Abstract
In this study, we investigated the potential for aflatoxin B1 (AFB1) and B2 (AFB2) production in rice grain by 127 strains of Aspergillus flavus isolated from rice grains collected from China. These strains were inoculated onto rice grains and incubated at 28 °C for 21 days. AFB1 and AFB2 were extracted and quantified by high-performance liquid chromatography coupled with fluorescence detection. Among the tested strains, 37% produced AFB1 and AFB2 with levels ranging from 175 to 124 101 μg kg−1 for AFB1 and from not detected to 10 329 μg kg−1 for AFB2. The mean yields of these isolates were 5884 μg kg−1 for AFB1 and 1968 μg kg−1 for AFB2. Overall, most of the aflatoxigenic strains produced higher levels of AFB1 than AFB2 in rice. The obtained information is useful for assessing the risk of aflatoxin contamination in rice samples.
Keywords: Aspergillus flavus, Rice grain, Aflatoxin, High performance liquid chromatography, China
1. Introduction
Aflatoxins (AFs) are a group of mycotoxins produced as secondary metabolites by the spoilage of Aspergillus fungi, particularly Aspergillus flavus and Aspergillus parasiticus (Davis and Diener, 1983; Miguel and Guillermo, 1986; Yu et al., 2003; Klich, 2007). These fungi can grow on various agricultural commodities and generate aflatoxins before and during harvest, handling, shipment and storage (Peraica et al., 1999; Giray et al., 2007; Reddy et al., 2009a). The most important members are aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1) and aflatoxin G2 (AFG2). They are highly toxic and carcinogenic compounds that cause disease in livestock and humans (Richard, 2007). The International Agency for Research on Cancer (IARC) has clarified AFB1, AFB2, AFG1 and AFG2 in the group I as human carcinogens (IARC, 1993).
Rice (Oryza sativa L.) is one of the most important staple foods in the world. Especially in Asian countries, large amounts of rice are consumed per capita per year. According to the Food and Agriculture Organization (FAO), in 2008 the worldwide rice production is about 68 501.3 million tons. The main rice producing countries are China, India, Indonesia, Bangladesh, Myanmar, Thailand and Vietnam (http://www.fao.org/newsroom/common/ecg/1000820/en/Rmprod0308.pdf). Rice cultivation is usually conducted in subtropical environments, which are characteristically warm and humid. Rice is generally dried after harvesting. Due to inappropriate storage conditions, rice can be an ideal substrate for mycotoxin-producing fungi. In recent years, numerous studies have revealed high levels of aflatoxins and fungal contamination in rice in many countries (Tanaka et al., 2007; Reddy et al., 2008; Reddy et al., 2009a,b; Reiter et al., 2010; Aydin et al., 2011; Bansal et al., 2011; Hussaini et al., 2011; Almeida et al., 2012; Elena et al., 2013; Ok et al., 2014). However, mycotoxin-producing fungi are less commonly reported for rice than for many other cereal crops (Tanaka et al., 2007).
In China, rice is the most important cereal, the staple food for more than 65% of the population (Zhang et al., 2005), and it is the subsistence crop for the most resource-poor rice farmers and consumers in rural areas. China ranks first in total annual rice production and produced 29% of the world’s rice in 2006 (www.fao.org). Some studies have reported that rice in China has been contaminated with mycotoxins such as aflatoxins and fumonisins (Tang, 1999; Trucksee, 2000; Liu et al., 2006; Wang and Liu, 2007; Sun et al., 2011; Lai et al., 2014). However, only limited data are available on the aflatoxigenic fungal contamination of rice in China and on the ability of local fungal strains to produce mycotoxins (Liu et al., 1981).
In our preliminary study, rice intended for human consumption in China in 2009–2011 was extensively collected from different geographical areas and a number of A. flavus strains have been isolated from the rice samples. The aim of this study was to evaluate the ability of A. flavus strains to produce aflatoxins on rice grains in vitro.
