Abstract
Background: Although evidence shows that reduced sodium intake lowers blood pressure, some studies suggest that sodium reduction may adversely affect insulin resistance and glucose tolerance.
Objectives: The objectives were to assess the effects of sodium reduction on glucose tolerance, evaluate strengths and weaknesses of the relevant scientific literature, and provide direction for future research.
Methods: We searched The Cochrane Library, MEDLINE, EMBASE, CINAHL, and Web of Science through August 2014. Both randomized and nonrandomized intervention trials were included in our meta-analyses. The effects of sodium reduction on glucose tolerance were evaluated in 37 articles, but because of a lack of comparable data, 8 trials were excluded from the meta-analyses.
Results: Participants were 10–79 y old, either primarily healthy or with hypertension. In meta-analyses of 20 randomized, crossover trials (n = 504 participants) and 9 nonrandomized crossover trials (n = 337), circulating glucose concentrations of fasting participants were not affected by reduction in sodium intake. In contrast, in meta-analyses of 19 of the 20 randomized, crossover trials (n = 494), fasting insulin concentrations were 9.53 pmol/L higher (95% CI: 5.04, 14.02 pmol/L higher) with sodium reduction. In 9 nonrandomized trials (n = 337), fasting insulin did not differ with reduced sodium intake. Results differed little when the analyses were restricted to studies with a low risk of bias and duration of ≥7 d.
Conclusions: This meta-analysis revealed no evidence that, in trials with a short intervention and large reductions in sodium, circulating glucose concentrations differed between groups. Recommendations for future studies include extending intervention durations, ensuring comparability of groups at baseline through randomization, and assessing sodium intakes relevant to population sodium reduction. In addition, analyses on other metabolic variables were limited because of the number of trials reporting these outcomes and lack of consistency across measures, suggesting a need for comparable measures of glucose tolerance across studies.
Keywords: fasting glucose, insulin resistance, meta-analysis, sodium reduction, systematic review
Introduction
Although evidence shows that reduced sodium intake lowers blood pressure, some studies suggest that sodium reduction may adversely affect insulin resistance (IR)9 and glucose tolerance (1–9). IR is a condition in which the body produces insulin sufficiently, but the insulin-mediated disposal of glucose is impaired. This leads to impaired glucose tolerance or impaired fasting glucose, conditions that are risk factors for type 2 diabetes (10–13). Sodium restriction, as is true with medications that lower blood pressure, can increase the activity of plasmin renin, which is associated with IR (14). The Institute of Medicine’s (IOM’s) Dietary Reference Intakes for water, potassium, sodium, chloride, and sulfate, however, which reviewed studies on sodium reduction and IR, concluded that the evidence linking sodium intake and IR was sparse and inconsistent (14). Particularly, the IOM noted a need for longer-term studies at relevant sodium intakes. Later, a 2008 systematic review by a Portuguese group examined the effects of sodium restriction on the metabolic syndrome (15) and found that sodium restriction was associated with increased IR in 2 articles but decreased IR in 3 others (9 articles were reviewed in all), underscoring the need to conduct further studies. Since 2008, several new studies were published, but they have not been systematically reviewed or included in available meta-analyses.
Given the lack of consistent evidence on sodium restriction and IR, we need to evaluate studies that incorporate sodium intakes that are relevant to population initiatives or recommendations aimed at reducing such intake. In the United States, the Healthy People 2020 objectives aim for a reduction in daily mean sodium intake among the population aged ≥2 y from 3641 mg in 2010 to 2300 mg in 2020, about a 40% reduction over 10 y (16). This agrees with a recommendation in the 2010 Dietary Guidelines for Americans that individuals should consume <2300 mg of sodium/d (17). Adults aged ≥51 y, African Americans, and individuals with hypertension, diabetes, or chronic kidney disease should reduce their intake even more, to 1500 mg/d (17). The latter recommendation applies to ∼50% of Americans and more adults (people aged ≥20 y) (17). It is not known how many studies published after the release of the IOM’s Dietary Reference Intakes examined the impact on glucose intolerance of sodium restriction of ≤40% or, alternatively, evaluated sodium restriction consistent with the recommendations in the 2010 Dietary Guidelines of 1500 mg/d for what would amount to 57% of adults.
In the present study we performed a systematic review and meta-analysis of previously published randomized and nonrandomized prospective intervention trials to examine the effect of sodium reduction on glucose tolerance. Additional objectives were to evaluate the strengths and weaknesses of these trials and to provide direction for future research.
Methods
To prepare a study protocol that included eligibility criteria and an informed approach to the analysis we followed the methods of the Cochrane Collaboration (18). We report our results in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (19).
Data sources and searches.
We searched 5 databases that covered the period of January 1950 to August 2014: The Cochrane Library, MEDLINE, EMBASE, CINAHL, and Web of Science. To obtain additional studies we reviewed the reference lists of the articles found in our initial search. That search included the terms sodium reduction, insulin resistance, and glucose intolerance and related entry terms (Supplemental Table 1). The search included a filter to limit the results to human trials. No language or date restrictions before August 2014 were applied.
Study selection.
