Abstract
A critical role for vitamin A (VA) in development is well established, but still relatively little is known about whole-body VA metabolism in early postnatal life. Recently, methods of mathematical modeling have begun to shed light on retinol kinetics in the postnatal growth period and on the effect of retinoid supplementation on retinol kinetics. Comparison of kinetic parameters from tracer studies in neonatal rats with those previously determined in models of VA metabolism in the adult suggests both similarities and differences in the relative transfer rates of plasma retinol to extrahepatic tissues, resulting in similarities and differences in kinetic parameters and inferences about physiologic processes. Similarities between neonatal and adult models include the capacity for efficient digestion and absorption of VA; characteristics of a high-response system; extensive retinol recycling among liver, plasma, and extrahepatic tissues; and comparable VA disposal rates. Differences between neonatal and adult models include that, in neonates, retinol turnover is faster and retinol recycling is much more extensive; there is a greater role for extrahepatic tissues in the uptake of chylomicron VA; and the intestine plays an important role in chylomicron VA uptake, especially in neonatal rats treated with a supplement containing VA. In summary, retinol kinetic modeling in the neonatal rat has provided a first view of whole-body VA metabolism in this age group and suggests that VA kinetics in neonatal rats differs in many ways from that in adults, perhaps reflecting an adaption to the lower VA concentration found in neonates compared with adults.
Keywords: retinol kinetics, mathematical modeling, neonate, chylomicron vitamin A uptake, extrahepatic vitamin A metabolism, vitamin A supplementation
Introduction
Vitamin A (VA)6 plays indispensable roles in development both prenatally and postnatally (1, 2). Despite the critical roles that VA plays in neonatal development, little is known about VA metabolism in this age group. However, it is known that neonates begin life with low levels of VA, and comparisons of VA (retinol) levels in plasma, liver, and extrahepatic tissues have consistently shown lower levels in neonates than in adults (3–10), even in developed countries not known for having VA deficiency (10). Low VA stores and plasma retinol concentrations are also common in infants of low birth weight and in preterm newborns (10–12). Despite this understanding, little is known about the metabolism of VA at the whole-body level in neonates. It is the purpose of this review to discuss recent advances in understanding VA metabolism in the neonate with the use of compartmental analysis of tracer kinetic data in a neonatal rat model and the effect of retinoid supplementation on neonatal VA metabolism. We also compare similarities and differences between the neonate and the adult, based on studies in both age groups that used similar approaches of compartmental analysis. First, we briefly review concepts of VA metabolism that form the basis for a physiologic model, and then we focus on how mathematical modeling in neonates has helped to elucidate the metabolism of newly absorbed VA and plasma retinol and provide insights into similarities and differences between the neonate and adult in retinol dynamics.
Characteristics of VA Transport and Metabolism in the Absorptive and Postabsorptive Phases
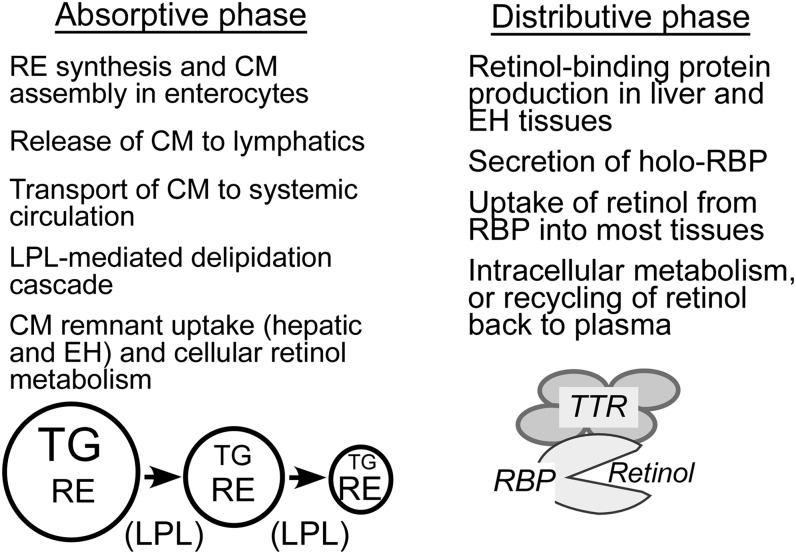
VA transport and metabolism are studied most extensively in adult animals and humans. Nevertheless, most studies focus on specific processes, and gaps still exist in developing and integrated understanding of whole-body VA metabolism. The transport and metabolism of VA can be conceptualized into 2 physiologic phases (Figure 1), an initial absorptive (postprandial) phase that is limited to a short time after the consumption of VA, and a distributive phase that takes place continually in both the postprandial and postabsorptive periods and is kinetically slower. These phases become important in the compartmental modeling process, because they help to set up the initial physiologic model to be tested. In the absorptive phase, the principal form of VA is retinyl esters (REs) and the major transport vehicle is the chylomicron. The TG component of the chylomicron is metabolized by lipoprotein lipase (LPL) situated on the capillary endothelial cells of numerous tissues (13). During this process, most chylomicron RE molecules remain within the chylomicron, which becomes known as a chylomicron remnant after sufficient delipidation. However, some VA may be transferred to tissues during the lipolytic process (14). After lipolysis, the chylomicron remnant with its remaining lipids is rapidly removed. In numerous studies, most of which are conducted in adult rats and mice, the liver is shown to be the main organ of chylomicron remnant clearance. However, chylomicron remnants are also cleared to some extent by extrahepatic tissues (13, 15, 16). Overall, the absorption of preformed VA is efficient, because studies in adult rats indicate that ∼70–90% of VA is absorbed when there is sufficient fat in the meal (17).
FIGURE 1.
Main characteristics of the absorptive, postprandial phase of chylomicron VA incorporation and metabolism and the distributive phase of VA transport to tissues by retinol-binding protein. CM, chylomicron; EH, extrahepatic; LPL, lipoprotein lipase; RE, retinyl ester (the main form of VA in CM); RBP, retinol-binding protein; TTR, transthyretin; VA, vitamin A.
In the distributive phase, the main form of VA is unesterified retinol and its transport vehicle is retinol-binding protein (RBP), a protein mainly synthesized in the liver and functionally associated with the mobilization of stored VA. Note, however, that the percentage of VA in liver compared with extrahepatic tissues is lower in rats with low VA status (18, 19). In studies in adult rats with adequate VA status, most newly absorbed VA is transferred to hepatic stellate cells for storage, whereas in the VA-deficient situation most newly absorbed VA is released back into the plasma bound to RBP (20). Nearly all holo-RBP present in the circulation is complexed to a second protein called transthyretin (TTR), which is present in molar excess to RBP (21). Retinol bound to RBP is delivered to a large number of VA target tissues for function. A portion of the retinol is oxidized to generate functional metabolites, retinal and retinoic acid, and inactive products that are eliminated.
