Abstract
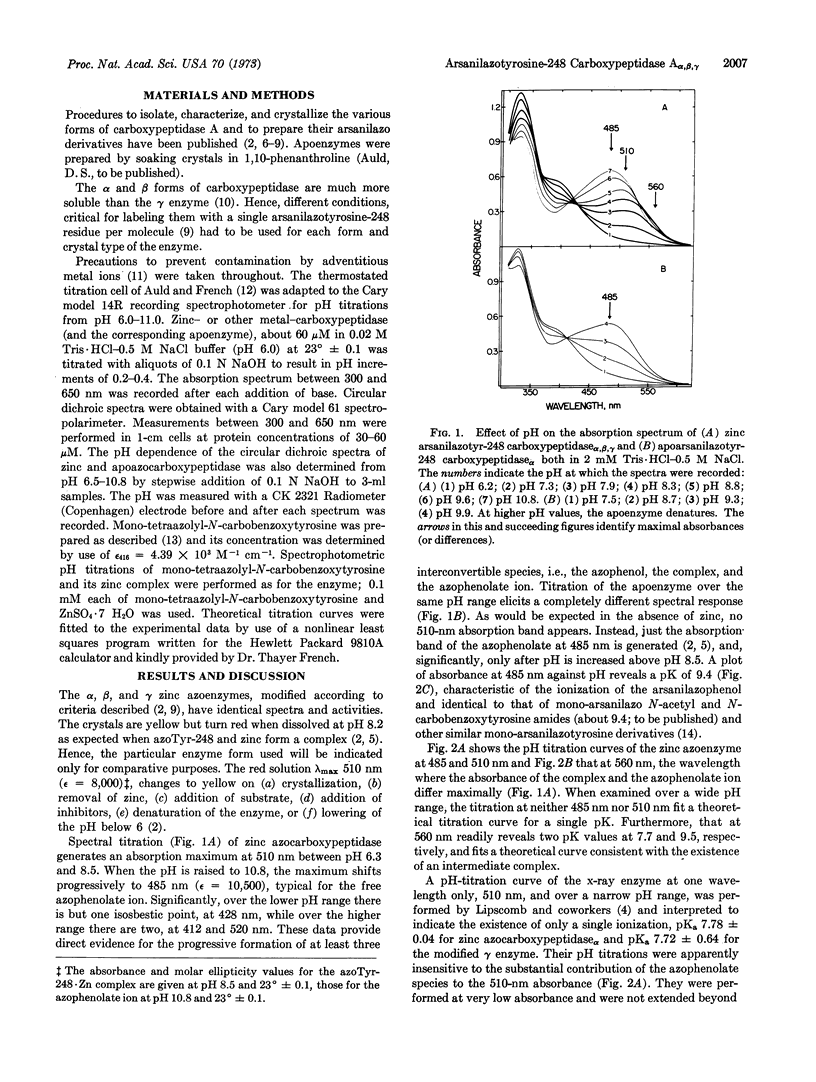
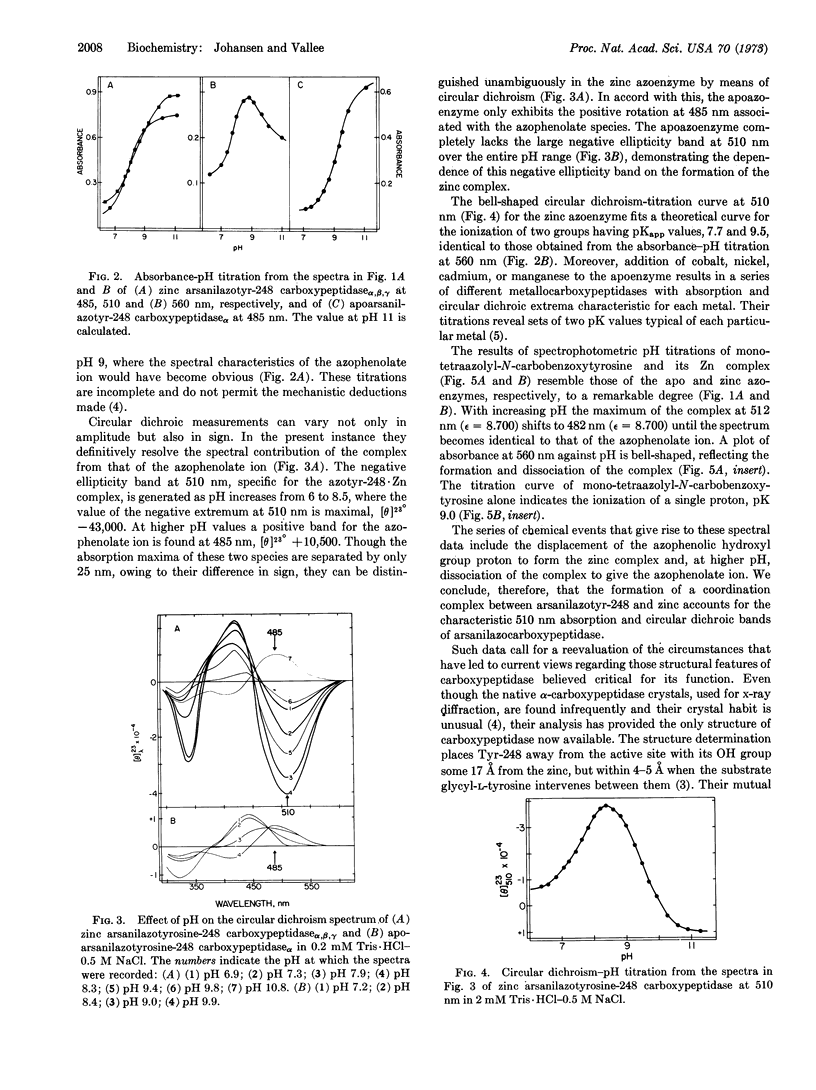
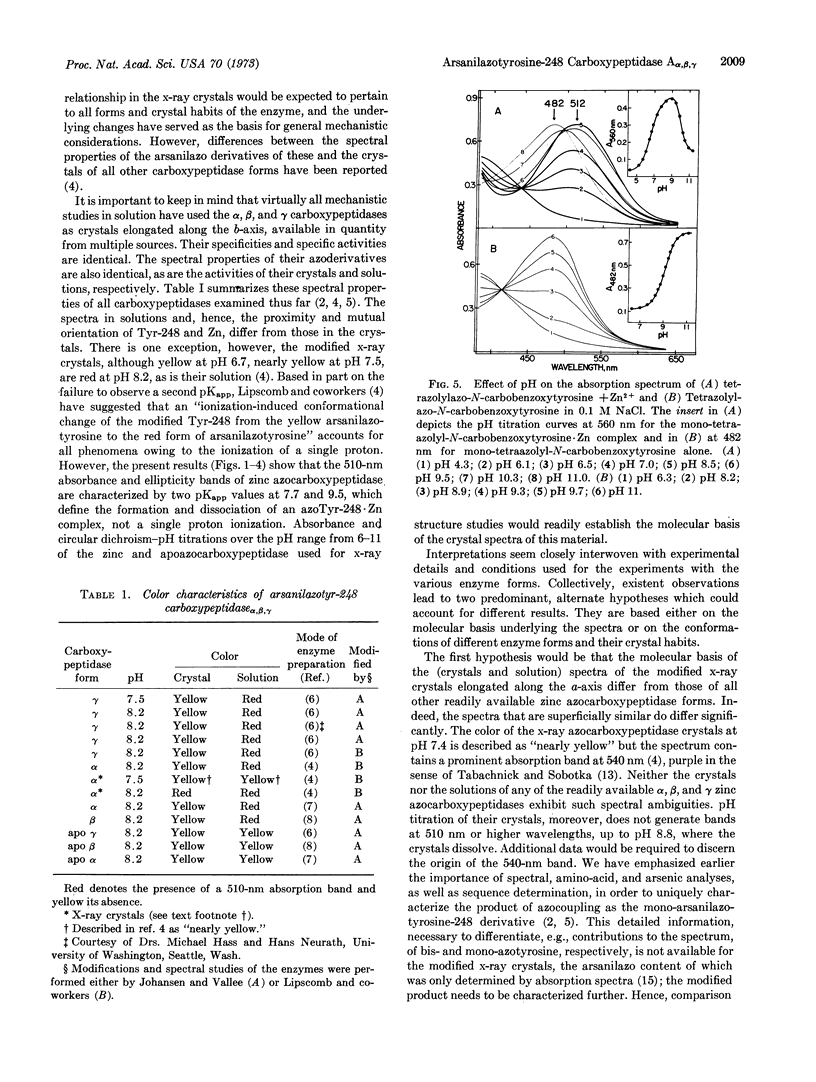
The spectra of the α, β, and γ forms of zinc monoarsanilazotyrosine-248 carboxypeptidase A are indistinguishable. At pH 8.2 their crystals are yellow, while their solutions are red, λmax 510 nm. Absorption and circular dichroism-pH titrations of the modified zinc and apoenzymes demonstrate that the absorption band at 510 nm is due to a complex between arsanilazotyrosine-248 and the active-site zinc atom. Two pKapp values, 7.7 and 9.5, characterize the formation and dissociation of this arsanilazotyrosine-248·Zn complex. On titrations of the apoenzyme, the absorption band at 510 nm is completely absent at all pH values. Instead, there is a single pKapp, 9.4, due to the ionization of the azophenol, λmax 485 nm. Substitution of other metals for zinc results in analogous intramolecular coordination complexes with absorption maxima and circular dichroism extrema characteristic of the particular metal. Similar data and conclusions have been derived from studies of heterocyclic azophenol·metal complexes.
