Abstract
Multiple lines of evidence indicate that mood disorders are associated with abnormalities in the brain's cellular composition, especially in glial cells. Considered inert support cells in the past, glial cells are now known to be important for brain function. Treatments for mood disorders enhance glial cell proliferation, and experimental stimulation of cell growth has antidepressant effects in animal models of mood disorders. These findings suggest that the proliferation and survival of glial cells may be important in the pathogenesis of mood disorders and may be possible targets for the development of new treatments.
In this chapter, we will review the evidence for glial abnormalities in mood disorders. We will discuss glial cell biology and evidence from postmortem studies of mood disorders. This is not carry out a comprehensive review; rather we selectively discuss existing evidence in building an argument for the role of glial cells in mood disorders.
Keywords: oligodendrocyte, astrocyte, microglia, postmortem, prefrontal
INTRODUCTION
Neuroimaging and postmortem studies have now demonstrated that mood disorders, including both major depressive disorder (MDD) and bipolar disorder (BD), are associated with significant abnormalities in the cellular composition of brain areas involved in processing emotional stimuli and setting mood1, 2. Among these abnormalities are altered densities of neurons and a class of support cells collectively referred to as glia3, 4. The term glia is derived from the Greek word for glue, which reflects the now-classic conceptualization of the primary role of these cells in the brain5. Recent conceptualizations propose more active roles for glia in normal brain function, as well as the pathophysiology and relief of psychiatric illness. For example, currently known treatments for mood disorders enhance cell proliferation, including the proliferation of glia, and experimental stimulation of cell growth has antidepressant effects in animal models of mood disorders 6. These findings imply that signaling cascades or processes regulating cell proliferation and survival may be targets for existing and new treatments of mood disorders 7.
Despite the recent excitement in the field there are many unanswered questions regarding the relationship between specific anomalies of cellular configuration and function and the pathophysiology of mood disorders. In this article, we will review the evidence for cellular abnormalities in mood disorders, with a focus on glial rather than neuronal cells. We will discuss glial cell biology and postmortem studies relevant to glial cell number and function. Given space limitations, we will not carry out a comprehensive review of the topic; rather we will speculate in this context on the consequences of glial cell dysfunction for brain activity in general and mood regulation in particular. As a consequence of this approach, the selection of papers cited in this paper was not all-inclusive and focused only on those required for the discussion at hand. For a more complete assessment, the reader is referred to other comprehensive reviews of the topic by other authors3.
