Abstract
Background: Intake of added sugar has been shown to correlate with many human metabolic diseases, and rodent models have characterized numerous aspects of the resulting disease phenotypes. However, there is a controversy about whether differential health effects occur because of the consumption of either of the two common types of added sugar—high-fructose corn syrup (fructose and glucose monosaccharides; F/G) or table sugar (sucrose, a fructose and glucose disaccharide).
Objectives: We tested the equivalence of sucrose- vs. F/G-containing diets on mouse (Mus musculus) longevity, reproductive success, and social dominance.
Methods: We fed wild-derived mice, outbred mice descended from wild-caught ancestors, a diet in which 25% of the calories came from either an equal ratio of F/G or an isocaloric amount of sucrose (both diets had 63% of total calories as carbohydrates). Exposure lasted 40 wk, starting at weaning (21 d of age), and then mice (104 females and 56 males) were released into organismal performances assays—seminatural enclosures where mice competed for territories, resources, and mates for 32 wk. Within enclosures all mice consumed the F/G diet.
Results: Females initially fed the F/G diet experienced a mortality rate 1.9 times the rate (P = 0.012) and produced 26.4% fewer offspring than females initially fed sucrose (P = 0.001). This reproductive deficiency was present before mortality differences, suggesting the F/G diet was causing physiologic performance deficits prior to mortality. No differential patterns in survival, reproduction, or social dominance were observed in males, indicating a sex-specific outcome of exposure.
Conclusion: This study provides experimental evidence that the consumption of human-relevant levels of F/G is more deleterious than an isocaloric amount of sucrose for key organism-level health measures in female mice.
Keywords: added sugar, carbohydrate metabolism, fitness assay, fructose, intraspecific competition, sugar, toxicity assessment
See corresponding commentary on page 385
Introduction
Added sugars are sugars that are added to foods during processing or preparation (1). The most common forms of added sugar in America are table sugar (sucrose) and high-fructose corn syrup [HFCS7; fructose and glucose monosaccharides (F/G)], which make up 44% and 42%, respectively, of consumption annually (2). HFCS comes in two main forms, one is 42% and the other is 55% fructose, with the remaining percentage glucose (3). Because of the wide use of both varieties of HFCSs, the fructose-to-glucose ratio consumed by the public is ∼1:1 (4). Conversely, even though consumption of HFCS is high in America, its total consumption globally is only about 8% that of sucrose (5).
Consumption of added sugar is linked to numerous diseases, including cardiovascular disease, fatty liver disease, metabolic syndrome, obesity, type 2 diabetes, and even mortality [(4, 6–10) but see Rippe (11)]. Consumption of added sugar increased in the United States by ∼70% from the late 1970s to 2000; although, from this high point rates have declined modestly (12). Most of this increase came through elevated consumption of HFCS, which has led to speculation that the fructose monosaccharide in HFCS may be more responsible for patterns of disease than that present as sucrose (4). Supporting correlative data for this idea are reported by Goran et al. (13) who showed that populations from countries that consume HFCS have higher rates of type 2 diabetes than populations only consuming table sugar.
The fructose component of added sugar is more detrimental than glucose, and mechanisms for fructose’s contributions to de novo lipogenesis, insulin resistance, lipid dysregulation, and obesity are reviewed (14). Rodent models support these mechanisms in which fructose consumption, typically >40% kcal, has increased adiposity, increased levels of cholesterol and TGs, and impaired glucose tolerance (15–17). In addition, fructose consumption increases portal vein concentrations of bacterial endotoxin, a postulated trigger of metabolic abnormalities (18). It also has been shown that many of the deleterious effects of high-carbohydrates diets may in part be because of the metabolic conversion of glucose within these diets into fructose (19).
Few experiments have attempted to compare the health consequences of consuming a mixture of the monosaccharides fructose and glucose with that of the disaccharide sucrose. Many studies have compared diets containing only fructose with diets containing sucrose (3). Unfortunately, these studies do not control for the amount of fructose between treatments and often use fructose in isolation of glucose, a condition that is rarely experienced (20). Of studies that compare diets with F/G to those containing sucrose, only 4 to our knowledge have found differences. In rodent studies, rats fed high levels of F/G required a lower glucose infusion rate to maintain euglycemia, experienced increased weight gain, and had more severe signs of fatty liver disease than rats fed sucrose (17, 21, 22). In humans, it was observed that people absorb more total fructose when consuming beverages sweetened with F/G than from sucrose-sweetened beverages, though this difference is at least in part due to slightly different fructose levels of the treatments. This difference in absorption was associated with elevated blood pressure (23). However, two clinical studies that looked for differential consequences of these sugars did not find differences in blood measures (gherlin, insulin, leptin, and TGs), and another study did not detect differences in hepatic and skeletal muscle fat depositions (24–26). Importantly, none of these studies, regardless of achieving positive or negative results, quantify actual organismal adversity in the ultimate sense but focus only on proximate measures related to specific human diseases.
To assess whether the consumption of F/G decreases mouse health relative to the consumption of sucrose at human-relevant levels, we used organismal performance assays (OPAs). OPAs are defined as sensitive phenotyping approaches that use seminatural conditions to challenge the physiologic performance of differentially treated animals (i.e., treatment and control) in direct competition with each other. OPAs have detected mating preferences because of major histocompatibility genes and quantified adverse consequences of both inbreeding and harboring a t complex allele, a classic selfish genetic element (27–30). In all cases, OPAs quantified substantial health effects that were missed by previous studies, which assessed animals with standard laboratory methodologies. More recently, OPAs were used to show that added sugar at the moderate level of 25% of calories decreases survival and reproduction of mice, another finding undetected by conventional methodologies (31).
Here, we use OPAs to specifically test if a 1:1 ratio of F/G at a level of 25% kcal (hereafter referred to as the F/G diet), decreases mouse health compared with consumption of an isocaloric sucrose diet. The added sugar exposure level was selected because it is consumed by 13–25% of Americans (32, 33). OPA endpoint measures include survival, male competitive ability, and reproductive success. In addition, we monitored food intake, weight, and glucose tolerance of mice to determine whether these measures predicted OPA outcomes.
Methods
Animals
Outbred, wild-derived house mice (Mus musculus) were used because many laboratory strains do not possess the natural and functional behaviors required for OPA assessment (34). Mice in this study were from the 10th and 11th generations of a colony that was previously described (29). Inbreeding coefficients of individuals from the 11th generation were found to be comparable to those of wild populations using 11 generations of pedigree data (35). Before mice were released into OPAs, they were housed according to standard protocols under a 12:12 h light:dark cycle with food and water available ad libitum. At the termination of experiments mice were killed via CO2 inhalation. All protocols were approved by the Institutional Animal Use and Care Committee at the University of Utah.