We included both randomized and nonrandomized intervention trials that compared ≥2 levels of sodium intake in relation to at least 1 measure of IR as an outcome. Studies either reduced or increased sodium intake via supplements, diet, or both. We excluded those studies that assessed combined interventions (e.g., medications and sodium restriction) whereby the effects of changes in sodium intake could not be disaggregated. The measures of IR included values for glucose (fasting, disposal rate, plasma, AUC, uptake, effectiveness) and insulin (fasting, plasma, immune-reactive, and acute insulin response) and measures such as insulin sensitivity, insulin c-peptide, HOMA-IR, and glucose-to-insulin ratio. Eligible studies included males or nonpregnant females of any age, regardless of hypertension or diabetes status. We excluded studies of subjects with chronic diseases other than hypertension or diabetes, and we did not use the abstracts of conferences. When multiple publications were available for an included study, we used 1 publication as a primary publication, with others used as companion publications.
Data extraction and quality assessment.
Two independent reviewers (SMP and 1 of 5 other coauthors) screened titles and abstracts of all references. Articles not published in English were translated with an online language translation service or by native speakers. Reviewers were not blinded to investigators, institutions, or journal of publication. After the screening of titles and abstracts, 2 independent reviewers, using a full-article screening form, assessed inclusion criteria. Disagreements in assessments were resolved by a third reviewer or by the consensus of group discussions. Two independent reviewers abstracted data from the included studies by using the same abstraction form. In trials not reporting the SD of outcomes, we estimated the SD according to standard formulas from the SE, 95% CI, or P value (18).
Two independent reviewers assessed study quality by using a form adapted from the Cochrane risk of bias tool and the National Heart, Lung, and Blood Institute (Supplemental Table 2). Disagreements in assessments were resolved by a third reviewer or by the consensus of group discussions. Within our study quality criteria, we assessed risk of bias. Our criteria for ruling out bias were as follows: evidence of random sequence generation, concealment of allocation, blinding (of study participants, study personnel, and outcome assessors), equivalence of the study groups in the numbers and reasons for dropouts and withdrawals, <20% attrition, prespecified outcomes, baseline comparability between exposure groups (e.g., intervention and control), and having an intent-to-treat analysis. We characterized a study as having a low risk of bias if it met at least the following 4 criteria: outcomes were prespecified, <20% attrition, an intent-to-treat analysis was present, and the study arms did not differ in demographic or other baseline characteristics. Additional factors to determine study quality were by assessing whether participants adhered to the intervention, there was similarity of intakes and avoidance of other interventions, an outcome was specified at the start of the study, and both outcome reliability and validity were acceptable (Supplemental Table 2).
Some researchers have concluded that sodium homeostasis and a full response in blood pressure, aldosterone, and other analytes require several days (20–24). He et al. (25) suggest that at least 28 d are required to establish homeostasis. Whether the full 28 d are required or 14 d are adequate is still unknown. We evaluated duration of the studies, and, in separate sensitivity analyses that considered previous research on sodium homeostasis, we compared the subset of studies with interventions lasting ≥7 d against studies with an intervention of <7 d.
To determine the relevance of studies for specific levels of sodium reduction, we used information from the Healthy People 2020 objectives for sodium intake and recommendations on sodium from the Dietary Guidelines (16, 17). We considered studies with an average difference in sodium reduction between comparison groups of ≤40% or with an average sodium intake in the lowest intervention arm of ≥1500 mg/d to be relevant to recommendations for population sodium reduction.
Data synthesis and analysis.
We summarized the demographic characteristics and attrition rate for each study and also summarized the comparability of the outcome measures and data reported across studies. We recorded whether the investigators used ≥1 of the following tests: oral glucose tolerance test, fasting blood sample, euglycemic clamp, continuous infusion of glucose with model assessment, or frequently sampled intravenous glucose tolerance test. In studies that reported using these tests we collected data on fasting glucose, fasting insulin, and AUC. For analysis, the data had to use the same test and reference time and present complete numeric outcome data, such as reporting the mean and some measure of variation of the mean.
Data were analyzed with Cochrane Review Manager, version 5.1.7 (The Nordic Cochrane Centre, The Cochrane Collaboration). For the meta-analysis, a minimum of 2 studies was required of a specific type with complete numeric data on comparable outcome measures. In the crossover studies, in which each participant acted as his or her own control, we conducted an approximated paired analysis by imputing missing SDs on the basis of the methods presented by the Cochrane Collaboration (18). By assuming a baseline to follow-up correlation of 0.75, we estimated the SD of the difference between baseline and follow-up measures if the SD was not given. In addition, we performed sensitivity analyses to examine the impact of different correlation values (0.25, 0.5, and 1.0) on the distribution of the mean difference. We used random effects models to generate pooled estimates, with each study weighted by the inverse of the study variance. We analyzed the data in Review Manager by using the generic inverse variance method (18) and defined statistical significance as P < 0.05. We used the I2 statistic to assess heterogeneity, with values of ≤25% considered low; 26–74% considered moderate, and ≥75% considered high (18).
We stratified the analyses by whether the interventions were randomized. In addition, we conducted sensitivity analyses to compare studies having a high risk of bias with those in which the risk of bias was low. Among studies with a low risk, subgroup analyses were based on duration of the intervention (≥7 vs. <7 d) and stratification of the low-sodium intervention arm by sodium intake (≥1500 vs. <1500 mg/d) or percentage of sodium reduction (>40% vs. ≤40%). Stratifications for sodium intake were based on urinary sodium excretion, but when this information was not available (26–28), intended doses were used.
Results
Thirty-seven articles, representing 38 trials, fulfilled our inclusion criteria. These trials included 27 crossover randomized controlled trials [RCTs; 1 article (4) had 2 crossover groups], 10 nonrandomized trials (9 of them using crossover), and 1 RCT with a parallel group design (Supplemental Figure 1, Supplemental Table 3) (1–8, 26–54).
