Abstract
Introduction
Extranodal lymphoma (ENL) in the muscles is a rare manifestation of non-Hodgkin lymphoma (NHL). The aim of this case report is to describe and evaluate the clinical presentation and important radiologic features of ENL affecting the musculoskeletal system.
Presentation of case
We present a 52-year-old female with a 3-week history of left gluteal pain. Computed tomography (CT) showed a non-uniformly early enhancing mass in the left gluteal muscle, the tumor demonstrating central necrosis and adjacent bone involvement. Fluorine-18 fluorodeoxyglucose positron emission tomography (18F-FDG PET)/CT showed areas of increased 18F-FDG uptake in the left gluteal musculature, pelvic bones, para-aortic and mediastinal lymph nodes and both lungs. Histopathological examination showed a diffuse large B cell lymphoma (DLBCL). After 8 cycles of R-CHOP chemotherapy, the mass in the left gluteal muscle has completely disappeared
Discussion
Although destructive tumor originating in the gluteal muscle with adjacent bone involvement is more common in soft tissue sarcoma, lymphoma should be regularly included in the differential diagnosis. While CT is a useful modality for assessing soft tissue masses, disruption and injury of the surrounding tissues, PET/CT fusion is superior for the detection of unexpected extranodal sites of disease, or for exclusion of disease in the presence of nonspecific extranodal CT findings.
Conclusion
A rapid growth pattern and destructive masses that invade adjacent structures on CT are key findings of DLBCL, and 18F-FDG PET/CT is a useful imaging modality to accurately determine the disease stage and disease aggressiveness of NHL.
Keyword: Gluteal mass, Extranodal lymphoma, Diffuse large B cell lymphoma, PET
1. Introduction
Malignant lymphomas are a heterogeneous group of malignancies of the B cells or T cells that usually originate in the lymph nodes, although they can originate in any part of the body [1–3]. However, extranodal lymphoma (ENL) in the muscles as an isolated manifestation is very rare, accounting for 1.5–8.3% of non-Hodgkin lymphoma (NHL) cases [4–6]. Clinically, the presence of cortical bone destruction in association with large soft-tissue masses arising in the skeletal muscle, favors the diagnosis of soft tissue sarcoma, malignant musculoskeletal tumor such as malignant fibrous histiocytoma (MFH), malignant neurogenic tumor (MPNST) or metastasis rather than malignant lymphoma [3,4,7,8].
Despite advances in imaging devices over the past decade, accurate diagnosis of soft tissue tumors remains a challenge for clinicians, requiring a close team approach between the surgical oncologist, musculoskeletal radiologist and pathologist. Here we present a rare case of extranodal lymphoma originating in the gluteal muscle, with aggressive bone erosion, indicative of primary bone involvement. We believe that our case adds an important piece of evidence to the clinically relevant problem of appropriate diagnostic strategy for an unusual presentation of malignant lymphoma.
2. Case report
A 52-year-old female with a 3-week history of progressive left gluteal pain was referred to our outpatient surgical department. She was previously healthy but had a history of night sweats and weight loss over the prior 2 months. Upon physical examination, a large firm mass was observed in the left buttock, with no inflammation of the skin. No peripheral lymphadenopathy was detected despite thorough examination of the whole body. Standard biochemistry and hematology studies revealed normal results, except for mild elevation of serum lactic dehydrogenase (LDH) and C-reactive protein (CRP). Contrast-enhanced computed tomography (CT) scan of the pelvis revealed a non-uniformly early enhancing mass, approximately 51 × 64 mm in size, in the left gluteal muscle (Fig. 1A). The tumor demonstrated central necrosis and adjacent bone involvement, with destruction of the sacroiliac joint (Fig. 1B). CT scan of the chest showed patchy consolidation in the lower lobes of both lungs. Fluorine-18 fluorodeoxyglucose positron emission tomography (18F-FDG PET)/CT demonstrated a large area of increased 18F-FDG uptake in the left gluteal musculature [maximum standard uptake value (SUVmax) = 34], the posterior aspect of the left ileum and the sacrum (Fig. 2A). Whole-body 18F-FDG PET/CT identified intense 18F-FDG uptake by the para-aortic and mediastinal lymph nodes, and faint scattered 18F-FDG uptake by both lungs (Fig. 2B). Based on these findings, the clinical and radiographic differential diagnosis was soft tissue sarcoma, malignant lymphoma or metastasis derived from small cell carcinoma of the lung. We then proceeded with an open biopsy of the left gluteal mass for further diagnosis and treatment planning. Pathological studies of the specimen demonstrated dense and diffuse infiltration and proliferation of large atypical lymphoid cells, accompanied by small lymphocytes (Fig. 3A). Immunohistochemical studies showed that the large atypical lymphoid cells were positive for LCA, CD20 and bcl-6, and were negative for cytokeratin, S-100, alpha-SMA and bcl-2 (Fig. 3B). Further, approximately 45% of the large atypical lymphoid cells were positive for MIB-1(Ki-67). Thus, from these results, we diagnosed diffuse large B cell lymphoma (DLBCL) of the left buttock. After staging work-up, including bone marrow biopsy, the patient was finally diagnosed with stage IV DLBCL, low-intermediate risk of the international prognostic index (IPI). Immediately after diagnosis, the patient has received 8 cycles of R-CHOP chemotherapy, and as a result the mass in the left gluteal muscle has completely disappeared (Fig. 4A, B). The patient achieved complete remission after chemotherapy and is currently under the regular follow-up evaluation.