Dietary exposure
Ad libitum exposure to specified diets began at weaning and continued until mice were released into OPAs ∼40 wk later. At the time of weaning, 73 litters were separated according to sex, and then both the females and males were subdivided into 2 equal-sized groups and ascribed randomly to either the F/G or sucrose group. The F/G diet (TD.05668; Harlan Teklad) contained 25% kcal from a 1:1 mixture of F/G. The sucrose diet (TD.05667; Harlan Teklad) was identical except for the component coming from the F/G was replaced by sucrose and had slightly less fiber to offset weight differences, both diets had 63% of total calories as carbohydrates. For a makeup of each diet see Supplemental Table 1 and for compositions of the mineral (TD.80318) and vitamin (TD.81125) mixes see Supplemental Table 2. On OPA entrance all mice consumed the F/G diet. Although leaving mice on their original diet would be preferable, we had no means to keep mice on their respective diets while they ranged freely together. The F/G diet was selected as the base diet in OPAs for both groups because we hypothesized that, if the F/G-fed mice experienced negative effects during the precompetition exposure, they would be maintained/exacerbated in OPAs by continued exposure. The alternative, feeding the sucrose diet in OPAs, could make differential performance difficult to detect because of potential recovery of mice pre-exposed to the F/G diet.
Metabolic cage food intake and weight measures
To assess if differential weight gain or food intake of the F/G and sucrose diets occurred 10 male mice were individually housed in metabolic cages (Lab Products Inc.) on weaning and exposed to either the F/G or sucrose diet ad libitum for 7 weeks. Males were selected because sex ratios in OPAs were asymmetrical, leading to an excess of male production in breeding cages. Mice were from sibling pairs that were split between treatments to control for home-cage factors. One mouse from the F/G diet group was removed from the study and excluded from analysis because it did not take to its new environment. Body and food weight measures were taken weekly for the first 2 weeks and then increased to 2–3 times weekly as intake increased. After measurement, available food was brought to a level of at least 5 full pellets (∼20g).
OPA enclosures
OPA enclosures were 30 m2 and were subdivided into 6 subsections by wire mesh to create environmental complexity and to promote territorial formation. Subsections have food and water sources provided ad libitum that were associated with housing in either 1 of 4 “optimal” territories, which contain nest boxes in enclosed structures or 2 “suboptimal” territories with exposed nest boxes. OPAs mimicked habitat and social environments experienced by mice in nature, and the population density was representative of wild levels (36). A photograph of OPA enclosures can be seen in Supplemental Figure 1, and an additional photograph and description can be found elsewhere (31).
Six OPA populations were founded by 24–30 mice, 8–10 males and 14–20 females, for a total of 160 mice (56 male; 104 female). Equal numbers of F/G- and sucrose-fed mice were represented in each sex within populations. To prevent confounding behaviors associated with relatedness no male mouse was related at the cousin level or above to any other mouse within a given population. Likewise, relatedness between female founders was also avoided, although in several populations a single pair of sisters was included (common in wild populations); when this was the case, sister pairs were balanced across diets. Mean ± SD age of founders was 44.4 ± 5.7 wk for males and 44.3 ± 5.9 wk for females at the time of release. To prevent incidental breeding before the establishment of male territories, we released nonexperimental females with the experimental males at the onset of each population to allow male territory formation before experimental female release. After 1 wk, the nonexperimental females were removed and the experimental females were released, marking the start (week 1) of the study. OPA populations ran for 32 wk.
Survivorship
Survivorship of population founders was determined by periodic checks in each enclosure. Deceased founders were identified by passive integrated transponder (PIT) tags and ear markings. Date of death was estimated on the basis of 3 factors: date of last check, the last date mouse was recorded feeding, and corpse condition. To avoid altering territorial dynamics and to decrease infanticide, researchers entered OPAs only to rotate PIT tag readers between pens and to conduct pup sweeps (described in the next paragraph). Corpses were collected in a variety of conditions that precluded necropsies.
Reproductive success
To determine the reproductive success of founders, samples (offspring per treatment per population) were gathered during pup sweeps in which pups born during the previous cycle were removed, killed, and had tissue taken for genetic analysis. The first sweep occurred during week 8 of the study, and additional sweeps followed every 6 wk. This schedule prevented offspring born in enclosures from breeding. In all 6 populations 5 pup sweeps occurred. A total of 1397 individual samples were collected with 235 ± 96 samples per population.
Population level reproductive success was determined for the F/G and sucrose groups as described previously (29). Briefly, in each competition enclosure male and female founders of each treatment were categorized by a common allelic variant on the Y chromosome and mitochondrial genome, respectively. Allelic assignments were reversed across populations to avoid possible confounding effects. We obtained 1336 mitochondrial (95.6% of total) and 667 Y-chromosome (99.9% of total assuming a 1:1 sex ratio) genotypes.
Male competitive ability
One week before entrance, founders were implanted with unique PIT tags (TX1400ST; BioMark). A set of PIT antennae and readers (FS2001F-ISO; BioMark) were rotated through the populations throughout the study and placed at each of the feeders; data were streamed to a computer with data-logging software (Minimon). Male dominance was assigned when a male had >80% of the PIT tag reads at a single location over a multiday reader session.
Body weight
Weight was assessed in the 160 mice that founded OPA populations at the time they were released into populations and at each pup sweep, for a total of 6 time points.
Glucose clearance
Intraperitoneal glucose tolerance tests (IPGTTs) were conducted on 8 females of each treatment at the end of the exposure period by giving an intraperitoneal injection of 1.5 mg D-glucose/g body weight after an 8-h deprivation of food. Only females were assessed because previous work on this population has shown that male clearance rates are not affected by this level of added sugar consumption (31). Blood was collected from the retro-orbital sinus before glucose injection and at 5, 10, 30, 60, and 120 min after injection. The 8-h period of food deprivation and bleeding technique were selected because our wild-derived mice do not tolerate food deprivation or handle stress as well as laboratory strains. Blood samples were immediately centrifuged at 10,000 g for 10 min after which time 8–10 μL of plasma was decanted and flash frozen. Samples were shipped on dry ice to the Children’s Hospital of Oakland Research Institute, and glucose levels were assessed by the hexokinase method (37).
Statistical methods
Weight gain and food intake.