Characteristics of the trials
The 38 trials included 1402 participants, with ages ranging from 10 to 79 y. Thirty-seven of the 38 trials were conducted in a single country or region as follows: Europe (n = 16), the United States (n = 11), Japan (n = 3), South America (n = 3), Canada (n = 2), New Zealand (n = 1), and Zimbabwe (n = 1); the remaining study included participants in both Europe and the United States (42). In most trials the participants were characterized as healthy or hypertensive (Supplemental Table 3). The nonrandomized trials were more likely to include participants who were hypertensive (8 of the 10 nonrandomized trials vs. 14 of 27 RCTs). The reported attrition rates ranged from 0% to 38%.
The most commonly used outcome measure was fasting glucose concentration (n = 34 trials), followed by fasting insulin concentration (n = 31) (Table 1). Seven trials included data on glucose AUC and 6 on insulin AUC. Because of variation in the time points of measurement (0–120 vs. 0–180 min), however, and lack of information on the methods of calculating AUC, we were unable to combine data on AUC for either variable. Accordingly, we did not include these measures in our meta-analyses (Table 2). Seven trials measured insulin sensitivity, but they were not comparable on either the measure used (e.g., insulin sensitivity index, M-value, or the time point of measurement); thus, we did not include them in our meta-analysis. For 5 of the measures, including glucose uptake, glucose disposal, glucose-to-insulin ratio, glucose effectiveness, and homeostasis model assessment, ≤2 trials presented data; thus, the results for these measures were not combined (and could not be used in the meta-analyses). Further, the lack of comparability in the type and time point of measure used ruled out their inclusion.
TABLE 1.
Outcome and intervention characteristics of trials that examined sodium intake and glucose tolerance in adults and children1
| Outcomes |
Intervention |
Actual |
||||||
| First author of study (reference) | Measure | Data reported | Type2,3 | Duration, d | Intended low Na dose, mg/d | Intended high Na dose, mg/d | Low 24-h UNa, mg/d | High 24-h UNa, mg/d |
| Crossover RCTs | ||||||||
| Ames (1) | OGTT | FG, FI, AUCG, AUCI | Diet + Supp | 28 | NR | 3145 | 3080 | 5981 |
| Boero (29) | Fasting blood sample | FG | Diet + Supp | 14 | 1150 | 5750 | 1403 | 6210 |
| Del Rio (30) | Fasting blood sample | FG, FI | Diet + Suppb | 14 | 786 | 4718 | 1095 | 4575 |
| Dengel (31) | FSIVGTT | FG, FI, ISI | Dieta | 7 | 460 | 4600 | 642 | 4485 |
| Donovan (32) | Clamp | FG, FI | Diet | 5 | 230 | 4600 | 184 | 3680 |
| Egan (33) | Fasting blood sample | FG, FI | Diet + Suppb | 7 | 460 | 4785 | 483 | 4922 |
| Facchini (34) | OGTT | SSPG, SSPI | Diet + Supp | 5 | 575 | 4600 | 292 | 4002 |
| Feldman (2) | Fasting blood sample | FG, FI | Diet + Supp | 7 | 460 | 5060 | 280 | 4356 |
| Feldman (3) | Fasting blood sample | FG, FI | Diet + Supp | 7 | 1725 | 5405 | 1104 | 4761 |
| Fliser (4) | Clamp, fasting blood sample | FG, FI | Diet + Supp | 3 | 460 | 4600 | 575 | 4462 |
| Fliser 3-d (4) | Clamp, fasting blood sample | FG, FI | Diet + Supp | 7 | 460 | 4600 | 529 | 4669 |
| Foo (35) | Clamp, fasting blood sample | FG, FI | Diet + Suppc | 6 | 920 | 5060 | 1787 | 5223 |
| Fotherby (36) | Fasting blood sample | FG | Diet + Supp | 35 | 1840–2300 | 3680–4140 | 2185 | 4002 |
| Garg (5) | Fasting blood sample | HOMA-IR | Diet | 7 | <460 | >3450 | 173 | 5469 |
| Gomi (6) | Clamp, fasting blood sample | FG, FI | Diet | 7 | 690 | 2300 | 566 | 2171 |
| Gray (37) | CIGMA | FG, FI, IS | Diet + Supp | 7 | 1840 | 4600 | 1196 | 4255 |
| Inoue (39) | OGTT | FG, FI | Diet + Supp | 7 | 230 | 8050 | 828 | 7567 |
| Iwaoka (38) | OGTT | FG, FI, AUCG, AUCI | Diet | 7 | 782 | 7866 | 745 | 6859 |
| Meland (41) | OGTT | FG, FI | Diet + Suppc | 56 | NR | Diet (NR) + 1150 | 2875 | 4393 |
| Mufunda (26) | OGTT | FG, FI | Dieta | 4 | 575 | 6900 | ||
| Perry (7) | Clamp, fasting blood sample | FG, FI, ISI | Diet + Suppa | 5 | >1840 | ∼4140 | 1610 | 4025 |
| Raji (42) | Fasting blood sample | HOMA-IR | Diet | 7 | 230 | 4600 | NR | NR |
