Abstract
Background
In the UK, the care of young people with diabetes has focused predominantly on type 1 diabetes (T1D). However, young-onset T2D has become increasingly prevalent. At present, it is unclear which type of diabetes represents the more adverse phenotype to develop complications. This study aims to determine the complication burden and its predictive factors in young-onset T2D compared with T1D.
Methods
A cross-sectional study using a hospital diabetes register to identify patients with young-onset T2D and T1D. Young-onset T2D was defined as age of diagnosis below 40 years. The T1D cohort with a similar age of diagnosis was used as a comparator. Data from the last clinic visit was used for analysis. Clinical characteristics and diabetes complications were evaluated at diabetes durations <10, 10–20, and >20 years. Predictive factors for diabetes complications (age, sex, glycated hemoglobin, creatinine, diabetes duration, hypertension, dyslipidemia, and body mass index >25) were determined by logistic regression analysis.
Results
Data were collected on 1287 patients, of which 760 and 527 had T1D and T2D, respectively. In all diabetes durations, the T2D cohort had an older age of onset (p<0.0005) with a higher prevalence of obesity, hypertension, and dyslipidemia (all p<0.0005) while glycemic control was similar in both groups. Cardiovascular disease (p<0.005) and neuropathy (p<0.05) were more prevalent in the young-onset T2D cohort in all diabetes durations. There was no difference in retinopathy. Cardiovascular disease was predominantly due to ischemic heart disease. Stroke and peripheral vascular disease became significantly higher in T2D after 20 years duration. After controlling for traditional risk factors, young-onset T2D was an independent predictor for cardiovascular disease (p<0.005) and neuropathy (p<0.05) but not for retinopathy.
Conclusions
Young-onset T2D is a more aggressive phenotype than T1D to develop diabetes complications, particularly for ischemic heart disease and neuropathy.
Keywords: Young Adult, Type 1, Type 2, Complication(s)
Key messages.
Although type 1 diabetes is the most common form of diabetes diagnosed below age 40, type 2 diabetes has become increasingly prevalent among the young population in the UK.
It remains unknown whether there is any difference in the propensity to develop complications between these two types of young-onset diabetes.
Young-onset type 2 diabetes represents the more adverse phenotype with a worse cardiometabolic profile and greater risk to develop microvascular and macrovascular complications than type 1 diabetes.
Background
Type 2 diabetes (T2D) in young adults has become increasingly prevalent. The proportion of newly diagnosed patients with T2D below the age of 40 years has increased significantly from 5.9% to 12.4% between 1991 and 2010 in the UK.1 It heralds a prolonged lifetime exposure to an adverse diabetic milieu with complications occurring at an economically productive young age.
In the UK, the clinical care of young people with diabetes has focused predominantly on T1D for a variety of reasons. First, it is the most common form of diabetes in this age group. Second, T1D is often perceived as more severe than T2D given its florid clinical presentation and the absolute need for life-saving insulin treatment from diagnosis. This perception is further strengthened by the adverse mortality outcome for young people with T1D.2 Third and most importantly, intensive intervention has been shown to improve the complication outcomes in this young cohort.3
Given the susceptibility of young-onset T2D for premature complications,4 it will be clinically pertinent to determine which type of diabetes is more deleterious when its onset occurs at a young age. There is evidence to suggest that young-onset T2D has worse complication and prognostic outcomes compared with patients with T1D with a similar age of diagnosis.5–8 At present, it is unknown whether the findings from these studies also apply to a UK population. The objective of this study is to address this knowledge gap, which may challenge the current focus in the care of young people with diabetes.
Methods
Study population
The study population was identified from a hospital electronic diabetes register which recorded clinical details of patients with T1D and T2D who attended clinics at the Northern General Hospital and Royal Hallamshire Hospital in Sheffield, UK. Clinical data were routinely entered into the database each time the patients were seen in these clinics. This diabetic population was referred by general practitioners predominantly to improve poor diabetes control and for optimization of risk factor management. Other reasons include those on hospital follow-up for chronic diabetes-related complications and newly diagnosed diabetes identified from hospital admissions.
Definition of study population
Young-onset T2D was defined as age of diagnosis below 40 years as stipulated in the National Institute for Health and Care Excellence (NICE) guideline.9 Since the youngest age of diagnosis for T2D was 15 years, the age of diabetes onset between 15 and 39 years was chosen to define this cohort. Patients with T1D with a similar age of diagnosis were used as a comparator. This is to minimize the bias associated with difference in the age of diabetes onset. T2D was defined as those whose diabetes was controlled by oral hypoglycemic agents without the need for continuous insulin therapy within the first year of diagnosis and/or with negative autoantibody status (glutamic acid decarboxylase, islet cell antibody, and islet-antigen-2). T1D was defined by the absolute requirement for insulin therapy within 1 year of diagnosis and/or with positive autoantibody status. Those with diabetes etiology due to maturity onset diabetes of the young, gestational diabetes and secondary diabetes were excluded.