An important feature of retinol metabolism that was learned from tracer kinetic studies and mathematical modeling is the extensive recycling of retinol that occurs among plasma, liver, and other tissues before the retinol is irreversibly disposed of from the body (19, 22). As discussed further later, the recycling of retinol contributes to the highly dynamic state of retinol trafficking. From modeling studies conducted in adult rats, the average retinol molecule circulates through the plasma compartment 12 to 13 times before undergoing irreversible degradation (22). On the basis of a study of a small number of humans, the recycling number is ∼3 (23).
The “Machinery” for VA Metabolism Is Expressed Early in Life
The metabolism of VA as outlined in the previous section is performed by numerous proteins that function as chaperones, transport proteins, enzymes, and receptors; collectively, they can be considered the “machinery” for VA metabolism. Previous research has shown that a number of these factors are expressed early in life, in temporally and spatially regulated patterns (24, 25). In a study of the embryonic development of the mouse, Ulven et al. (26) showed that the mRNA for all the retinoid nuclear receptors (retinoic acid receptor α, β, and γ and retinoid receptor X α, β, and γ), RBP, cellular RBP (CRBP)1 and CRBP2, and 2 of the enzymes able to convert retinol to retinal were detected as early as 7.5 d after coitus. Retinal dehydrogenase type 2, an enzyme capable of oxidizing the final step in all-trans-retinoic acid synthesis, was also detected (26). Thus, it can be inferred that the neonate is born with the capability of metabolizing retinoids and responding to them. In addition, research on the ontogeny of expression of proteins needed for normal VA metabolism in the small intestine of the rat show that levels of important proteins involved in intestinal RE formation, including CRBP2 and lecithin:retinol acyltransferase, are detectable by gestational days 18–19, increase at birth, and reach elevated levels in the middle of the suckling period, and then decline after weaning to adult levels, whereas CRBP1 is present by the 16th day of gestation, declines by 70% at birth, and then is maintained to adulthood (27). Research by Quadro and colleagues (28) has shown that genes required for β-carotene metabolism, and those for retinol transport, are expressed during gestation. RBP is detectable in liver of the fetal rat around gestational day 16 (29). LPL activity in rat lung, skeletal muscle, heart muscle, and brown adipose tissue emerges substantially during the first 24 h after birth, and LPL activity in brown adipose tissue and skeletal muscle is highest during the period of suckling (30). Other studies show that lecithin:retinol acyltransferase; cytochrome P450 enzyme 26 family that oxidize retinoids; and stimulated by retinoic acid 6, a putative receptor for RBP-retinol, are expressed in lung and liver of the neonatal rat (31). Although these data are not inclusive of all possible retinoid-metabolizing genes, they suggest that, despite the immaturity of the neonate, the stage is well set before birth for important processes in retinol trafficking and metabolism.
Using Compartmental Modeling to Understand VA Dynamics
The methods of tracer kinetics and model-based compartmental analysis are applied to VA kinetic data in adults for >30 y (15, 18, 19, 22, 32, 33). These studies contribute much to our understanding of VA metabolism and kinetics in adult animals and humans, including prediction of extensive recycling of retinol among plasma, liver, and extrahepatic tissues before its irreversible loss and estimates of the transit, turnover, storage, and utilization of retinol (19, 22, 33–36). Although the rat is studied most often and under a wider variety of circumstances, studies in adult humans reveal a qualitatively similar picture in terms of model structure and confirm aspects of retinol metabolism such as the recycling of retinol among plasma, liver, and other tissues that were first understood from modeling data from the rat (23, 37).
In compartmental analysis, a system is represented by a finite number of homogenous states and lumped processes, called compartments, which interact by material exchange (33), whereby a compartment is a mathematical construct in which material acts in a kinetically homogeneous way. A compartment may or may not define a discrete physiologic or biochemical space; for example, several organs may act kinetically as 1 compartment or, within a given organ, there may be several kinetically distinguishable compartments. L(I,J), the fractional transfer coefficient, is an important parameter that quantifies the interconnectivities between compartments. It is defined as the fraction of the material in compartment J transferred to compartment I per unit of time. L(I,J)s are parameters that define the behavior of the system. In model-based compartmental analysis, the investigator first fits kinetic data obtained from plasma and/or tissues to a hypothesized compartmental model on the basis of previous models or knowledge of the system. Then, the model parameters and/or structure are adjusted until a model is identified that is compatible both with the observed data and the known characteristics of the system (38). The Windows version of the Simulation, Analysis and Modeling software (WinSAAM) is used for such analysis (39). Tracer data (calculated as fraction of dose vs. time) and other relevant information, such as the initial estimates for L(I,J)s, can be entered into a WinSAAM input file. WinSAAM solves the input file, and solution results are evaluated by comparing the observed and calculated data. A close fit is judged by visual inspection of the simulated tracer data plot and by statistical analysis, including the sum of squares from nonlinear regression analysis and the estimated fractional SD for each kinetic parameter. Once a satisfactory fit is achieved, the final estimates of the L(I,J)s and their statistical uncertainties are generated by nonlinear regression analysis in WinSAAM. Other parameters are then calculated from the L(I,J)s. If organ data are also available for modeling, the “forcing function” option in WinSAAM can be applied, through which one can “uncouple” the system, organ by organ, because all organs are linked via the blood, but not directly to each other (34, 40). Once a model is developed for each individual organ, then all of the data can be put together, the forcing function released, and a final integrated model established.
Challenges to Studying VA Kinetics in Neonates
Because neonates grow rapidly, a main challenge to modeling in this age group is the inherently nonsteady metabolic state, which could bring difficulties and uncertainty for the modeling process. In addition, because neonatal rodents are small, dose administration itself can be challenging. Note that, in previous studies in adult animals, the retinol tracer is nearly always introduced directly into the plasma compartment, for example, by injecting plasma that contained [3H]retinol-labeled RBP that is harvested from donor rats into the venous system of the recipients (18, 22). This route of delivery results in a maximum plasma radioactivity at time 0, followed by a rapid decay, then a plateau indicative of recycling, and, finally, a terminal slope that indicates the disposal (22). In the neonate, we specifically wanted to model the fate of orally administered VA (40, 41), because this represents the most common route of VA supplementation. Thus, the initial model needed to include both an initial absorptive phase and a later distributive phase. Other challenges in the neonate included the need to kill the animals to collect sufficient blood, and tissues if desired, for analysis. In the studies of neonatal rats by Tan et al. (40, 41) described in “Retinol kinetics in neonates”, plasma and individual tissues were collected at each time point and used for retinol analysis, whereas pooled data (means of data for plasma or tissues from groups of pups killed at the same time point) were subjected to modeling. This approach, which was used previously (33), is referred to as a “super-rat” or, in the case of neonates, a “super-pup” approach.