| Ruppert (43) | Fasting blood sample | FG, FI | Diet + Supp | 7 | 460 | 6440 | 391 | 6665 |
| Schorr (44) | OGTT | FG, FI, AUCG, AUCI | Diet + Suppb | 28 | 2302 | 3595 | 2406 | 4030 |
| Sharma (27) | OGTT | FG, FI | Diet + Supp | 7 HS, 14 LS | 460 | 5980 | ||
| Suzuki (48) | OGTT | AUCG, AUCI, SSPG, SSPI | Diet | 7 | 1150 | 5865 | 1164 | 3848 |
| Townsend (8) | Clamp, fasting blood sample | FG, FI | Diet + Suppc | 6 | 460 | 4600 | 529 | 4462 |
| Randomized parallel group trial | ||||||||
| Meland (40) | OGTT | FG | Diet + Supp | 56 | NR | Diet (NR) + 1150 | 1909 | 2898 |
| Nonrandomized trials | ||||||||
| Melander (54) | Clamp, fasting blood sample | FG, FI | Diet + Supp | 7 | 230 | 5520 | 228 | 5221 |
| Egan (48) | OGTT | FG, FI | Diet + Supp | 14 | 460 | 4785 | 322 | 4301 |
| Egan (49) | OGTT | FG, FI AUCG, AUCI | Diet + Supp | 7 | 500 | 4746 | 398 | 5191 |
| Dziwura (47) | Fasting blood sample | FG, FI, HOMA-IR | Diet | 7 | 230–460 | 5060–5520 | 451 | 5032 |
| Rocchini (53) | Fasting blood sample | FG, FI | Diet + Supp | 14 | 460–690 | 5750 | 763 | 5055 |
| Fuenmayor (50) | Fasting blood sample | SSPG, SSPI | Diet + Supp | 7 | 920–1380 | 6440–6900 | 1541 | 7452 |
| Lima (51) | Fasting blood sample | FG, FI, HOMA-IR | Diet + Supp | 7 LS, 91 HS | NR | NR | 1611 | 6778 |
| Nakandakare (52) | Fasting blood sample | FG, FI, HOMA-IR4 | Diet | 7 HS, 21 LS | 1380 | 3680 | 1748 | 3910 |
| Dengel (46) | OGTT, clamp | FG, FI, AUCG, AUCI | Dieta | 14 | 3000 | 10,000 | 2567 | 8370 |
| Lind (28) | IVGTT | FI, IS | Diet + Supp | 7 | 1179 | 7863 | ||
Three studies (26–28) did not collect or report on 24-h urine samples. AUCG, AUC glucose; AUCI, AUC insulin; CIGMA, continuous infusion of glucose with model assessment; Clamp, euglycemic clamp; FG, fasting glucose; FI, fasting insulin; FSIVGTT, frequently sampled intravenous glucose tolerance test; HS, high sodium; IS, insulin sensitivity (%); ISI, insulin sensitivity index; IVGTT, intravenous glucose tolerance test; LS, low sodium; NR, not reported; OGTT, 75-g oral glucose tolerance test; RCT, randomized control trial; SSPG, steady state plasma glucose; SSPI, steady state plasma insulin; Supp, supplement; UNa, urinary sodium excretion.
Diet + Supp interventions were performed with a prescribed diet and sodium supplement capsules.
Studies with a washout period (between intervention arms) of a≤1 wk, b2 wk, or c4 wk.
Used a computer model to calculate HOMA-IR.
TABLE 2.
Pooled effects of sodium restriction on fasting glucose or fasting insulin concentrations in adults and children1
| Fasting glucose |
Fasting insulin |
|||||||
| Trials, n | Participants, n | Pooled difference,mmol/L,mean (95% CI) | I2, % | Trials, n | Participants, n | Pooled difference,pmol/L,mean (95% CI) | I2, % | |
| Randomized controlled trials | 20 | 504 | 0.01 (−0.04, 0.05) | 18 | 19 | 494 | 9.53 (5.04, 14.0) | 89 |
| Risk of bias | ||||||||
| Low | 14 | 356 | 0.01 (−0.03, 0.06) | 0 | 14 | 359 | 10.8 (7.32, 14.2) | 52 |
| High | 6 | 148 | 0.01 (−0.09, 0.11) | 46 | 5 | 135 | 4.4 (−2.51, 11.3) | 82 |
| Low risk of bias plus duration of intervention | ||||||||
| <7 d | 4 | 57 | −0.05 (−0.16, 0.05) | 18 | 5 | 76 | 8.75 (3.04, 14.5) | 49 |
| ≥7 d | 10 | 299 | 0.04 (−0.02, 0.10) | 0 | 9 | 283 | 12 (7.45, 16.6) | 56 |
| Low risk of bias plus intake of sodium-restricted group | ||||||||
| ≥1500 mg/d | 3 | 48 | −0.04 (−0.16, 0.08) | 0 | 2 | 31 | 1.1 (−5.46, 7.65) | 0 |
| <1500 mg/d | 11 | 308 | 0.02 (−0.03, 0.08) | 9 | 12 | 328 | 11.8 (8.35, 15.2) | 46 |
| Nonrandomized trials | 9 | 337 | 0.05 (−0.03, 0.14) | 72 | 9 | 337 | 3.87 (−4.62, 12.4) | 92 |
| Risk of bias | ||||||||
| Low | 2 | 143 | 0.01 (−0.18, 0.20) | 93 | 2 | 143 | −7.26 (−26.3, 11.8) | 98 |
| High | 7 | 194 | 0.07 (0.00, 0.15) | 16 | 7 | 194 | 7.67 (−0.44, 15.8) | 79 |
| Low risk of bias plus duration of intervention ≥7 d | 2 | 143 | 0.01 (−0.18, 0.20) | 93 | 2 | 143 | −7.26 (−26.3, 11.8) | 98 |
| Low risk of bias plus intake of sodium-restricted group <1500 mg/d | 2 | 143 | 0.01 (−0.18, 0.20) | 93 | 2 | 143 | −7.26 (−26.3, 11.8) | 98 |
I2, percentage of total variation across studies that is due to heterogeneity.
