR (43) and FRET (44–46) for intrinsic destabilization even before spin labeling. The peak widths (full width at half height) of the interlabel distributions diminished in the order SLAB, SLCD, SLEF from 7.0 to 5.2 to 4.3 Å. The broader interprobe distance distribution of SLAB, compared to SLCD and SLEF, is consistent by its implication of disorder with the greater entropic destabilization in the lower stem. In conclusion, the DEER technique provided a comparison of the disorder in separate oligonucleotide domains.
In the absence of NCp7 and complementary RNA, the doubly labeled SLAB showed an interlabel distance of 27.5 Å (Fig. 5), whereas SLCD and SLEF mini TAR DNA constructs (Fig. 6, a and b), both showed slightly smaller interlabel distances of 26.8 and 26.3 Å, respectively. All of these interlabel distances are similar to each other and within an 1.5 Å of NASNOX predictions (Table S1). NASNOX was used with the starting point of an intact duplex DNA structure, which is not varied throughout the NASNOX algorithm (13,14). If the interlabel distances between diametrically opposite labels in stem regions had markedly differed from NASNOX predictions and from each other, the strong implication would have been for a marked perturbation to the duplex stem-loop structure due to the spin labels. There was no such structural perturbation.
NCp7-mediated destabilization of doubly labeled mini TAR DNA
In the ambient temperature 9.5 GHz ESR work of Sun et al. (7), done at a ratio of 1 mini TAR 27-mer per 4 NCp7 (i.e., ∼6–7 TAR bases per NCp7), the tumbling times of the spin labels in stem, loop, and bulge of mini TAR DNA markedly increased from ∼1 ns to times longer than ∼5 ns. This was attributed to the formation of an NCp7-mini TAR DNA condensate, naturally favored at the 1:4 ratio by the 1–55 NCp7 (55-mer) with its highly basic 1–11 tail. At this ratio of NCp7 coverage significant annealing was known to occur (6). This 1:4 ratio of mini TAR DNA to NCp7 was chosen here to probe the biophysical structural implications of annealing under condensing conditions which are not amenable to NMR or FRET.
DEER evidence for NCp7-induced fraying and disorder of the doubly labeled SLAB was previously reported in the lower stem; see Fig. 8 C of Sun et al. (7). In this work, DEER spectroscopy of doubly labeled SLCD mini TAR DNA with a 1:4 mini TAR DNA to NCp7 coverage and no TAR RNA present showed that the upper stem also became frayed and disordered (Fig. 4). Although as found in unperturbed SLCD there was a substantial peak at 27 Å in the interlabel distribution, this distribution was considerably broader in the presence of fourfold NCp7. There were also conformers having longer interprobe distances of ∼35 Å and 45 Å. The previously studied SLAB was in an intrinsically more unstable region than the upper stem where SLCD is located. There were relatively more conformers with interprobe distances >35 Å for SLAB than for SLCD.
This DEER study notably showed that the upper stem can also be destabilized by NCp7. Overall, DEER measurements show the structural nature of destabilization under conditions where annealing happens. The aggregating sample conditions here of 4 NCp7 per 1 mini TAR 27-mer demonstrate how DEER is able to resolve structure and structural distributions that only could be inferred by fluorescence techniques or by FRET. (See detailed discussion and comparison of DEER and FRET findings in the Supporting Material.)
Annealing doubly labeled mini TAR DNA with mini TAR RNA
For SLAB, SLCD, and SLEF, the presence of mini TAR DNA, mini TAR RNA, and NCp7 in a ratio of 1:1:4 led to a duplex RNA-DNA form. The comparison of interlabel distributions for the NCp7-annealed duplex to the distributions for a thermally annealed mini TAR DNA-mini TAR RNA duplex showed at least as complete a conversion by NCp7 annealing as did thermal annealing. One obvious implication of this comparison is that the ∼5 min time for allowing mini TAR DNA, mini TAR RNA, and NCp7 to react was sufficient for conversion to the duplex. The biophysical importance is that detailed structural evidence at Ångstrom resolution is provided by DEER for the stem-loop to duplex conversion, and the NCp7-induced conversion to the DNA-RNA duplex is compared to conversion by thermal annealing.
