Highlights
-
•
A rare type of primary mesenteric neoplasm.
-
•
No definitive guidelines for the diagnosis.
-
•
Histopathology and immunology is the key to diagnosis and prognosis.
-
•
Radical excision is the only reliable treatment option.
-
•
Overall survival rate is poor for this malignant neoplasm.
Keywords: Keywords, Leiomyosacoma, Mesenchymal tumour, Gastrointestinal stromal tumour
Absract
Leiomyosarcoma is extremely rare with a reported incidence of 1:350,000. So accurate preoperative diagnosis of mesenteric soft tissue tumor is generally difficult. Leiomyosarcoma is a malignant tumour arising from smoothcell lineage. Mesenteric leiomyosarcoma occurs most commonly in middle-aged individuals. We describe a case of leiomyosarcoma arising from the ileal mesentery in a 33 year old male patient. The diagnosis was based on histopathologic evaluation using immunohistochemical staining.
1. Introduction
Leiomyosarcoma is a malignant mesenchymal tumor that derives from the smooth muscle lineage [1]. The underlying genetic mechanisms remain unclear and complex. Unbalanced karyotypic defects are the only shared features observed across the different leiomyosarcoma subtypes. Cell-cycle perturbations, mainly through RB1 defects, and phosphatidylinositol 3 kinase/Akt pathway activation caused by PTEN genomic deletion are the two most consistent drivers commonly observed in sarcomas, including leiomyosarcoma [1].
Mesenteric leiomyosarcoma likely derives from the smooth muscle of blood vessels in the mesentery [3], and little information is available regarding clinical presentation, pathology, treatment and prognosis. The occurrence of primary mesenteric leiomyosarcoma is extremely rare and very few cases appear to have been reported. So accurate preoperative diagnosis of mesenteric tumor is generally difficult.
2. Presentation of case
A 33 year old male patient presented with diffuse abdominal pain and difficulty in passing stools for the past 3 years . Pain was diffuse dull aching in character, mainly aggravated during night and relieved on lying in prone position. Later pain shifted to left side of abdomen. He complains of passing mucous in stools. He has loss of appetite and early satiety with occasional episodes of nausea for the past 6 months. No history of malena, fever, vomiting, loss of weight . He was treated as a case of irritable bowel syndrome for past 3 years.
On examination, a well defined firm tender mass of 10 × 10 cms was felt in the left lumbar and umbilical region. It was freely mobile in the direction perpendicular to mesentery, with nodular surface. No movement with respiration and on percussion an impaired resonant note was heard. Per rectal examination was with in normal limits.
Based on the clinical findings a provisional diagnosis of mesenteric mass was made and further investigated.
USG abdomen showed lobulated hypoechoic intra abdominal mass lesion. GIST, with areas of vascularity. CECT abdomen revealed large neoplasm 8.6 × 9.7 × 9.0 cms in abdomen related to mesentry displacing the adjacent intestine. No invasion to adjacent organs. No metastasis to liver. No enlarged lymph nodes.
Intra operatively there was a large lobulated 15 × 15 cm tumor in the small bowel mesentry at the transition zone of jejunum and ileum without any breach the peritoneal covering. There is no mesenteric lymphadenopathy, no infiltration to bowel, no ascites and no liver/pelvic/peritoneal metastasis. So resection of tumour with a segment of small bowel was done (Fig. 1 and 2).
Fig. 1.

CECT picture of leiomyosarcoma.
Fig. 2.
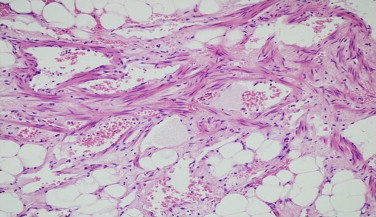
Microscopic picture of resected tumour.
Histopathology came as leiomyosarcoma, FNCLCC grade 3, involving mesentery, resected small bowel are unremarkable (Fig. 3 and 4).
Fig. 3.

Intraoperative picture of tumour.
Fig. 4.