The interventions in 71% (n = 27) of the trials included both diet and supplements to achieve the desired sodium intake (Table 1). The average duration was 12.3 d (range: 3–56 d) for the lower-sodium diet and 14.2 d (range: 3–91 d) for the high-sodium diet. In 4 RCTs and the 1 trial with a randomized parallel group design (but in no nonrandomized trials) the low-sodium intervention lasted ≥28 d. Overall, the duration of the intervention was ≥7 d (20 RCTs, 10 nonrandomized, crossover trials, the 1 parallel-group trial) in 31 trials and <7 d in 7 trials. Intended sodium restriction varied widely across studies. Most trials (n = 34) measured and reported 24-h urine sodium excretion by using Association of Official Analytical Chemists International methods at the end of the intervention period to assess adherence to intake. When 24-h urine sodium excretion was available, we used it to assess sodium intake in both the intervention and control arms. The mean 24-h values ranged from 173 to 3080 mg/d for the lower-sodium intervention arms and from 2171 to 8370 mg/d for the higher-sodium intervention arms. Among 34 trials with a low-sodium intervention, the actual mean 24-h sodium excretion was <1500 mg/d in 23 trials, 1500 to <2300 mg/d in 7 trials, and ≥2300 mg/d in 4 trials.
Risk of bias and quality of the studies
Risk of bias and study quality varied across trials. Nineteen of the RCTs, the 1 study with the parallel group design, and 2 of the nonrandomized, crossover trials had a low risk of bias on the basis of our 4 criteria (Supplemental Table 4). For the RCTs, the 2 reasons for not meeting these criteria were ≥20% attrition and absence of an intent-to-treat analysis or a lack of clarity about whether this was done. For the nonrandomized trials, the most common reason was lack of baseline comparability of the intervention groups.
The blinding of participants, providers, and assessors was difficult to determine. All of the nonrandomized trials lacked sufficient information to determine the presence of blinding. Only 5 of the RCTs reported having enough statistical power to detect a difference in the outcomes.
Twenty-two trials (20 randomized, 2 nonrandomized) met all 4 of our specified criteria for study quality; 16 of these 22 trials had a low risk of bias (results not shown in supplemental tables). The most common reason for not meeting all 4 criteria for quality was an absence of the information needed to make sure the outcome was not present at the start of the study (this was true for 8 RCTs and 4 nonrandomized trials).
Only 2 studies performed reductions of ≤40%, 1 of which had a high risk of bias; thus we did not perform sensitivity analyses on the basis of this classification.
Quantitative analysis
Effects of reduced sodium intake.
Of the 34 trials with data on fasting glucose concentrations, 4 did not present numeric data and thus were not included in the analyses (8, 27, 39, 50). Twenty crossover RCTs and 9 nonrandomized, crossover trials contributed data on fasting glucose to the meta-analyses (Table 2). We examined results from the 1 RCT with a parallel group design separately (40).
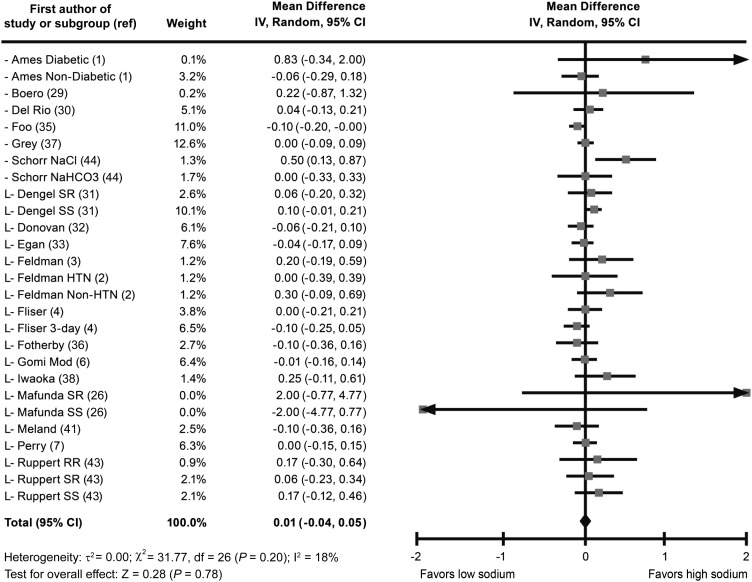
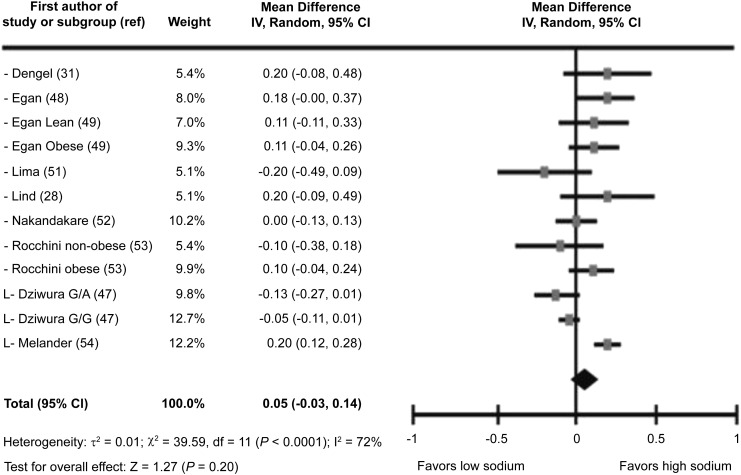
Among the 504 participants in the RCTs, sodium intake did not have a significant effect on fasting glucose concentrations, and the pooled mean difference between low- and high-sodium diets was 0.01 mmol/L (95% CI, −0.04, 0.05 mmol/L; P = 0.78)(Figure 1, Table 2). Heterogeneity was low (I2 = 18%). Among the 337 participants in the nonrandomized trials, the pooled mean difference between the low- and high-sodium diets was 0.05 (95% CI: −0.03, 0.14; P = 0.20) (Table 2, Figure 2). For the 46 participants in the 1 trial with a parallel group design (which lasted 56 d), the mean 24-h sodium excretion at the end of the intervention (data not shown in tables or figures) was 2898 mg for the group receiving salt capsules vs. 1909 mg for the group receiving placebo capsules; the investigators concluded that salt restriction did not influence glucose concentrations (P = 0.50) (40).