Retinol Kinetics in Neonates
To begin to fill the gap in the understanding of VA metabolism in neonates, we conducted a tracer kinetic study in which model-based compartmental analysis was used to investigate whole-body VA metabolism and kinetics in neonatal rats (40, 41). In this study, rat dams were fed a diet with a marginal level of VA (0.35 mg retinol equivalents/kg diet) throughout the study period, because it was reasoned that marginal status in the mothers would best resemble the real-world situation in which infants would be most likely to receive VA supplementation. In addition, because it is known that VA concentration in rat milk reflects the dam’s recent dietary intake of VA (15), controlling maternal VA intake was expected to help stabilize the VA intake of the neonates. [3H]Retinol was orally administered to neonatal rats on postnatal day 4, and thereafter 3–4 pups per group were killed by isoflurane euthanasia at 14 time points after dosing: 1, 2.5, 4, 6, 8, 11, 15, and 24 h and 2, 4, 6, 8, 11, and 14 d. Blood, liver, lung, stomach, intestine, kidney, thymus, spleen, heart, and the remaining carcass were collected. On the basis of the tracer responses in collected tissues, 2 types of models were developed: in the first model (41), which we referred to as a multicompartmental model of VA kinetics as viewed from the plasma space, only plasma data were used. This approach most closely resembled the approach that would be feasible in human studies; therefore, it was considered translational, whereas it also produced results that were important for subsequent modeling. The second model that was developed resulted in compartmental models for individual tissues and an integrated compartmental model that incorporated plasma and all tissues (40). This type of model was more comprehensive but would not be feasible in human studies; thus, the animal model fulfilled an important predictive role. From the models, information was obtained on the absorption, transfer, recycling, storage, distribution, and disposal of retinol and the contribution of different tissues to whole-body VA kinetics. In this study (40, 41), a group of neonatal rats were also supplemented with a high dose of the combination of VA and retinoic acid (referred to as VARA; 6 mg/kg body weight of retinyl palmitate, plus 1/10 of retinoic acid) on postnatal day 4 to investigate the effects of VARA supplementation on whole-body VA kinetics in neonatal rats.
In the next part of this review, VA kinetics in adult rats and in neonatal rats are discussed and compared on the basis of our study in neonatal rats (40, 41) and studies conducted by Green and colleagues (19, 22) in adult rats and are related to the more limited studies of retinol kinetics in human subjects (23, 37).
Compartmental Models Developed for VA Kinetics
Two types of compartmental models were developed to describe VA kinetics. For the first type, only plasma kinetic data were used to describe whole-body VA kinetics ("plasma view model"). In this approach, processes with similar kinetics were lumped into the same compartment. For the second type, referred to as "an integrated model", tissue data from various tissues (anatomic compartments) were used, in addition to plasma data.
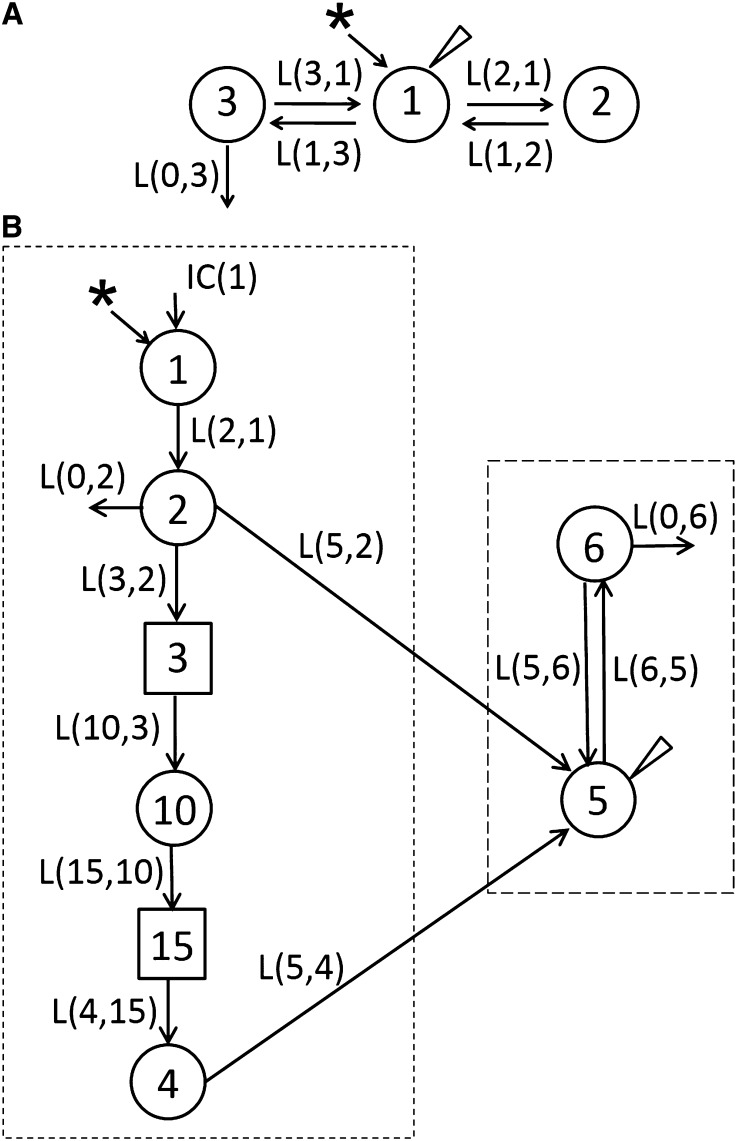
A 3-compartment model that was based on plasma [3H]retinol data collected in adults rats that were injected with [3H]retinol/RBP/TTR-labeled plasma is shown (42) (Figure 2A). Compartment 1 represents plasma retinol, which was the site of dietary retinol input and tracer input. Compartment 1 exchanges VA with 2 extravascular compartments, 2 and 3. Compartment 2 is a small, rapidly turning-over retinol pool, consisting mainly of retinol in interstitial fluid and some intracellular pools and retinol filtered by the kidneys, whereas pool 3 is a large, slowly turning-over pool that includes RE stores in liver and extrahepatic tissues. This model was able to fit VA kinetics in adult rats with both marginal and depleted liver VA stores (35, 42). In both VA-marginal and VA-deficient adult rats, retinol turnover rate was much higher than its disposal rate, and retinol recycled among plasma, liver, and other tissues extensively before irreversible disposal.
FIGURE 2.
Proposed multicompartmental model for VA kinetics in adult (A) and neonatal (B) rats on the basis of plasma tracer response data vs. time. (A) Compartment 1 represents plasma retinol, which exchanges VA with 2 extravascular compartments, 2 and 3. (B) Components 3 and 15 are delay elements. Compartments/component 1–3 represent VA digestion and absorption. Compartments/component 10, 15, and 4 represent CM metabolism, the uptake of CM remnants, and the processing of VA in extravascular tissues, respectively. Compartment 5 represents plasma retinol bound to RBP; this retinol exchanges with VA in 1 extravascular pool (compartment 6). Arrows indicate interconnectivities between compartments; asterisk, the site of input of [3H]retinol; circles, compartments; long-dashed box, aspects of the neonatal model that are similar to the adult model; short-dashed box, unique aspects of the model for neonates, to which the dose was administered orally; triangle, the site of sampling. CM, chylomicron; IC, initial condition; L(I,J), fractional transfer coefficients, or the fraction of the material transferred to compartment I in compartment J per day; RBP, retinol-binding protein; VA, vitamin A. Adapted from reference 42 (A) and reproduced from reference 42 (B) with permission.