For SLEF and SLCD the presence of mini TAR DNA, mini TAR RNA, and NCp7 in a 1:1:1 ratio was sufficient to convert most of the mini TAR DNA and mini TAR RNA to duplex form. However, a detailed comparison of the interlabel distributions resulting from the 1:1:1 and 1:1:4 ratios showed that the 1:1:1 ratio left more of the original stem-loop unreacted, as shown by retention of a feature at 27 Å. The 1:1:4 ratio also gave rise to a broad feature extending up to ∼50 Å that implies the existence of unwound, nonduplex mini TAR DNA and possibly indicated that the duplex form may have been somewhat destabilized at the 1:1:4 ratio.
A number of stem-loop RNA and DNA constructs with specifically bound NCp7 complexes have been characterized by NMR study at a 1:1 oligonucleotide/NCp7 ratio (43,47–49). One additional NCp7 bound to mini TAR DNA beyond the first was inferred by isothermal calorimetry, but not verified by NMR (43), although that study used 11–55 NCp7 lacking the basic 1–10 tail. For binding of basic 1–55 NCp7 elsewhere than to unpaired loop and bulge regions, NCp7 has been proposed to bind nonspecifically (24,47–49). NCp7 may well operate as annealing catalyst under condensing conditions without the necessity for forming stoichiometric complexes (24). The study of NCp7 binding to model oligonucleotides by numerous spectroscopic techniques and mass spectrometry showed a complicated compendium of species exhibiting multivalency, i.e., 1:1:, 2:1, 2:2, 1:2, and higher order complexes (50). It is not clear at either the 1:1:1 or 1:1:4 DNA/RNA/NCp7 ratio, where NCp7 is an effective catalyst for annealing, that there is or should be a well-ordered stoichiometric complex of NCp7, especially to the final duplex RNA-DNA product. Regardless of the precise mode of action of NCp7, the critical point shown by our DEER study is that NCp7 does indeed convert stem-loops to duplex DNA-RNA having the definitive structural signature expected for a duplex.
Conclusions
With strategically placed nitroxide double labels, we have obtained evidence via DEER distance measurements for the structural changes due to NCp7-induced fraying of mini TAR DNA and for NCp7-induced annealing of mini TAR DNA with complementary mini TAR RNA. Using double labels within the upper and lower mini TAR DNA stem, DEER provided interprobe distributions that showed NCp7-induced fraying, that is, evidence at Ångstrom resolution for a structural change of the mini TAR DNA itself. DEER showed the large 25 to 80 Å structural change undergone by double labels on the mini TAR DNA as the DNA conformation changed in the presence of complementary mini TAR RNA from a DNA hairpin to DNA in an RNA-DNA duplex. Other techniques provide qualitative insight, e.g., by FRET efficiency changes (discussed in the Supporting Material) or by gel retardation assays. Detail of the structural change is shown more explicitly by quantitative distance measurements using DEER spectroscopy. Even though this study was carried out on just a 27-mer, it points to the emergence of PDS as a major contributor in elucidating larger looped and knotted tertiary structures from biologically relevant oligonucleotide and protein-oligonucleotides complexes. An application of PDS to another RNA-protein system has just been reported (51).
Supporting Material
Protocols are provided for the HPLC purification, the denaturing gel assay, and the gel-shift annealing assay of doubly labeled mini TAR, including, 1) Fig. S1, which shows the HPLC purification trace for doubly labeled mini TAR DNA, Fig. S2, which shows analytical gel traces for doubly labeled mini TAR DNA, and Fig. S3, which shows gel shift annealing assays of mini TAR DNA with complementary mini TAR RNA; 2) Figs. S4–S6, which show the dipolar evolution transients that respectively lead to the interlabel distances for SLCD in Fig. 4, SLAB in Fig. 5, and SLCD and SLEF in Fig. 6; 3) Fig. S7 shows the similarity in SLA-SLB interlabel distances between NCp7-annealed and thermally annealed 1:1 mixtures of doubly labeled mini DNA and mini TAR RNA; 4) details of the application of the NASNOX method and a tabulated comparison of NASNOX findings with DEER findings; 5) Figs. S8 and S9, respectively, provide the comparison of the experimental DEER internitroxide distributions of SLCD (Fig. 6 a) and SLEF (Fig. 6 b) to internitroxide histograms computed from NASNOX; and 7) a discussion of the complementary nature of PDS and FRET findings.
Acknowledgments
We are grateful to Prof. Carla Theimer, Department of Chemistry, University at Albany, for providing lab space and technical advice for the preparation and characterization of oligonucleotides and to Prof. Peng Chen, Department of Chemistry and Chemical Biology, Cornell University, for a helpful discussion on FRET.