Fig. 1.

Contrast-enhanced computed tomography (CT) of the pelvis demonstrating the left gluteal mass (arrow). (A) CT showed a non-uniformly enhancing hypervascular mass with central necrosis in the left gluteal muscle. (B) The tumor demonstrated adjacent bone involvement with destruction of the sacroiliac joint.
Fig. 2.
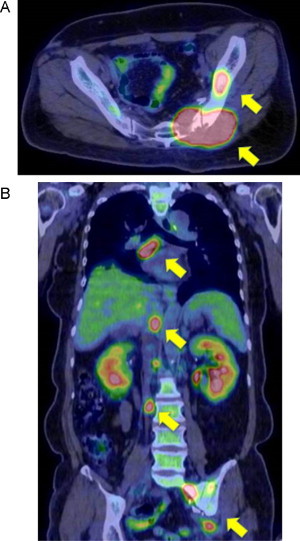
Fluorine-18 fluorodeoxyglucose positron emission tomography (18F-FDG PET)/CT images. (A) Axial 18F-FDG PET/CT view demonstrated large areas of increased 18F-FDG uptake in the left gluteal musculature [maximum standard uptake value (SUVmax) = 34], the posterior aspect of the left ileum (arrow) and the sacrum. (B) Whole-body 18F-FDG PET/CT identified strong 18F-FDG uptake by the para-aortic and mediastinal lymph nodes (arrow), and faint scattered 18F-FDG uptake by both lungs (SUVmax = 3.8).
Fig. 3.
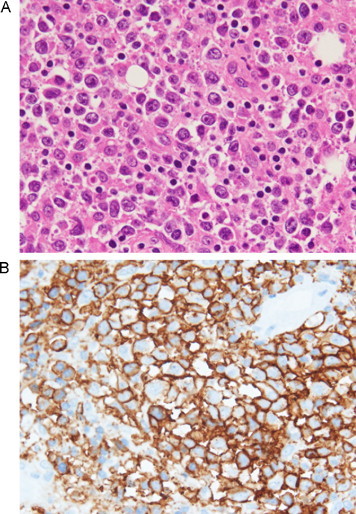
Histological and immunohistochemical examination of the resected specimen of the left buttock. (A) Hematoxylin and eosin (H–E) staining revealed diffuse infiltration with large atypical lymphoid cells with prominent nucleoli (×400). (B) CD20 immunohistochemical staining demonstrated the presence of large atypical lymphoid cells on the membrane (×400).
Fig. 4.
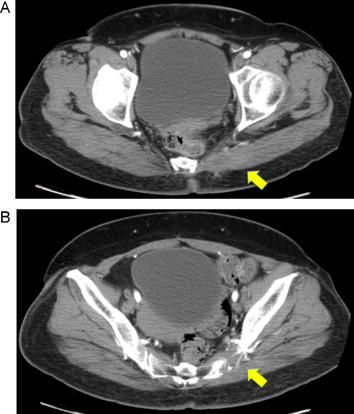
Contrast-enhanced CT of the pelvis after chemotherapy. (A) CT showed the mass in the left gluteal muscle has completely disappeared (arrow). (B) CT showed the erosion of the sacroiliac joint still remained (arrow), showing the destructive characteristics of the tumor.
3. Discussion
Malignant lymphomas in the musculoskeletal organs are predominantly a manifestation of disseminated lymphoma [3,4]. Common sites for the development of ENL are the skin, head, neck and gastrointestinal tract; primary lymphoma of the skeletal muscle is extremely rare [1–4]. To date, only a few cases of malignant lymphoma originating in the gluteal muscle have been reported in the English literature [5,7,9,10]. Textbooks present the common aspects of ENL in detail, but the published literature on the unexpected manifestations of NHL, such as soft tissue masses invading adjacent structures, is inadequate. These tumors can have an atypical presentation, which can lead to diagnostic difficulty.
The most meaningful radiologic features of ENL affecting the musculoskeletal system are permeative lytic destruction, although mixed lytic and blastic (sclerotic) lesions have been reported [3,11,12]. Patients with primitive or poorly differentiated tumors were more likely to have bone involvement than patients with well differentiated lymphomas [12]. Although the compartmental location of a lesion usually provides a differential diagnosis, the intrinsic imaging characteristics, including signal intensity, often add specificity. While CT is a useful modality for assessing soft tissue masses, disruption and injury of the surrounding tissues, periosteal reaction and sequestration [2,3,11–13], it has some limitations in terms of staging of the disease, because of its low sensitivity in detecting lymphomatous involvement of normal-sized lymph nodes, bone marrow and spleen [13,14]. A recently published systematic review article showed that PET/CT fusion is superior to both CT alone and PET alone in the initial staging and restaging after treatment [15]. Regarding extranodal involvement, the sensitivity and specificity were 88% and 100% for PET/CT and 50% and 90% for contrast enhanced CT, respectively [14]. A previous study in NHL patients reported that the disease was up-staged by PET/CT in 31% (mostly in stages I and II) and down-staged in only 1% of patients compared with CT alone. As a result, the treatment strategy was modified in 25% of patients according to CT versus PET/CT findings [16]. Although its diagnostic efficacy for indolent lymphoma remains unclear, PET/CT can serve as a standard imaging modality for the detection of unexpected extranodal sites of disease, or for exclusion of disease in the presence of nonspecific extranodal CT findings [2–4,11,13].
In addition to imaging studies, a definitive diagnosis of lymphoma requires histological examination of the specimen. For ENL patients, DLBCL is among the most common histological types, with expression of pan-B-cell markers, such as CD20 [1,17,18]. DLBCL often shows a rapid growth pattern and typically presents as large destructive masses that may infiltrate and invade adjacent structures [1,17,18]. In a recent report which analyzed NHL patients, the relationship between SUVmax on 18F-FDG PET and disease aggressiveness was evaluated [19,20]. For patients with DLBCL, the 3-year progression-free survival rates for patients with low and high SUVmax were 90% and 39%, respectively, indicating that low SUVmax is a good independent prognostic factor [20]. Therefore, 18F-FDG PET is a useful imaging modality to study the hallmarks of progression of the disease and its response to treatment [19,20].
Conflict of interest
The authors have no conflicts of interest.
Funding
This research has not been sponsored by any organization.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. There is no approval of ethics committee in our hospital because this study is retrospective observational study without patient’s cell.
Author contribution
MK and HN carried out review of medical record and collected the information. MK reviewed the literature and drafted the manuscript. TN carried out the pathological studies and provided the histological figure. YS interpreted and provided the radiological images.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Guarantor
All authors read and approved the final manuscript. Corresponding author of this article is Morihiro Katsura.
Acknowledgements
The authors would like to thank all the attending surgeons at Okinawa Prefectural Hokubu Hospital for their advice in managing this case. We gratefully acknowledge the help of Mr. Tsukasa Oshiro for making the PET images available to us, and Dr. Mariko Oshiro for reviewing the pathological contents. This research has not been sponsored by any organization.
Contributor Information
Morihiro Katsura, Email: morihiro@bj8.so-net.ne.jp.
Hirokazu Nishina, Email: carrot_and_whip_hiro@yahoo.co.jp.
Yasushi Shigemori, Email: shigemori-sin@umin.ac.jp.
Takaya Nakanishi, Email: h097509@med.u-ryukyu.ac.jp.
References
- 1.Zucca E. Extranodal lymphoma: a reappraisal. Annals of Oncology. 2008;19(iv):77–80. doi: 10.1093/annonc/mdn204. [DOI] [PubMed] [Google Scholar]
- 2.Chua S.C., Rozalli F.I., O’Connor S.R. Imaging features of primary extranodal lymphomas. Clin. Radiol. 2009;64:574–588. doi: 10.1016/j.crad.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Thomas A.G., Vaidhyanath R., Kirke R., Rajesh A. Extranodal lymphoma from head to toe: part 2, the trunk and extremities. Am. J. Roentgenol. 2011;197:357–364. doi: 10.2214/AJR.11.6738. [DOI] [PubMed] [Google Scholar]
- 4.Ilica A.T., Kocacelebi K., Savas R., Ayan A. Imaging of extranodal lymphoma with PET/CT. Clin. Nucl. Med. 2011;36:e127–138. doi: 10.1097/RLU.0b013e31821c99cd. [DOI] [PubMed] [Google Scholar]
- 5.Sonnino R., Kambouris A.A. Malignant lymphoma presenting in gluteal muscles: case report and brief review of the literature. Henry Ford Hosp. Med. J. 1988;36:61–63. [PubMed] [Google Scholar]
- 6.Freeman C., Berg J.W., Cutler S.J. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–260. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Ariad S., Hatskelzon L., Benharroch D., Geffen D.B. Gluteal manifestation of advanced Hodgkin’s disease. Skeletal Radiol. 1997;26:622–625. doi: 10.1007/s002560050298. [DOI] [PubMed] [Google Scholar]
- 8.Singh R., Al Wattar B.H., Mohanty K. A rare case of primary bone lymphoma mimicking a pelvic abscess. Ann. R. Coll. Surg. Engl. 2011;93:e141–143. doi: 10.1308/147870811X602168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utkan G., Buyukcelik A., Yalcin B., Tek I., Doruk H., Dincol D. Extranodal Hodgkin disease presenting with gluteal mass and hypercalcemia. South Med. J. 2006;99:1149–1150. doi: 10.1097/01.smj.0000240720.24001.77. [DOI] [PubMed] [Google Scholar]
- 10.Scally J., Garrett A. Primary extranodal lymphoma in muscle. Br. J. Radiol. 1989;62:81. doi: 10.1259/0007-1285-62-733-81. [DOI] [PubMed] [Google Scholar]
- 11.Kashyap R., Rai Mittal B., Manohar K., Balasubramanian Harisankar C.N., Bhattacharya A. Extranodal manifestations of lymphoma on [18F]FDG-PET/CT: a pictorial essay. Cancer Imaging. 2011;11:166–174. doi: 10.1102/1470-7330.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braunstein E.M., White S.J. Non-Hodgkin lymphoma of bone. Radiology. 1980;135:59–63. doi: 10.1148/radiology.135.1.6892659. [DOI] [PubMed] [Google Scholar]
- 13.Even-Sapir E., Lievshitz G., Perry C., Herishanu Y., Lerman H., Metser U. Fluorine-18 fluorodeoxyglucose PET/CT patterns of extranodal involvement in patients with Non-Hodgkin lymphoma and Hodgkin’s disease. Radiol. Clin. North Am. 2007;45:697–709. doi: 10.1016/j.rcl.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer N.G., Hany T.F., Taverna C., Seifert B., Stumpe K.D., von Schulthess G.K. Non-Hodgkin lymphoma and Hodgkin disease: coregistered FDG PET and CT at staging and restaging–do we need contrast-enhanced CT? Radiology. 2004;232:823–829. doi: 10.1148/radiol.2323030985. [DOI] [PubMed] [Google Scholar]
- 15.Kwee T.C., Kwee R.M., Nievelstein R.A. Imaging in staging of malignant lymphoma: a systematic review. Blood. 2008;111:504–516. doi: 10.1182/blood-2007-07-101899. [DOI] [PubMed] [Google Scholar]
- 16.Raanani P., Shasha Y., Perry C., Metser U., Naparstek E., Apter S. Is CT scan still necessary for staging in Hodgkin and non-Hodgkin lymphoma patients in the PET/CT era? Ann. Oncol. 2006;17:117–122. doi: 10.1093/annonc/mdj024. [DOI] [PubMed] [Google Scholar]
- 17.Ferry J.A. Extranodal lymphoma. Arch. Pathol. Lab. Med. 2008;132:565–578. doi: 10.5858/2008-132-565-EL. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui M.T., Pitelka L.A., Gattuso P. Extranodal lymphomas: review of clinicopathologic and cytologic features. Diagn. Cytopathol. 2009;37:220–229. doi: 10.1002/dc.21045. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe R., Tomita N., Takeuchi K., Sakata S., Tateishi U., Tanaka M. SUVmax in FDG-PET at the biopsy site correlates with the proliferation potential of tumor cells in non-Hodgkin lymphoma. Leuk. Lymphoma. 2010;51:279–283. doi: 10.3109/10428190903440953. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki Y., Nawa Y., Miyagawa M., Kohashi S., Nakase K., Yasukawa M. Maximum standard uptake value of 18F-fluorodeoxyglucose positron emission tomography is a prognostic factor for progression-free survival of newly diagnosed patients with diffuse large B cell lymphoma. Ann. Hematol. 2013;92:239–244. doi: 10.1007/s00277-012-1602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