The plasma view model developed for VA kinetics in neonatal rats is shown in Figure 2B (41). Because [3H]retinol was given orally, the model necessarily included the flow from compartment 1 (the site of input of [3H]retinol and dietary VA) to compartment 4, which represents the processes of VA digestion, absorption, chylomicron production, and metabolism; delay element 3 corresponds to chylomicron production before chylomicrons are secreted into plasma; compartment 10 represents newly absorbed REs in plasma chylomicrons, and delay element 15 corresponds to chylomicron metabolism before the uptake of REs into liver and other tissues, which is represented by compartment 4. After the initial processing of VA, retinol-RBP is secreted into plasma (compartment 5). Compartment 5 represents the retinol-RBP pool in plasma; this VA exchanges with 1 extravascular VA pool (compartment 6). As mentioned, the part of the neonatal model representing VA digestion, absorption, chylomicron production, and metabolism (indicated in Figure 1B, short-dashed box) was not included in the adult model because of different routes of dose administration. The part describing the exchange of retinol between plasma and extravascular tissues and the disposal of retinol from the system (Figure 1B, long-dashed box) was similar to that in the adult model; compartment 5 in the neonatal model is comparable with compartment 1 in the adult model. However, to fit plasma VA kinetic data in neonatal rats, only 1 extravascular VA pool was required, compared with both a rapidly turning-over pool and a slowly turning-over pool in adults.
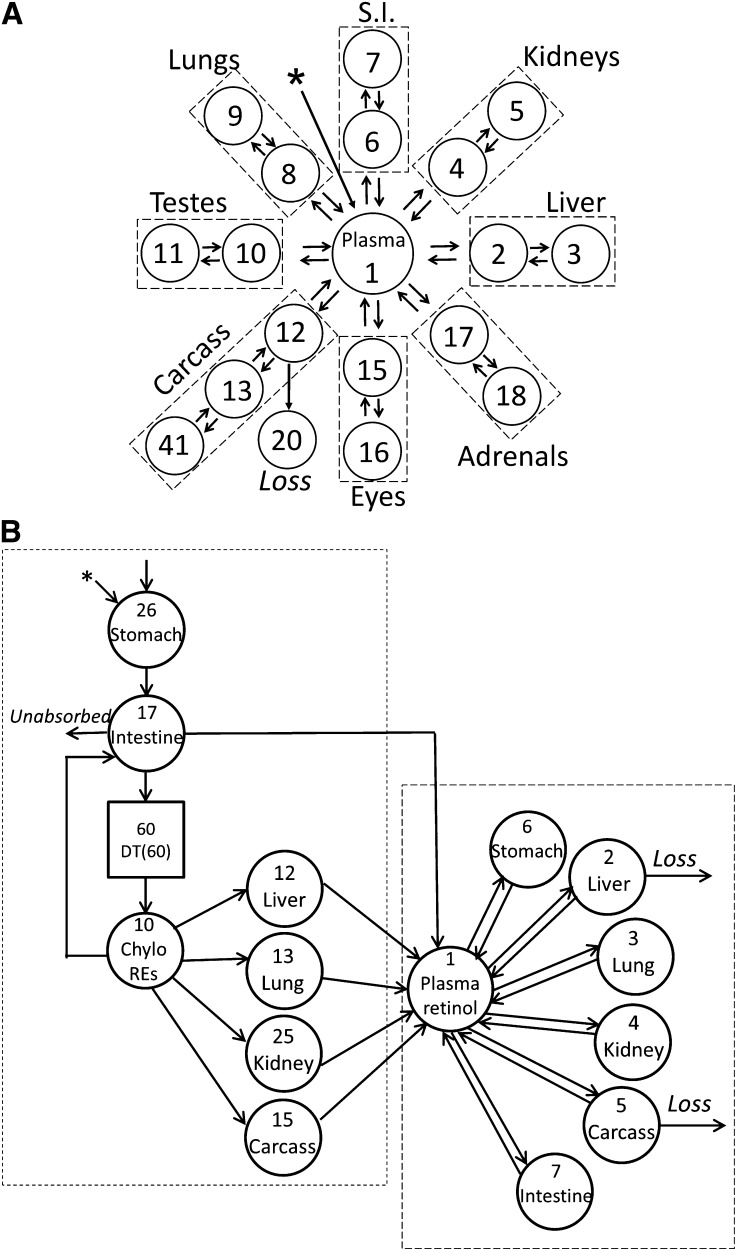
An integrated compartmental model, including individual organs, of VA kinetics in [3H]retinol/RBP/TTR-labeled plasma-injected adult rats with low VA status is shown (33) (Figure 3A). The model was developed on the basis of kinetic data collected for both plasma and tissues. Compartment 1 represents the plasma retinol pool. It exchanges VA with compartments 2, 4, 6, 8, 10, 12, 15, and 17, which represent rapidly turning-over retinol pools in liver, kidney, small intestine, lung, testes, carcass, eyes, and adrenals, respectively. The other compartments in the tissues represent slowly turning-over retinol pools that exchange VA with the rapidly turning-over pool in that tissue. A similar type of integrated model was developed for neonatal rats; this is shown in Figure 3B (40). [3H]Retinol was given orally in our study of neonates, so the model included the processes of VA digestion and absorption and chylomicron production and metabolism (Figure 3B, short-dashed box), which were not included in the adult model. Compartment 26 represents the processing of newly ingested VA in the stomach, and compartment 17 represents its processing in intestine. Compartment 10 represents plasma REs in chylomicron/chylomicron remnants. Delay element 60 describes the time needed for the production of chylomicron before their appearance in plasma. Compartments 12, 13, 25, and 15 represent the uptake of plasma REs from chylomicron/chylomicron remnants into liver, lung, kidney, and carcass, respectively. The model indicated that, after being processed, plasma retinol, presumably as holo-RBP, was secreted from these tissues into compartment 1, the plasma retinol pool. The parts of the model describing retinol exchange between plasma and extravascular tissues (Figure 3B, long-dashed box) were similar to those in the adult model. Compartments 2, 3, 4, 5, 6, and 7 represent VA pools in liver, lung, kidney, carcass, stomach, and intestine, respectively, that exchange VA with retinol in compartment 1. However, although both rapidly turning-over retinol pools and slowly turning-over retinol pools were necessary to fit the organ data in adults rats, only 1 compartment representing tissue retinol was required for fitting the data for all tissues in neonatal rats. A single compartment may be adequate because of the much faster turnover of retinol found in neonatal rats than in adult rats, which is discussed in the next paragraph. The tracer data for plasma, liver, lung, kidney, carcass, stomach, and intestine were assigned to compartments 10 plus 1, 12 plus 2, 13 plus 3, 25 plus 4, 15 plus 5, 26 plus 6, and 17 plus 7, respectively. Liver (compartment 2) and carcass (compartment 5) were 2 sites of irreversible loss of retinol. A good fit to plasma and organ/tissue tracer data were obtained only when the output was from both compartment 2 and compartment 5. In the adult model, however, carcass was the only site of irreversible loss of retinol (Figure 3A). What leads to this difference between neonatal and adult rats is currently unknown.