2. Materials and methods
2.1. Strains and mycotoxin production
A total of 127 strains of A. flavus, which were isolated from rice samples collected from twelve provinces in China, were selected to test for aflatoxin production in cultures of rice grain. Spore suspensions of the individual A. flavus strains were prepared by growing the fungi on Petri dishes for 5 days with potato dextrose agar. After incubation at 25 °C, spores were harvested by adding sterilized distilled (10 mL for each plate) water on each plate. The spore suspension thus obtained was filtered using cheesecloth, and spores were counted using a hemocytometer and brought to a final concentration of 107 conidia per mL. All treatments were replicated two times.
2.2. Rice grain cultures
Twenty-five grams of polished rice grain was placed in Erlenmeyer flasks (150 mL), 25 mL of distilled water was added, and they were autoclaved at 121 °C for 1 h and allowed to stand overnight. Each flask was inoculated with 2 mL of the spore suspension described above, incubated at 28 ± 1 °C in dark/night of 12/12 h for 21 days and shaken once or twice daily for 3 days to aid in even distribution of the inocula. After incubation, the moldy rice grains were autoclaved at 100 °C for 30 min, then dried overnight at 60 °C and used for the extraction of AFB1 and AFB2.
2.3. Extraction of aflatoxins from moldy rice grains
Aflatoxins were extracted from moldy rice grains by the method described in our previous study (Lai et al., 2014) with minor modifications. The homogenized moldy rice samples (1.5 g, dry weight) were extracted with 6 mL of a 79/20/1 (v/v/v) mixture of MeCN/H2O/AcOH in an ultrasonic cleaner for 15 min. After centrifugation for 5 min at 3000 rpm, the upper layer was filtered through a filter paper (Whatman No 44) and then processed by dispersive liquid–liquid microextraction (DLLME). Two hundred microliters of CHCl3 was added to a 1 mL aliquot of 79% MeCN extract and was mixed with a vortex mixer for 30 s. The mixture was then rapidly injected into a 15 mL screw-cap glass centrifuge tube with a conical bottom that contained 5 mL of 2% NaCl solution (pH 3.0). This ternary component system was mixed for few seconds with a vortex mixer. After centrifugation for 5 min at 3000 rpm, the upper aqueous phase was removed with a Pasteur pipette, and the sedimented CHCl3 phase was quantitatively transferred to a small vial with a microsyringe. The sample was blown dry with a gentle stream of nitrogen gas at room temperature. Derivatives of AFB1 and AFB2 were prepared by adding 200 μL n-hexane and 100 μL trifluoroacetic acid to the residue, heating the mixture at 40 °C in a water bath for 15 min, evaporating to dryness and then dissolving the residue with 200 μL of MeCN/MeOH/1% H3PO4 (17 + 13 + 70) and vortexing for 30 s. The solution was filtered with a 0.22 μm nylon membrane filter, transferred to the sample vial and analyzed by HPLC.
2.4. HPLC-FLD analysis
Aflatoxins (AFB1 and AFB2) were quantified according to the method described in our previous study (Lai et al., 2014) with minor modifications. HPLC analysis was performed with an Agilent 1260 HPLC system (Agilent Technologies, Germany) equipped with a quaternary pump, an automatic sample injector, a degasser, and a fluorescence detector. Separations were conducted with a KR100-10 C18 column (5 μm, 150 mm × 4.6 mm, Kromasil Limited). Acetonitrile (MeCN) was used as mobile phase A, methanol (MeOH) was used as mobile phase B, and 1% phosphoric acid (H3PO4) was used as mobile phase C. At first, the mobile phase was maintained for 13 min at a ratio of 17/13/70 (v/v/v) A/B/C, then changed to 45/15/40 (v/v/v) over a 2 min gradient and maintained for 1 min, then it was switched back to 17/13/70 (v/v/v) over a 2 min gradient and maintained for 5 min. The flow rate was set at 1.0 mL/min, and the injection volume was 50.0 μL. The detection wavelengths for AFB1 and AFB2 were 360 nm and 440 nm for excitation and emission, respectively. The retention time was 5.95 min and 13.98 min for AFB1 and AFB2, respectively (Fig. 1).