This work was supported by the National Institutes of Health (NIH) (GM066253-01A1 and 3RO1GM06625304S1 to C.P.S.), the NIH/National Institute of General Medical Sciences (NIGMS) (grant P41GM103521 to J.H.F.), and a Faculty Research Award Program-Category A grant from the University at Albany to C.P.S.
Footnotes
William K. Myers’s present address is Inorganic Chemistry Laboratory, Centre for Advanced Electron Spin Resonance (CAESR), University of Oxford, South Parks Road, Oxford OX1 3QR, United Kingdom.
Supporting Material
Supporting Citations
References (52–62) appear in the Supporting Material.
References
- 1.Polyhach Y., Bordignon E., Jeschke G. High sensitivity and versatility of the DEER experiment on nitroxide radical pairs at Q-band frequencies. Phys. Chem. Chem. Phys. 2012;14:10762–10773. doi: 10.1039/c2cp41520h. [DOI] [PubMed] [Google Scholar]
- 2.Borbat P.P., Georgieva E.R., Freed J.H. Improved sensitivity for long-distance measurements in biomolecules: five-pulse double electron-electron resonance. J. Phys. Chem. Lett. 2013;4:170–175. doi: 10.1021/jz301788n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borbat P.P., Freed J.H. Pulse dipolar ESR: distance measurements. In: Harmer J.R., Timmel C.R., editors. Structural Information from Spin-Labels and Intrinsic Paramagnetic Centers in the Biosciences (Structure and Bonding) Springer Heidelberg; Germany; New York: 2014. pp. 1–82. [Google Scholar]
- 4.Johnson P.E., Turner R.B., Summers M.F. A mechanism for plus-strand transfer enhancement by the HIV-1 nucleocapsid protein during reverse transcription. Biochemistry. 2000;39:9084–9091. doi: 10.1021/bi000841i. [DOI] [PubMed] [Google Scholar]
- 5.Vo M.N., Barany G., Musier-Forsyth K. HIV-1 nucleocapsid protein switches the pathway of transactivation response element RNA/DNA annealing from loop-loop “kissing” to “zipper”. J. Mol. Biol. 2009;386:789–801. doi: 10.1016/j.jmb.2008.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vo M.N., Barany G., Musier-Forsyth K. Mechanistic studies of mini-TAR RNA/DNA annealing in the absence and presence of HIV-1 nucleocapsid protein. J. Mol. Biol. 2006;363:244–261. doi: 10.1016/j.jmb.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y., Zhang Z., Scholes C.P. The internal dynamics of mini c TAR DNA probed by electron paramagnetic resonance of nitroxide spin-labels at the lower stem, the loop, and the bulge. Biochemistry. 2012;51:8530–8541. doi: 10.1021/bi301058q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiemann O., Weber A., Sigurdsson S.T. Nanometer distance measurements on RNA using PELDOR. J. Am. Chem. Soc. 2003;125:3434–3435. doi: 10.1021/ja0274610. [DOI] [PubMed] [Google Scholar]
- 9.Borbat P.P., Davis J.H., Freed J.H. Measurement of large distances in biomolecules using double-quantum filtered refocused electron spin-echoes. J. Am. Chem. Soc. 2004;126:7746–7747. doi: 10.1021/ja049372o. [DOI] [PubMed] [Google Scholar]
- 10.Schiemann O., Piton N., Prisner T.F. A PELDOR-based nanometer distance ruler for oligonucleotides. J. Am. Chem. Soc. 2004;126:5722–5729. doi: 10.1021/ja0393877. [DOI] [PubMed] [Google Scholar]
- 11.Cai Q., Kusnetzow A.K., Qin P.Z. Site-directed spin labeling measurements of nanometer distances in nucleic acids using a sequence-independent nitroxide probe. Nucleic Acids Res. 2006;34:4722–4730. doi: 10.1093/nar/gkl546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Q., Kusnetzow A.K., Qin P.Z. Nanometer distance measurements in RNA using site-directed spin labeling. Biophys. J. 2007;93:2110–2117. doi: 10.1529/biophysj.107.109439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price E.A., Sutch B.T., Haworth I.S. Computation of nitroxide-nitroxide distances in spin-labeled DNA duplexes. Biopolymers. 2007;87:40–50. doi: 10.1002/bip.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin P.Z., Haworth I.S., He H. Measuring nanometer distances in nucleic acids using a sequence-independent nitroxide probe. Nat. Protoc. 2007;2:2354–2365. doi: 10.1038/nprot.2007.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiemann O., Prisner T.F. Long-range distance determinations in biomacromolecules by EPR spectroscopy. Q. Rev. Biophys. 2007;40:1–53. doi: 10.1017/S003358350700460X. [DOI] [PubMed] [Google Scholar]
