Abstract
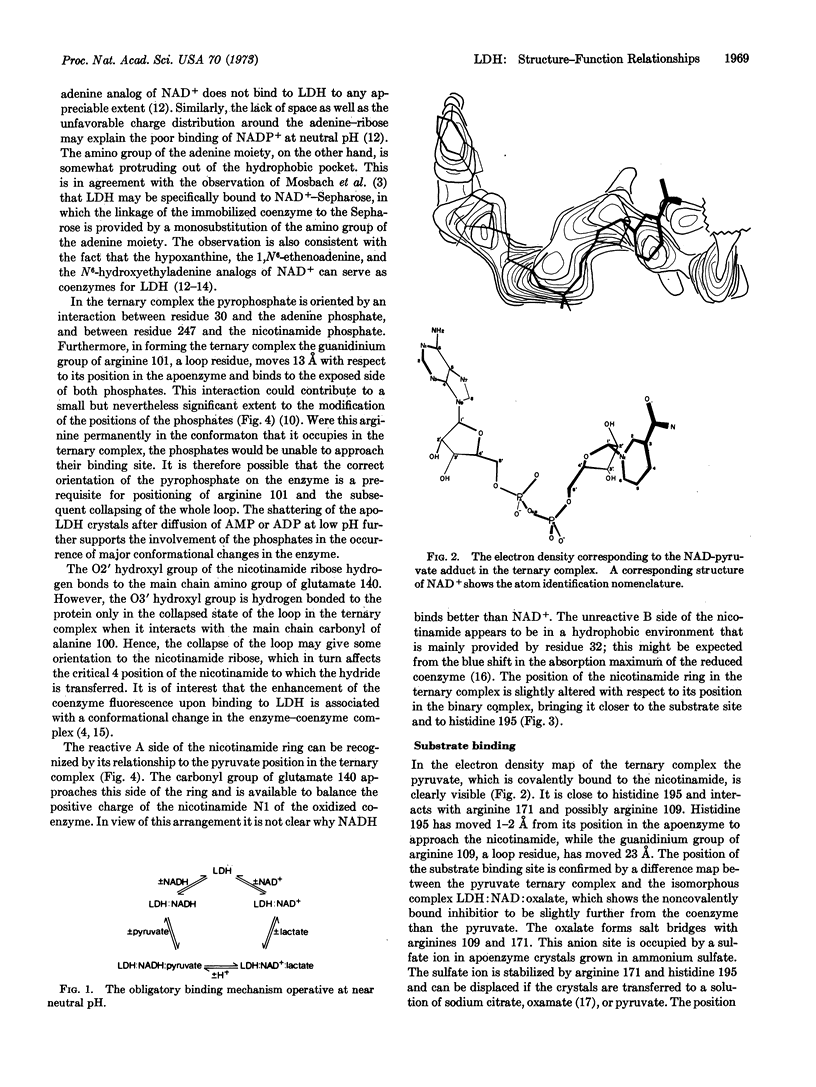
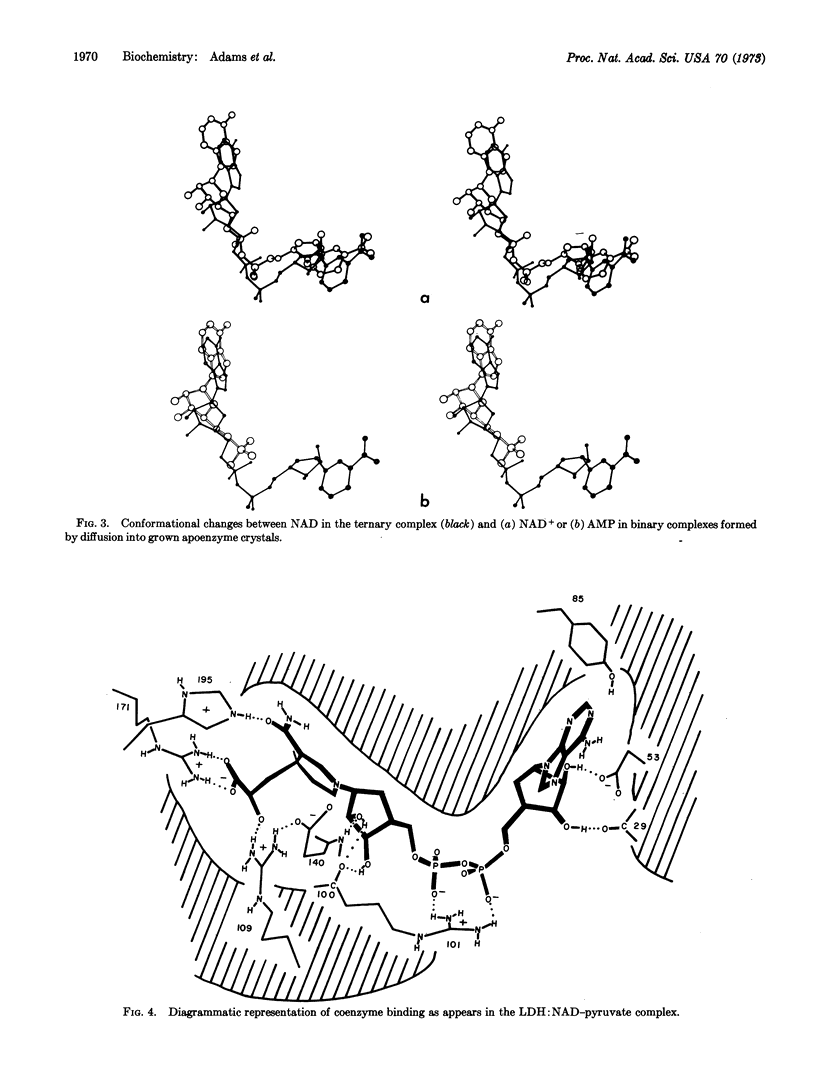
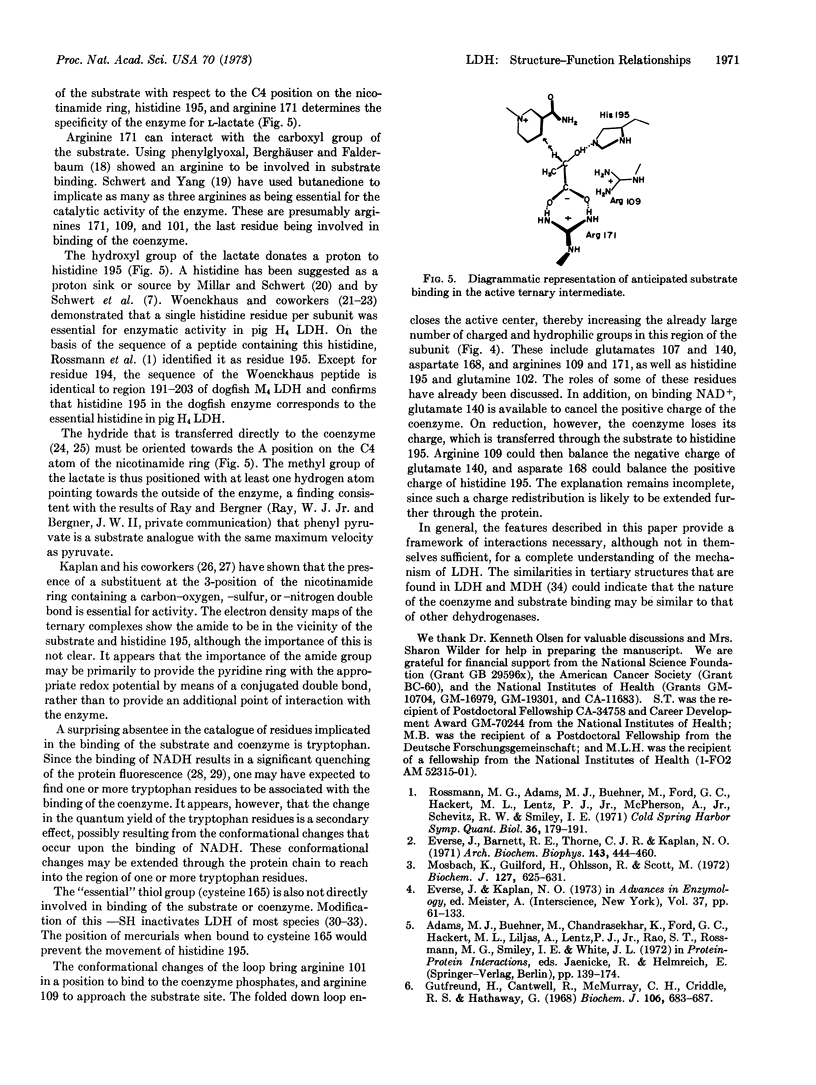
The binding of coenzyme and substrate are considered in relation to the known primary and tertiary structure of lactate dehydrogenase (EC 1.1.1.27). The adenine binds in a hydrophobic crevice, and the two coenzyme phosphates are oriented by interactions with the protein. The positively charged guanidinium group of arginine 101 then folds over the negatively charged phosphates, collapsing the loop region over the active center and positioning the unreactive B side of the nicotinamide in a hydrophobic protein environment. Collapse of the loop also introduces various charged groups into the vicinity of the substrate binding site. The substrate is situated between histidine 195 and the C4 position on the nicotinamide ring, and is partially oriented by interactions between its carboxyl group and arginine 171. The spatial arrangements of these groups may provide the specificity for the L-isomer of lactate.