Figure 1.
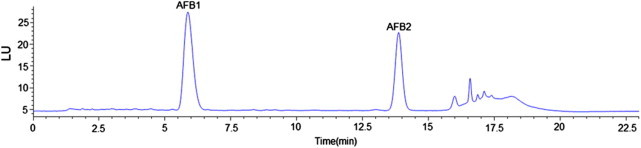
HPLC Chromatograms of AFB1 and AFB2 in polished rice grain culture. The retention times of AFB1 and AFB2 were 5.95 min and 13.98 min, respectively.
3. Results and discussion
Analysis of the aflatoxin-producing activity of A. flavus showed that 47 (37%) of 127 tested isolates produced aflatoxins, and the levels ranged from 175 to 124 101 μg kg−1 for AFB1 and 0 to 10 329 μg kg−1 for AFB2 (Table 1). Lisker et al. (1993) reported that A. flavus is often the most frequently isolated aflatoxin-producing species. Our results have supported the opinions, in which 37% of the tested A. flavus strains were found to produce aflatoxins on a rice substrate. Reddy et al. (2009c) also reported that 50.5% (43) of the tested A. flavus strains (85) isolated from discolored rice grains in India were identified as AFB1 producers. Our results showed that the percentage of aflatoxigenic strains differs significantly in different provinces, for example, 29% in Guangdong, 40.6% in Hunan, 18.2% in Jiangsu, 66.7% in Sichuan, 12.5% in Guizhou, and 37.5% in Fujian province. However, none of the A. flavus strains in Henan province was an aflatoxigenic strain. We think that this was due to different brands and sources of the rice samples and the culture conditions. So, more strains need to be analyzed for their ability to produce aflatoxins.
Table 1.
Aflatoxin production (μg kg−1 dry rice grain) on polished rice grains by A. flavus strains isolated from rice collected from twelve provinces, China.
| Source of places (province) | All tested isolates (No.) | Aflatoxigenic strains (No.) | Aflatoxins yields of aflatoxigenic strains |
|||
|---|---|---|---|---|---|---|
| Maximum of AFB1 | Minimum of AFB1 | Maximum of AFB2 | Minimum of AFB2 | |||
| Guangdong | 31 | 9 | 30 797 | 1513 | 3856 | NDa |
| Hunan | 32 | 13 | 124 101 | 175 | 10 329 | 311 |
| Jiangsu | 11 | 2 | 5081 | 1409 | 5203 | 279 |
| Yunnan | 3 | 1 | 3257 | 3257 | 631 | 631 |
| Sichuan | 15 | 10 | 35 263 | 473 | 9471 | 70 |
| Jiangxi | 2 | 1 | 2314 | 2314 | 1583 | 1581 |
| Henan | 4 | 0 | /b | / | / | / |
| Guizhou | 8 | 1 | 2020 | 2020 | 336 | 336 |
| Anhui | 3 | 2 | 21 363 | 2372 | 8461 | 2030 |
| Hubei | 4 | 2 | 9292 | 2466 | 2109 | 866 |
| Guangxi | 6 | 3 | 11 387 | 4799 | 6517 | 1450 |
| Fujian | 8 | 3 | 30 156 | 1882 | 3079 | 1956 |
| Total | 127 | 47 | 124 101 | 175 | 10 329 | ND |
| Average (mean) | 47 | 10 906 (5884) | 2573 (1968) | |||
ND, not detected.
Means that there is no aflatoxigenic strains in Henan province.