FIGURE 1.
Forest plot showing all randomized crossover trials for fasting glucose (mmol/L) mean difference (low sodium to high sodium) in adults and children (n = 504). HTN, hypertension; IV, inverse variance; L, studies with a low risk of bias; Mod, moderate sodium intake; ref, reference; RR, reverse reactors; SR, salt-resistant; SS, salt-sensitive.
FIGURE 2.
Forest plot showing all nonrandomized trials for fasting glucose (mmol/L) mean difference (low sodium to high sodium) in adults and children (n = 337). G/A, glycine/arginine carriers; G/G, glycine/glycine carriers; IV, inverse variance; L, studies with a low risk of bias; ref, reference.
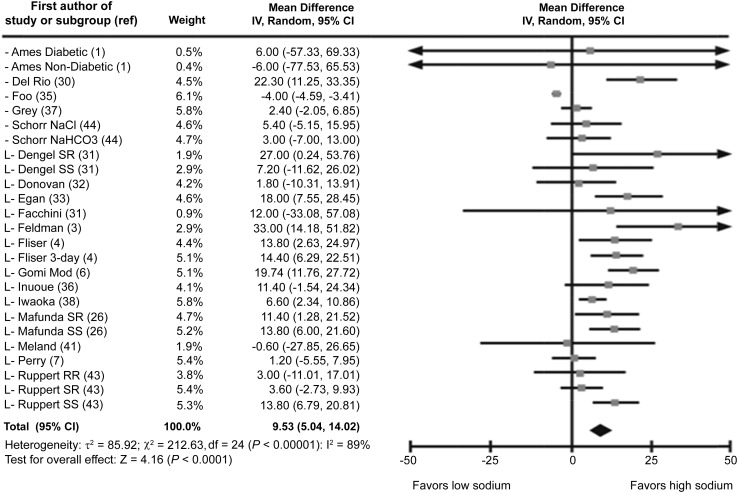
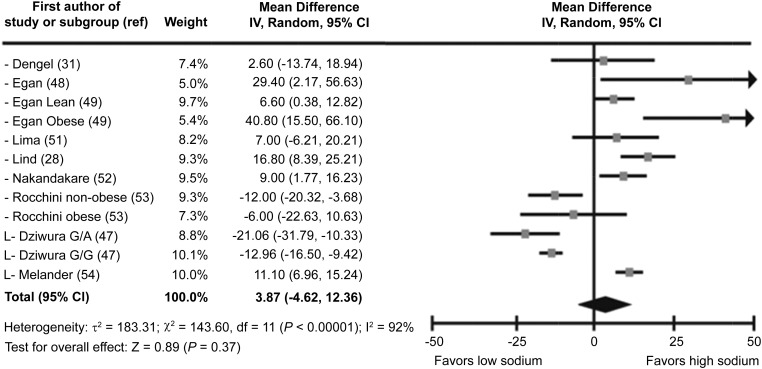
Of the 31 trials presenting information on fasting insulin concentrations, 3 were excluded because they lacked numeric data (8, 27, 50). In all, 19 RCTs and 9 nonrandomized, crossover trials were included in the meta-analyses. Among the 494 participants in the RCTs, fasting insulin concentrations differed significantly between the low- and high-sodium groups (mean difference: 9.53 pmol/L; 95% CI: 5.04, 14.02 pmol/L) (Figure 3, Table 2). Among the 337 participants in the nonrandomized trials, fasting insulin concentrations did not differ significantly (P = 0.37) between intervention groups (Table 2, Figure 4). In both of these analyses, heterogeneity was high.
FIGURE 3.
Forest plot showing all randomized crossover trials for fasting insulin (pmol/L) mean difference (low sodium to high sodium) in adults and children (n = 494). IV, inverse variance; L, studies with a low risk of bias; Mod, moderate sodium intake; ref, reference; RR, reverse reactors; SR, salt-resistant; SS, salt-sensitive.
FIGURE 4.
