Abstract
The current study was undertaken to evaluate the protective activity of olive and rosemary leaves extracts on experimental liver cirrhosis induced by thioacetamide (TAA) in Wistar male rats. Highly significant decline in the values of body weight gain and highly statistically increase of liver/body weight ratio were noted in rats treated with TAA. Furthermore, the levels of serum alanine aminotransferase, aspartate aminotransferase, gamma glutamyl transferase, alkaline phosphatase and total bilirubin were statistically increased. Additionally, light microscopic examination of liver sections from rats treated with TAA showed a marked increase in the extracellular matrix collagen content and bridging fibrosis was prominent. There were bundles of collagen surrounding the lobules that resulted in large fibrous septa and distorted tissue architecture. Interestingly, the findings of this experimental study indicated that the extracts of olive and rosemary leaves and their combination possess hepatoprotective properties against TAA-induced hepatic cirrhosis by inhibiting the physiological and histopathological alterations. Moreover, these results suggest that the hepatoprotective effects of these extracts may be attributed to their antioxidant activities.
Keywords: Liver cirrhosis, Thioacetamide, Olive leaves, Rosemary leaves, Rats
1. Introduction
Liver or hepatic cirrhosis affects hundreds of millions of patients worldwide. Liver cirrhosis is the terminal stage of various chronic liver diseases (Gressner, 1996; Schuppan and Afdhal, 2008). Moreover, the majority of patients worldwide with hepatocellular carcinoma (HCC) have underlying liver cirrhosis, supported by the fact that in 80% of autopsies of patients with HCC, cirrhosis is found (Simonetti et al., 1991).
Thioacetamide (TAA), also known as thioacetimidic acid, or acetothioamide (CH3CSNH2), was originally used as a fungicide (Vadi and Neal, 1981). Moreover, many experimental studies showed that TAA induced liver fibrosis and cirrhosis in experimental animals (Al-Attar, 2011, 2012; Wang et al., 2012; Fatima and Mahboob, 2013; Shao et al., 2014).
Recently, World Health Organization (WHO) defined traditional medicine (including herbal drugs) as therapeutic practices that have been in existence, often for hundreds of years, before the development and spread of modern medicine and are still in use today (Kashaw et al., 2011). The olive tree (Olea europaea L.), family: Oleaceae, and in particular, its leaves have been used for the treatment of wounds, fever, diabetes, gout, atherosclerosis and hypertension since ancient times (Jänicke et al., 2003). Rosemary (Rosmarinus officinalis Linn.), mint (Labiatae) family, is a common household plant grown in many parts of the world. It is commonly used as a spice and flavoring agent in food processing (Saito et al., 2004). However, rosemary and its constituents have a therapeutic potential in treatment or prevention of many physiological, biochemical and histopathological alterations (al-Sereiti et al., 1999; Osakabe et al., 2004; Sancheti and Goyal 2006; Gaya et al., 2013). The purpose of the present study is to compare the effects of olive and rosemary leaves extracts on experimental liver cirrhosis induced by TAA in rats.
2. Materials and methods
2.1. Animals
Male albino rats of the Wistar strain (Rattus norvegicus), weighing 72.6–103.4 g were used in the present study. The experimental animals were obtained from the Experimental Animal Unit of King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. Rats were acclimatized to the laboratory conditions for 10 days prior to the initiation of experimental treatments. The experimental animals were housed in standard plastic cages and maintained under controlled laboratory conditions of humidity (65%), temperature (20 ± 1 °C) and 12:12 h light:dark cycle. Rats were fed ad libitum on normal commercial chow and had free access to water. The experimental treatments were conducted in accordance with ethical guidelines of the Animal Care and Use Committee of King Abdulaziz University.