In our study, the mean yields of the aflatoxin-producing isolates were 5884 μg kg−1, 1968 μg kg−1 and 7852 μg kg−1 for AFB1, AFB2, and total AFBs (AFB1 + AFB2), respectively (Table 1). Our results are in accordance with other reports indicating that many strains of A. flavus isolated from corn and other widely varying substrates have a high potential for aflatoxin production (Joffe, 1969; Graciela et al., 2003; Reddy et al., 2009a,c, 2011). However, the production of aflatoxins by different strains of A. flavus varies widely. The A. flavus strains tested in our study produced AFB1 in the range from 175 to 124 101 μg kg−1 and AFB2 from not detected to 10 328 μg kg−1. These results are in agreement with Abbas et al. (2005) who observed greater variations in aflatoxin production by A. flavus. Different aflatoxin production capabilities of the A. flavus strains would be influenced by different sources of the strains and environmental conditions.
The results of our study showed that 91.5% (43) of aflatoxigenic A. flavus strains produced higher levels of AFB1 than AFB2 in rice cultures. Only four isolates, which were isolated from Hunan, Sichuan and Fujian provinces, produced more AFB2 (1251, 311, 514 and 1956 μg kg−1) than AFB1 (1166, 175, 474 and 1881 μg kg−1) in rice culture. Among the tested A. flavus isolates, none of the four strains collected from the Henan province had the ability to produce aflatoxins. However, in the other eleven provinces, there has been at least one strain which can produce aflatoxins.
Rice and its products are primary foods for human consumption throughout the world, especially in China. Rice represents a very good substrate for fungal growth and toxinogenesis because it is used as an ideal culture growth medium to test the toxigenic potential of isolated strains (Bars and Bars, 1992). Our results showed that rice as a substrate is susceptible to AFB1 and AFB2 accumulation from A. flavus strains, and the mean yield of AFB1 in rice grains from the A. flavus strains was 5884 μg kg−1. One isolate of A. flavus (HN 65), which was isolated from Hunan province, produced the highest amount of AFB1 (124 101 μg kg−1) and AFB2 (10 329 μg kg−1) in rice cultures. In other studies, Reddy et al. (2009c) reported that one isolate of A. flavus produced AFB1 in the range 386 000–415 000 μg kg−1 on four rice cultures. Fouzia and Samajpati (2000) reported AFB1 production by A. flavus on rice grains ranging from 555 to 10 416 μg kg−1 from India. Reddy et al. (2011) observed AFB1 accumulation from A. flavus on rice grains ranging from 3125.2 to 15 645.2 μg kg−1. All these results show that rice grain has a high risk of contamination by aflatoxins. In fact, many studies have reported that certain levels of aflatoxins have been detected in rice in many countries (Trucksee, 2000; Tanaka et al., 2007; Reddy et al., 2008).
4. Conclusion
The results of this study show that 37% (47) of the tested A. flavus strains produced aflatoxins, and most of the aflatoxigenic A. flavus strains showed a high capacity for aflatoxin production in rice grain. The high production of aflatoxins in rice grain cultures by A. flavus strains isolated from rice in China may have been due to incubation under optimal conditions. In fact, the levels of aflatoxins on rice samples in China are very low (unpublished data).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 31071546).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbas H.K., Weaver M.A., Zablotowicz R.M., Horn B.W., Shier W.T. Relationships between aflatoxin production and sclerotia formation among isolates of Aspergillus section Flavi from the Mississippi Delta. Eur. J. Plant Pathol. 2005;112:283–287. [Google Scholar]