Data collection
This was a cross-sectional study focused on the hospital diabetes population. Data were extracted from the diabetes register in 2009 as part of the service evaluation exercise. The most recent data obtained from the last clinic visit were used for analysis. Patients with incomplete data were excluded. For each patient, complete data on clinical characteristics, laboratory tests (glycated hemoglobin (HbA1c), full lipid profile (total cholesterol, high-density lipoprotein (HDL), and triglyceride), and kidney function) and diabetes-related complications pertaining to microvascular disease (retinopathy and peripheral neuropathy) and cardiovascular disease (CVD; ischemic heart disease, peripheral vascular disease, and stroke) were collected. These medical conditions were entered into the database using the International Classification of Diseases 10 and Read codes. At the time of data extraction, microalbuminuria was not routinely screened in patients with T2D; hence, these data were not included in the study analysis. However, screening for other microvascular complications (retinopathy and neuropathy) was routinely performed in all patients. Apart from age of onset, comparing the outcomes of T1D and T2D is often confounded by the differences in disease duration. To minimize this bias and to assess the impact of diabetes duration, study subjects were stratified into three categories of diabetes duration, namely, <10, 10–20, and >20 years. Clinical characteristics and the diabetes complication burden were analyzed in each group.
Retinopathy was detected from digital photography which was performed as part of the city-wide diabetes eye screening program. Peripheral neuropathy was detected by clinical examination (monofilament test, vibration sense), nerve conduction test, or presence of sensory symptoms. Ischemic heart disease was defined as myocardial infarction (ST and non-ST elevation), stable and unstable angina and/or coronary intervention. Peripheral vascular disease was defined as a history of leg amputation for ischemia, peripheral revascularization, or ankle-brachial index <0.9. Stroke was defined as ischemic stroke proven on a CT scan or history of transient ischemic attack. Hypertension was defined as blood pressure >140/80 mm Hg or >130/80 mm Hg with diabetic complications or on antihypertensive treatment while dyslipidemia was defined as fasting triglyceride >1.7 mmol/L and/or HDL <1.03 mmol/L for male or <1.29 mmol/L for female or on specific treatment for this condition. Overweight and obesity were defined by body mass index (BMI) greater than 25 kg/m2.
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Science V.16.0 for Windows (SPSS Inc, Chicago, Illinois, USA). Continuous data were expressed as mean±SD, while categorical data were expressed as percentage. The normality of continuous data distribution was assessed with the Kolmogorov-Smirnov test to determine the appropriate method for parametric or non-parametric statistical analysis. Continuous data were analyzed by Student's t test or the Mann Whitney U test, while the χ2 test was used to compare categorical variables between two groups. The χ2 value for the trend was used to determine the significance of trends in frequency across different groups. Logistic regression analysis was conducted to estimate the odds of diabetes complications associated with the type of diabetes (type 2 vs 1), controlled for variables found to be significant in the univariate analysis. The independent variables used were age, sex, HbA1c, creatinine, diabetes duration, hypertension, dyslipidemia, and BMI>25 kg/m2. A p value of <0.05 was considered significant.
Results
Subject characteristics
There were 2681 patients with age of diagnosis below 40 years for both types of diabetes, of which 1394 had an incomplete data set and were excluded from the analysis. A total of 1287 patients with a complete data set were included in this analysis, of which 760 and 527 had T1D and T2D, respectively. The clinical characteristics of the study subjects are shown in table 1. Overall, patients with T1D had a younger age of diabetes onset and longer disease duration. The mean age of diagnosis was 25.8±6.9 and 32.5±5.9 years (p<0.0005) while diabetes duration was 20.4±12.9 and 15.0±10.3 years (p<0.0005) for T1D and T2D, respectively. There was an excess of males, particularly in the T1D cohort (60.5% vs 50.7%, p<0.005). Glycemic control was similar but suboptimal (>7% (53 mmol/mol)) in both groups in all categories of diabetes duration.
Table 1.