FIGURE 3.
Model structure for integrated compartmental models for VA metabolism in adult (A) and neonatal (B) rats. (A) Compartment 1 represents the plasma retinol pool. Compartments 2, 4, 6, 8, 10, 12, 15, and 17 represent rapidly turning-over retinol pools in liver, kidney, small intestine, lung, testes, carcass, eyes, and adrenals, respectively. Compartments 3, 5, 7, 9, 11, 13 and 14, 16, and 18 represent slowly turning-over retinol pools in these same organs. Note irreversible loss is predicted to come entirely from carcass compartment 12. The organ compartments are grouped inside dashed rectangles. (B) In the neonatal model, compartment 26 represents the processing of newly ingested VA in the stomach, and compartment 17 represents its processing in the intestine. Compartment 10 represents plasma REs in Chylo/Chylo remnants. DT(60) describes the time needed for the production of Chylo before their appearance in the plasma. Compartments 12, 13, 25, and 15 represent the uptake of plasma REs from Chylo/Chylo remnants into liver, lung, kidney, and carcass, respectively. Compartment 1 represents the plasma retinol pool. Compartments 2, 3, 4, 5, 6, and 7 represent VA pools in liver, lung, kidney, carcass, stomach, and intestine, respectively, that exchange VA with retinol in compartment 1. Note irreversible loss is predicted to come from liver compartment 2 and carcass compartment 5. Initial condition (26) represents the newly ingested dose that enters the stomach. Compartments within the long-dashed box indicate aspects of the neonatal model that are similar to the adult model; components within the short-dashed box, unique aspects of the model for neonates, to which the dose was administered orally. Arrows indicate the L(I,J) values that connect the kinetically identified compartments; asterisk, site of input of [3H]retinol; circles, compartments. Chylo, chylomicron; DT(60), delay element 60; L(I,J), fractional transfer coefficient; RE, retinyl ester; S.I., small intestine; VA, vitamin A. Compartments are numbered according to reference 34 (A) and reference 40 (B). Adapted from references 34 (A) and 40 (B) with permission.
Similarities and Differences in VA Kinetics in Adult and Neonatal Rats
From the plasma view models (Figure 2A, B), parameters characterizing the absorption, transfer, turnover (recycling), uptake, and disposal (utilization) of VA could be derived. From the integrated models that incorporate tissues (Figure 3A, B), VA kinetics in various tissues and the contributions of different tissues to whole-body VA kinetics could be estimated. VA kinetics were compared between neonatal rats in our study (40, 41), which had a normal plasma retinol level but marginal liver VA status, and adult rats studied by Green et al. (22, 42), which also had normal plasma retinol concentration and marginal liver VA stores. Both similarities and differences were found.
The similarities included the following. First, there was extensive recycling of retinol among liver, plasma, and extrahepatic tissues in both adults and neonates, and the plasma retinol turnover rate was much higher than the retinol disposal rate. The system of VA metabolism in neonates is a high response system (22), as it is in adults. Because plasma retinol turnover rate is much higher than the disposal rate, if tissue demands for retinol increase, recycling could decrease without an appreciable change in plasma retinol level. Changes in retinol recycling allow for adjustment in VA distribution in response to changes in nutritional, metabolic, or physiologic conditions (22). Second, of the plasma retinol turnover, a considerable amount goes to extrahepatic tissues; significant amounts of VA were predicted to be present in extrahepatic tissues. Approximately 51% of whole-body VA was extrahepatic in neonatal rats (4–6 d old) and 44% was extrahepatic in adult rats with marginal VA status. These results suggest that extrahepatic tissues may play a more important role in VA homeostasis when VA status is marginal, compared with the adequate state (18, 32, 42). Third, despite differences in body size, VA disposal rates were quite comparable between adult and neonatal rats. Disposal rate was ∼20–30 nmol/d in neonates and ∼24 nmol/d in adult rats. Although plasma retinol levels in the neonatal rats in the study of Tan et al. (41) and the adult rats in the study by Green et al. (22) were within the normal range, the average level in neonatal rats (∼1 μM) was somewhat lower than that in adults (∼1.7 μM). This might indicate that, even though neonates have a lower plasma VA concentration than adults, they still are able to maintain a comparable retinol utilization rate to meet their VA requirements. Fourth, the digestion and absorption of VA was efficient in both neonatal and adult rats. The absorption efficiency of VA was estimated to be ∼75% in adult rats (43). The calculation that was based on our plasma view model in neonates indicated that the efficiency is ∼97% in neonatal rats. VA digestion depends on adequate pancreatic function and adequate fat intake for micellization of retinol. The pancreas is functionally immature at birth in newborns (44). Moreover, neonates are deficient in pancreatic co-lipase–dependent TG lipase (also known as bile salt-stimulated lipase), which can digest REs, compared with older infants and adults. However, bile salt-stimulated lipase is also expressed in the mammary gland of some species during lactation, and it is secreted into the milk (45). Because fat, which is present in large amounts in milk, is digested well by neonates, it seems likely that bile salt-stimulated lipase and/or other lipases, such as carboxyl ester lipase, in milk, or some combination of lipases from the pancreas, intestine, and/or breast milk, are sufficient for the digestion of REs by the neonate (46). In addition, neonates use lingual lipase for fat digestion (46). As mentioned, it was shown that levels of CRBP2 and lecithin:retinol acyltransferase, important proteins involved in intestinal RE formation, increase at birth (27). The capacity for absorption of VA appears to be well developed in the neonate.
A number of differences were also found between VA kinetics in neonatal and adult rats. The key differences are summarized in Table 1. The first notable difference in retinol kinetics in neonatal rats is that retinol turnover was faster and that recycling was much more extensive than in adults. The recycling number was ∼12–13 in adult rats and 144 in neonatal rats. The time that retinol spends during a single transit in plasma was 1.9 h in adults, whereas it was 0.4 h in neonates. The molecular mechanism involved in the faster retinol turnover in neonates is unknown. One possible explanation is that perhaps TTR is not synthesized at the adult rate during the neonatal stage; therefore, retinol-RBP in plasma is not coupled to TTR to the extent seen in adults and thus turns over faster. The faster turnover of retinol might be an adaptive mechanism to the neonatal life stage. It might make retinol more available when tissue demands for retinol increase.