- 16.Singh V., Azarkh M., Drescher M. Human telomeric quadruplex conformations studied by pulsed EPR. Angew. Chem. Int. Ed. Engl. 2009;48:9728–9730. doi: 10.1002/anie.200902146. [DOI] [PubMed] [Google Scholar]
- 17.Kim N.K., Bowman M.K., DeRose V.J. Precise mapping of RNA tertiary structure via nanometer distance measurements with double electron-electron resonance spectroscopy. J. Am. Chem. Soc. 2010;132:8882–8884. doi: 10.1021/ja101317g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krstić I., Frolow O., Prisner T.F. PELDOR spectroscopy reveals preorganization of the neomycin-responsive riboswitch tertiary structure. J. Am. Chem. Soc. 2010;132:1454–1455. doi: 10.1021/ja9077914. [DOI] [PubMed] [Google Scholar]
- 19.Sicoli G., Wachowius F., Höbartner C. Probing secondary structures of spin-labeled RNA by pulsed EPR spectroscopy. Angew. Chem. Int. Ed. Engl. 2010;49:6443–6447. doi: 10.1002/anie.201000713. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen P., Qin P.Z. RNA dynamics: perspectives from spin labels. Wiley Interdiscip. Rev. RNA. 2012;3:62–72. doi: 10.1002/wrna.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romainczyk O., Endeward B., Engels J.W. The RNA-DNA hybrid structure determined by EPR, CD and RNase H1. Mol. Biosyst. 2011;7:1050–1052. doi: 10.1039/c0mb00258e. [DOI] [PubMed] [Google Scholar]
- 22.Wunnicke D., Strohbach D., Steinhoff H.J. Ligand-induced conformational capture of a synthetic tetracycline riboswitch revealed by pulse EPR. RNA. 2011;17:182–188. doi: 10.1261/rna.2222811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X., Tung C.S., Qin P.Z. Global structure of a three-way junction in a phi29 packaging RNA dimer determined using site-directed spin labeling. J. Am. Chem. Soc. 2012;134:2644–2652. doi: 10.1021/ja2093647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vo M.N., Barany G., Musier-Forsyth K. Effect of Mg(2+) and Na(+) on the nucleic acid chaperone activity of HIV-1 nucleocapsid protein: implications for reverse transcription. J. Mol. Biol. 2009;386:773–788. doi: 10.1016/j.jmb.2008.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paoletti A.C., Shubsda M.F., Borer P.N. Affinities of the nucleocapsid protein for variants of SL3 RNA in HIV-1. Biochemistry. 2002;41:15423–15428. doi: 10.1021/bi026307n. [DOI] [PubMed] [Google Scholar]
- 26.Chiang Y.W., Borbat P.P., Freed J.H. Maximum entropy: a complement to Tikhonov regularization for determination of pair distance distributions by pulsed ESR. J. Magn. Reson. 2005;177:184–196. doi: 10.1016/j.jmr.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Chiang Y.W., Borbat P.P., Freed J.H. The determination of pair distance distributions by pulsed ESR using Tikhonov regularization. J. Magn. Reson. 2005;172:279–295. doi: 10.1016/j.jmr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Theimer C.A., Wang Y., Giedroc D.P. Non-nearest neighbor effects on the thermodynamics of unfolding of a model mRNA pseudoknot. J. Mol. Biol. 1998;279:545–564. doi: 10.1006/jmbi.1998.1812. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z., Xi X., Karim C.B. Rotational dynamics of HIV-1 nucleocapsid protein NCp7 as probed by a spin label attached by peptide synthesis. Biopolymers. 2008;89:1125–1135. doi: 10.1002/bip.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xi X., Sun Y., Scholes C.P. HIV-1 nucleocapsid protein NCp7 and its RNA stem loop 3 partner: rotational dynamics of spin-labeled RNA stem loop 3. Biochemistry. 2008;47:10099–10110. doi: 10.1021/bi800602e. [DOI] [PubMed] [Google Scholar]