- Almeida M.I., Almeida N.G., Carvalho K.L., Goncalves G.A.A., Silva C.N., Santos E.A., Garcia J.C., Vargas E.A. Co-occurrence of aflatoxins B1, B2, G1 and G2, ochratoxin A, zearalenone, deoxynivalenol, and citreoviridin in rice in Brazil. Food Addit. Contam. Part A. 2012;29:694–703. doi: 10.1080/19440049.2011.651750. [DOI] [PubMed] [Google Scholar]
- Aydin A., Aksu H., Gunsen U. Mycotoxin levels and incidence of mould in Turkish rice. Environ. Monit. Assess. 2011;178:271–280. doi: 10.1007/s10661-010-1688-9. [DOI] [PubMed] [Google Scholar]
- Bansal J., Pantazopoulos P., Tam J., Cavlovic P., Kwong K., Turcotte A.M., Lau B.P.Y., Scott P.M. Surveys of rice sold in Canada for aflatoxins, ochratoxin A and fumonisins. Food Addit. Contam. Part A. 2011;28:767–774. doi: 10.1080/19440049.2011.559279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bars L.J., Bars L.P. Fungal contamination of aromatic herbs, aflatoxinogenesis and residues in infusions. Microbiol. Aliment. Nutr. 1992;10:267–271. [Google Scholar]
- Davis N.D., Diener U.L. Some characteristics of toxigenic and nontoxigenic isolates of A. flavus and A. parasiticus. In: Diener U.L., Asquith R.I., Dickens J.W., editors. Aflatoxin and A. flavus in Corn. Vol. 279. 1983. pp. 1–5. (Southern Coop Ser Bull Alabama Agric Exp Stavol). [Google Scholar]
- Elena S.B., Magda C., Ignacio M.R., Pavel C.U., Josefina C.E., Sandra G.A., Jose M.M.V. Aflatoxin (B1, B2, G1, and G2) contamination in rice of Mexico and Spain from local sources or imported. J. Food Sci. 2013;78:1822–1829. doi: 10.1111/1750-3841.12291. [DOI] [PubMed] [Google Scholar]
- Fouzia B., Samajpati N. Mycotoxin production on rice, pulses and oil seeds. Naturwissenschaften. 2000;87:275–277. doi: 10.1007/s001140050720. [DOI] [PubMed] [Google Scholar]
- Giray B., Girgin G., Engin A.B., Aydm S., Sahin G. Aflatoxin levels in wheat samples consumed in some regions of Turkey. Food Control. 2007;18:23–29. [Google Scholar]
- Graciela V., Andrea P., Virginia F.P., Ricardo C., Claudia D. Variability of aflatoxin and cyclopiazonic acid production by Aspergillus section flavi from different substrates in Argentina. Int. J. Food Microbiol. 2003;8:79–84. doi: 10.1016/s0168-1605(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Hussaini A.M., Michael F.D., Patrick B.N., Mulunda M., Adamu Y.K. Natural multi-occurrence of mycotoxins in rice from Niger state, Nigeria. Mycotoxin Res. 2011;27:97–104. doi: 10.1007/s12550-010-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC . Vol. 56. International Agency for Research on Cancer; 1993. Food items and constituents, heterocyclic aromatic amines and mycotoxins. (IARC monographs on the evaluation of carcinogenic risks to humans). (pp. 489-521) [Google Scholar]
- Joffe A.Z. Aflatoxin produced by 1626 isolates of Aspergillus flavus from groundnut kernels and soils in Israel. Nature. 1969;221:492. doi: 10.1038/221492a0. [DOI] [PubMed] [Google Scholar]
- Klich M.A. Aspergillus flavus: the major producer of aflatoxin. Mol. Plant Pathol. 2007;8:713–722. doi: 10.1111/j.1364-3703.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Lai X.W., Sun D.L., Ruan C.Q., Zhang H., Liu C.L. Rapid analysis of aflatoxins B1, B2 and ochratoxin A in rice samples using dispersive liquid-liquid microextraction combined with HPLC. J. Sep. Sci. 2014;37:92–98. doi: 10.1002/jssc.201300970. [DOI] [PubMed] [Google Scholar]
- Lisker J., Michaeli R., Frank Z.R. Mycotoxigenic potential of Aspergillus flavus strains isolated from groundnuts growing in Israel. Mycopathologia. 1993;122:177–183. doi: 10.1007/BF01103479. [DOI] [PubMed] [Google Scholar]