2.2. Olive and rosemary leaf extraction
Olive and rosemary leaves of fine quality were obtained from a commercial market, Jeddah, Saudi Arabia. The leaves were thoroughly washed and dried at room temperature. The methods of Sakr and Lamfon (2012), and Al-Attar and Abu Zeid (2013) were used to prepare the extracts with some modifications. The dried olive leaves (50 g) were powdered and added to 2 liters of hot water in a flask. After 6 h, the mixture was slowly boiled for 1 h. After boiling period, the mixture was cooled at room temperature and it was gently subjected to an electric mixer for 10 min. Also, the dried rosemary leaves (50 g) were powdered and added to 2 liters of hot water in a flask. After 6 h, the mixture was slowly boiled for 1 h. After boiling period, the mixture was cooled at room temperature and it was gently subjected to an electric mixer for 10 min. Thereafter the solutions of olive and rosemary leaves were filtered. Finally, the filtrates were evaporated in an oven at 40 °C to produce dried residues (active principles). With references to the powdered samples, the yields means of the olive and rosemary extracts were 18.7% and 20.6% respectively. Furthermore, these extracts were prepared every 2 weeks and stored in a refrigerator for subsequent experiments.
2.3. Experimental design
A total of forty-eight rats were randomly divided into eight experimental groups, six of rats each. The experimental groups were treated as follows:
-
1.
Rats of group 1 were served as controls and intraperitoneally injected with saline solution (0.9% NaCl), twice weekly for twelve weeks.
-
2.
Rats of group 2 were given 300 mg/kg body weight of TAA (Sigma–Aldrich Corp., St. Louis, MO, USA) by intraperitoneal injection, twice weekly for twelve weeks.
-
3.
Rats of group 3 were intraperitoneally injected with TAA at the same dose given to group 2 and were orally supplemented with olive leaves extract at a dose of 200 mg/kg body weight/day for twelve weeks.
-
4.
Rats of group 4 were intraperitoneally injected with TAA at the same dose given to group 2 and were orally supplemented with rosemary leaves extract at a dose of 200 mg/kg body weight/day for twelve weeks.
-
5.
Animals of group 5 were intraperitoneally injected with TAA at the same dose given to group 2 and were orally supplemented with olive leaves extract (100 mg/kg body weight/day) and rosemary leaves extract (100 mg/kg body weight/day) for twelve weeks.
-
6.
Rats of group 6 were intraperitoneally received saline solution at the same dose given to group 1 and were orally supplemented with olive leaves extract at the same dose given to group 3 for twelve weeks.
-
7.
Animals of group 7 were intraperitoneally received saline solution at the same dose given to group 1 and were orally supplemented with rosemary leaves extract at the same dose given to group 4 for twelve weeks.
-
8.
Rats of group 8 were intraperitoneally received saline solution at the same dose given to group 1 and were supplemented with olive and rosemary leaves extracts at the same dose given to group 5 for twelve weeks.
2.4. Body weight determinations
The body weights of rats were determined at the start of the experimental period and after twelve weeks using a digital balance. These weights were measured at the same time during the morning (Al-Attar and Zari, 2010). Moreover, the experimental animals were observed for signs of abnormalities throughout the period of study.
2.5. Blood serum analyses
After twelve weeks, the experimental animals were fasted for 12 h, water was not restricted, and then anaesthetized with diethyl ether. Blood samples were collected from orbital venous plexus in non-heparinized tubes, centrifuged at 2500 rpm for 15 min and blood sera were then collected and stored at 4 °C prior immediate determination of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT), alkaline phosphatase (ALP) and total bilirubin. The method of Reitman and Frankel (1957) was used to determine the levels of serum ALT and AST. Serum GGT level was measured according to the method of Szasz (1969). The method of MacComb and Bowers (1972) was carried out to determine the level of serum ALP. Total bilirubin concentration was determined using the method of Doumas et al. (1973).
2.6. Histopathological examinations
After blood sampling, rats were dissected and the liver tissues were preserved in 10% buffered formalin immediately after removal from the animals, embedded with paraffin. After routine processing, paraffin sections of each tissue were cut into 4 μm thickness and stained with hematoxylin and eosin. Moreover, liver sections were subjected to Masson’s trichrome stain. All liver sections were examined using a light microscope and photographed.
2.7. Weight changes of liver
After twelve weeks, livers were carefully excised and weighed for the evaluation of their ratios/body weight. The ratio was calculated by the following equation:
2.8. Statistical analysis
The data were analyzed using the Statistical Package for Social Sciences (SPSS for windows, version 12.0). Each value is expressed as mean ± standard deviation (S.D.) and values were analyzed using two-way analysis of variance (ANOVA) to determine differences between the mean values of experimental groups. P-values of less than 0.05 were considered as significant.