- 31.Karim C.B., Kirby T.L., Thomas D.D. Phospholamban structural dynamics in lipid bilayers probed by a spin label rigidly coupled to the peptide backbone. Proc. Natl. Acad. Sci. USA. 2004;101:14437–14442. doi: 10.1073/pnas.0402801101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karim C.B., Zhang Z., Thomas D.D. Synthesis of TOAC spin-labeled proteins and reconstitution in lipid membranes. Nat. Protoc. 2007;2:42–49. doi: 10.1038/nprot.2007.2. [DOI] [PubMed] [Google Scholar]
- 33.Tummino P.J., Scholten J.D., Hupe D. The in vitro ejection of zinc from human immunodeficiency virus (HIV) type 1 nucleocapsid protein by disulfide benzamides with cellular anti-HIV activity. Proc. Natl. Acad. Sci. USA. 1996;93:969–973. doi: 10.1073/pnas.93.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borbat P.P., Crepeau R.H., Freed J.H. Multifrequency two-dimensional Fourier transform ESR: an X/Ku-band spectrometer. J. Magn. Reson. 1997;127:155–167. doi: 10.1006/jmre.1997.1201. [DOI] [PubMed] [Google Scholar]
- 35.Borbat P.P., Freed J.H. Measuring distances by pulsed dipolar ESR spectroscopy: spin-labeled histidine kinases. Methods Enzymol. 2007;423:52–116. doi: 10.1016/S0076-6879(07)23003-4. [DOI] [PubMed] [Google Scholar]
- 36.Pannier M., Veit S., Spiess H.W. Dead-time free measurement of dipole-dipole interactions between electron spins. J. Magn. Reson. 2000;142:331–340. doi: 10.1006/jmre.1999.1944. [DOI] [PubMed] [Google Scholar]
- 37.Jeschke G., Polyhach Y. Distance measurements on spin-labelled biomacromolecules by pulsed electron paramagnetic resonance. Phys. Chem. Chem. Phys. 2007;9:1895–1910. doi: 10.1039/b614920k. [DOI] [PubMed] [Google Scholar]
- 38.Georgieva E.R., Roy A.S., Freed J.H. Effect of freezing conditions on distances and their distributions derived from Double Electron Electron Resonance (DEER): a study of doubly-spin-labeled T4 lysozyme. J. Magn. Reson. 2012;216:69–77. doi: 10.1016/j.jmr.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leporc S., Mauffret O., Fermandjian S. An NMR and molecular modelling analysis of d(CTACTGCTTTAG). d(CTAAAGCAGTAG) reveals that the particular behaviour of TpA steps is related to edge-to-edge contacts of their base-pairs in the major groove. Nucleic Acids Res. 1999;27:4759–4767. doi: 10.1093/nar/27.24.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hantz E., Larue V., Huynh Dinh T. Solution conformation of an RNA–DNA hybrid duplex containing a pyrimidine RNA strand and a purine DNA strand. Int. J. Biol. Macromol. 2001;28:273–284. doi: 10.1016/s0141-8130(01)00123-4. [DOI] [PubMed] [Google Scholar]
- 41.Gyi J.I., Lane A.N., Brown T. Solution structures of DNA.RNA hybrids with purine-rich and pyrimidine-rich strands: comparison with the homologous DNA and RNA duplexes. Biochemistry. 1998;37:73–80. doi: 10.1021/bi9719713. [DOI] [PubMed] [Google Scholar]
- 42.Saenger W. Springer-Verlag; New York: 1984. Principles of Nucleic Acid Structure. [Google Scholar]
- 43.Bazzi A., Zargarian L., Mauffret O. Structural insights into the cTAR DNA recognition by the HIV-1 nucleocapsid protein: role of sugar deoxyriboses in the binding polarity of NC. Nucleic Acids Res. 2011;39:3903–3916. doi: 10.1093/nar/gkq1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernacchi S., Stoylov S., Mély Y. HIV-1 nucleocapsid protein activates transient melting of least stable parts of the secondary structure of TAR and its complementary sequence. J. Mol. Biol. 2002;317:385–399. doi: 10.1006/jmbi.2002.5429. [DOI] [PubMed] [Google Scholar]
- 45.Cosa G., Harbron E.J., Barbara P.F. Secondary structure and secondary structure dynamics of DNA hairpins complexed with HIV-1 NC protein. Biophys. J. 2004;87:2759–2767. doi: 10.1529/biophysj.104.043083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zargarian L., Kanevsky I., Mauffret O. Structural and dynamic characterization of the upper part of the HIV-1 cTAR DNA hairpin. Nucleic Acids Res. 2009;37:4043–4054. doi: 10.1093/nar/gkp297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Summers M.F., Henderson L.E., Hare D.R. Nucleocapsid zinc fingers detected in retroviruses: EXAFS studies of intact viruses and the solution-state structure of the nucleocapsid protein from HIV-1. Protein Sci. 1992;1:563–574. doi: 10.1002/pro.5560010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Guzman R.N., Wu Z.R., Summers M.F. Structure of the HIV-1 nucleocapsid protein bound to the SL3 psi-RNA recognition element. Science. 1998;279:384–388. doi: 10.1126/science.279.5349.384. [DOI] [PubMed] [Google Scholar]
- 49.Amarasinghe G.K., Zhou J., Summers M.F. Stem-loop SL4 of the HIV-1 psi RNA packaging signal exhibits weak affinity for the nucleocapsid protein. structural studies and implications for genome recognition. J. Mol. Biol. 2001;314:961–970. doi: 10.1006/jmbi.2000.5182. [DOI] [PubMed] [Google Scholar]
- 50.Fisher R.J., Fivash M.J., Rein A. Complex interactions of HIV-1 nucleocapsid protein with oligonucleotides. Nucleic Acids Res. 2006;34:472–484. doi: 10.1093/nar/gkj442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duss O., Yulikov M., Allain F.H. EPR-aided approach for solution structure determination of large RNAs or protein-RNA complexes. Nat. Commun. 2014;5:3669. doi: 10.1038/ncomms4669. [DOI] [PubMed] [Google Scholar]
- 52.Wang H., Musier-Forsyth K., Barbara P.F. Single-molecule spectroscopic study of dynamic nanoscale DNA bending behavior of HIV-1 nucleocapsid protein. J. Phys. Chem. B. 2013;117:4183–4196. doi: 10.1021/jp3018259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan B., Weidemaier K., Musier-Forsyth K. Intra-tRNA distance measurements for nucleocapsid protein-dependent tRNA unwinding during priming of HIV reverse transcription. Proc. Natl. Acad. Sci. USA. 1999;96:459–464. doi: 10.1073/pnas.96.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong M.K., Harbron E.J., Musier-Forsyth K. Nucleic acid conformational changes essential for HIV-1 nucleocapsid protein-mediated inhibition of self-priming in minus-strand transfer. J. Mol. Biol. 2003;325:1–10. doi: 10.1016/s0022-2836(02)01177-4. [DOI] [PubMed] [Google Scholar]
- 55.Liu H.W., Cosa G., Barbara P.F. Single-molecule FRET studies of important intermediates in the nucleocapsid-protein-chaperoned minus-strand transfer step in HIV-1 reverse transcription. Biophys. J. 2005;89:3470–3479. doi: 10.1529/biophysj.105.065326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu H.W., Zeng Y., Barbara P.F. Insights on the role of nucleic acid/protein interactions in chaperoned nucleic acid rearrangements of HIV-1 reverse transcription. Proc. Natl. Acad. Sci. USA. 2007;104:5261–5267. doi: 10.1073/pnas.0700166104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng Y., Liu H.W., Barbara P.F. Probing nucleation, reverse annealing, and chaperone function along the reaction path of HIV-1 single-strand transfer. Proc. Natl. Acad. Sci. USA. 2007;104:12651–12656. doi: 10.1073/pnas.0700350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H., Yeh Y.S., Barbara P.F. HIV-1 nucleocapsid protein bends double-stranded nucleic acids. J. Am. Chem. Soc. 2009;131:15534–15543. doi: 10.1021/ja9070046. [DOI] [PubMed] [Google Scholar]
- 59.Beltz H., Piémont E., Mély Y. Role of the structure of the top half of HIV-1 cTAR DNA on the nucleic acid destabilizing activity of the nucleocapsid protein NCp7. J. Mol. Biol. 2004;338:711–723. doi: 10.1016/j.jmb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 60.Beltz H., Azoulay J., Mély Y. Impact of the terminal bulges of HIV-1 cTAR DNA on its stability and the destabilizing activity of the nucleocapsid protein NCp7. J. Mol. Biol. 2003;328:95–108. doi: 10.1016/s0022-2836(03)00244-4. [DOI] [PubMed] [Google Scholar]
- 61.Godet J., Mély Y. Biophysical studies of the nucleic acid chaperone properties of the HIV-1 nucleocapsid protein. RNA Biol. 2010;7:687–699. doi: 10.4161/rna.7.6.13616. [DOI] [PubMed] [Google Scholar]
- 62.Gopich I.V., Szabo A. Single-Molecule Biophysics. John Wiley & Sons; Hoboken, NJ: 2011. Theory of single-molecule FRET efficiency histograms; pp. 245–297. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