GLIAL CELL BIOLOGY
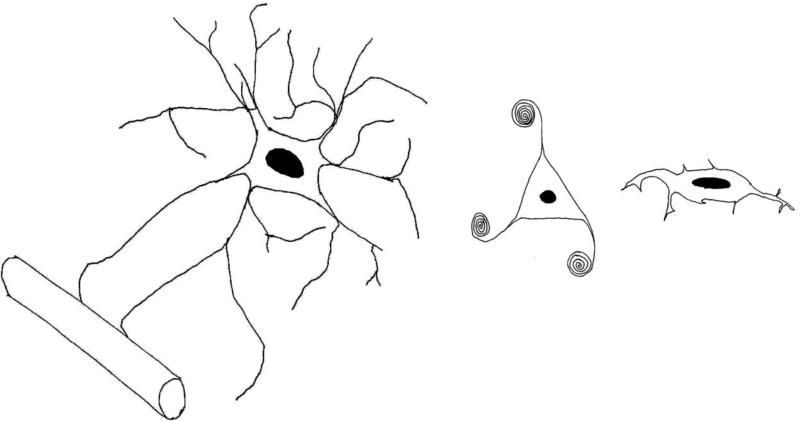
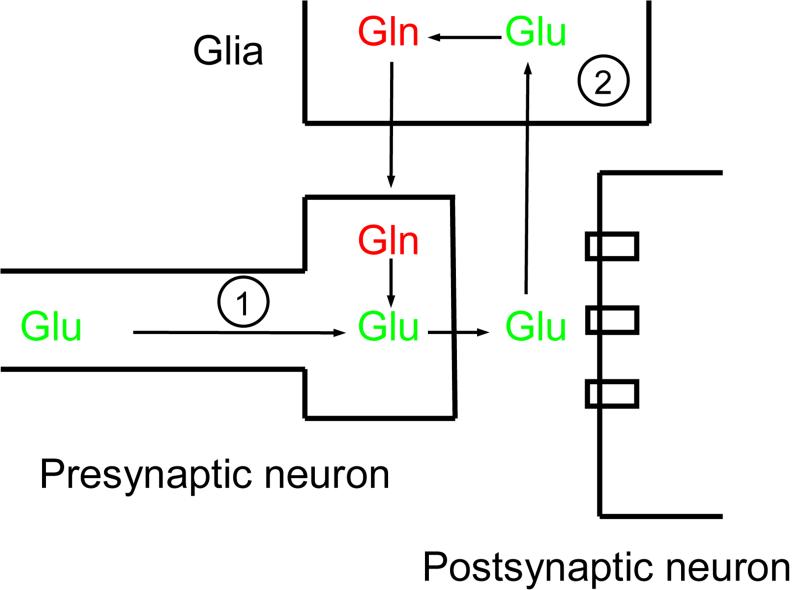
In the brain, glia are usually divided into three main sub-types: astrocytes, oligodendrocytes and microglia (Figure 1). Particularly enriched in the grey matter, near synapses, astrocytes are metabolically and morphologically activated by a variety of signals 8. These cells are essential for numerous processes in the brain including, but not limited to: gliotic response to brain injury 9; the coupling of neuronal activity with cerebral metabolism 10, 11; and the synthesis of ion channels and neurotransmitter transporters 12. Astrocytes are frequently found ensheathing synapses, and they modulate neurotransmission by taking up glutamate and GABA from the synaptic cleft 13. This close apposition has led to the coining of the term “tripartite synapse”, acknowledging the astrocyte as an essential part of the synapse along with the presynaptic and postsynaptic neurons (Figure 2) 14. In addition to being modulators of neurotransmission, astrocytes also modulate synapse numbers in cell culture, indicating that they play a role in inducing and stabilizing synapses 15. Notably, astrocytes synthesize a number of molecules with neuromodulatory effects, including d-serine, a partial agonist at the NMDA glutamate receptor site 16, adenosine, a tonic suppressor of synaptic transmission 17, and glutathione, the main antioxidant in the brain 18.
Figure 1. The major glial cell subtypes.
Line drawings of the three glial subtypes, drawn approximately to scale and depicting key features of each cell type. On the left is an astrocyte, depicted in close apposition to a blood vessel. Astrocytes have multiple processes extending in multiple directions, giving these cells a “star-like” appearance. The middle panel depicts an oligodendrocyte with a few processes wrapping around and myelinating nearby axons. Oligodendrocytes have smaller cell bodies and are more numerous than astrocytes in the brain. The panel on the right shows a microglial cell, which is oblong in appearance, with short processes extending from the cell body.
Figure 2. The role of glia at the glutamatergic synapse.
Glutamate (Glu) is synthesized in the presynaptic neuron (1) from metabolic sources, and it is released into the synaptic cleft when the action potential arrives in the presynaptic bouton. The action of glutamate on postsynaptic glutamate receptors is terminated by uptake into the glial cell (2), which converts it to glutamine (Glu). Glutamine is then shuttled back to neurons where it is converted back to glutamate. The levels of glutamate in the synapse are thus determined by the balance of interactions between neurons (1) and glial cells (2).