Keywords: amino-acid sequence, crystallographic structure
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON B. M., CIOTTI C. J., KAPLAN N. O. Chemical properties of 3-substituted pyridine analogues of diphosphopyridine nucleotide. J Biol Chem. 1959 May;234(5):1219–1225. [PubMed] [Google Scholar]
- ANDERSON B. M., KAPLAN N. O. Enzymatic studies with analogues of diphosphopyridine nucleotide. J Biol Chem. 1959 May;234(5):1226–1232. [PubMed] [Google Scholar]
- Berghäuser J., Falderbaum I. Modifizierung eines essentiellen Argininrestes in Lactat-Dehydrogenase mit Phenylglyoxal. Hoppe Seylers Z Physiol Chem. 1971 Sep;352(9):1189–1194. [PubMed] [Google Scholar]
- Berghäuser J., Falderbaum I., Woenckhaus C. Zuordnung eines essentiellen Histidinrestes der Lactat-Dehydrogenase zur Substratbindungsstelle. Hoppe Seylers Z Physiol Chem. 1971 Jan;352(1):52–58. doi: 10.1515/bchm2.1971.352.1.52. [DOI] [PubMed] [Google Scholar]
- CHANCE B., NEILANDS J. B. Studies on lactic dehydrogenase of heart. II. A compound of lactic dehydrogenase and reduced pyridine nucleotide. J Biol Chem. 1952 Nov;199(1):383–387. [PubMed] [Google Scholar]
- CORNFORTH J. W., RYBACK G. Stereochemistry of enzymic hydrogen transfer to pyridine nucleotides. Biochem Biophys Res Commun. 1962 Nov 27;9:371–375. doi: 10.1016/0006-291x(62)90018-9. [DOI] [PubMed] [Google Scholar]
- Everse J., Barnett R. E., Thorne C. J., Kaplan N. O. The formation of ternary complexes by diphosphopyridine nucleotide-dependent dehydrogenases. Arch Biochem Biophys. 1971 Apr;143(2):444–460. doi: 10.1016/0003-9861(71)90230-x. [DOI] [PubMed] [Google Scholar]
- FAWCETT C. P., KAPLAN N. O. Preparation and properties of some nicotinamide adenine dinucleotide analogues with pentose and purine modifications. J Biol Chem. 1962 May;237:1709–1715. [PubMed] [Google Scholar]
- Fondy T. P., Everse J., Driscoll G. A., Castillo F., Stolzenbach F. E., Kaplan N. O. The comparative enzymology of lactic dehydrogenases. IV. Function of sulfhydryl groups in lactic dehydrogenases and the sequence around the essential group. J Biol Chem. 1965 Nov;240(11):4219–4234. [PubMed] [Google Scholar]
- Gold A. H., Segal H. L. A peptide containing the essential sulfhydryl group of beef heart lactic dehydrogenase. Biochemistry. 1965 Aug;4(8):1506–1511. doi: 10.1021/bi00884a007. [DOI] [PubMed] [Google Scholar]
- Gutfreund H., Cantwell R., McMurray C. H., Criddle R. S., Hathaway G. The kinetics of the reversible inhibition of heart lactate dehydrogenase through the formation of the enzyme-oxidized nicotinamide-adenine dinucleotide-pyruvate compounds. Biochem J. 1968 Feb;106(3):683–687. doi: 10.1042/bj1060683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E., Tsernoglou D., Webb L., Banaszak L. J. Polypeptide conformation of cytoplasmic malate dehydrogenase from an electron density map at 3.0 angstrom resolution. J Mol Biol. 1972 Dec 30;72(3):577–589. doi: 10.1016/0022-2836(72)90176-3. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Stinson R. A. Reactivity of the essential thiol group of lactate dehydrogenase and substrate binding. Biochem J. 1970 Nov;120(2):289–297. doi: 10.1042/bj1200289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J. The importance of SH-groups for enzymic activity. V. The coenzyme-binding capacity of pig heart lactate dehydrogenase, isozyme I, after inhibition by various maleinimides. Biochem Z. 1966 Mar 28;344(2):141–152. [PubMed] [Google Scholar]