Clinical characteristics of study subjects
| Diabetes duration (years) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–10 |
10–20 |
>20 |
|||||||
| Type 1 (n=197) | Type 2 (n=211) | p Value | Type 1 (n=190) | Type 2 (n=151) | p Value | Type 1 (n=373) | Type 2 (n=165) | p Value | |
| Current age (years) | 33.9 (7.7) | 39.5 (6.3) | <0.0005 | 41.6 (6.9) | 48.4 (6.4) | <0.0005 | 57.8 (10.7) | 61.4 (8.3) | <0.0005 |
| Age of diagnosis (years) | 27.5 (7.3) | 32.6 (5.5) | <0.0005 | 25.9 (6.5) | 32.7 (5.3) | <0.0005 | 24.9 (6.8) | 32.3 (6.8) | <0.0005 |
| DM duration (years) | 5.2 (2.6) | 5.6 (2.4) | NS | 14.4 (2.8) | 14.2 (2.9) | NS | 31.4 (8.4) | 27.6 (7.4) | <0.0005 |
| Systolic BP (mm Hg) | 127.4 (16.9) | 133.3 (19.5) | 0.001 | 130.4 (17.1) | 138.1 (19.1) | <0.0005 | 138.8 (17.5) | 141.2 (20.6) | NS |
| Diastolic BP (mm Hg) | 75.0 (12.4) | 79.8 (10.8) | <0.0005 | 77.2 (9.8) | 76.8 (11.8) | NS | 73.7 (9.8) | 71.3 (8.7) | 0.005 |
| BMI (kg/m2) | 26.6 (5.0) | 34.7 (8.6) | <0.0005 | 27.6 (4.8) | 34.9 (7.1) | <0.0005 | 27.6 (4.7) | 33.4 (6.9) | <0.0005 |
| HbA1c (%) (mmol/mol) |
8.7 (1.9) 72 (21) |
8.8 (2.0) 74 (22) |
NS | 8.9 (1.8) 74 (18) |
9.2 (2.0) 77 (21) |
NS | 8.6 (1.3) 70 (14) |
8.9 (1.7) 74 (18) |
NS |
| Creatinine (µmol/L) | 77.3 (55.6) | 73.0 (20.1) | NS | 80.6 (26.9) | 80.7 (48.7) | NS | 94.7 (69.1) | 103.8 (60.9) | NS |
| Total cholesterol (mmol/L) | 4.8 (1.1) | 4.7 (1.5) | 0.002 | 4.7 (1.0) | 4.4 (1.4) | <0.0005 | 4.4 (0.9) | 4.1 (0.9) | <0.0005 |
| HDL (mmol/L) | 1.7 (0.4) | 1.0 (0.3) | <0.0005 | 1.5 (0.5) | 1.0 (0.3) | <0.0005 | 1.6 (0.5) | 1.2 (0.3) | <0.0005 |
| Triglyceride (mmol/L) | 1.7 (4.3) | 2.8 (3.3) | <0.0005 | 1.3 (0.9) | 2.7 (3.4) | <0.0005 | 1.1 (0.9) | 2.0 (1.3) | <0.0005 |
| BMI>25 kg/m2 (%) | 57.4 | 90.5 | <0.0005 | 70.0 | 96.0 | <0.0005 | 70.0 | 90.9 | <0.0005 |
| Hypertension (%) | 26.9 | 55.9 | <0.0005 | 43.2 | 78.1 | <0.0005 | 71.0 | 87.3 | <0.0005 |
| Dyslipidemia (%) | 36.5 | 80.6 | <0.0005 | 31.1 | 82.1 | <0.0005 | 23.3 | 71.5 | <0.0005 |
| Risk factors >2 (%) | 37.6 | 82.5 | <0.0005 | 46.8 | 90.7 | <0.0005 | 58.4 | 92.1 | <0.0005 |
| Antihypertensive (%) | 10.6 | 38.3 | <0.0005 | 28.6 | 67.1 | <0.0005 | 57.7 | 75.9 | <0.0005 |
| Statin (%) | 24.5 | 59.0 | <0.0005 | 48.1 | 73.5 | <0.0005 | 76.6 | 73.1 | NS |
BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; NS, not significant.
There were some differences in the treatment between young-onset T1D and T2D. Overall, the use of antihypertensive and statin medications was significantly higher among the T2D cohort, apart from those with diabetes duration >20 years, where the statin treatment was similar (table 1). As expected, the proportion of patients with T2D on insulin treatment increased with disease duration with almost all the patients being insulin-dependent after 20 years duration (<10 vs 10–20 vs >20 years; 44.9% vs 76.8% vs 93.1%, p<0.0005 for trend).
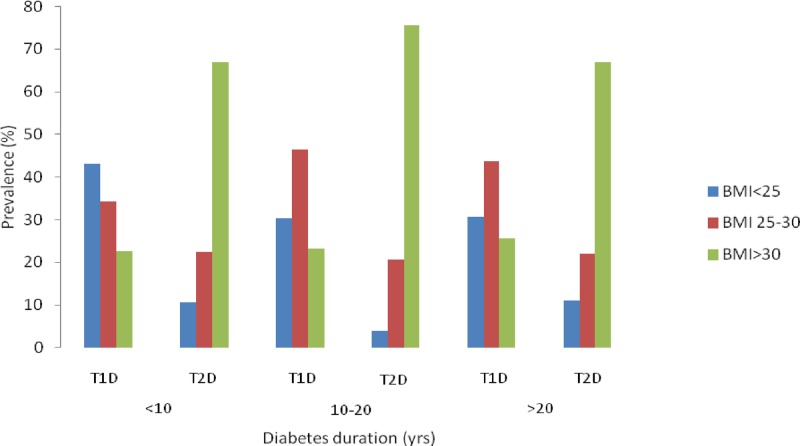
The importance of obesity in young-onset T2D was illustrated by the progressive rise in its prevalence with increasing BMI regardless of diabetes duration, in contrast to T1D (p<0.0005 vs T1D for all diabetes durations; figure 1).
Figure 1.
Distribution of body mass index (BMI) between young-onset type 1 diabetes (T1D) and T2D at different diabetes durations.