Oligodendrocytes are smaller than astrocytes, enriched in the white matter, and primarily responsible for myelin synthesis in the brain. The interaction between oligodendrocytes and the axons they ensheath is complex; these glia can help generate sprouting of axons, while axonal signals are needed in turn for oligodendrocyte survival 19. Abnormalities of oligodendrocytes have been reported in studies of schizophrenias, but there are few reports in the literature of studies in mood disorders20, 21. Oligodendrocyte precursor cells (OPCs) positive for the proteoglycan NG2 are less-differentiated cells found in the grey matter, and are capable of proliferating into new oligodendrocytes 22. NG2-positive OPC processes receive synaptic contacts from pyramidal neurons in the hippocampus, and generate excitatory post-synaptic currents 23. This is the only known example of a non-neuronal postsynaptic cell in the brain, indicating that OPCs monitor and modulate ongoing neuronal activity. Several but not all studies have identified NG2-positive OPCs as a major proliferating cell type in response to antidepressant treatments (see below).
Microglia are derived from peripheral blood macrophages, and mediate the inflammatory response in the brain 24. Although they have been implicated in a variety of neuropsychiatric conditions including Alzheimer's Disease25, autism spectrum disorders26, and AIDS-related dementia, they have not yet been the focus of study in mood disorders.
In summary, glial cells serve multiple important purposes in the brain, and glial cell dysfunction can impact synaptic transmission, cerebral metabolism, neuroplasticity, and myelination. In fact, neuronal-glial interactions are ubiquitous in health and disease states 27 and there may be no brain functions that would not be affected by glial cell anomalies. As discussed below, there are clues from postmortem and animal studies that glia are involved in the pathogenesis of mood disorders. However, it appears that multiple glial cell types are involved (see below), and much work needs to be done to deepen our insight into glial cell function in mood disorders.
HISTOPATHOLOGY IN MOOD DISORDERS
Studies of brain tissue from patients with MDD and BD were not widely reported until the last decade. In very promising findings, much recent work has provided consistent evidence that there are substantial reductions in the density and number of glial cells in areas of the prefrontal cortex (PFC) and in the amygdala in mood disorders 1, 20, 28-31. In one study, researchers attempted to subtype glial cells in mood disorders and concluded that it was oligodendrocytes that were selectively reduced in the amygdala 32. Similar reductions in glial number were not seen in somatosensory area 3b, an area not implicated in emotional processing 29. Gene expression studies in postmortem brain have led to findings that suggest glial abnormalities, including reduced expression of astrocyte related genes in the cerebral cortex of individuals with MDD 33 and oligodendrocyte related transcripts in BD 34. Postmortem studies have also led to reports of reductions in glial number in the hippocampus 35 and dorsolateral PFC in alcoholism, with and without comorbid mood disorder 36. No abnormalities were reported in one study documenting cell numbers in the hippocampus in MDD 37 and a second suggested loss of neuropil without cell loss 38.
These results suggest that patients with affective disorders have a perturbation in glial cells in some parts of the brain implicated in emotional processing and, further, that these perturbations are not seen throughout the brain. The selectivity of these cellular changes raises the possibility that they are causally relevant to the pathophysiology of mood disorders. Note that this is not a comprehensive review of the relevant literature. For that purpose, refer to work by Rajkowska 39, Cotter 40, and others 1.
FUTURE DIRECTIONS
Although multiple lines of evidence indicate that glial cells are abnormal in the brains of patients with mood disorders and that treatments for these conditions enhance glial cell proliferation, multiple questions need to be answered before these insights can be translated into diagnostic and therapeutic advances.
One basic question has to do with the meaning of reduced glial cell numbers in vivo. Reduced cell number could be either a cause or an effect of illness. For example, reductions may be due to a lack of adequate cell growth during development or to the death of existing cells, reduced proliferation of new cells, or reduced survival rates of proliferating cells. In addition, it is not known whether fewer glial cells actually means reduced glial cell function. As discussed above, we only have indirect evidence of glial cell functional abnormalities in mood disorders, from postmortem work. 13C magnetic resonance spectroscopy (MRS) is a non-radioactive, noninvasive MRI methodology which tracks the movement of 13C labeled molecules through metabolic pathways in brain cells. Studies of glial cell metabolism using this approach, which can indirectly quantitate astrocyte number and activity, will be invaluable to study this issue, as will a more detailed characterization of glial subtype related transcript expression in the human brain. In addition, glial cell numbers may be just one facet of glial cell abnormalities present in mood disorders. Other possible factors include cell size, length of cell processes, cell activity, or the relationship between glial cells and synaptic function and plasticity. Genomic studies are underway to determine if genes involved in determining glial number and function may also be associated with determining risk of illness.