Forest plot showing all nonrandomized trials for fasting insulin (pmol/L) mean difference (low sodium to high sodium) in adults and children (n = 337). G/A, glycine/arginine carriers; G/G, glycine/glycine carriers; IV, inverse variance; L, studies with a low risk of bias; ref, reference.
Among the studies excluded from the meta-analyses because of a lack of numeric data on glucose or insulin concentrations among fasting participants, results based on other indicators were inconsistent. In 3 of these studies (n = 18–236 participants) (5, 8, 42), the investigators concluded that sodium restriction resulted in an increase in IR. In 3 other studies (n = 16–23 participants) (27, 45, 50), the investigators concluded that responses to sodium restriction differed on the basis of whether the participant was salt sensitive or resistant. In 2 of the studies, (n = 14–19 participants) (34, 39), the investigators concluded that sodium restriction did not influence glucose tolerance or IR. Furthermore, in 3 of the excluded studies (5, 42, 50), the risk of bias was categorized as high; in 2 of these 3 studies, the investigators concluded that sodium restriction increased IR (5, 42), but the loss to follow-up was >30%.
Sensitivity analyses.
Among RCTs categorized as having a low risk of bias, mean urinary sodium excretion for the low-sodium interventions ranged from 184 to 2875 mg/d (weighted mean: 738 mg/d), vs. 2171–6859 mg/d (weighted mean: 5569 mg/d) for the high-sodium interventions. The results of the sensitivity analysis on fasting glucose concentrations remained nonsignificant when analysis was limited to these trials (mean difference: 0.01 mmol/L; 95% CI: −0.03, 0.06 mmol/L) (Table 2).
Among trials with a low risk of bias, we separately stratified the analysis for fasting glucose by duration of intervention and amount of sodium restriction. Among 299 participants in 10 RCTs with interventions lasting ≥7 d and a low risk of bias, sodium restriction did not significantly affect the glucose concentrations (mean difference: 0.04 mmol/L; 95% CI: −0.02, 0.10 mmol/L). Similarly, sodium restriction did not significantly affect these concentrations among 48 participants in 3 trials with a low risk of bias who were in a lower-sodium intervention arm with intake of ≥1500 mg/d (Table 2).
Among the 143 participants in 2 nonrandomized trials with a low risk of bias, sodium restriction did not affect fasting glucose (mean difference: 0.01 mmol/L; 95% CI: −0.18, 0.20 mmol/L; P = 0.90). There was evidence of high heterogeneity in these studies (I2 = 93%), however. One of these trials (n = 115) found that sodium restriction did not have an effect on glucose concentrations (46), whereas the investigators in the other nonrandomized trial (n = 28) concluded that fasting glucose concentrations were significantly higher with salt restriction (P < 0.001) (54).
Sodium restriction remained associated with fasting insulin concentrations among participants in the RCTs with a low risk of bias (Table 2). Similarly, sodium restriction remained associated with fasting insulin among participants in such trials lasting ≥7 d but not in trials with sodium intake of ≥1500 mg/d. However, only 2 trials with a total of 31 participants were in this group. Finally, when analyses of nonrandomized trials were limited to the 2 studies with a low risk of bias, insulin concentrations did not differ with sodium restriction. Heterogeneity was moderate or high for these analyses.
Discussion
In our meta-analysis of studies on sodium restriction and glucose tolerance, we found that sodium reduction did not lead to higher average fasting glucose concentrations either overall or in sensitivity analyses on the basis of risk of bias, duration of the intervention, or sodium intake level in the reduction arm. Sodium reduction was associated with higher average insulin concentrations, but this finding was not consistent across sensitivity analyses, and the significant heterogeneity in results across studies suggests caution in their interpretation. In an analysis that combined the 2 studies (n = 31 participants) with a low risk of bias and sodium restriction to ≥1500 mg/d, average insulin concentrations did not differ between intervention arms.
One of the notable strengths of this review is the comprehensive and systematic search of the literature from the beginning of each database forward. In addition, in contrast to a previous review by Portuguese researchers of 9 articles published between 2004 and 2008 on sodium restriction and the metabolic syndrome (15), we included only studies with healthy or hypertensive participants and were able to perform a meta-analysis of data on glucose and insulin concentrations among fasting participants. The crossover design of most of the included studies allowed, in almost all cases, for each participant to serve as his or her own control, thus eliminating between-subject variability (55) and increasing statistical power. In addition, because methods of measuring fasting glucose and insulin were comparable across most studies, we were able to conduct an analysis without converting measures of effect to an alternative measure.
Its numerous strengths notwithstanding, however, we should note that our study has several limitations. First, the analyses on the effects of sodium reduction on other metabolic variables, including glycated hemoglobin and other longer-term measures of glucose tolerance, were limited because of the small number of trials that reported these outcomes and the lack of consistency across measures. Second, the data were also limited on whether there was control for hypertension. In addition, because of the limited data we were unable to perform separate analyses that were based on health background (e.g., healthy vs. hypertensive patients). Overall, the quality of the included studies varied. Most of the RCTs did not provide detailed information about the randomization process, concealment of allocation, or blinding. Lack of data about these attributes may have led to exaggerated estimates of the effect.
Other possible limitations of the present review are publication bias and heterogeneity among studies in methods and results. Examination of the funnel plots for the meta-analyses on fasting glucose (Supplemental Figure 2) reveals some clear evidence of asymmetry in the RCTs, suggesting publication bias, whereas the nonrandomized trials were more evenly dispersed. Given the small number of trials, our overall fasting glucose estimate for RCTs had low heterogeneity (I2 value of 18%), whereas in the estimate for fasting insulin the I2 value was 89% (18). For fasting glucose, in sensitivity analyses, the studies we classified as having a low risk of bias had no observed heterogeneity.