Cardiovascular risk factors
An adverse cardiovascular risk profile was observed among the T2D cohort in all categories of diabetes duration with a higher prevalence of obesity, hypertension, dyslipidemia, and clustering of cardiovascular risk factors (obesity, hypertension, dyslipidemia) compared with T1D (p<0.0005 vs T1D for all risk factors; table 1). To explore whether these risk factors had a significant presence in the early stages of the diabetes condition, the cardiovascular risk profile was analyzed within 5 years of diagnosis. Clinical data were available for 81 and 72 patients with T1D and T2D, respectively. Despite the short diabetes duration (T1D vs T2D; 2.5±1.2 vs 2.7±1.2 years, p=NS), the prevalence of obesity (T1D vs T2D; 50.6 vs 91.7%, p<0.0005), hypertension (T1D vs T2D; 27.2 vs 44.4%, p=0.029), dyslipidemia (T1D vs T2D; 38.3 vs 76.4%, p<0.0005), and risk factor clustering (T1D vs T2D; 38.3 vs 80.6%, p<0.0005) were significantly greater in T2D. Among those who were recently diagnosed (diabetes duration <1 year), the adverse cardiovascular risk profile was again more prevalent in young-onset T2D (T1D (n=19) vs T2D (n=14): risk factor clustering; 42.1 vs 85.7%, p=0.015).
Obesity increased the burden of hypertension and dyslipidemia in both types of diabetes (p<0.0005), but to a greater extent in the T2D cohort (p<0.005 vs T1D for hypertension and dyslipidemia with BMI>25 kg/m2; table 2). More patients with T2D had dyslipidemia regardless of the BMI. In contrast, there was no difference in the prevalence of hypertension when the BMI was less than 25 kg/m2.
Table 2.
The differing impact of increased BMI on cardiovascular risk factors in young-onset type 1and type 2 diabetes
| Type 1 diabetes |
Type 2 diabetes |
|||||
|---|---|---|---|---|---|---|
| BMI<25 kg/m2 (n=259) | BMI>25 kg/m2 (n=501) | p Value | BMI<25 kg/m2 (n=46) | BMI>25 kg/m2 (n=481) | p Value | |
| Hypertension (%) | 36.7 | 60.9 | <0.0005 | 39.1* | 75.3† | <0.0005 |
| Dyslipidemia (%) | 19.3 | 33.5 | <0.0005 | 56.5‡ | 80.2§ | 0.001 |
*p=NS versus type 1 diabetes with BMI<25 kg/m2.
†p<0.005 versus type 1 diabetes with BMI>25 kg/m2.
‡p<0.005 versus type 1 diabetes with BMI<25 kg/m2.
§p<0.005 versus type 1 diabetes with BMI>25 kg/m2.
BMI, body mass index; NS, not significant.
Diabetes complications
The relationship between diabetes duration and CVD and microvascular complications is shown in table 3. As expected, the complication burden increased with disease duration in both types of diabetes. Within 10 years of diagnosis, CVD and neuropathy were significantly higher in the T2D cohort and this trend persisted for the remainder of diabetes duration. The burden of CVD was driven predominantly by ischemic heart disease. In contrast, retinopathy was similar in both groups across all diabetes durations and this differential burden in neuropathy and retinopathy complications occurred despite similar glycemic control. Stroke and peripheral vascular disease became significantly higher only among the T2D cohort after 20 years duration. Compared with T1D, the CVD and neuropathic complications occurred earlier in the diabetic disease process in T2D. For an equivalent prevalence of CVD complication (∼14%), the T2D cohort was much younger (T2D vs T1D; 48.4 vs 57.8 years, p<0.0005) and had shorter diabetes duration (T2D vs T1D; 14.2 vs 31.4 years, p<0.0005). For neuropathy (prevalence ∼12–15%), the T2D cohort had a much shorter diabetes duration (T2D vs T1D; 5.6 vs 14.2 years, p<0.0005).
Table 3.
Prevalence of diabetes-related complications as stratified by diabetes duration
| Diabetes duration (years) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–10 |
10–20 |
>20 |
|||||||
| Type 1 | Type 2 | p Value | Type 1 | Type 2 | p Value | Type 1 | Type 2 | p Value | |
| IHD (%) | 0 | 5.7 | <0.0005 | 2.6 | 9.9 | 0.005 | 9.1 | 28.5 | <0.0005 |
| Stroke (%) | 0.5 | 1.4 | NS | 1.1 | 4.0 | NS | 2.7 | 7.9 | 0.01 |
| PVD (%) | 0.5 | 1.4 | NS | 1.6 | 1.3 | NS | 4.3 | 13.3 | <0.0005 |
| CVD (%) | 1.0 | 8.1 | 0.001 | 4.7 | 14.6 | 0.002 | 13.7 | 38.2 | <0.0005 |
| Retinopathy (%) | 1.5 | 3.8 | NS | 28.9 | 29.1 | NS | 53.9 | 60.0 | NS |
| Neuropathy (%) | 6.1 | 12.3 | 0.04 | 15.3 | 29.1 | 0.002 | 35.9 | 52.1 | 0.001 |
CVD, cardiovascular disease; IHD, ischemic heart disease; NS, not significant; PVD, peripheral vascular disease.