To assess differential weight gain a linear mixed-effects model (LMM) was used to assess the main effects of diet, time, and their interaction on the weight of the 9 male mice in metabolic cages; daily food intake was also included as a covariate. An LMM was selected because it accounted for repeated measures of individuals, was multivariate, did not require balanced design, and was appropriate for continuous data. The intercept was set at the day of weaning (day 0). Body weight and food intake were observed 136 times and grouped across the 9 mice. Diet, time, their interaction, and food intake were modeled as fixed effects, whereas individual was modeled as a random effect with an independent intercept for each calculated to account for repeated measures. A reciprocal LMM was used to assess if daily food intake was influenced by diet, time, or their interaction, with body weight as a covariate. The intercept was set at day 6, because this was the first time all mice were measured. Diet, time, their interaction, and body weight were modeled as fixed effects, whereas individual was modeled as a random effect with an independent intercept and slope for each calculated.
Survival.
Survivorship of the 160 founders was analyzed by Cox proportional hazard (PH) models with male and female mice assessed separately because of different mortality rates. A PH model was selected because it was a standard time-to-event test that allow for multivariate analysis. Week 1 was defined as when mice entered OPA enclosures. A multivariate model was used to assess the effects of precompetition diet, population, and their interaction. Mice that survived the duration of the trial or were removed from the study were censored. In the male data set there were 32 events (deaths) and 24 censorings, whereas for females there were 40 events and 64 censorings.
Male competitive ability.
To assess the effects of precompetition diet and their interaction on competitive ability, we used a generalized linear mixed model (GLMM) to predict the probability of ownership. A GLMM was used because it accounted for repeated measures of populations, was multivariate, and was appropriate for binomial data. Because a territory can only be defended or not, we used a binomial distribution with a logit link to estimate probability of ownership. Territorial control within populations by each dietary treatment was assessed multiple times throughout the study for a total of 112 observations. Six territories are in each population, and territories were occupied (by a mouse of either treatment) or unoccupied. The intercept of the model was set at week 0 when males were released into enclosures. Time, precompetition diet, and their interaction were treated as fixed effects, and population was modeled as a random effect with a random intercept calculated for each.
Reproduction.
Because reproduction data are discrete counts, for each sex we modeled offspring counts over time in a GLMM with a Poisson distribution and a logarithmic link. A GLMM was used because it accounted for repeated measures of populations, was multivariate, and was appropriate for count data. The model assessed the main effects of precompetition diet and time and their interaction on population-level fitness across the 6 populations. Reproductive output of each dietary treatment was measured 5 times at 6-wk intervals for a total of 60 observations. Time, precompetition diet, and their interaction were modeled as fixed effects, and population was modeled as a random effect with both a random slope and intercept calculated. The intercept was set at week 8 because this was the first time point for which data were available. Male and female reproduction data were analyzed separately because they were based on separate measurements.
Weight.
Because weight data are continuous, a LMM was used to assess the main effects of precompetition diet, sex, and time, and their respective interactions on the weight of the 160 population founders. An LMM was selected because it accounted for repeated measures of individuals nested within populations, was multivariate, did not require balanced design, and was appropriate for continuous data. Precompetition diet, sex, time, and their interactions were modeled as fixed effects, and individual and population were modeled as random effects with a random intercept. The intercept was set at week 0. Founders were weighed at week 0, and surviving mice were weighed across the 5 pup sweeps for a total of 706 observations; this large number of observations allowed the sexes to be assessed together.
IPGTT.
The area under the curve (AUC) was calculated for plasma glucose levels over time by using the trapezoid rule. AUC values were calculated for 8 mice of each treatment, and comparisons were made between groups by using a Mann-Whitney U test because the distribution of the sucrose data set was found to vary significantly from that of a normal distribution by using a Shapiro-Wilk normality test.
All mixed-effects models were fit in R, using the glmer or lmer functions of the lme4 library (38, 39). Degrees of freedom and resulting P values for LMMs were determined with a Satterthwaite approximation by using the lmerTest library (40). Because controversy exists on how to best calculate degrees of freedom in LMMs, it should be noted that all effects deemed statistically significant through their resulting P values also possess a t value > |2|, a conservative criterion for significance recommended by the library’s authors. For all mixed-effects models several candidate models for the random effect terms were generated, including models estimating both intercept and/or slope for random effects. In all cases the model that explained at least some of the variance with random effects and had the lowest Akaike information criterion score was selected. Proportional hazard models were performed in JMP version 9.0.3 (SAS institute Inc.). All α values for statistical tests were 0.05, and all tests were two-tailed.
Results
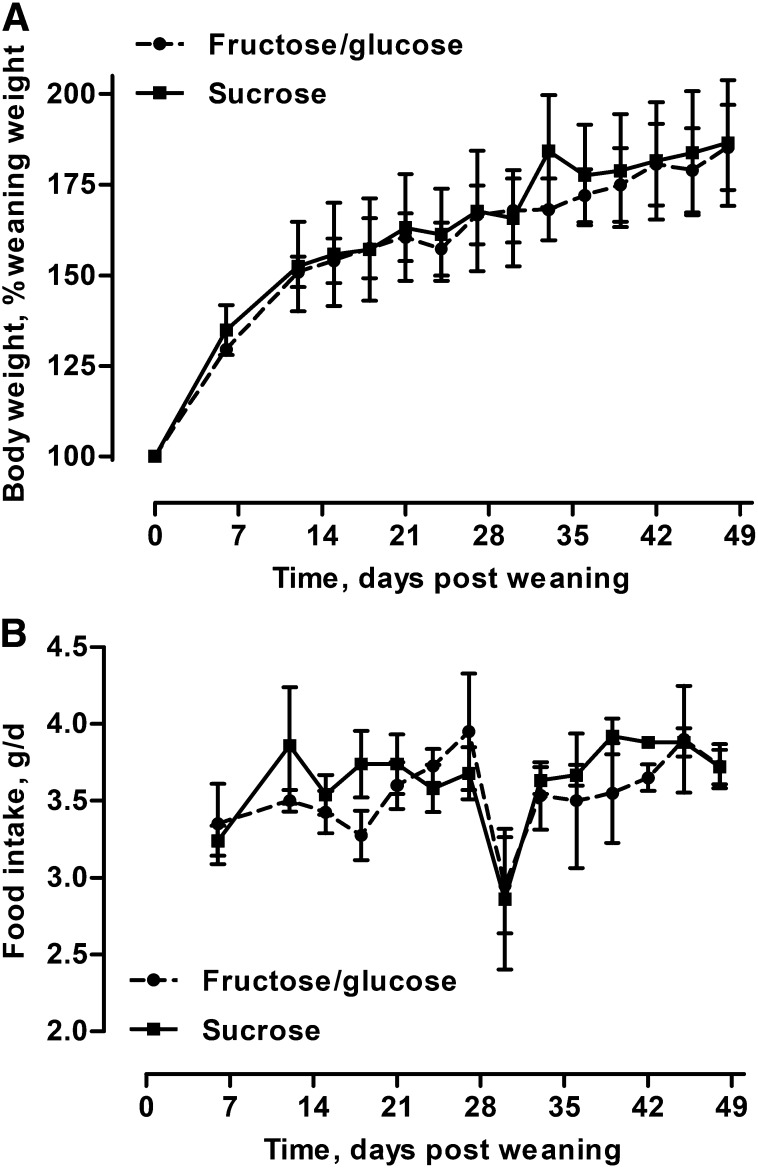
No evidence of differential weight gain or food intake between dietary treatments was observed in male mice within metabolic cages. At weaning mice ascribed to each dietary treatment did not differ in weight (LMM; P = 0.20) nor did their rate of weight gain differ across the 48-d trial (LMM; P = 0.60; Figure 1A). Mice gained weight as they aged (LMM; P < 0.001), and larger mice consumed more food (LMM; P < 0.001). Likewise, food intake was not influenced by diet (LMM; P = 0.63), time (LMM, P = 0.82), or their interaction (LMM; P = 0.90; Figure 1B). For a complete readout of weight gain and food intake LMM results, see Supplemental Table 3.