Our review merits further research to confirm its findings. Because current guidelines recommend that individuals consume <2300 mg/d of sodium but just 1500 mg/d if they are members of specific populations, trials restricting sodium intake to an amount between 1500 and 2300 mg/d are necessary to determine the potential adverse effects of such reductions on glucose tolerance (17). In addition, interventions need to be longer to assess the gradual changes that are recommended for population sodium reduction (56). Previous researchers included trials with duration of salt reduction of ≥4 weeks (25), but others have indicated that as little as 2 wk would be adequate on the basis of current research on sodium homeostasis (20–24). Yet only 13 trials, with varying risks of bias, included interventions of ≥2 weeks. Of the 4 randomized crossover trials with interventions ≥28 d, only 2 had a low risk of bias (36, 41). Analyses of these 2 studies indicated that there are no effects of sodium reduction on fasting glucose concentrations.
To limit potential biases, trials should report their randomization procedures, ensure adequate randomization to permit comparability between intervention groups, and have <20% loss to follow-up. An average difference of 0.55 mmol/L is larger than the SD of fasting plasma glucose observed in many groups, indicating this may represent a true change in glucose concentrations (57, 58). Crossover studies should conduct trials with larger samples to ensure adequate statistical power to detect differences. For example, a single RCT should have 53 participants to detect a difference between interventions of 0.55 mmol/L for fasting glucose with an α of 0.05 and statistical power of 0.80; of the studies included in this review only 5 had samples with >53 participants. As one of the first studies in this topic, this meta-analysis should serve as a useful tool for those scholars interested in summarizing the relevant study results and identifying the strengths and weaknesses of the scientific literature to provide directions for future research. Furthermore, studies should report comprehensive results for glycemic control and insulin sensitivity and use valid and reliable measurement tools. Finally, studies could include measures of glycated hemoglobin to assess the longer-term influence of sodium on glucose concentrations.
Investigators should consider these suggestions when designing future studies. Our study suggests that large, short-term reductions in sodium intake might not affect fasting glucose concentrations, a measure of IR. Assuming that no differences in fasting glucose concentrations would be observed even with an average of >4000 mg/d of sodium restriction, it is unlikely that a 40% reduction (∼1200 mg/d) would have an effect. Still, an average reduction of just 400 mg/d in US sodium intake could prevent 28,000 deaths annually from any cause and save 7 billion health care dollars annually, most of these benefits resulting from reductions in cardiovascular events (59). In general, the findings of this systematic review and meta-analysis suggest that the current recommendations to reduce sodium intake in the US population are unlikely to cause harm related to hyperglycemia or to IR.
Supplementary Material
Acknowledgments
All authors designed the research; all authors conducted the research (reviewed articles for inclusion); SMP and PC abstracted data from the included articles; SMP, PC, and XZ analyzed data and performed statistical analysis; all authors wrote sections of the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: IOM, Institute of Medicine; IR, insulin resistance; RCT, randomized controlled trial.
References
- 1.Ames RP. The effect of sodium supplementation on glucose tolerance and insulin concentrations in patients with hypertension and diabetes mellitus. Am J Hypertens 2001;14:653–9. [DOI] [PubMed] [Google Scholar]
- 2.Feldman RD, Logan AG, Schmidt ND. Dietary salt restriction increases vascular insulin resistance. Clin Pharmacol Ther 1996;60:444–51. [DOI] [PubMed] [Google Scholar]
- 3.Feldman RD, Schmidt ND. Moderate dietary salt restriction increases vascular and systemic insulin resistance. Am J Hypertens 1999;12:643–7. [DOI] [PubMed] [Google Scholar]
- 4.Fliser D, Fode P, Arnold U, Nowicki M, Kohl B, Ritz E. The effect of dietary salt on insulin sensitivity. Eur J Clin Invest 1995;25:39–43. [DOI] [PubMed] [Google Scholar]
- 5.Garg R, Williams GH, Hurwitz S, Brown NJ, Hopkins PN, Adler GK. Low-salt diet increases insulin resistance in healthy subjects. Metabolism 2011;60:965–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomi T, Shibuya Y, Sakurai J, Hirawa N, Hasegawa K, Ikeda T. Strict dietary sodium reduction worsens insulin sensitivity by increasing sympathetic nervous activity in patients with primary hypertension. Am J Hypertens 1998;11:1048–55. [DOI] [PubMed] [Google Scholar]
- 7.Perry CG, Palmer T, Cleland SJ, Morton IJ, Salt IP, Petrie JR, Gould GW, Connell JM. Decreased insulin sensitivity during dietary sodium restriction is not mediated by effects of angiotensin II on insulin action. Clin Sci (Lond) 2003;105:187–94. [DOI] [PubMed] [Google Scholar]
- 8.Townsend RR, Kapoor S, McFadden CB. Salt intake and insulin sensitivity in healthy human volunteers. Clin Sci (Lond) 2007;113:141–8. [DOI] [PubMed] [Google Scholar]
- 9.Petrie JR, Morris AD, Minamisawa K, Hilditch TE, Elliott HL, Small M, McConnell J. Dietary sodium restriction impairs insulin sensitivity in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1998;83:1552–7. [DOI] [PubMed] [Google Scholar]
- 10.Sung KC, Jeong WS, