Abstract
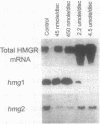
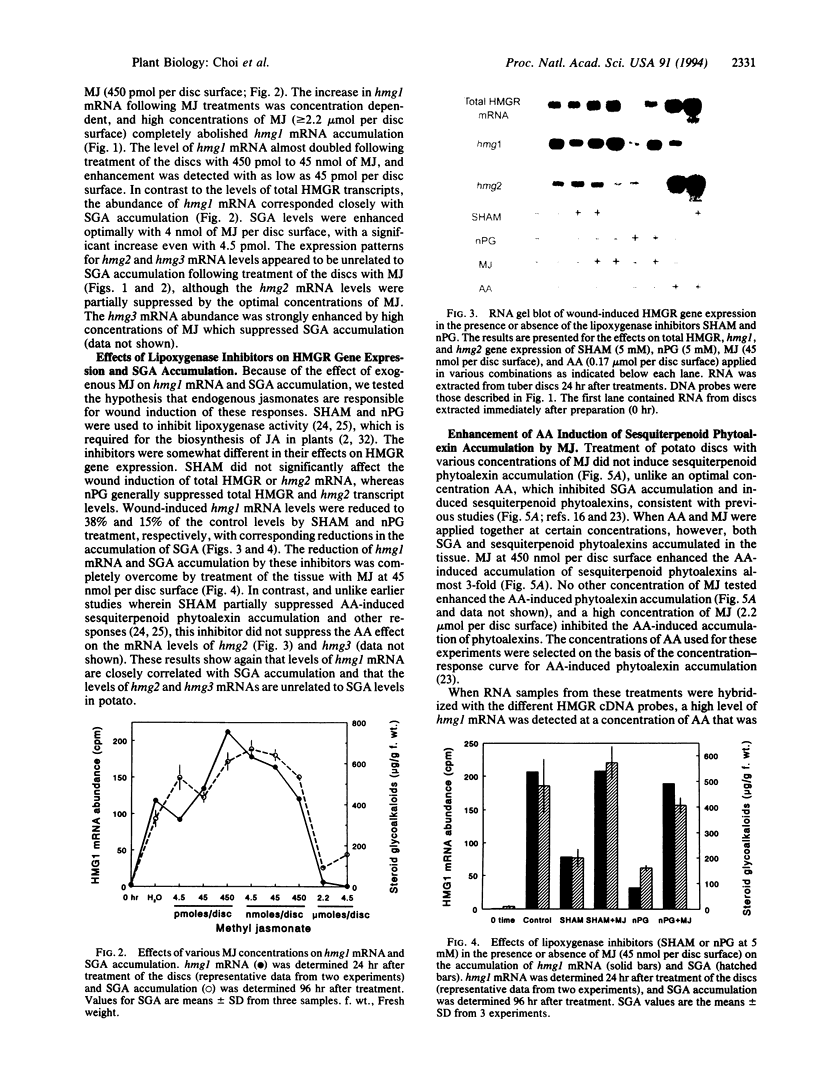
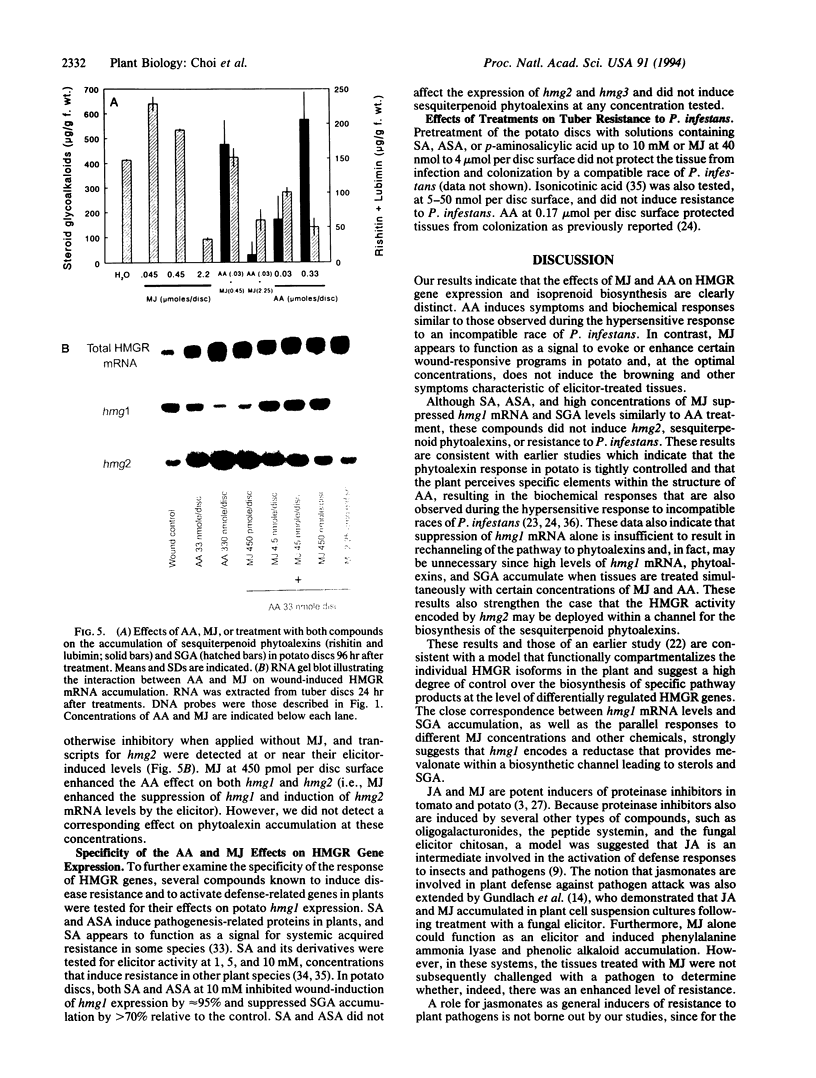
Induction of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR; EC 1.1.1.34) is essential for the synthesis of steroid derivatives and sesquiterpenoid phytoalexins in solanaceous plants following mechanical injury or pathogen infection. Gene-specific probes corresponding to different HMGR genes (hmg1 and hmg2) were used to study HMGR expression in potato tissue following treatment with methyl jasmonate, a lipoxygenase product of linolenic acid, or arachidonic acid, an elicitor present in the lipids of the potato late blight fungus Phytophthora infestans. Treatment of potato discs (2.2 cm in diameter) with low concentrations (0.45-45 nmol per disc surface) of methyl jasmonate nearly doubled the wound-induced accumulation of hmg1 transcripts and steroid-glycoalkaloid (SGA) accumulation, reduced the abundance of hmg2 transcripts, and did not induce phytoalexins. High concentrations of methyl jasmonate (2-4.5 mol per disc surface) suppressed hmg1 mRNA and SGA accumulation but did not affect hmg2 mRNA abundance or induce phytoalexins. In contrast, arachidonate treatment strongly suppressed hmg1 and strongly induced hmg2 mRNA in a concentration-dependent manner. There was a corresponding suppression of SGA accumulation and an induction of sesquiterpene phytoalexin accumulation by this elicitor. Lipoxygenase inhibitors reduced the wound-induced accumulation of hmg1 transcripts and suppressed SGA levels, effects that were overcome by exogenous methyl jasmonate (45 nmol per disc surface). The results (i) suggest that methyl jasmonate can function as a signal for hmg1 expression and SGA induction following wounding and (ii) indicate that the arachidonate- and jasmonate-response pathways are distinct in relation to HMGR gene expression and isoprenoid product accumulation. The results also are consistent with placement of the HMGR activities encoded by hmg1 and hmg2 within discrete steroid and sesquiterpenoid biosynthetic channels.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bostock R. M., Kuc J. A., Laine R. A. Eicosapentaenoic and Arachidonic Acids from Phytophthora infestans Elicit Fungitoxic Sesquiterpenes in the Potato. Science. 1981 Apr 3;212(4490):67–69. doi: 10.1126/science.212.4490.67. [DOI] [PubMed] [Google Scholar]
- Bostock R. M., Yamamoto H., Choi D., Ricker K. E., Ward B. L. Rapid stimulation of 5-lipoxygenase activity in potato by the fungal elicitor arachidonic Acid. Plant Physiol. 1992 Nov;100(3):1448–1456. doi: 10.1104/pp.100.3.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadle L. S., Stelzig D. A., Harper K. L., Young R. J. Thin-layer chromatographic system for identification and quantitation of potato tuber glycoalkaloids. J Agric Food Chem. 1978 Nov-Dec;26(6):1453–1454. doi: 10.1021/jf60220a033. [DOI] [PubMed] [Google Scholar]
- Chappell J., Vonlanken C., Vögeli U. Elicitor-inducible 3-hydroxy-3-methylglutaryl coenzyme a reductase activity is required for sesquiterpene accumulation in tobacco cell suspension cultures. Plant Physiol. 1991 Oct;97(2):693–698. doi: 10.1104/pp.97.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D., Ward B. L., Bostock R. M. Differential induction and suppression of potato 3-hydroxy-3-methylglutaryl coenzyme A reductase genes in response to Phytophthora infestans and to its elicitor arachidonic acid. Plant Cell. 1992 Oct;4(10):1333–1344. doi: 10.1105/tpc.4.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Tierney M. L., Mullet J. E. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich H., Kutchan T. M., Zenk M. H. The jasmonate precursor, 12-oxo-phytodienoic acid, induces phytoalexin synthesis in Petroselinum crispum cell cultures. FEBS Lett. 1992 Aug 31;309(1):33–36. doi: 10.1016/0014-5793(92)80733-w. [DOI] [PubMed] [Google Scholar]
- Farmer E. E., Johnson R. R., Ryan C. A. Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic Acid. Plant Physiol. 1992 Mar;98(3):995–1002. doi: 10.1104/pp.98.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Octadecanoid Precursors of Jasmonic Acid Activate the Synthesis of Wound-Inducible Proteinase Inhibitors. Plant Cell. 1992 Feb;4(2):129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Grimes H. D. Induction of soybean vegetative storage proteins and anthocyanins by low-level atmospheric methyl jasmonate. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6745–6749. doi: 10.1073/pnas.88.15.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach H., Müller M. J., Kutchan T. M., Zenk M. H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Gardner H. W. Oxylipin pathway to jasmonates: biochemistry and biological significance. Biochim Biophys Acta. 1992 Nov 11;1165(1):1–18. doi: 10.1016/0005-2760(92)90069-8. [DOI] [PubMed] [Google Scholar]
- Hildmann T., Ebneth M., Peña-Cortés H., Sánchez-Serrano J. J., Willmitzer L., Prat S. General roles of abscisic and jasmonic acids in gene activation as a result of mechanical wounding. Plant Cell. 1992 Sep;4(9):1157–1170. doi: 10.1105/tpc.4.9.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason H. S., Dewald D. B., Creelman R. A., Mullet J. E. Coregulation of soybean vegetative storage protein gene expression by methyl jasmonate and soluble sugars. Plant Physiol. 1992 Mar;98(3):859–867. doi: 10.1104/pp.98.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisig C. L., Kuć J. A. Arachidonic acid-related elicitors of the hypersensitive response in potato and enhancement of their activities by glucans from Phytophthora infestans (Mont.) deBary. Arch Biochem Biophys. 1985 Jan;236(1):379–389. doi: 10.1016/0003-9861(85)90638-1. [DOI] [PubMed] [Google Scholar]
- Preisig C. L., Kuć J. A. Inhibition by Salicylhydroxamic Acid, BW755C, Eicosatetraynoic Acid, and Disulfiram of Hypersensitive Resistance Elicited by Arachidonic Acid or Poly-l-Lysine in Potato Tuber. Plant Physiol. 1987 Jul;84(3):891–894. doi: 10.1104/pp.84.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J. B., Hammerschmidt R., Zook M. N. Systemic Induction of Salicylic Acid Accumulation in Cucumber after Inoculation with Pseudomonas syringae pv syringae. Plant Physiol. 1991 Dec;97(4):1342–1347. doi: 10.1104/pp.97.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987 Sep 4;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Staswick P. E., Huang J. F., Rhee Y. Nitrogen and methyl jasmonate induction of soybean vegetative storage protein genes. Plant Physiol. 1991 May;96(1):130–136. doi: 10.1104/pp.96.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Jasmonate, genes, and fragrant signals. Plant Physiol. 1992 Jul;99(3):804–807. doi: 10.1104/pp.99.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E., Su W., Howell S. H. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6837–6840. doi: 10.1073/pnas.89.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stermer B. A., Bostock R. M. Involvement of 3-hydroxy-3-methylglutaryl coenzyme a reductase in the regulation of sesquiterpenoid phytoalexin synthesis in potato. Plant Physiol. 1987 Jun;84(2):404–408. doi: 10.1104/pp.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjamos E. C., Kucacute J. A. Inhibition of steroid glycoalkaloid accumulation by arachidonic and eicosapentaenoic acids in potato. Science. 1982 Aug 6;217(4559):542–544. doi: 10.1126/science.217.4559.542. [DOI] [PubMed] [Google Scholar]
- Tranbarger T. J., Franceschi V. R., Hildebrand D. F., Grimes H. D. The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell. 1991 Sep;3(9):973–987. doi: 10.1105/tpc.3.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vögeli U., Chappell J. Induction of sesquiterpene cyclase and suppression of squalene synthetase activities in plant cell cultures treated with fungal elicitor. Plant Physiol. 1988 Dec;88(4):1291–1296. doi: 10.1104/pp.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. R., Uknes S. J., Williams S. C., Dincher S. S., Wiederhold D. L., Alexander D. C., Ahl-Goy P., Metraux J. P., Ryals J. A. Coordinate Gene Activity in Response to Agents That Induce Systemic Acquired Resistance. Plant Cell. 1991 Oct;3(10):1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N., Silverman P., Wilson T. M., Kleier D. A., Raskin I. Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell. 1991 Aug;3(8):809–818. doi: 10.1105/tpc.3.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]