Another important issue is identifying the specific glial subtypes that are abnormal in mood disorders. Detailed immunohistochemistry studies on postmortem brains, as well as more detailed phenotyping of newly generated cells in the PFC and amygdala following treatments for mood disorders can provide a more complete description of glial cell dynamics. Genomics can again help by identifying a role for genes specific to subtypes of glia. If one glial subtype is selectively implicated, this could change the direction of glial cell research in mood disorders and generate new vistas for treatment development focused on the properties of that specific cell type.
It appears that the identification of factors leading to glial dysfunction in mood disorders is essential if we are to gain a complete understanding of these illnesses. Glial proliferation in the brain is controlled by a variety of molecular factors depending on glial subtype, inciting stimulus, and developmental stage 41-45. Specific developmental/genetic factors must be relevant to glial cell health and function. Toxic factors, too, may dysregulate glial proliferation and function. For example, it is known that corticosteroids released from the adrenal gland under stressful conditions are chronically over-secreted in many patients with mood disorders and they inhibit proliferation of multiple glial cell types 46, 47. It is likely that glial cell numbers reflect the state of balance between multiple trophic and toxic factors acting on the brain during development of the nervous system and throughout adult life. Defining these factors and their relationship to health and illness should lead to new treatments for those already suffering and to measures to prevent mood disorders in those at risk.
REFERENCES
- 1.Harrison PJ. The neuropathology of primary mood disorder. Brain. 2002 Jul;125(Pt 7):1428–1449. doi: 10.1093/brain/awf149. [DOI] [PubMed] [Google Scholar]
- 2.Banasr M, Dwyer JM, Duman RS. Cell atrophy and loss in depression: reversal by antidepressant treatment. Curr Opin Cell Biol. 2011 Dec;23(6):730–737. doi: 10.1016/j.ceb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007 Jun;6(3):219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull. 2001 Jul 15;55(5):585–595. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- 5.Golgi C. On the structure of nerve cells. 1898. J Microsc. 1989 Jul;155(Pt 1):3–7. doi: 10.1111/j.1365-2818.1989.tb04294.x. [DOI] [PubMed] [Google Scholar]
- 6.Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004 Aug 1;56(3):140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003 Apr 24;38(2):157–160. doi: 10.1016/s0896-6273(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 8.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005 Jun;50(4):427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 9.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005 Oct;11(5):400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 10.Magistretti PJ, Pellerin L. Astrocytes couple synaptic activity to glucose utilization in the brain. News Physiol Sci. 1999 Oct;14:177–182. doi: 10.1152/physiologyonline.1999.14.5.177. [DOI] [PubMed] [Google Scholar]
- 11.Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004 Jul 2;305(5680):99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- 12.Gallo V, Ghiani CA. Glutamate receptors in glia: new cells, new inputs and new functions. Trends Pharmacol Sci. 2000 Jul;21(7):252–258. doi: 10.1016/s0165-6147(00)01494-2. [DOI] [PubMed] [Google Scholar]
- 13.Araque A, Sanzgiri RP, Parpura V, Haydon PG. Astrocyte-induced modulation of synaptic transmission. Can J Physiol Pharmacol. 1999 Sep;77(9):699–706. [PubMed] [Google Scholar]
- 14.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999 May;22(5):208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 15.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001 Jan 26;291(5504):657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 16.Boehning D, Snyder SH. Novel neural modulators. Annu Rev Neurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- 17.Pascual O, Casper KB, Kubera C, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005 Oct 7;310(5745):113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 18.Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000 Aug;267(16):4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002 Aug;67(6):451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 20.Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002 Sep 1;52(5):404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 21.Edgar N, Sibille E. A putative functional role for oligodendrocytes in mood regulation. Transl Psychiatry. 2012;2:e109. doi: 10.1038/tp.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001 Jan;24(1):39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- 23.Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000 May 11;405(6783):187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz M. Macrophages and microglia in central nervous system injury: are they helpful or harmful? J Cereb Blood Flow Metab. 2003 Apr;23(4):385–394. doi: 10.1097/01.WCB.0000061881.75234.5E. [DOI] [PubMed] [Google Scholar]