- Liu X., Yin X., Li Y., Meng Z. Investigation on toxic-producing of A. flavus in grains in different region, China. Chin. J. Med. Academician. 1981;3:266–269. (in Chinese) [PubMed] [Google Scholar]
- Liu Z., Gao J., Yu J. Aflatoxins in stored maize and rice grains in Liaoning Province, China. J. Stored Prod. Res. 2006;42:468–479. [Google Scholar]
- Miguel A.M.R., Guillermo S.F. Aflatoxin-producing potential of Aspergillus flavus strains isolated from Spanish poultry feeds. Mycopathologia. 1986;95:129–132. doi: 10.1007/BF00437115. [DOI] [PubMed] [Google Scholar]
- Ok H.E., Kim D.M., Kim D., Chung S.H., Chung M.S., Park K.H., Chun H.S. Mycobiota and natural occurrence of aflatoxin, deoxynivalenol, nivalenol and zearalenone in rice freshly harvested in South Korea. Food Control. 2014;37:284–291. [Google Scholar]
- Peraica M., Radic B., Lucic A., Pavlovic M. Toxic effects of mycotoxins in humans. Bull. World Health Org. 1999;77:754–766. [PMC free article] [PubMed] [Google Scholar]
- Reddy K.R.N., Reddy C.S., Abbas H.K., Abel C.A., Muralidharan K. Mycotoxigenic fungi, mycotoxins, and management of rice grains. Toxin Rev. 2008;27:287–317. [Google Scholar]
- Reddy K.R.N., Reddy C.S., Kumar P.N., Reddy C.S., Muralidharan K. Genetic variability of aflatoxin B1 producing Aspergillus flavus strains isolated from discolored rice grains. World J. Microbiol. Biotechnol. 2009;25:33–39. [Google Scholar]
- Reddy K.R.N., Reddy C.S., Muralidharan K. Detection of Aspergillus spp. and aflatoxin B1 in rice in India. Food Microbiol. 2009;26:27–31. doi: 10.1016/j.fm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Reddy K.R.N., Saritha P., Reddy C.S., Muralidharan K. Aflatoxin B1 producing potential of Aspergillus flavus strains isolated from stored rice grains. Afr. J. Biotechnol. 2009;8:3303–3308. [Google Scholar]
- Reddy K.R.N., Raghavender C.R., Salleh B., Reddy C.S., Reddy B.N. Potential of aflatoxin B1 production by Aspergillus flavus strains on commercially important food grains. Int. J. Sci. Technol. 2011;46:161–165. [Google Scholar]
- Reiter E.V., Vouk F., Bohm J., Ebrahim R.F. Aflatoxins in rice – a limited survey of products marketed in Austria. Food Control. 2010;21:988–991. [Google Scholar]
- Richard J.L. Some major mycotoxins and their mycotoxicoses – an overview. Int. J. Food Microbiol. 2007;119:3–10. doi: 10.1016/j.ijfoodmicro.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Sun G., Wang S., Hu X., Su J., Zhang Y., Xie Y., Zhang H., Tang L., Wang J.S. Co-contamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit. Contam. Part A. 2011;28:461–470. doi: 10.1080/19440049.2010.544678. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Sago Y., Zheng Y.Z., Nakagawa H., Kushiro M. Mycotoxins in rice. Int. J. Food Microbiol. 2007;119:59–66. doi: 10.1016/j.ijfoodmicro.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Tang J. Research progress of aflatoxin in Guangxi. Chin. J. Vet. Sci. 1999;5:33–35. (in Chinese) [Google Scholar]
- Trucksee M.W. Joint Mycotoxin committee report. J. AOAC Int. 2000;83:536–541. [Google Scholar]
- Wang J., Liu X.M. Contamination of aflatoxins in different kinds of food in China. Bio. Environ. Sci. 2007;20:483–487. [PubMed] [Google Scholar]
- Yu J., Mohawed S.M., Bhatnagar D., Cleveland T.E. Substrate-induced lipase gene expression and aflatoxin production in Aspergillus parasiticus and Aspergillus flavus. J. Appl. Microbiol. 2003;95:1334–1342. doi: 10.1046/j.1365-2672.2003.02096.x. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang D., Fang F., Zhen Y., Liao X. Food safety and rice production in China. Res. Agric. Modern. 2005;26:85–88. (in Chinese) [Google Scholar]