The present studies demonstrate that the conformation of the crystals of all generally available α, β, and γ forms of the arsanilazoenzyme differs from that of their solutions. The spectra of the modified x-ray crystals, however, differ from those of all other carboxypeptidase forms and crystal habits studied. The internal consistency of their data, their interpretation, and the conclusions of Lipscomb and coworkers [Proc. Nat. Acad. Sci. USA (1972) 69, 2850-2854] are examined. Dissimilar chemical modification or conformation is thought to underlie these differences.
The arsanilazotyrosine-248·zinc complex is a sensitive, dynamic probe of environmental conditions. Its response to changes in pH and physical state of the enzyme suggest different orientation of the arsanilazotyrosine-248 side chain in solution from that in the crystal. This finding calls for reexamination of the basis of the substrate-induced conformation change which has been thought to be critical to the mechanism, postulated on the basis of the x-ray structure analysis performed at pH 7.5.
Keywords: intramolecular coordination, circular dichroism and absorbance-pH titration
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLAN B. J., KELLER P. J., NEURATH H. PROCEDURES FOR THE ISOLATION OF CRYSTALLINE BOVINE PANCREATIC CARBOXYPEPTIDASE A. I. ISOLATION FROM ACETONE POWDERS OF PANCREAS GLANDS. Biochemistry. 1964 Jan;3:40–43. doi: 10.1021/bi00889a007. [DOI] [PubMed] [Google Scholar]
- Auld D. S., French T. C. Cell for spectrophotometric titrations with small volumes. Anal Biochem. 1970 Mar;34:262–274. doi: 10.1016/0003-2697(70)90104-1. [DOI] [PubMed] [Google Scholar]
- COX D. J., BOVARD F. C., BARGETZI J. P., WALSH K. A., NEURATH H. PROCEDURES FOR THE ISOLATION OF CRYSTALLINE BOVINE PANCREATIC CARBOXYPEPTIDASE A. II. ISOLATION OF CARBOXYPEPTIDASE A-ALPHA FROM PROCARBOXYPEPTIDASE A. Biochemistry. 1964 Jan;3:44–47. doi: 10.1021/bi00889a008. [DOI] [PubMed] [Google Scholar]
- Johansen J. T., Livingston D. M., Vallee B. L. Chemical modification of carboxypeptidase A crystals. Azo coupling with tyrosine-248. Biochemistry. 1972 Jul 4;11(14):2584–2588. doi: 10.1021/bi00764a005. [DOI] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Differences between the conformation of arsanilazotyrosine 248 of carboxypeptidase A in the crystalline state and in solution. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2532–2535. doi: 10.1073/pnas.68.10.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petra P. H., Hermodson M. A., Walsh K. A., Neurath H. Characterization of bovine carboxypeptidase A (Allan). Biochemistry. 1971 Oct 26;10(22):4023–4025. doi: 10.1021/bi00798a600. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., McMurray C. H., Lipscomb W. N. Similarities between the conformation of arsanilazotyrosine 248 of carboxypeptidase A in the crystalline state and in solution. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2850–2854. doi: 10.1073/pnas.69.10.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovsky M., Vallee B. L. The reaction of diazonium-1H-tetrazole with proteins. Determination of tyrosine and histidine content. Biochemistry. 1966 Nov;5(11):3574–3581. doi: 10.1021/bi00875a028. [DOI] [PubMed] [Google Scholar]
- Steitz T. A., Ludwig M. L., Quiocho F. A., Lipscomb W. N. The structure of carboxypepidase A. V. Studies of enzyme-substrate and enzyme-inhibitor complexes at 6 A resolution. J Biol Chem. 1967 Oct 25;242(20):4662–4668. [PubMed] [Google Scholar]
- TABACHNICK M., SOBOTKA H. Azoproteins. I. Spectrophotometric studies of amino acid azo derivatives. J Biol Chem. 1959 Jul;234(7):1726–1730. [PubMed] [Google Scholar]
- TABACHNICK M., SOBOTKA H. Azoproteins. II. A spectrophotometric study of the coupling of diazotized arsanilic acid with proteins. J Biol Chem. 1960 Apr;235:1051–1054. [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F., Johansen J. T., Livingston D. M. Spectro-chemical probes for protein conformation and function. Cold Spring Harb Symp Quant Biol. 1972;36:517–531. doi: 10.1101/sqb.1972.036.01.066. [DOI] [PubMed] [Google Scholar]