TABLE 1.
Differences in characteristics of VA kinetics between adult rats and neonatal rats1
| Characteristic | Adult rats | Control neonatal rats | VARA-supplemented neonatal rats |
| Recycling number2 | ∼12–13 | 144 | 100 |
| Transit time in plasma,3 h | 1.90 | 0.40 | 0.14 |
| Plasma REs cleared by liver, % | ∼75 | 52 | 22 |
| Plasma REs cleared by intestine, % | NA | 4.7 | 32 |
| Plasma REs cleared by carcass, % | NA | 40 | 43 |
| Plasma retinol turnover to liver, % | 48 | 76 | 56 |
Data for adult rats are from reference 22, and data for neonatal rats are from reference 41. NA, not available because of administration of retinol tracer directly into plasma (Figures 2A and 3A); RE, retinyl ester; VA, vitamin A; VARA, vitamin A and retinoic acid.
Average number of times a retinol molecule recycles through plasma before it irreversibly exits it.
Mean of the time that a retinol molecule spends in plasma during a single transit before leaving reversibly or irreversibly.
The second distinguishing feature of retinol kinetics in neonates is that neonatal tissues other than liver are important in taking up plasma REs in chylomicrons. Fifty-two percent of plasma REs was cleared by the liver, whereas as much as 40% was taken up by carcass. The role of extrahepatic tissues in clearing plasma REs from chylomicron in neonatal rats was more prominent in VARA-supplemented pups, in which liver took up only 22% of plasma REs, whereas the remaining 78% was taken up by extrahepatic tissues. In adult rats, liver is known to be the main organ that clears REs from chylomicron remnants, although it is also reported that extrahepatic tissues active in metabolizing chylomicron TGs, such as adipose tissue and the mammary gland during lactation and skeletal muscle, heart, and lungs, may also acquire newly absorbed VA during lipolysis of chylomicron TGs by LPL (13, 15, 16). Because neonates are born with a low VA level but presumably have a high requirement for it, it may be speculated that the uptake of dietary REs by extrahepatic tissues is an adaptive mechanism in neonates to make VA more readily available for use in tissues. Chylomicron REs might serve as a direct precursor for retinol and the synthesis of retinoic acid in tissues where retinoic acid is needed. It is reported that, in adult rats, LPL contributes to extrahepatic clearance of chylomicron VA. The level of LPL expression in skeletal muscle, heart, and/or adipose tissue influences the amount of [3H]retinoid taken up from chylomicron and/or chylomicron remnants (13). It is still not clear how chylomicron REs are taken up by neonatal extrahepatic tissues. However, it is reasonable to hypothesize that LPL is involved, as in adults. As mentioned, a previous report indicates that LPL activity in rat skeletal muscle, brown adipose tissue, lung, and heart muscle emerges substantially during the first 24 h after birth, and the activity in brown adipose tissue and skeletal muscle is highest during the suckling period (30).
A third different feature of VA kinetics in neonatal rats is that some chylomicron remnants are taken up by the intestine, which is not found in adults (13). To obtain a good fit to the data in our study, it was necessary to include this process in the model. The uptake was low in the control group; however, the VARA treatment stimulated the uptake dramatically, with the percentage uptake of plasma REs by intestine estimated as ∼30%. This finding suggests the novel viewpoint that, in neonates, the intestine is not only involved in chylomicron formation but in chylomicron RE clearance as well.
Fourth, the turnover of plasma retinol, representing holo-RBP, into tissues also differs between control neonatal rats and adult rats both with marginal VA status. The percentages of plasma retinol turnover were 75.6% to liver and 24.4% to extrahepatic tissues in control neonatal rats, whereas, by comparison in adult rats, the percentages were 48% to liver and 52% to extrahepatic tissues (22). In neonatal VARA-treated rats, however, the percentages were 56% to liver and 44% to extrahepatic tissues, which was similar to that in adult rats. This may indicate that VA status plays an important role in determining extrahepatic uptake.
Note that, for liver and lung, both model-predicted and direct measurements of total retinol content were available for the control group in the first 2 d of the study when the pups’ weight and plasma retinol levels were nearly constant (steady-state conditions). The predicted and directly measured values were 12.7 and 15.0 nmol, respectively, for liver, and 0.50 and 3.5 nmol, respectively, for lung (40). The close agreement between these estimates lends confidence that the model that was developed is robust in predicting tissue retinol masses.
VA Kinetics in Humans
Studies of retinol kinetics in human subjects are limited; however, some available data provide insights into VA dynamics in the human body. Green and Green (23) used model-based compartmental modeling to analyze long-term kinetic data on plasma retinol in 3 human subjects who were administrated an intravenous dose of autologous plasma labeled in vitro with [14C]retinol. These data were collected by Goodman and colleagues in 1965–1966 (23). Blood samples were collected between ∼20 h and either 115 d or 240 d. Data for each subject were fit to a 3-compartment model similar to the one shown in Figure 1A. By applying the multiple studies feature in WinSAAM, a final population model was developed. Average parameters were predicted from the model. Most parameters were similar to those obtained in adult rats. Differences included the transit time of retinol in plasma and the recycling number of retinol through plasma. In adult rats, the transit time was ∼1.9–2.7 h and the recycling number was ∼9–13 regardless of VA status (32). In neonatal rats with marginal VA status in our study, the transit time was 0.4 h and the recycling number was 144 (Table 1). In the human subjects in (23), the transit time was 5.4 h, which was longer than that for adult and neonatal rats, and the recycling number was 3, which was lower than that in adult rats and much lower than that in neonatal rats. Whether these are species differences or related to the difference in body size between humans and rodents is not clear, but in general metabolic rates in rodents are faster than in humans.
Conclusions and Opportunities for Future Research
Our models predicted several features of neonatal VA metabolism which were not anticipated from previous studies in the adult, including that neonates have a much more extensive recycling of retinol; that neonatal extrahepatic tissues play an important role in clearing chylomicron REs, especially in VARA-treated pups; and that a significant amount of extrahepatic VA is present in neonatal rats. The neonatal intestine and the remaining carcass were found to play important roles in retinol kinetics in neonates. These predictions that are based on compartmental models suggest directions and hypotheses for further studies. In future research, lymph that contains chylomicrons labeled with [3H]REs (32) could be injected into neonatal rats for investigation of chylomicron metabolism. Although this would be challenging technically, the assessment of chylomicron VA metabolism in the early absorptive and postprandial stages of metabolism would provide new data on the distribution of VA and could be especially useful in determining the role of the intestine, which is predicted to be significant in neonatal chylomicron VA uptake. Alternatively, the use of the oral route of administration, as in the study already conducted (40, 41), with added early time points, could provide this needed information. Although the intestine was identified as a target of chylomicron VA metabolism, the regions of the intestine were not separated and studied individually. Different parts of the small intestine and tissues in the carcass such as skin, bones, and brown adipose tissue that might be critical for neonatal VA metabolism could be separated and analyzed for more detailed investigation. In addition, the compartmental models established in our study can be used as the initial models for developing compartmental models of VA kinetics in neonatal rats under different conditions, such as different VA statuses, various VA or non-VA supplementation strategies, perturbation by exogenous factors, and diseases that affect VA metabolism. Studies in the neonatal animal may also help to inform public health. Our modeling studies predicted that the daily disposal rate for retinol in the neonatal rat is similar (after adjustment for body weight) (41) to the range of VA that human breast milk provides as previously calculated by Zachman (47), but the estimated disposal rate is slightly higher than the current Adequate Intake (400 μg/d) set by the Institute of Medicine (48). Tracer studies of a similar design but using stable isotope technologies could help to clarify the retinol disposal rate and hence the actual biological requirement for retinol in infants, and thus be useful for development of Estimated Average Requirements and an RDA in this age group.