- LEVY H. R., VENNESLAND B. The stereospecificity of enzymatic hydrogen transfer from diphosphopyridine nucleotide. J Biol Chem. 1957 Sep;228(1):85–96. [PubMed] [Google Scholar]
- MCKAY R. H., KAPLAN N. O. STUDIES OF PROTEIN AND BOUND COENZYME FLUORESCENCE OF LACTATE DEHYDROGENASES. Biochim Biophys Acta. 1964 Mar 30;79:273–283. [PubMed] [Google Scholar]
- MILLAR D. B., SCHWERT G. W. LACTIC DEHYDROGENASE. IX. EFFECT OF PHOTO-OXIDATION UPON ACTIVITY AND COMPLEX FORMATION. J Biol Chem. 1963 Oct;238:3249–3255. [PubMed] [Google Scholar]
- McPherson A., Jr Interaction of lactate dehydrogenase with its coenzyme, nicotinamide-adenine dinucleotide. J Mol Biol. 1970 Jul 14;51(1):39–46. doi: 10.1016/0022-2836(70)90268-8. [DOI] [PubMed] [Google Scholar]
- Mosbach K., Guilford H., Ohlsson R., Scott M. General ligands in affinity chromatography. Cofactor-substrate elution of enzymes bound to the immobilized nucleotides adenosine 5'-monophosphate and nicotinamide-adenine dinucleotide. Biochem J. 1972 May;127(4):625–631. doi: 10.1042/bj1270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVOA W. B., SCHWERT G. W. Lactic dehydrogenase. VIII. Binding of oxamate and of oxalate by enzyme-coenzyme complexes. J Biol Chem. 1961 Jul;236:2150–2153. [PubMed] [Google Scholar]
- Rossmann M. G., Adams M. J., Buehner M., Ford G. C., Hackert M. L., Lentz P. J., Jr, McPherson A., Jr, Schevitz R. W., Smiley I. E. Structural constraints of possible mechanisms of lactate dehydrogenase as shown by high resolution studies of the apoenzyme and a variety of enzyme complexes. Cold Spring Harb Symp Quant Biol. 1972;36:179–191. doi: 10.1101/sqb.1972.036.01.025. [DOI] [PubMed] [Google Scholar]
- Schwert G. W., Miller B. R., Peanasky R. J. Lactic dehydrogenase. X. A re-evaluation of the effects of pH upon the kinetics of the reaction. J Biol Chem. 1967 Jul 25;242(14):3245–3252. [PubMed] [Google Scholar]
- WINDMUELLER H. G., KAPLAN N. O. The preparation and properties of N-hydroxyethyl derivatives of adenosine, adenosine triphosphate, and nicotinamide adenine dinucleotide. J Biol Chem. 1961 Oct;236:2716–2726. [PubMed] [Google Scholar]
- Woenckhaus C., Berghäuser J., Pfleiderer G. Markierung essentieller Aminosäurereste der Lactat-Dehydrogenase aus Schweineherz mit (Carbonyl-14C)3-(2-Brom-acetyl)-pyridin. Hoppe Seylers Z Physiol Chem. 1969 Apr;350(4):473–483. [PubMed] [Google Scholar]
- Woenckhaus C., Schättle E., Jeck R., Berghäuser J. Spezifische Modifizierung der Coenzymbindungsstelle von Dehydrogenasen mit dem NAD-ähnlichen Inaktivator (3-(4-Bromacetylpyridinio)propyl)-adenosin-pyrophosphat. Hoppe Seylers Z Physiol Chem. 1972 Apr;353(4):559–564. [PubMed] [Google Scholar]
- Yang P. C., Schwert G. W. Inactivation of lactate dehydrogenase by butanedione. Biochemistry. 1972 Jun 6;11(12):2218–2224. doi: 10.1021/bi00762a002. [DOI] [PubMed] [Google Scholar]