3. Results
Fig. 1 demonstrates the changes of body weights of all experimental groups after twelve weeks. Significant decreases in the values of body weight gain were observed in rats treated with TAA, TAA plus olive leaves extract, TAA plus rosemary leaves extract, and TAA plus olive and rosemary leaves extracts. The minimum body weight gain was noted in TAA-intoxicated rats (220.1%). The maximum body weight gain was noted in normal control rats (381.0%). Supplementation with the tested extracts showed remarkable lowering effect on the percentage changes of body weight in rats treated with TAA plus olive leaves extract, TAA plus rosemary leaves extract, and TAA plus olive and rosemary leaves extracts which amounted 251.5%, 266.9% and 254.9% respectively. Oral administration of tested extracts to normal rats caused significant increases in body weight gain. The change of body weight gain was 286.9% in normal rats supplemented with olive leaves extract. Supplementation with rosemary leaves extract in normal rats showed a remarkable increase of the percentage change of body weight (301.4%). The percentage change of body weight gain in normal rats fed with olive and rosemary leaves extracts is 297.6%. In comparison with normal control rats, notably increases in the values of liver/body weight ratio were observed in TAA (73.7%), TAA plus olive leaves extract (57.8%), TAA plus rosemary leaves extract (46.0%), and TAA plus olive and rosemary leaves extracts (58.5%) treated rats. The values of liver/body weight ratio were statistically unchanged in normal rats supplemented with olive leaves extract, rosemary leaves extract, and olive and rosemary leaves extracts (Fig. 2).
Figure 1.
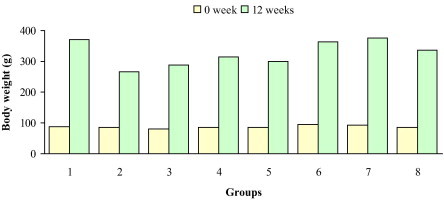
Changes of body weight in control (group 1), TAA (group 2), TAA plus olive leaves extract (group 3), TAA plus rosemary leaves extract (group 4), TAA plus olive and rosemary leaves extracts (group 5), olive leaves extract (group 6), rosemary leaves extract (group 7), and olive and rosemary leaves extracts (group 8) treated rats.
Figure 2.
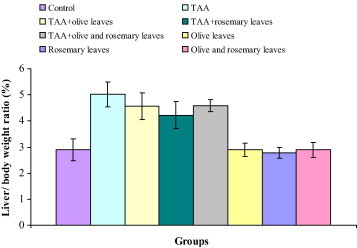
Changes of liver /body weight ratio in control, TAA, TAA plus olive leaves extract, TAA plus rosemary leaves extract, TAA plus olive and rosemary leaves extracts, olive leaves extract, rosemary leaves extract, and olive and rosemary leaves extracts treated rats.
TAA administration to normal rats significantly elevated the level of serum ALT (1521.5%) compared with control rats and other treated groups. The levels of this parameter were statistically increased in rats with TAA plus olive leaves extract (401.3%), TAA plus rosemary leaves extract (308.4%), and TAA plus olive and rosemary leaves extracts (359.1%). As shown in Table 1, significant elevations in the level of serum AST were noted in TAA (1113.8%), TAA plus olive leaves extract (258.3%), TAA plus rosemary leaves extract (131.4%), and TAA plus olive and rosemary leaves extracts (187.4%) treated rats compared with control rats. Remarkable elevations in the level of serum GGT were observed in rats treated with TAA (374.9%), TAA plus olive leaves extract (71.0%), TAA plus rosemary leaves extract (56.3%), and TAA plus olive and rosemary leaves extracts (43.2%) when compared to control rats. The levels of serum ALP were statistically increased in rats treated with TAA (604.7%), TAA plus olive leaves extract (43.0%), TAA plus rosemary leaves extract (25.7%), and TAA plus olive and rosemary leaves extracts (32.6%). Notable increases in the level of serum total bilirubin were observed in rats treated with TAA (215.2%), TAA plus olive leaves extract (55.6%), TAA plus rosemary leaves extract (56.2%), and TAA plus olive and rosemary leaves extracts (77.9%) as compared with control group. On the other hand, insignificant changes of serum ALT, AST, GGT, ALP and total bilirubin levels were observed in olive leaves extract, rosemary leaves extract, and olive and rosemary leaves extracts treated rats as compared with control rats (Table 1).