FIGURE 1.
Weight gain (A) and food intake (B) of fructose/glucose- and sucrose-fed male mice within metabolic cages over 7 wk. No differences were detected for weight gain between dietary treatments (P = 0.60). Likewise, no difference in daily food intake was observed between treatments (P = 0.63). Values are means ± SEMs, n = 9.
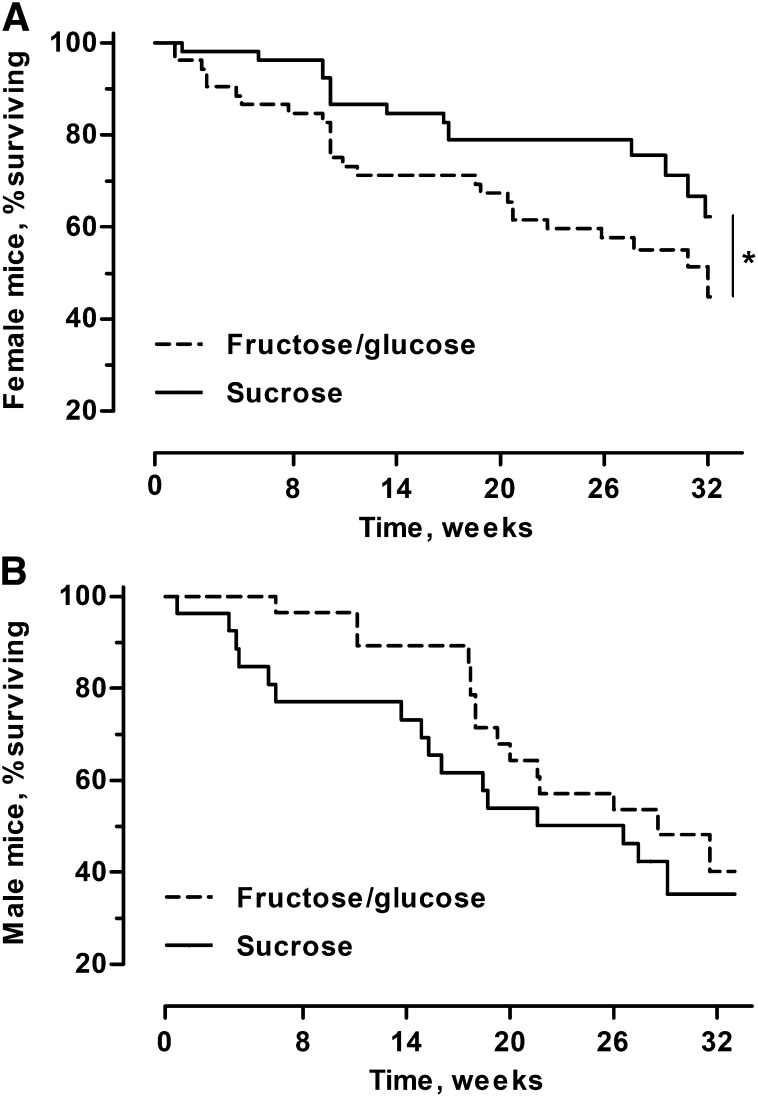
Survival of female mice within OPA enclosures was affected by precompetition diet, with females initially fed the F/G diet experiencing death rates 1.87 times the rates of mice initially fed sucrose (PH; χ2 = 6.38, P = 0.012; Figure 2A). No difference was found in survival among populations (PH; χ2 = 3.88, P = 0.57) nor did the impact of precompetition diet differ among populations (PH; χ2 = 8.16, P = 0.15).
FIGURE 2.
Survival of female (A) and male (B) mice fed the fructose/glucose and sucrose diets for 40 wk before release into enclosures wherein all mice consumed the fructose/glucose diet during the 32-wk OPA competition. (A) Females initially fed the fructose/glucose diet experienced mortality at twice the level of females fed the sucrose diet before OPA release (P = 0.012). (B) No overall dietary pattern was seen in males because the influence of precompetition diet differed significantly across populations (P = 0.040). Values are totals across replicates; n = 104 females, n = 56 males. *Indicates a P value < 0.05. OPA, organismal performance assay.
For male survival, no relation between precompetition diet and survival was detected (PH; χ2 = 2.66; Figure 2B). Overall survival did not differ among populations (PH; χ2 = 4.16); however, survival of the 2 treatments did significantly vary across populations [PH; χ2 = 11.63, P = 0.040 (P values are not provided for precompetition diet and population effects because they have a significant interaction)].
Male competitive ability was not affected by precompetition diet, with mice initially fed the F/G diet controlling approximately the same number of territories as mice initially fed sucrose at week 0, model intercept (GLMM; P = 0.28). Males initially fed the F/G diet controlled 39.2% and mice fed sucrose initially had 32.8% of territories throughout the study, leaving 28.0% unoccupied at any time. No effect of time (GLMM; P = 0.18) or precompetition diet by time (GLMM; P = 0.75) was detected on territorial acquisition. For a complete readout of GLMM (competitive ability and reproduction) and LMM (weight) founder results see Supplemental Table 4.
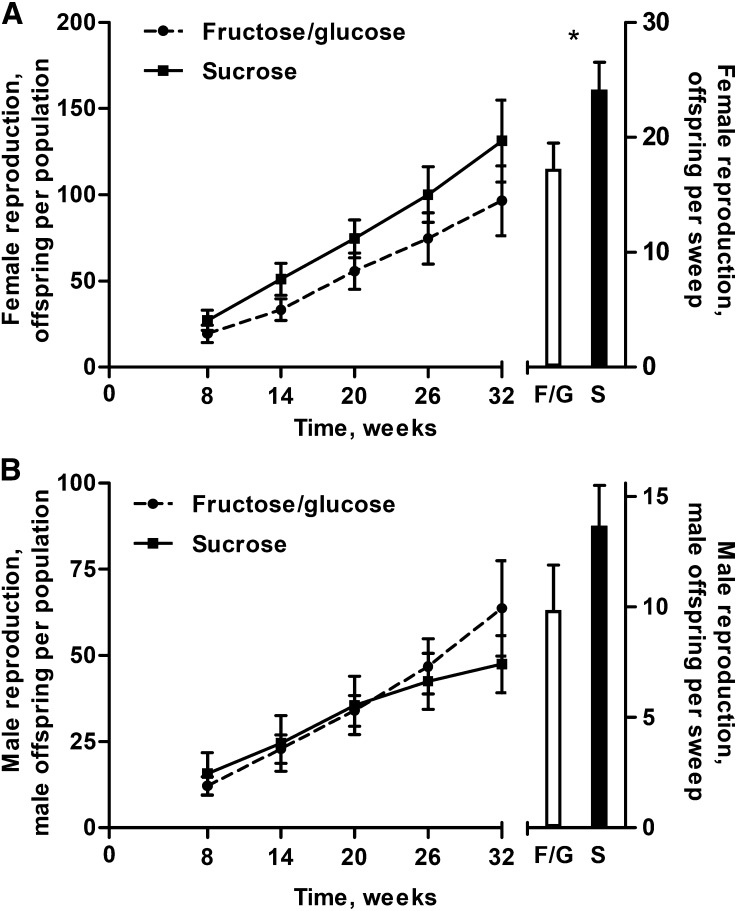
Female reproductive success was affected by precompetition diet with females initially fed the F/G diet producing 26.4% fewer offspring than females initially fed sucrose (Figure 3A). At week 8 (the model intercept) mean reproduction of females initially fed the F/G diet was 17.1 (SEM: +2.4, −2.1) offspring per population, whereas females initially fed sucrose produced 24.0 (SEM: +2.5, −2.2) offspring per population (reported SEMs are asymmetric because values were back-transformed from logarithmic data; see Supplemental Table 4 for symmetric SEMs); this difference was found to be statistically significant (GLMM; P = 0.001). Because there was no effect of time (GLMM; P = 0.83) or a time-by-precompetition diet interaction (GLMM; P = 0.69), the deficiency in offspring production experienced by females initially fed the F/G diet at the intercept persisted throughout the duration of the study.
FIGURE 3.
Cumulative reproductive success of female (A) and male (B) mice fed the fructose/glucose and sucrose diets for 40 wk before release into enclosures wherein all mice consumed the fructose/glucose diet during the 32-wk OPA competition. (A) Females initially fed the fructose/glucose diet produced 26.4% fewer offspring throughout the study than females fed sucrose before OPA release (P = 0.001) because of a consistent deficiency in offspring across pup sweeps. (B) No overall effect of initially consuming the fructose/glucose diet was seen in males. At the first pup sweeps males initially fed sucrose had higher reproduction, but this pattern was inverted for the second half of the study; post hoc tests indicated no significant differences at any given pup sweep. Values are means ± SEMs, n = 6. Because of a significant interaction between precompetition diet and time, the bar graphs are not interpretable for male data. *Indicates a P value < 0.05. F/G, fructose/glucose; OPA, organismal performance assay; S, sucrose.
No consistent pattern emerged for male reproductive success and precompetition diet. At week 8 (model intercept) mean reproduction of males initially fed the F/G diet was 9.8 (SEM: +2.1, −1.2) male offspring per population, whereas males initially fed sucrose sired 13.6 (SEM: +1.9, −1.7) male offspring per population (GLMM; Figure 3B). Conversely, males initially fed the sucrose diet had decreased reproduction over time by −0.06 ± 0.01 (mean ± SEM) offspring per week per population relative to those males initially fed the F/G diet [GLMM, P < 0.001 (reported slope data are log transformed)]. No overall effect of time on reproduction was observed [GLMM (P values are not provided for precompetition diet and time effects because they have a significant interaction)]. Because precompetition diet had significant and opposing effects for the intercept and slope of the linear model, post hoc t tests were conducted at each pup sweep, and no significant differences were detected.