Finally, as noted above, the development of a compartmental model as viewed from the plasma space is an approach that can be used in translational research to investigate nutrient metabolism in humans, which could be performed with stable isotopic tracers or minimal radioactivity, as used with [2H8]retinyl acetate in studying VA kinetics, storage, and disposal rate in well-nourished Chinese and US adults (37) and with [13C10]β-carotene and [13C10]retinyl acetate methods as in Oxley et al. (49), for example, for determining VA status and bioavailability/bioconversion of β-carotene in human subjects (50). This approach has the potential of also being useful in studying VA metabolism and kinetics in infants and children.
Acknowledgments
LT, MHG, and ACR wrote and edited the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CRBP, cellular retinol-binding protein; L(I,J), fractional transfer coefficient; LPL, lipoprotein lipase; RBP, retinol-binding protein; RE, retinyl ester; TTR, transthyretin; VA, vitamin A; VARA, vitamin A and retinoic acid; WinSAAM, Windows version of the Simulation, Analysis and Modeling software.
References
- 1.Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu Rev Nutr 2002;22:347–81. [DOI] [PubMed] [Google Scholar]
- 2.Altucci L, Gronemeyer H. Nuclear receptors in cell life and death. Trends Endocrinol Metab 2001;12:460–8. [DOI] [PubMed] [Google Scholar]
- 3.Yeum K-J, Ferland G, Patry J, Russell RM. Relationship of plasma carotenoids, retinol and tocopherols in mothers and newborn infants. J Am Coll Nutr 1998;17:442–7. [DOI] [PubMed] [Google Scholar]
- 4.Godel JC, Basu TK, Pabst HF, Hodges RS, Hodges PE, Ng ML. Perinatal vitamin A (retinol) status of northern Canadian mothers and their infants. Biol Neonate 1996;69:133–9. [DOI] [PubMed] [Google Scholar]
- 5.Berggren Söderlund M, Fex GA, Nilsson-Ehle P. Concentrations of retinoids in early pregnancy and in newborns and their mothers. Am J Clin Nutr 2005;81:633–6. [DOI] [PubMed] [Google Scholar]
- 6.Dahro M, Gunning D, Olson JA. Variations in liver concentrations of iron and vitamin A as a function of age in young American children dying of the sudden infant death syndrome as well as of other causes. Int J Vitam Nutr Res 1983;53:13–8. [PubMed] [Google Scholar]
- 7.Davila ME, Norris L, Cleary MP, Ross AC. Vitamin A during lactation: relationship of maternal diet to milk vitamin A content and to the vitamin A status of lactating rats and their pups. J Nutr 1985;115:1033–41. [DOI] [PubMed] [Google Scholar]
- 8.Zachman RD, Kakkad B, Chytil F. Perinatal rat lung retinol (vitamin A) and retinyl palmitate. Pediatr Res 1984;18:1297–9. [DOI] [PubMed] [Google Scholar]
- 9.Zachman RD. Role of vitamin A in lung development. J Nutr 1995;125:1634S–8S. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal K, Dabke AT, Phuljhele NL, Khandwal OP. Factors affecting serum vitamin A levels in matched maternal-cord pairs. Indian J Pediatr 2008;75:443–6. [DOI] [PubMed] [Google Scholar]
- 11.Chan V, Greenough A, Cheeseman P, Gamsu HR. Vitamin A status in preterm and term infants at birth. J Perinat Med 1993;21:59–62. [DOI] [PubMed] [Google Scholar]
- 12.Shenai JP, Chytil F, Stahlman MT. Liver vitamin A reserves of very low birth weight neonates. Pediatr Res 1985;19:892–3. [DOI] [PubMed] [Google Scholar]
- 13.van Bennekum AM, Kako Y, Weinstock PH, Harrison EH, Deckelbaum RJ, Goldberg IJ, Blaner WS. Lipoprotein lipase expression level influences tissue clearance of chylomicron retinyl ester. J Lipid Res 1999;40:565–74. [PubMed] [Google Scholar]
- 14.Blaner WS, Obunike JC, Kurlandsky SB, Al-Haideri M, Piantedosi R, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase hydrolysis of retinyl ester. Possible implications for retinoid uptake by cells. J Biol Chem 1994;269:16559–65. [PubMed] [Google Scholar]
- 15.Ross AC, Pasatiempo AM, Green MH. Chylomicron margination, lipolysis, and vitamin A uptake in the lactating rat mammary gland: implications for milk retinoid content. Exp Biol Med (Maywood) 2004;229:46–55. [DOI] [PubMed] [Google Scholar]
- 16.Goodman DW, Huang HS, Shiratori T. Tissue distribution and metabolism of newly absorbed vitamin A in the rat. J Lipid Res 1965;6:390–6. [PubMed] [Google Scholar]
- 17.Blomhoff R, Green MH, Norum KR. Vitamin A: physiological and biochemical processing. Annu Rev Nutr 1992;12:37–57. [DOI] [PubMed] [Google Scholar]
- 18.Green MH, Green JB, Lewis KC. Model-based compartmental analysis of retinol kinetics in organs of rats at different levels of vitamin A status. In: Livrea M, Packer L, editors. Retinoids: new trends in research and clinical applications. New York: Marcel Dekker; 1993, p. 185–204. [Google Scholar]
- 19.Lewis KC, Green MH, Green JB, Zech LA. Retinol metabolism in rats with low vitamin A status: a compartmental model. J Lipid Res 1990;31:1535–48. [PubMed] [Google Scholar]
- 20.Blomhoff R, Helgerud P, Rasmussen M, Berg T, Norum KR. In vivo uptake of chylomicron [3H] retinyl ester by rat liver: evidence for retinol transfer from parenchymal to nonparenchymal cells. Proc Natl Acad Sci USA 1982;79:7326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muto Y, Goodman DS, Vitamin A. Transport in rat plasma. J Biol Chem 1972;247:2533–41. [PubMed] [Google Scholar]
- 22.Green MH, Uhl L, Green JB. A multicompartmental model of vitamin A kinetics in rats with marginal liver vitamin A stores. J Lipid Res 1985;26:806–18. [PubMed] [Google Scholar]
- 23.Green MH, Green JB. Dynamics and control of plasma retinol. In: Blomhoff R, editor. Vitamin A in health and disease. London: CRC Press; 1994; p. 119–33. [Google Scholar]
- 24.Sonneveld E, van der Saag PT. Metabolism of retinoic acid: implications for development and cancer. Int J Vitam Nutr Res 1998;68:404–10. [PubMed] [Google Scholar]