Table 1.
The levels of ALT, AST, GGT, ALP and total bilirubin in serum from control, TAA, TAA plus olive leaves extract, TAA plus rosemary leaves extract, TAA plus olive and rosemary leaves extracts, olive leaves extract, rosemary leaves extract, and olive and rosemary leaves extracts treated rats (n = 6). Percentage changes are included in parentheses.
| Treatments | Parameters |
||||
|---|---|---|---|---|---|
| ALT (U/L) | AST (U/L) | GGT (U/L) | ALP (U/L) | Total bilirubin (μmol/L) | |
| Control | 49.17 ± 3.56 | 98.50 ± 5.60 | 4.03 ± 0.22 | 314.90 ± 23.92 | 6.30 ± 0.72 |
| TAA | 797.27 ± 50.6 (+1521.5) | 1195.54 ± 158.45 (+1113.8) | 19.14 ± 1.42 (+374.9) | 2219.00 ± 135.62 (+604.7) | 19.86 ± 4.34 (+215.2) |
| TAA + olive leaves | 264.51 ± 29.21 (+401.3) | 352.90 ± 20.75 (+258.3) | 6.89 ± 1.51 (+71.0) | 450.33 ± 127.86 (+43.0) | 9.80 ± 0.84 (+55.6) |
| TAA + rosemary leaves | 200.80 ± 25.95 (+308.4) | 227.95 ± 19.91 (+131.4) | 6.30 ± 0.47 (+56.3) | 395.92 ± 62.61 (+25.7) | 9.84 ± 1.09 (+56.2) |
| TAA + olive and rosemary leaves | 225.75 ± 19.12 (+359.1) | 283.13 ± 58.32 (+187.4) | 5.77 ± 1.01 (+43.2) | 417.58 ± 71.55 (+32.6) | 11.21 ± 1.68 (+77.9) |
| Olive leaves | 49.45 ± 4.29 (+0.6) | 94.81 ± 3.72 (−3.8) | 4.18 ± 0.17 (+3.7) | 307.05 ± 11.32 (−2.5) | 6.34 ± 0.85 (+0.6) |
| Rosemary leaves | 49.11 ± 2.44 (−0.1) | 94.17 ± 8.08 (−4.4) | 4.03 ± 0.22 (0.0) | 314.10 ± 25.53 (−0.3) | 6.26 ± 0.62 (−0.6) |
| Olive and rosemary leaves | 50.30 ± 1.97 (+2.3) | 93.88 ± 9.71 (+4.7) | 3.89 ± 0.19 (−3.47) | 298.12 ± 12.39 (−5.3) | 6.31 ± 0.65 (+0.2) |
Light microscopic examination indicated a normal structure of the liver in the control rats (Fig. 3A) as well as the rats treated with olive leaves extract (Fig. 3J), rosemary leaves extract (Fig. 3K), and olive and rosemary leaves extracts (Fig. 3L). In the rats treated with only TAA, there was a marked increase in the extracellular matrix collagen content and bridging fibrosis was prominent. There were bundles of collagen surrounding the lobules that resulted in large fibrous septa and distorted tissue architecture. The administration of TAA induced nodular transformations in liver parenchyma similar to those found in human nodular cirrhosis. Moreover, the parenchyma nodules were surrounded by extensive of fibrous septae which divided the liver into pseudolobules. TAA administration induced centrilobular necrosis, hepatic cells surrounding central vein showed various degenerative changes like cloudy swelling, hydropic degeneration and necrosis with loss of nucleus (Fig. 3B and C). In rats treated with TAA plus olive leaves extract (Fig. 3D and E), TAA plus rosemary leaves extract (Fig. 3F and G), TAA plus olive and rosemary leaves extracts (Fig. 3H and I), liver sections showed that the degree of fibrosis was substantially less than the TAA-alone treated rats. Furthermore, the liver cells showed slight alterations compared with liver cells structure of rats treated with only TAA. Additionally, there are no any obvious features of hepatic cirrhosis in rats treated with TAA plus olive leaves extract, TAA plus rosemary leaves extract, and TAA plus olive and rosemary leaves extracts.
Figure 3.