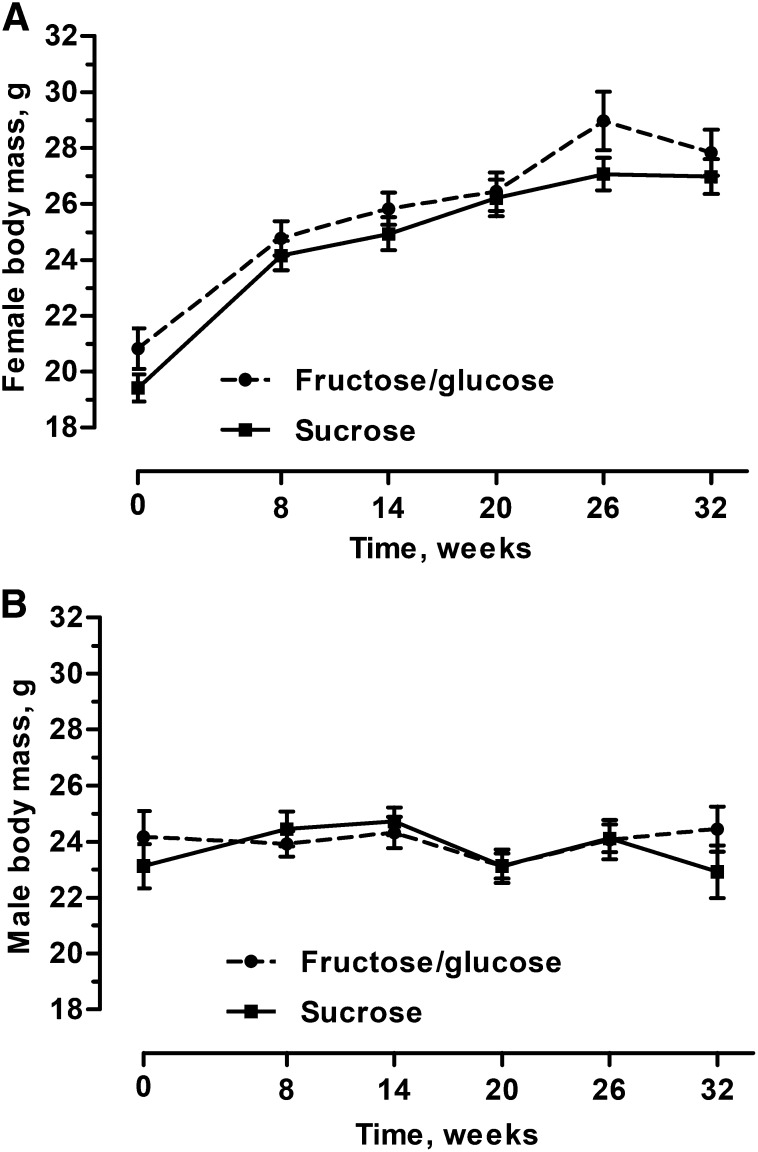
Precompetition diet did not affect the weight of population founders at the model intercept (LMM; P = 0.14; Figure 4). In addition, precompetition diet had no affect over time (LMM; P = 0.51), nor did it differentially affect the sexes (LMM; P = 0.63).
FIGURE 4.
Body weight of female (A) and male (B) mice fed the fructose/glucose and sucrose diets for 40 wk before release into enclosures wherein all mice consumed the fructose/glucose diet during the 32-wk OPA competition. No weight differences between dietary treatments were observed in population founders (P = 0.14). Founders did gain weight over time with males gaining at a decreased rate compared with females (P < 0.001). Values are means ± SEMs; n = 104 females, n = 56 males. OPA, organismal performance assay.
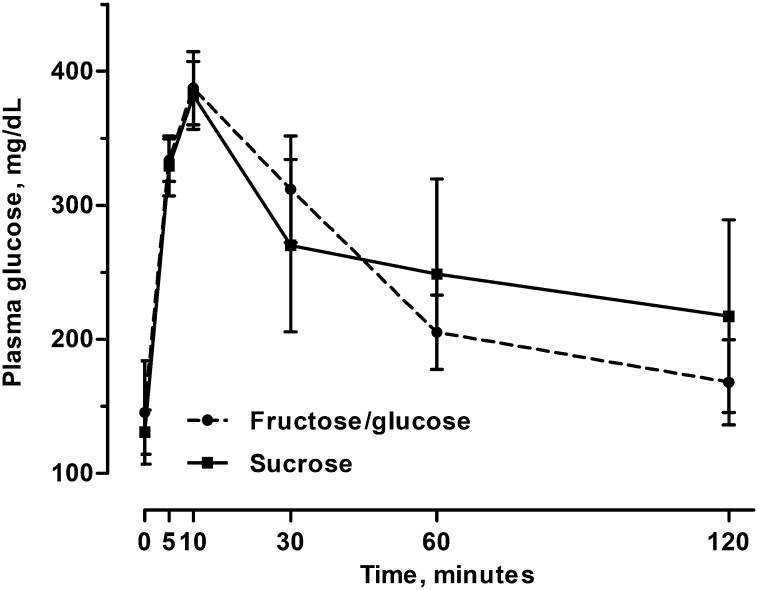
Female glucose tolerance, as assessed by IPGTTs, was not altered by diet at the end of the exposer period with the F/G-fed females having a mean ± SEM AUC score of 28.9 ± 2.81 g/dL × 120 min and sucrose-fed females having an AUC score of 31.1 ± 7.23 g/dL × 120 min (Mann-Whitney U test; n = 16; U = 25.0; P = 0.51; Figure 5).
FIGURE 5.
Glucose tolerance curves of female mice fed the fructose/glucose and sucrose diets for 40 wk. No difference in glucose clearance rates between treatment groups were observed (P = 0.51). Values are means ± SEMs, n = 16.
Discussion
Within OPAs, females on the precompetition F/G diet were dramatically outperformed by females initially fed sucrose, as demonstrated by a death rate 2 times that of initially sucrose-fed females and a 26% relative reduction in reproductive output. Interestingly, the reproductive disadvantage of females initially fed the F/G diet is present as soon as they are introduced into OPAs, indicating that even before the unequal mortality rates were manifest, reproduction differentials were present; this suggests that the F/G diet was causing health and physiologic performance problems. The increased female death rate observed here (1.9-fold) is remarkably similar to the one (2.0-fold) we detected in a previous OPA competition between mice exposed to the same F/G diet vs. a starch-based control diet, indicating that perhaps the increased mortality in the previous study was because of free fructose and not total fructose as originally concluded (31). However, to assess this question, a direct OPA comparison between sucrose and starch-fed control mice is needed.
Unlike females, males on the F/G precompetition diet were not outcompeted by their sucrose-fed counterparts. Males initially fed the F/G diet gained equivalent numbers of territories and experienced similar levels of mortality. Interestingly, a significant interaction between the precompetition diet and population was detected for survival within OPAs, indicating that in some populations one dietary treatment had higher rates while in other populations the alternative treatment had higher rates; thus, for males, there was far more interpopulation variation than there was variation between dietary groups, making any effect of precompetition diet uninterpretable. This pattern is likely because of heritable components of social dominance that we have previously detected, which influence mortality, combined with the situation wherein brothers in separate populations were on opposing dietary treatments (35). Furthermore, OPAs have never detected a difference in male survival rates between treatments that was not accompanied by differences in territorial acquisition. Likewise, no overall pattern emerged for reproduction with males initially fed sucrose producing more offspring early on, whereas those initially fed the F/G diet sired more offspring toward the end of the study. A possible explanation for this pattern is that once mice enter OPAs they all consume the F/G diet, which could lead to convergence in reproduction because differences between the treatments decrease over time. These findings, in conjunction with our previous study, indicate that, although both male competitive ability and reproduction in OPAs is decreased by the consumption of 25% kcal from added sugar (compared with starch), the form of that added sugar, as either F/G or the disaccharide sucrose, appears inconsequential (31); although this should be confirmed with direct competition experiments between starch- and sucrose-fed mice.