Resected part of the tumour.
Immunohistochemistry was strongly positive for SMA and CD99 and negative for CD117, CD 34, abd bcl-2 [2]. The mitotic rate was 8/10HPF with atypical mitosis.
3. Discussion
Primary mesenteric leiomyosarcoma in adolescent patients is exceedingly rare, with little known about its pathology. Mesenteric leiomyosarcoma in an adult was first described by Yannopoulos and Stout et al. [3]. The differential diagnosis includes leiomyosarcoma and other mesenteric tumors such as GIST, malignant myofibroblastic tumor, solitary fibroustumor, liposarcoma, and lymphoma [2]. Generally, percutaneous biopsy is contraindicated because skin metastasis and peritoneal dissemination may be induced by biopsy. A definitive diagnosis of leiomyosarcoma can only be made by histopathologic study, including immunohistochemical staining and genetic analysis.
Treatment strategies for mesenteric leiomyosarcoma vary. Surgical excision with a wide margin of normal tissue is most effective since adjuvant chemotherapy or radiotherapy are unreliable [4]. In our case enblock resection was done and the margins were free from tumor. Lee noted that surgeons should attempt to improve the survival rate by radical removal of all smooth muscle tumors, including the removal of at least 4 inches of healthy tissue on all sides of the tumor, plus the corresponding mesentery [6].
Patients with leiomyosarcoma of the bowel mesentery generally display a poor prognosis. The overall 5-year survival rate for this tumor is only 20%–30% [5], and complete primary surgical resection is critical for achieving the best outcome. Conversely, recurrence can occur within 5 years, so that close and long term follow-up of such patients for 5 years or more, with particular attention to the gastrointestinal tract, liver, and lung, is necessary.
Pathologically, leiomyosarcoma exhibits high mitotic activity. Survival is influenced by the histological grade of mesenteric leiomyosarcomas, based on cell differentiation, cellularity of the tumor, anaplasia, and number of mitoses per HPF. Ranchod and Kempson showed frequency of mitoses to be the most useful indicator of malignant potential [7]. Simpson et al. introduced histological grading of intestinal leiomyosarcomas [8]. Grade 3 has marked cellularity anaplasia, and a mitotic index more than 10 mitoses per 10HPFs. As our histopathology showed FNCLCC grade 3 and mitotic rate of 8/10HPF, patient was put on radiotherapy for further management.
Guidelines for the management of gastrointestinal leiomyosarcoma or mesenteric leiomyosarcoma are unclear, probably because of the small number of patients reported. Earlier detection using ultrasonography and computed tomography, as well as complete surgical resection, may improve long-term prognosis in patients with primary leiomyosarcoma of mesenteric origin.
4. Conclusion
Primary mesenteric leiomyosarcoma is a rare entity. Because of its rarity there is no uniform treatment modality for the same. Diagnosis can only be confirmed by histopathology and immunological staining. Even though surgery is the main modality of treatment recurrence is common with this. Early detection and wide surgical resection will improve the prognosis of this type of lesion.
Conflict of interest
No conflict of interest.
Funding
No source of funding or no sponsors.
Ethical approval
As this is a case report got the approval from an institutional ethical commitee.
Authors contribution
| Contributor 1 | Contributor 2 | Contributor 3 | Contributor 4 | |
|---|---|---|---|---|
| Concepts | ✓ | |||
| Design | ✓ | |||
| Definition of intellectual content | ✓ | |||
| Literature search | ✓ | ✓ | ||
| Clinical studies | ||||
| Experimental studies | ||||
| Data acquisition | ||||
| Data analysis | ||||
| Statistical analysis | ||||
| Manuscript preparation | ✓ | ✓ | ||
| Manuscript editing | ✓ | ✓ | ||
| Manuscript review | ✓ | |||
| Guarantor | ✓ |
Concent
As this is a case report got the informed consent from the patient.
References
- 1.Serrano, MDa,b C., George MDb S. Hematol. Oncol. Clin. N. Am. 2013;27:957–974. doi: 10.1016/j.hoc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto H., Tsuneyoshi M., Enjoji M. Malignant smooth muscletumors of the retroperioneum and mesentery: a clinicopathologicanalysis of 44 cases. J. Surg. Oncol. 1985;28:177–186. doi: 10.1002/jso.2930280307. [DOI] [PubMed] [Google Scholar]
- 3.Yannopoulos K., Stout A.P. Primary solid tumors of the mesentery. Cancer. 1963;16:914–927. doi: 10.1002/1097-0142(196307)16:7<914::aid-cncr2820160708>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Simonovich C.J., Hardman J.M., Navin J.J. An unusual abdominaltumor—leiomyosarcoma of the mesentery: a case report. Hawaii Med. J. 2006;65:18–20. [PubMed] [Google Scholar]
- 5.Fukunaga M. Neuron-specific enolase-producing leiomyosarcoma ofthe mesentery. Acta Pathol. Microbiol. Immunol. Scand. 2004;112:805–808. doi: 10.1111/j.1600-0463.2004.apm1120204.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y.-T.N. Leiomyosarcomas of the gastrointestinal tract: general pattern of metastases and recurrence. Cancer Treat. Rev. 1984;10:91–101. doi: 10.1016/0305-7372(83)90007-5. [DOI] [PubMed] [Google Scholar]
- 7.Ranchod M., Kempson R.L. Smooth muscle tumors of the gastrointestinal tract and retroperitoneum. Cancer. 1977;39:255–262. doi: 10.1002/1097-0142(197701)39:1<255::aid-cncr2820390139>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Simpson B.B., Reynolds E.M., Kim S.H., Ferguson W.S., Graeme-Cook F., Doody D.P. Infantile intestinal leiomyosarcoma: surgical resection (without adjuvant therapy) for cure. J. Pediatr. Surg. 1996;31:1577–1580. doi: 10.1016/s0022-3468(96)90184-0. [DOI] [PubMed] [Google Scholar]