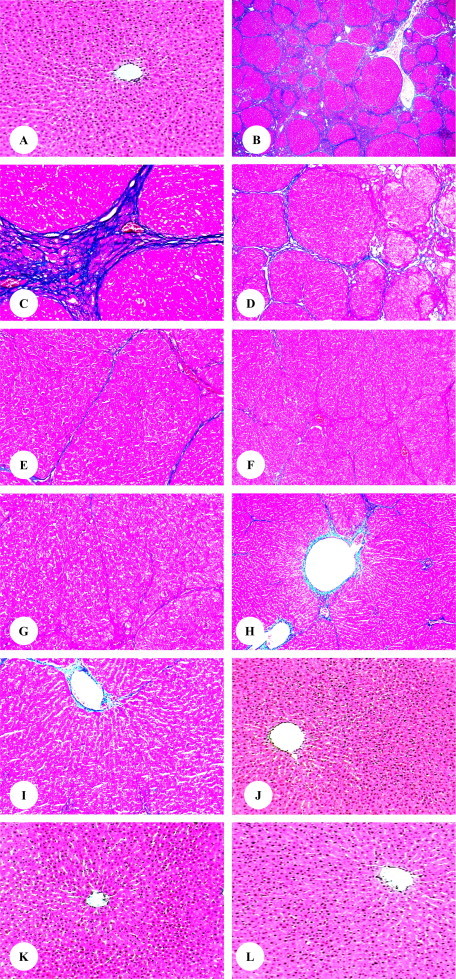
(A–L) Photomicrographs of liver sections in each group. (A) control (200×), (B and C) TAA (40× and 200×), (D and E) TAA plus olive leaves extract (100× and 200×), (F and G) TAA plus rosemary leaves extract (100× and 200×), (H and I) TAA plus olive and rosemary leaves extracts (100× and 200×), (J) olive leaves extract (200×), (K) rosemary leaves extract (200×), and (L) olive and rosemary leaves extracts (200×) treated rats.
4. Discussion
Hepatic injuries lead to attenuation of metabolic functions regulated by liver, and has remained one of the serious health problems (Wolf, 1999) threatening the human society. Cirrhosis is the endpoint of the fibrogenic process that accompanies chronic liver injury, and complications arising from cirrhosis are the ultimate cause of death in many patients with chronic liver disease (Friedman, 2006). Despite recent therapeutic advances, many liver diseases remain relentlessly progressive because specific therapies to target the underlying etiologies of the liver diseases are not available. Therefore, the demand for liver transplantation is likely to increase unless more effective therapeutic and antifibrotic agents are developed.
The present study demonstrated that TAA at a dose of 300 mg/kg body weight, twice weekly for a period of 12 weeks induced hepatic cirrhosis with many histopathological alterations in male Wistar rats. TAA intoxication has shown a dramatic decrease in the percentage of body weight gain and significant increases in the relative weights of body liver. Moreover, the obtained results showed an elevation in the levels of serum ALT, AST, GGT, ALP and total bilirubin. Similar observations were noted in experimental animals treated with TAA (Al-Attar, 2011, 2012; Salama et al., 2013; Zargar, 2014; Kim et al., 2014).
The obtained results showed that the treatment of rats with olive and rosemary leaves extracts or their combination improved the physiological and histopathological alterations induced by TAA intoxication. This indicated the effectiveness of these extracts in prevention of TAA toxicity. Hierarchically, the current study revealed that the most effective treatment was rosemary leaves extract followed by the combination of olive and rosemary leaves extracts and olive leaves extract The possible mechanism of the studied extracts as hepatoprotective factors may be due to its antioxidant effect which impair the activation of TAA into the reactive form. Bruck et al. (2004) stated that the chronic of TAA administration induced liver cirrhosis and oxidative stress. Aydin et al. (2010) showed that TAA administration resulted in hepatic fibrosis, significant increases in plasma transaminase activities as well as hepatic hydroxyproline and lipid peroxide levels, while liver glutathione (GSH) and superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) protein expressions and activities decreased. Al-Attar (2012) reported that the levels of liver SOD and GSH were significantly decreased in mice treated with TAA. Additionally, many investigations indicated that TAA caused a significant decrease in the levels of liver SOD and GSH (Uskoković-Marković et al., 2007; Mansour and El-Kabany, 2009; Kantah et al., 2011; Fatima and Mahboob, 2013).