The sex-specific nature of our OPA findings is surprising, but not unprecedented, because this system had revealed a similar mortality pattern in 2 previous experiments. First, females harboring the selfish genetic element known as the t complex experienced an increased risk of mortality, whereas males with the t complex did not (30). Second, females fed the F/G diet had increased mortality compared with controls fed starch, whereas males showed no differences between groups (31). It is inappropriate to assume that experimental treatments will affect the sexes in exactly the same way, because major differences in life histories of female and male mice are well established. For example, a female when pregnant consumes 18–25% more calories than when she is not pregnant; therefore, a female mouse is likely to respond differentially to a nutritional treatment than a male mouse (41). Even without considering pregnancy status, sex-specific differences are well established in metabolic processes crucial to fructose toxicity, such as the insulin response, for both mice and humans (42, 43).
We found no evidence of differential weight gain (both sexes), food intake (males), or glucose tolerance (females) between mice fed the F/G and the sucrose diets. Our weight findings are consistent with our previous work in which mice were fed the same F/G diet, but stand in contrast to another rodent study possibly because of rat/mouse differences or variation in sugar dosage and exposure methods (21, 31). Our intake data indicate that mice consume these diets in equal amounts and are consistent with other studies that evaluate liquid fructose intake in male mice (17); however, because only males were assessed, it remains possible that differential intake could occur in females, although this was evaluated in rodent models before and not observed (21). Furthermore, our intake results are similar to those for weight-matched mice on standard rodent diets, implying our mice regardless of dietary treatment are not increasing consumption because of fructose content (44). Glucose clearance rates did not differ between females raised on either diet and were similar to those of female mice fed the same F/G diet in a previous study (31). This observation is in accord with data from a previous study, which showed glucose clearance rates do not differ between mice on a much higher F/G diet vs. a sucrose diet, although differences in the glucose infusion rate required to maintain euglycemia were detected (17); this latter aspect of glucose metabolism was not measured in our present study. In the future, reducing the duration of food deprivation before the IPGTT could improve the ability to measure differences in glucose tolerance that are not as readily observed otherwise.
OPAs are able to detect differential health consequences because of the consumption of different sources of added sugar because they evaluate an organism’s performance under the stressors of its natural social and physical environments. House mouse physiology did not evolve under the stresses, or lack thereof, associated with caged-based laboratory housing. Therefore, to understand if mouse physiology is impaired it is essential to assess it under ecologically relevant conditions in which physiologic systems are tested in the context of the selective forces that forged them. Evaluations in this manner allow the detection of differences that are masked in other systems but nonetheless cause substantial impairment (28–31). Furthermore, because of their sensitivity, OPAs allow for the use of moderate, human-relevant levels of nutritional (or toxicologic) exposures, and effects at these levels are likely to be more nuanced and possibly differ from those observed at extreme levels.
The mechanistic basis of female mortality and reproductive impairment because of the consumption of the F/G diet, as opposed to sucrose, is not known. We directly tested for differences in glucose homeostasis and weight gain, 2 outcomes that were previously reported as differentially affected by fructose-containing diets, similar to the ones used in this study, but no differences emerged. We did not directly assess the rate of fructose uptake after consumption, a third metric that was shown to differ between similar diets in humans, leaving open the possibility that our F/G-fed mice absorbed a higher amount of fructose. If not because of an increase in total fructose absorption, then it seems likely that whatever mechanism is at play is taking place at, or before, absorption of these sugars by enterocytes, because the bond connecting the monosaccharide components of sucrose is hydrolyzed at this time (45). One intriguing possibility is that free fructose, relative to that in sucrose and whose availability from the disaccharide is rate limited by sucrase activity, may lead to increased rates of intestinal permeability to bacterial endotoxin and therefore inflammation [as seen in Bergheim et al. (18)]. Another possibility is that the microbiomes of mice raised on the different sugar sources could differ and that these differences could lead to differential health status, especially because changes to microbiomes are linked with metabolic diseases and are sex specific (46, 47). These potential explanations are fascinating because they are both independent of the metabolism of fructose in the liver, where most scientific investigation has focused. Regardless of what mechanism is contributing to the increased mortality and decreased reproduction of females on the F/G diet, the organismal-level phenotype characterized herein should greatly aid in its elucidation.
The possibility of differential health affects arising from HFCS and sucrose diets are controversial (4). This study provides unique experimental evidence that the consumption of a 1:1 ratio of F/G can dramatically decrease female mammalian health compared with the intake of an isocaloric amount of sucrose. Moreover, the F/G diet used in this study contains these added sugars at levels that are consumed by 13–25% of Americans (32, 33). However, this study should not be seen as a vindication of sucrose, but rather as evidence of nonequivalence for health degradation of these 2 forms of fructose. Finally, our work highlights that, although many aspects of fructose toxicity are well described, there is still much to learn beyond the confines of our present biochemical understanding.
Supplementary Material
Acknowledgments
We thank B Ames for suggesting we apply OPAs to added sugar; D Dearing and A Torregrossa for aid in experimental design; S Laverty for statistical modeling assistance; D Cornwall for input on figures; J Donohoe, S Gentry, and A Stenquist for help in data collection. JSR, MKS, and WKP designed the experiment; JSR, SAH, AKS, MMS, RET, MEH, and LCM maintained OPAs and collected the associated data; JSR and AKS collected plasma; MEH gathered intake data; SHG obtained plasma measures; JSR analyzed data and wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: F/G, fructose and glucose monosaccharides; HFCS, high-fructose corn syrup; GLMM, generalized linear mixed model; IPGTT, intraperitoneal glucose tolerance test; LMM, linear mixed-effects model; OPA, organismal performance assay; PH, proportional hazard; PIT, passive integrated transponder.
References
- 1.A Report of the Panel on Macronutrients, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington (DC): The National Academies Press; 2005.
- 2.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 2009;139:1228S–35S. [DOI] [PubMed] [Google Scholar]
- 3.White JS. Straight talk about high-fructose corn syrup: what it is and what it ain't. Am J Clin Nutr 2008;88:1716S–21S. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537–43. [DOI] [PubMed] [Google Scholar]
- 5.Fereday N, Forber G, Girardello S, Midgley C, Nutt T, Powell N, Todd M. HFCS industry annual review-a year of changing expectations. In Sweetener analysis. Oxford (UK): LMC International Ltd; 2007:1–8. [Google Scholar]
- 6.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 2009;89:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 2008;48:993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007;116:480–8. [DOI] [PubMed] [Google Scholar]
- 9.Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr 2004;79:774–9. [DOI] [PubMed] [Google Scholar]
- 10.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med 2014;174:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rippe JM. The metabolic and endocrine response and health implications of consuming sugar-sweetened beverages: findings from recent randomized controlled trials. Adv Nutr 2013;4:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 2011;94:726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goran MI, Ulijaszek SJ, Ventura EE. High fructose corn syrup and diabetes prevalence: a global perspective. Glob Public Health 2013;8:55–64. [DOI] [PubMed] [Google Scholar]
- 14.Stanhope KL. Role of fructose-containing sugars in the epidemics of obesity and metabolic syndrome. Annu Rev Med 2012;63:329–43. [DOI] [PubMed] [Google Scholar]
- 15.Jürgens H, Haass W, Castañeda TR, Schürmann A, Koebnick C, Dombrowski F, Otto B, Nawrocki AR, Scherer PE, Spranger J, et al. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res 2005;13:1146–56. [DOI] [PubMed] [Google Scholar]
- 16.Kelley GL, Allan G, Azhar S. High dietary fructose induces a hepatic stress response resulting in cholesterol and lipid dysregulation. Endocrinology 2004;145:548–55. [DOI] [PubMed] [Google Scholar]
- 17.Thresher JS, Podolin DA, Wei Y, Mazzeo RS, Pagliassotti MJ. Comparison of the effects of sucrose and fructose on insulin action and glucose tolerance. Am J Physiol Regul Integr Comp Physiol 2000;279:R1334–40. [DOI] [PubMed] [Google Scholar]
- 18.Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol 2008;48:983–92. [DOI] [PubMed] [Google Scholar]
- 19.Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzycky P, Rivard C, Inaba S, Roncal-Jimenez CA, Bales ES, et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun 2013;4:2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White JS. Weak association between sweeteners or sweetened beverages and diabetes. J Nutr 2008;138:138; author reply 139. [DOI] [PubMed] [Google Scholar]
- 21.Bocarsly ME, Powell ES, Avena NM, Hoebel BG. High-fructose corn syrup causes characteristics of obesity in rats: increased body weight, body fat and triglyceride levels. Pharmacol Biochem Behav 2010;97:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez-Lozada LG, Mu W, Roncal C, Sautin YY, Abdelmalek M, Reungjui S, Le M, Nakagawa T, Lan HY, Yu X, et al. Comparison of free fructose and glucose to sucrose in the ability to cause fatty liver. Eur J Nutr 2010;49:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le MT, Frye RF, Rivard CJ, Cheng J, McFann KK, Segal MS, Johnson RJ, Johnson JA. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism 2012;61:641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melanson KJ, Zukley L, Lowndes J, Nguyen V, Angelopoulos TJ, Rippe JM. Effects of high-fructose corn syrup and sucrose consumption on circulating glucose, insulin, leptin, and ghrelin and on appetite in normal-weight women. Nutrition 2007;23:103–12. [DOI] [PubMed] [Google Scholar]
- 25.Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr 2008;87:1194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bravo S, Lowndes J, Sinnett S, Yu Z, Rippe J. Consumption of sucrose and high-fructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Appl Physiol Nutr Metab 2013;38:681–8. [DOI] [PubMed] [Google Scholar]
- 27.Potts WK, Manning CJ, Wakeland EK. Mating patterns in seminatural populations of mice influenced by MHC genotype. Nature 1991;352:619–21. [DOI] [PubMed] [Google Scholar]
- 28.Ilmonen P, Penn DJ, Damjanovich K, Clarke J, Lamborn D, Morrison L, Ghotbi L, Potts WK. Experimental infection magnifies inbreeding depression in house mice. J Evol Biol 2008;21:834–41. [DOI] [PubMed] [Google Scholar]
- 29.Meagher S, Penn DJ, Potts WK. Male-male competition magnifies inbreeding depression in wild house mice. Proc Natl Acad Sci USA 2000;97:3324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll LS, Meagher S, Morrison L, Penn DJ, Potts WK. Fitness effects of a selfish gene are revealed in an ecological context. Evolution 2004;58:1318–28. [DOI] [PubMed] [Google Scholar]
- 31.Ruff JS, Suchy AK, Hugentobler SA, Sosa MM, Schwartz BL, Morrison LC, Gieng SH, Shigenaga MK, Potts WK. Human-relevant levels of added sugar consumption increase female mortality and lower male fitness in mice. Nat Commun 2013;4:2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marriott BP, Olsho L, Hadden L, Connor P. Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003–2006. Crit Rev Food Sci Nutr 2010;50:228–58. [DOI] [PubMed] [Google Scholar]
- 33.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson AC, Cauceglia JW, Merkley SD, Youngson NA, Oler AJ, Nelson RJ, Cairns BR, Whitelaw E, Potts WK. Reintroducing domesticated wild mice to sociality induces adaptive transgenerational effects on MUP expression. Proc Natl Acad Sci USA 2013;110:19848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunningham CB, Ruff JS, Chase K, Potts WK, Carrier DR. Competitive ability in male house mice (Mus musculus): genetic influences. Behav Genet 2013;43:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sage RD. Wild mice. In: Foster HL, Smal JD, Fox JG, editors. The mouse in biomedical research. Vol. I New York: Academic Press; 1981: p. 40–90. [Google Scholar]
- 37.Slein MW. Methods of enzymatic analysis. New York: Academic Press; 1963. [Google Scholar]
- 38.R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 39.Bates D, Maechler M, Bolker B. R package; version 0.999375-42. lme4: linear mixed-effects models using S4 classes; 2013 [cited 2014 Aug 19]. Available from: http://cran.R-project.org/package=lme4.
- 40.Kuznetsova A, Brockhoff R, Christensen R. R package; version 2.0-20. lmerTest: tests for random and fixed effects for linear mixed effects models(lmer objects of lme4 package); 2013 [cited 2014 Aug 19]. Available from: http://cran.r-project.org/web/packages/lmerTest/.
- 41.Gittleman JL, Thompson SD. Energy allocation in mammalian reproduction. Am Zool 1988;28:863–76. [Google Scholar]
- 42.Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 2010;3:525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mittendorfer B. Insulin resistance: sex matters. Curr Opin Clin Nutr Metab Care 2005;8:367–72. [DOI] [PubMed] [Google Scholar]
- 44.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet 2002;32:435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riby JE, Fujisawa T, Kretchmer N. Fructose absorption. Am J Clin Nutr 1993;58:748S–53S. [DOI] [PubMed] [Google Scholar]
- 46.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010;328:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013;39:400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.