Predictors of diabetes complications
The relationship between independent risk factors for the development of diabetes complications was analyzed by logistic regression analysis (table 4). Age, diabetes duration, creatinine, hypertension, dyslipidemia, and obesity were significant predictors for CVD while for microvascular complications, the significant factors were age, male sex (for neuropathy), diabetes duration, creatinine, HbA1c, hypertension, and obesity. Interestingly, cardiovascular risk factors (obesity, hypertension, and dyslipidemia) were more strongly associated with neuropathy than retinopathy. After controlling for these variables, T2D was found to be a significant independent predictor for CVD and neuropathy but not for retinopathy. The association was strongest for CVD.
Table 4.
Logistic regression analysis for predictors of diabetes complications
| Cardiovascular disease |
Retinopathy |
Neuropathy |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | 1.08 (1.07 to 1.10) | <0.0005 | 1.08 (1.07 to 1.09) | <0.0005 | 1.08 (1.06 to 1.09) | <0.0005 |
| Sex | 1.04 (0.75 to 1.45) | NS | 1.10 (0.87 to 1.39) | NS | 1.35 (1.05 to 1.75) | 0.021 |
| Diabetes duration | 1.06 (1.05 to 1.07) | <0.0005 | 1.10 (1.08 to 1.11) | <0.0005 | 1.06 (1.05 to 1.08) | <0.0005 |
| HbA1c | 1.04 (0.95 to 1.14) | NS | 1.11 (1.03 to 1.18) | 0.003 | 1.15 (1.07 to 1.23) | <0.0005 |
| Creatinine | 1.006 (1.003 to 1.010) | <0.0005 | 1.016 (1.012 to 1.021) | <0.0005 | 1.016 (1.012 to 1.021) | <0.0005 |
| Hypertension | 7.75 (4.50 to 13.34) | <0.0005 | 3.22 (2.46 to 4.22) | <0.0005 | 3.36 (2.50 to 4.52) | <0.0005 |
| Dyslipidemia | 2.15 (1.53 to 3.03) | <0.0005 | 0.91 (0.72 to 1.15) | NS | 1.24 (0.96 to 1.59) | NS |
| BMI>25 kg/m2 | 3.28 (1.90 to 5.68) | <0.0005 | 1.39 (1.04 to 1.86) | 0.026 | 1.50 (1.09 to 2.06) | 0.012 |
| Type of diabetes (type 2 vs type 1) | 2.04 (1.26 to 3.28)* | 0.004 | 1.03 (0.74 to 1.44)† | NS | 1.47 (1.04 to 2.08)‡ | 0.028 |
*Adjusted for age, diabetes duration, creatinine, hypertension, dyslipidemia, and BMI>25 kg/m2.
†Adjusted for age, diabetes duration, creatinine, hypertension, BMI>25 kg/m2, and HbA1c.
‡Adjusted for age, sex, diabetes duration, creatinine, hypertension, BMI>25 kg/m2, and HbA1c.
BMI, body mass index; HbA1c, glycated hemoglobin; NS, not significant.
Discussion
The principal concern with diabetes diagnosed at a young age is the development of complications at an earlier stage of life. Comparative analysis of complication characteristics between the increasingly prevalent young-onset T2D and T1D, the most common form of diabetes in the young, is clinically relevant. This is the first study in the UK to specifically assess the burden of diabetes complications and its predictive risk factors in these two cohorts. The atherogenic milieu was present early in the natural history of young-onset T2D and obesity incurred a greater burden of cardiovascular risk factors in this cohort. CVD and neuropathy were substantially higher in T2D and occurred at an earlier stage than T1D. Ischemic heart disease accounted for the majority of CVD. Despite the similar glycemic control and diabetes duration, neuropathic complication was higher in T2D, in contrast to retinopathy, which was similar in both types of diabetes. After controlling for traditional risk factors, young-onset T2D emerged as an independent predictive factor for CVD and neuropathy, but not for retinopathy.
Recent population-based studies from Australia,5 Canada,6 the USA,7 and Hong Kong8 analyzed the differential burden of diabetes-related complications among young-onset T1D and T2D with the age of diabetes onset ranging from children to young adults. Constantino et al5 showed that young adults with T2D (age of diagnosis 25.6 years) had a higher CVD (ischemic heart disease and stroke), albuminuria, and neuropathy with a 2-fold increase in mortality predominantly from CVD but no difference in retinopathy. Dart et al6 and Jaiswal et al7 showed that young-onset T2D with age of diagnosis between 11 and 14 years had higher rates of neuropathy and albuminuria with no significant difference in retinopathy.6 In a Chinese population, Luk et al8 showed that CVD (coronary heart disease and stroke), nephropathy (albuminuria and end-stage renal disease), neuropathy, and retinopathy complications were higher in young-onset T2D (age of diagnosis 33 years). Taken altogether, these observations demonstrated the consistent pattern of a higher burden of CVD, neuropathy, and nephropathy in young-onset T2D compared with T1D with probable equivalence for retinopathy. With the exception of nephropathy due to lack of albuminuria data, the findings from this study are consistent with the published literature with a similar observation in young adults with T2D (age of diagnosis 32.5 years) in a UK population. It appears that young-onset T2D is universally a more adverse phenotype compared with T1D.