The main constituent of the olive leaves is oleuropein, one of iridoide monoterpenes, which is thought to be responsible for pharmacological effects. Furthermore, the olive leaves contain triterpenes (oleanolic and maslinic acid), flavonoides (e.g., luteolin, apigenine, rutin), and chalcones such as olivin, olivin-diglucoside (Meirinhos et al. 2005; Pereira et al. 2007). It was shown that total olive leaves extract had antioxidant activity higher than vitamin C and vitamin E, due to the synergy between flavonoids, oleuropeosides and substituted phenols (Benavente-Garcia et al., 2000). Khalil (2004) investigated the hepatoprotective activity of an aqueous extract of olive leaves against overdose paracetamol in male albino rats. She concluded that an aqueous extract of olive leaves has antioxidant property which can protect liver damage occurred by overdose paracetamol in male albino rats.
Rosemary, via one of its active constituents rosmarinic acid (RA), is often used by herbalists and naturopaths for its beneficial effects on the liver disease. Li et al. (2010) investigated the potential effects of RA on hepatic fibrosis, the consequence of sustained wound healing responses by the liver. Cultures of hepatic stellate cells (HSCs) were used for in vitro assessment and tetrachloride treated rats as in vivo subjects. They reported that RA could prevent hepatic fibrosis due to chronic liver damage and thus delay cirrhosis development. Abdel-Wahhab et al. (2011) investigated the chemoprotective effect of rosemary extract against CCl4-induced hepatotoxicity in adult male Wistar albino rats. Administration of rosemary extract before or during the treatment with CCl4 improved all biochemical parameters and histological picture of the liver. They reported that rosemary extract has a protective effect against hepatotoxicity and this extract inhibited and reduced the CCl4-induced hepatotoxicity in rats possibly by scavenging or blocking the formation of free radicals generated during CCl4 metabolism. These improving effects of rosemary could be attributed to the bioactive constituents that alleviated the deleterious effect of CCl4 either by the well-known scavenging action or the antioxidant properties that inhibited lipid peroxidation, stabilized the reactive radicals, preserve the cellular integrity and restrain the severity of CCl4.
One of the most important findings in the present study is the observation that the studied extracts of olive and rosemary leaves and their combination were effective in reducing the TAA induced liver cirrhosis, that were proven by physiological analysis and histopathological evaluation. Collectively, the results of this study suggest that the effects of these extracts against TAA-induced hepatic cirrhosis possibly due to antioxidant properties of their natural chemical constituents. Moreover, this study is from the first investigations that apply scientific methodology to looking at how these extracts exert its role in the protection action against physiological disturbances and histopathological alterations in hepatic cirrhosis cases and may be in its complications. Additional physiological, biochemical and histopathological investigations are needed to explore the possible use of different doses of these extracts and their constituents as potential natural therapeutic agents in therapy of hepatic cirrhosis against TAA and may be against other fibrogenic factors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Wahhab K.G.E., El-Shamy K.A., El-Beih N.A.E., Morcy F.A., Mannaa F.A.E. Protective effect of a natural herb (Rosmarinus officinalis) against hepatotoxicity in male albino rats. Com. Sci. 2011;2:9–17. [Google Scholar]
- Al-Attar A.M. Attenuating effect of Ginkgo biloba leaves extract on liver fibrosis induced by thioacetamide in mice. J. Biomed. Biotechnol. 2012;2012:1–9. doi: 10.1155/2012/761450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attar A.M. Hepatoprotective influence of vitamin C on thioacetamide-induced liver cirrhosis in Wistar male rats. J. Pharmacol. Toxicol. 2011;6:218–233. [Google Scholar]