- 25.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol 2006;46:451–80. [DOI] [PubMed] [Google Scholar]
- 26.Ulven SM, Gundersen TE, Weedon MS, Landaas VØ, Sakhi AK, Fromm SH, Geronimo BA, Moskaug JO, Blomhoff R. Identification of endogenous retinoids, enzymes, binding proteins, and receptors during early postimplantation development in mouse: important role of retinal dehydrogenase type 2 in synthesis of all-trans-retinoic acid. Dev Biol 2000;220:379–91. [DOI] [PubMed] [Google Scholar]
- 27.Ong DE, Lucas PC, Kakkad B, Quick TC. Ontogeny of two vitamin A-metabolizing enzymes and two retinol-binding proteins present in the small intestine of the rat. J Lipid Res 1991;32:1521–7. [PubMed] [Google Scholar]
- 28. Spiegler E, Kim YK, Wassef L, Shete V, Quadro L. Maternal-fetal transfer and metabolism of vitamin A and its precursor β -carotene in the developing tissues. Biochim Biophys Acta 2012;1821:88–98. [DOI] [PMC free article] [PubMed]
- 29.Takahashi YI, Smith JE, Goodman DS. Vitamin A and retinol-binding protein metabolism during fetal development in the rat. Am J Physiol 1977;233:E263–72. [DOI] [PubMed] [Google Scholar]
- 30.Cryer A, Jones HM. Developmental changes in the activity of lipoprotein lipase (clearing-factor lipase) in rat lung, cardiac muscle, skeletal muscle and brown adipose tissue. Biochem J 1978;174:447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu L, Ross AC. Acidic retinoids synergize with vitamin A to enhance retinol uptake and STRA6, LRAT, and CYP26B1 expression in neonatal lung. J Lipid Res 2010;51:378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley SK, Nilsson CB, Green MH, Green JB, Håkansson H. Use of model-based compartmental analysis to study effects of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin on vitamin A kinetics in rats. Toxicol Sci 1998;44:1–13. [DOI] [PubMed] [Google Scholar]
- 33.Cifelli CJ, Green JB, Green MH. Use of model-based compartmental analysis to study vitamin A kinetics and metabolism. Vitam Horm 2007;75:161–95. [DOI] [PubMed] [Google Scholar]
- 34.Cifelli CJ, Green JB, Green MH. Dietary retinoic acid alters vitamin A kinetics in both the whole body and in specific organs of rats with low vitamin A status. J Nutr 2005;135:746–52. [DOI] [PubMed] [Google Scholar]
- 35.Gieng SH, Green MH, Green JB, Rosales FJ. Model-based compartmental analysis indicates a reduced mobilization of hepatic vitamin A during inflammation in rats. J Lipid Res 2007;48:904–13. [DOI] [PubMed] [Google Scholar]
- 36.Kelley SK, Green MH. Plasma retinol is a major determinant of vitamin A utilization in rats. J Nutr 1998;128:1767–73. [DOI] [PubMed] [Google Scholar]
- 37.Cifelli CJ, Green JB, Wang Z, Yin S, Russell RM, Tang G, Green MH. Kinetic analysis shows that vitamin A disposal rate in humans is positively correlated with vitamin A stores. J Nutr 2008;138:971–7. [DOI] [PubMed] [Google Scholar]
- 38.Green MH, Green JB. The application of compartmental analysis to research in nutrition. Annu Rev Nutr 1990;10:41–61. [DOI] [PubMed] [Google Scholar]
- 39.Wastney ME, Patterson BH, Linares OA, Greif PC, Boston RC. WinSAAM. Investigating biological systems using modeling: strategies and software. San Diego: Academic Press; 1999.
- 40.Tan L, Wray AE, Green MH, Ross AC. Compartmental modeling of whole-body vitamin A kinetics in unsupplemented and vitamin A-retinoic acid-supplemented neonatal rats. J Lipid Res 2014;55:1738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan L, Wray AE, Green MH, Ross AC. Retinol kinetics in unsupplemented and vitamin A-retinoic acid supplemented neonatal rats: a preliminary model. J Lipid Res 2014;55:1077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green MH, Green JB. Vitamin A intake and status influence retinol balance, utilization and dynamics in rats. J Nutr 1994;124:2477–85. [DOI] [PubMed] [Google Scholar]
- 43.Allen LE, Green MH. Correspondence re: S.E. Dew et al., Effects of pharmacological retinoids on several vitamin A-metabolizing enzymes. Cancer Res, 53: 2965–2969, 1993. Cancer Res 1994;54:3319–20. [PubMed] [Google Scholar]
- 44.McClean P, Weaver LT. Ontogeny of human pancreatic exocrine function. Arch Dis Child 1993;68:62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Lindquist S, Lowe M, Noppa L, Hernell O. Bile salt–stimulated lipase and pancreatic lipase-related protein 2 are the dominating lipases in neonatal fat digestion in mice and rats. Pediatr Res 2007;62:537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamosh M. The role of lingual lipase in neonatal fat digestion. Development of mammalian absorptive processes. Amsterdam: Ciba Foundation Symposium; 1979. p. 69–92. [DOI] [PubMed]
- 47.Zachman RD. Retinol (vitamin A) and the neonate: special problems of the human premature infant. Am J Clin Nutr 1989;50:413–24. [DOI] [PubMed] [Google Scholar]
- 48.Institute of Medicine (US) Panel on Micronutrients, Institute of Medicine (US), Food and Nutrition Board. DRI, Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc: a report of the Panel on Micronutrients. Food and Nutrition Board, Institute of Medicine. Washington (DC): National Academies Press; 2001. [PubMed]
- 49.Oxley A, Berry P, Taylor GA, Cowell J, Hall MJ, Hesketh J, Lietz G, Boddy AV. An LC/MS/MS method for stable isotope dilution studies of β-carotene bioavailability, bioconversion, and vitamin A status in humans. J Lipid Res 2014;55:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang G, Qin J, Dolnikowski GG, Russell RM. Short-term (intestinal) and long-term (postintestinal) conversion of beta-carotene to retinol in adults as assessed by a stable-isotope reference method. Am J Clin Nutr 2003;78:259–66. [DOI] [PubMed] [Google Scholar]