There is other evidence to support the hypothesis of young-onset T2D being the less favorable phenotype prognostically. In a Swedish study of patients with diabetes aged 15–34 years compared with the general population, the standardized mortality ratio was higher for T2D than for the T1D cohort (2.9 vs 1.8).10 The probability for complication-free survival was significantly lower for youths aged up to 18 years with T2D versus T1D.6 Youths with T2D (mean age 17 years) have been shown to have increased arterial stiffness11 and a worse lipid profile12 than patients of similar age with T1D , indicating increased risk for premature CVD.
The differential burden of retinopathy and neuropathy between young-onset T1D and T2D is consistent with other studies.5 6 This difference is not attributable to glycemia and diabetes duration, given the equipoise in these variables in both types of diabetes. Since cardiovascular risk factors (obesity, hypertension, and dyslipidemia) were significantly higher in T2D and have a stronger association with neuropathy, these factors may play an etiological role in this complication. This hypothesis is supported by the findings from several large epidemiological studies implicating these cardiometabolic risk factors in the pathogenesis of diabetic neuropathy.13–15 This association has also been observed among young adults with T2D.7 In contrast, glycemia and disease duration are the main contributors to the evolution of retinopathy in both types of diabetes with cardiometabolic risk factors playing a less significant role.
Young adults with T2D have higher cardiovascular events and mortality occurring at shorter diabetes duration than T1D.5 The atherogenic metabolic milieu was already present early in its natural history (within 5 years of diagnosis) implying increased susceptibility to premature cardiovascular complications. This hypothesis was supported by the findings of this study which showed that patients with young-onset T2D experienced similar burden (∼14%) of CVD at a much younger age (by ∼10 years) and at shorter diabetes duration (by ∼17 years), compared to T1D. Glycemia was not a significant predictive factor for CVD and this is consistent with Luk et al8 who showed cardiometabolic risk factors (obesity, hypertension, and dyslipidemia), rather than hyperglycemia, being the principal driver for CVD in young adults with T2D.
In contrast, microvascular complications were strongly associated with hyperglycemia in this study cohort. Diabetes control was suboptimal in both types of young-onset diabetes and hyperglycemia would have played an important role in driving the burden of retinopathy and neuropathy, accentuated by the increasing diabetes duration. Intensive treatment of glycemia has long-term beneficial effects of reducing microvascular as well as macrovascular complications in T1D16 17 and older-onset T2D.18 These data are crucially lacking in young-onset T2D, particularly in regard to the legacy effect from intensive glucose control within the first few years of diagnosis. This issue is pertinent given its predisposition to prolonged exposure to the adverse diabetic milieu.4
Obesity with associated dyslipidemia and hypertension is a common feature in young-onset T2D which occurred to a greater degree than T1D. The mean BMI for T2D of 33–35 kg/m2 is consistent with that of other studies,5 19 and this is significantly higher than those of T1D (∼27 kg/m2). Since obesity drives the atherogenic metabolic syndrome components,20 the higher cardiovascular risk in young-onset T2D is most likely attributable to the greater degree of obesity. Obesity is instrumental to the rising incidence of T2D in young people.21 Among young adults (mean age ∼34 years) who were at risk of T2D, almost 20% had abnormal glucose metabolism and ∼90% were obese with a mean BMI of 34 kg/m2.22 In the UK, NICE recommended screening for diabetes in high-risk individuals aged 25–39 years particularly from ethnic minority groups and those with conditions that increase the risk of T2D,23 highlighting the importance of early detection in young people.
The care of young people with diabetes has predominantly focused on T1D, reflected by structured educational programs and national guidelines on care pathways specifically designed for this population.24 While young people with T1D have an increased risk of adverse outcome,3 this now holds true for T2D. The findings from this study and others support this. Given its increasing incidence, the emphasis of care of young people should also include T2D which is often inappropriately perceived as ‘mild 'diabetes due to its insidious onset. The asymptomatic period of dysglycemia prior to its diagnosis constitutes an unfavorable metabolic milieu that predisposes to vascular damage, which is further compounded by the prolonged exposure to the diabetic phase. By using mathematical modeling, Rhodes et al25 estimated that young-onset patients with T2D will experience severe chronic complications by their 40s and the observation from this study of the significant burden of microvascular and macrovascular disease in this age group concurs with their projection. Not surprisingly, the life expectancy of young people diagnosed with T2D between age 15 and 40 years is shortened by 8–15 years.25 26
The strengths of this study pertain to the relatively large number of the young-onset T1D and T2D cohorts, comparable with other studies.5 6 Moreover, steps were taken to minimize the confounding bias of the different age of onset and diabetes duration on complication burden, a common dilemma in assessing complication outcomes between T1D and T2D. However, there are some study limitations. First, the absence of mortality data precludes ascertainment of the cause of death, particularly from cardiovascular events. Second, it is difficult to determine the relative contributions of the various predictive factors on diabetes complications, given the cross-sectional nature of this study. It is plausible that young-onset T2D may not be an independent predictor of complications if longitudinal data on factors such as glycemia, hypertension, and dyslipidemia were available. Third, the possibility of high-risk patients with young-onset T2D being preferentially managed in hospital clinics cannot be excluded, implying that the findings of this study may not be extrapolated to the majority of patients with T2D whose care is delivered in the community. However, the complication risk profile of the young-onset T2D cohort in this study is similar to that of a large community-based study with more than 300 general practices in the UK,27 indicating that this study cohort is likely to be representative of the young-onset T2D population overall. Fourth, there were incomplete data on microalbuminuria and proteinuria for T2D. However, the findings from this study are consistent with those which included diabetic renal complication,5 6 8 indicating that the observations from this study most likely reflect the complication profile of young-onset T2D.
Several important clinical questions remain unanswered which can guide directions for future research. First, preventing T2D in the young is of utmost importance given the intimate relationship between obesity and its etiology. While there is good evidence for reducing the incidence of T2D among high-risk adults,28 there are no similar data in children, adolescents, and young adults. This issue is pertinent given that overweight or obese children who remained obese as adults are at risk of T2D and atherosclerotic complications.29 Second, there is a suggestion that lifestyle intervention has a minimal impact on weight loss, glycemic control, and dyslipidemia in young patients with T2D.30 31 Improving its effectiveness with an appropriate model for structured education to enhance patient empowerment and sustain behavioral change is crucial. Third, randomized clinical trials to assess the effectiveness of glycemia and risk factor intervention on clinical outcomes in young-onset T2D are lacking, in contrast to the older cohort32 and T1D.3 16 17 Given the suboptimal diabetes control observed in this study cohort and the importance of glycemia as well as other cardiometabolic risk factors in the etiology of microvascular and macrovascular complications, this is an important issue to address. Fourth, the observation from the TODAY study33 of greater decline in β cell function among adolescents with T2D compared with older-onset patients merits further investigation into its etiology. Fifth, the therapeutic effectiveness and safety of newer agents such as GLP-1 agonist and SGLT-2 inhibitor in reducing obesity and improving glycemic control and durability need to be explored in young-onset T2D, more so with the reduced therapeutic efficacy of metformin in this population.30 With the weight loss benefit, these medications can be an important component of the treatment armamentarium.
In conclusion, evidence is accumulating to support young-onset T2D being the more aggressive phenotype than T1D to develop complications. More emphasis should be placed on the care of young people with T2D focusing not only on glycemia and risk factor intervention but also improving adherence to treatment and a healthy lifestyle. Since the majority of patients with T2D are solely managed in the community, the challenge is to raise this awareness among the primary care physicians. Preventing T2D in high-risk young individuals is also a vital strategy in addressing this burgeoning problem. Robust evidence is lacking and more research is needed to formulate and guide evidence-based care of this increasingly prevalent population.
Acknowledgments
The author would like to thank Teresa Dodd for her excellent technical assistance in obtaining the relevant data for this study.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Holden SE, Barnett AH, Peters JR et al. The incidence of type 2 diabetes in the United Kingdom from 1991 to 2010. Diabetes Obes Metab 2013;15:844–52. 10.1111/dom.12123 [DOI] [PubMed] [Google Scholar]
- 2.Laing SP, Swerdlow AJ, Slater SD et al. The British Diabetic Association Cohort Study, II: cause-specific mortality in patients with insulin-treated diabetes mellitus. Diabet Med 1999;16:466–71. 10.1046/j.1464-5491.1999.00076.x [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 4.Song SH, Hardisty CA. Early onset type 2 diabetes mellitus: a harbinger for complication in later years—clinical observation from a secondary care cohort. QJM 2009;102:799–806. 10.1093/qjmed/hcp121 [DOI] [PubMed] [Google Scholar]
- 5.Constantino MI, Molyneaux L, Limacher-Gisler F et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care 2013;36:3863–9. 10.2337/dc12-2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dart AB, Martens PJ, Rigatto C et al. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care 2014;37:436–43. 10.2337/dc13-0954 [DOI] [PubMed] [Google Scholar]
- 7.Jaiswal M, Lauer A, Martin CL et al. , SEARCH for Diabetes in Youth Study Group. Peripheral neuropathy in adolescents and young adults with type 1 and type 2 diabetes from the SEARCH for Diabetes in Youth follow-up cohort: a pilot study. Diabetes Care 2013;36:3903–8. 10.2337/dc13-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luk AO, Lau ES, So WY et al. Prospective study on the incidences of cardiovascular-renal complications in Chinese patients with young-onset type 1 and type 2 diabetes. Diabetes Care 2014;37:149–57. 10.2337/dc13-1336 [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Clinical Excellence. Type 2 diabetes. The management of type 2 diabetes. NICE clinical guideline. London: NICE, 2008. [Google Scholar]
- 10.Waernbaum I, Blohmé G, Ostman J et al. Excess mortality in incident cases of diabetes mellitus aged 15–34 years at diagnosis: a population-based study (DISS) in Sweden. Diabetologia 2006;49:653–9. 10.1007/s00125-005-0135-x [DOI] [PubMed] [Google Scholar]
- 11.Wadwa RP, Urbina EM, Anderson AM et al. Measures of arterial stiffness in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care 2010;33:881–6. 10.2337/dc09-0747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petitti DB, Imperatore G, Palla SL et al. , SEARCH for Diabetes in Youth Study Group. Serum lipids and glucose control: the SEARCH for Diabetes in Youth study. Arch Pediatr Adolesc Med 2007;161:159–65. 10.1001/archpedi.161.2.159 [DOI] [PubMed] [Google Scholar]
- 13.Tesfaye S, Chaturvedi N, Eaton SE et al. , EURODIAB Prospective Complications Study Group. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–50. 10.1056/NEJMoa032782 [DOI] [PubMed] [Google Scholar]
- 14.Ziegler D, Rathmann W, Dickhaus T et al. , KORA Study Group. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008;31:464–9. 10.2337/dc07-1796 [DOI] [PubMed] [Google Scholar]
- 15.Forrest KY, Maser RE, Pambianco G et al. Hypertension as a risk factor for diabetic neuropathy: a prospective study. Diabetes 1997;46:665–70. 10.2337/diab.46.4.665 [DOI] [PubMed] [Google Scholar]
- 16.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of Intensive Therapy. N Engl J Med 2000;342:381–9. 10.1056/NEJM200002103420603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53. 10.1056/NEJMoa052187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holman RR, Paul SK, Bethel MA et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 19.Benhalima K, Song SH, Wilmot EG et al. Characteristics, complications and management of a large multiethnic cohort of younger adults with type 2 diabetes. Prim Care Diabetes 2011;5:245–50. 10.1016/j.pcd.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 20.Abbasi F, Brown BW Jr, Lamendola C et al. Relationship between obesity, insulin resistance and coronary heart disease. J Am Coll Cardiol 2002;40:937–43. 10.1016/S0735-1097(02)02051-X [DOI] [PubMed] [Google Scholar]
- 21.Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care 2001;24:1522–7. 10.2337/diacare.24.9.1522 [DOI] [PubMed] [Google Scholar]
- 22.Wilmot EG, Edwardson CL, Biddle SJ et al. Prevalence of diabetes and impaired glucose metabolism in younger ‘at risk ‘UK adults: insights from the STAND programme of research. Diabet Med 2013;30:671–5. 10.1111/dme.12173 [DOI] [PubMed] [Google Scholar]
- 23.NICE. Preventing type 2 diabetes: risk identification and interventions for individuals at high risk. London: National Institute for Health and Care Excellence, 2012. [Google Scholar]
- 24.NICE. Type 1 diabetes: diagnosis and management of type 1 diabetes in children, young people and adults. London: National Institute for Health and Care excellence, 2004. [Google Scholar]
- 25.Rhodes ET, Prosser LA, Hoerger TJ et al. Estimated morbidity and mortality in adolescents and young adults diagnosed with type 2 diabetes mellitus. Diabet Med 2012;29:453–63. 10.1111/j.1464-5491.2011.03542.x [DOI] [PubMed] [Google Scholar]
- 26.Roper NA, Bilous RW, Kelly WF et al. Excess mortality in a population with diabetes and the impact of material deprivation: longitudinal, population based study. BMJ 2001;322:1389–93. 10.1136/bmj.322.7299.1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunathilake W, Song S, Sridharan S et al. Cardiovascular and metabolic risk profiles in young and old patients with type 2 diabetes. QJM 2010;103:881–4. 10.1093/qjmed/hcq135 [DOI] [PubMed] [Google Scholar]
- 28.Knowler WC, Fowler SE, Hamman RF et al. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–86. 10.1016/S0140-6736(09)61457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juonala M, Magnussen CG, Berenson GS et al. Childhood adiposity, adult adiposity and cardiovascular risk factors. N Engl J Med 2011;365:1876–85. 10.1056/NEJMoa1010112 [DOI] [PubMed] [Google Scholar]
- 30.TODAY Study Group. A clinical trial to maintain glycaemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–56. 10.1056/NEJMoa1109333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.TODAY Study Group. Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: The TODAY clinical trial. Diabetes Care 2013;36:1758–64. 10.2337/dc12-2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaede P, Vedel P, Larsen N et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–93. 10.1056/NEJMoa021778 [DOI] [PubMed] [Google Scholar]
- 33.TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–57. 10.2337/dc12-2393 [DOI] [PMC free article] [PubMed] [Google Scholar]