- Al-Attar A.M., Abu Zeid I.M. Effect of tea (Camellia sinensis) and olive (Olea europaea L.) leaves extracts on male mice exposed to diazinon. BioMed Res. Int. 2013;2013:1–6. doi: 10.1155/2013/461415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attar A.M., Zari T.A. Influences of crude extract of tea leaves, Camellia sinensis, on streptozotocin diabetic male albino mice. Saudi J. Biol. Sci. 2010;17:201–295. doi: 10.1016/j.sjbs.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Sereiti M.R., Abu-Amer K.M., Sen P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J. Exp. Biol. 1999;37:124–130. [PubMed] [Google Scholar]
- Aydin A.F., Küskü-Kiraz Z., Doğru-Abbasoğlu S., Güllüoğlu M., Uysal M., Koçak-Toker N. Effect of carnosine against thioacetamide-induced liver cirrhosis in rat. Peptides. 2010;31:67–71. doi: 10.1016/j.peptides.2009.11.028. [DOI] [PubMed] [Google Scholar]
- Benavente-Garcia O., Castillo J., Lorente J., Ortuno A., Del Rio J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000;68:457–462. [Google Scholar]
- Bruck R., Schey R., Aeed H., Hochman A., Genina O., Pines M. A protective effect of pyrrolidine dithiocarbamate in a rat model of liver cirrhosis. Liver Int. 2004;24:169–176. doi: 10.1111/j.1478-3231.2004.00900.x. [DOI] [PubMed] [Google Scholar]
- Doumas B.T., Perry B.W., Sasse E.A., Straumfjord J.V. Standardization in bilirubin assays: evaluation of selected methods and stability of bilirubin solutions. Clin. Chem. 1973;19:984–993. [PubMed] [Google Scholar]
- Fatima S.N., Mahboob T. Role of selenium in protection of liver cirrhosis. Pak. J. Pharm. Sci. 2013;26:1097–1102. [PubMed] [Google Scholar]
- Friedman S.L. Transcriptional regulation of stellate cell activation. J. Gastroenter. Hepatol. 2006;21:S79–S83. doi: 10.1111/j.1440-1746.2006.04585.x. [DOI] [PubMed] [Google Scholar]
- Gaya M., Repetto V., Toneatto J., Anesini C., Piwien-Pilipuk G., Moreno S. Antiadipogenic effect of carnosic acid, a natural compound present in Rosmarinus officinalis, is exerted through the C/EBPs and PPARγ pathways at the onset of the differentiation program. Biochim. Biophys. Acta. 2013;1830:3796–3806. doi: 10.1016/j.bbagen.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Gressner A.M. Transdifferentiation of hepatic stellate cells (Ito cells) to myofibroblasts: a key event in hepatic fibrogenesis. Kidney Int. 1996;49:S39–S45. [PubMed] [Google Scholar]
- Jänicke C., Grünwald J., Brendler T. Wissenschaftliche Verlagsgesellschaft; Stuttgart: 2003. Handbuch Phytotherapie. [Google Scholar]
- Kantah M.K., Kobayashi R., Sollano J., Naito Y., Solimene U., Jains S., Catanzaro R., Minelli E., Polimeni A., Marotta F. Hepatoprotective activity of a phytotherapeutic formula on thioacetamide-induced liver fibrosis model. Acta Biomed. 2011;82:82–89. [PubMed] [Google Scholar]
- Kashaw V., Nemal A.K., Agarwal A. Hepatoprotective prospective of herbal drugs and their vesicular carriers – a review. Int. J. Res. Pharm. Biomed. Sci. 2011;2:360–374. [Google Scholar]
- Khalil E.A.M. Evaluation of the hepatoprotective activity of an aqueous extract of olive leaves in male albino rats. Egypt. J. Hosp. Med. 2004;15:118–123. [Google Scholar]
- Kim J.H., Jeong Y.J., Hong J.M., Kim H.R., Kang J.S., Lee W.J., Hwang Y.I. Chronic vitamin C insufficiency aggravated thioacetamide-induced liver fibrosis in gulo-knockout mice. Free Radic. Biol. Med. 2014;67:81–90. doi: 10.1016/j.freeradbiomed.2013.10.813. [DOI] [PubMed] [Google Scholar]
- Li G., Jiang W., Tian J., Qu G., Zhu H., Fu F. In vitro and in vivo antifibrotic effects of rosmarinic acid on experimental liver fibrosis. Phytomedicine. 2010;17:282–288. doi: 10.1016/j.phymed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- MacComb R.B., Bowers G.N., Jr. Study of optimum buffer conditions for measuring alkaline phosphatase activity in human serum. Clin. Chem. 1972;18:97–104. [PubMed] [Google Scholar]