- 25.Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013 Jan 11;339(6116):156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes V. Microglia: The constant gardeners. Nature. 2012 May 31;485(7400):570–572. doi: 10.1038/485570a. [DOI] [PubMed] [Google Scholar]
- 27.Hansson E, Ronnback L. Glial neuronal signaling in the central nervous system. Faseb J. 2003 Mar;17(3):341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- 28.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002 Apr;12(4):386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 29.Öngür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001 Jun;58(6):545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 31.Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001 May 1;49(9):741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 32.Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004 Mar 15;55(6):563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Choudary PV, Molnar M, Evans SJ, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005 Oct 25;102(43):15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tkachev D, Mimmack ML, Ryan MM, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003 Sep 6;362(9386):798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 35.Korbo L. Glial cell loss in the hippocampus of alcoholics. Alcohol Clin Exp Res. 1999 Jan;23(1):164–168. [PubMed] [Google Scholar]
- 36.Miguel-Hidalgo JJ, Rajkowska G. Comparison of prefrontal cell pathology between depression and alcohol dependence. J Psychiatr Res. 2003 Sep-Oct;37(5):411–420. doi: 10.1016/s0022-3956(03)00049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJ, Holsboer F, Swaab DF. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci. 2001 Nov;14(10):1603–1612. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- 38.Stockmeier CA, Mahajan GJ, Konick LC, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004 Nov 1;56(9):640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002 Oct;7(4):281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- 40.Cotter D, Hudson L, Landau S. Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord. 2005 Aug;7(4):358–369. doi: 10.1111/j.1399-5618.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 41.Anlar B, Sullivan KA, Feldman EL. Insulin-like growth factor-I and central nervous system development. Horm Metab Res. 1999 Feb-Mar;31(2-3):120–125. doi: 10.1055/s-2007-978708. [DOI] [PubMed] [Google Scholar]
- 42.Betsholtz C, Karlsson L, Lindahl P. Developmental roles of platelet-derived growth factors. Bioessays. 2001 Jun;23(6):494–507. doi: 10.1002/bies.1069. [DOI] [PubMed] [Google Scholar]
- 43.Bhat NR. Signal transduction mechanisms in glial cells. Dev Neurosci. 1995;17(5-6):267–284. doi: 10.1159/000111296. [DOI] [PubMed] [Google Scholar]
- 44.Sleeman MW, Anderson KD, Lambert PD, Yancopoulos GD, Wiegand SJ. The ciliary neurotrophic factor and its receptor, CNTFR alpha. Pharm Acta Helv. 2000 Mar;74(2- 3):265–272. doi: 10.1016/s0031-6865(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 45.Stevens B, Fields RD. Regulation of the cell cycle in normal and pathological glia. Neuroscientist. 2002 Apr;8(2):93–97. doi: 10.1177/107385840200800205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wennstrom M, Hellsten J, Ekstrand J, Lindgren H, Tingstrom A. Corticosterone-induced inhibition of gliogenesis in rat hippocampus is counteracted by electroconvulsive seizures. Biol Psychiatry. 2006 Jan 15;59(2):178–186. doi: 10.1016/j.biopsych.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 47.Crossin KL, Tai MH, Krushel LA, Mauro VP, Edelman GM. Glucocorticoid receptor pathways are involved in the inhibition of astrocyte proliferation. Proc Natl Acad Sci U S A. 1997 Mar 18;94(6):2687–2692. doi: 10.1073/pnas.94.6.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]