- Mansour S.Z., El-Kabany H. Effects of Fructus Piperis Longi extract on fibrotic liver of gamma-irradiated rats. Chin. Med. 2009;4:1–8. doi: 10.1186/1749-8546-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirinhos J., Silva B.M., Valentao P., Seabra R.M., Pereira J.A., Dias A., Andrade P.B., Ferreres F. Analysis and quantification of flavonoidic compounds from Portuguese olive (Olea europaea L.) leaf cultivars. Nat. Prod. Res. 2005;19:189–195. doi: 10.1080/14786410410001704886. [DOI] [PubMed] [Google Scholar]
- Osakabe N., Yasuda A., Natsume M., Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: anti-carcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004;25:549–557. doi: 10.1093/carcin/bgh034. [DOI] [PubMed] [Google Scholar]
- Pereira A.P., Ferreira I.C., Marcelino F., Valentao P., Andrade P.B., Seabra R., Estevinho L., Bento A., Pereira J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrancosa) leaves. Molecules. 2007;12:1153–1162. doi: 10.3390/12051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–58. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Saito Y., Shiga A., Yoshida Y., Furuhashi T., Fujita Y., Niki E. Effects of novel gaseous antioxidative system containing a rosemary extract on the oxidation induced by nitrogen dioxide and ultraviolet radiation. Biosci. Biotechnol. Biochem. 2004;68:781–786. doi: 10.1271/bbb.68.781. [DOI] [PubMed] [Google Scholar]
- Sakr S.A., Lamfon H.A. Protective effect of rosemary (Rosmarinus Officinalis) leaves extract on carbon tetrachloride-induced nephrotoxicity in albino rats. Life Sci. J. 2012;9:779–785. [Google Scholar]
- Salama S.M., Abdulla M.A., AlRashdi A.S., Ismail S., Alkiyumi S.S., Golbabapour S. Hepatoprotective effect of ethanolic extract of Curcuma longa on thioacetamide induced liver cirrhosis in rats. BMC Complement. Altern. Med. 2013;13:1–17. doi: 10.1186/1472-6882-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancheti G., Goyal P.K. Effect of Rosmarinus officinalis in modulating 7,12-dimethylbenz(a)anthracene induced skin tumorigenesis in mice. Phytother. Res. 2006;20:981–986. doi: 10.1002/ptr.1989. [DOI] [PubMed] [Google Scholar]
- Schuppan D., Afdhal N.H. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C.H., Chen S.L., Dong T.F., Chai H., Yu Y., Deng L., Wang Y., Cheng F. Transplantation of bone marrow-derived mesenchymal stem cells after regional hepatic irradiation ameliorates thioacetamide-induced liver fibrosis in rats. J. Surg. Res. 2014;186:408–416. doi: 10.1016/j.jss.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Simonetti R.G., Cammá C., Fiorello F., Politi F., D’Amico G., Pagliaro L. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Digest. Dis. Sci. 1991;36:962–972. doi: 10.1007/BF01297149. [DOI] [PubMed] [Google Scholar]
- Szasz G. A kinetic photometric method for serum gamma-glutamyl transpeptidase. Clin. Chem. 1969;15:124–136. [PubMed] [Google Scholar]
- Uskoković-Marković S., Milenković M., Topić A., Kotur-Stevuljević J., Stefanović A., Antić-Stanković J. Protective effects of tungstophosphoric acid and sodium tungstate on chemically induced liver necrosis in Wistar rats. J. Pharm. Pharm. Sci. 2007;10:340–349. [PubMed] [Google Scholar]
- Vadi H.V., Neal R.A. Microsomal activation of thioacetamide-S-oxide to a metabolite(s) that covalently binds to calf thymus DNA and other polynucleotides. Chem. Biol. Interact. 1981;35:25–38. doi: 10.1016/0009-2797(81)90061-2. [DOI] [PubMed] [Google Scholar]
- Wang H., Wu G., Park H.J., Jiang P.P., Sit W.H., van Griensven L.J., Wan J.M. Protective effect of Phellinus linteus polysaccharide extracts against thioacetamide-induced liver fibrosis in rats: a proteomics analysis. Chin. Med. 2012;7:23. doi: 10.1186/1749-8546-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P.L. Biochemical diagnosis of liver disease. Indian J. Clin. Biochem. 1999;14:59–90. doi: 10.1007/BF02869152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargar S. Protective effect of Trigonella foenum-graecum on thioacetamide induced hepatotoxicity in rats. Saudi J. Biol. Sci. 2014;21:139–145. doi: 10.1016/j.sjbs.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


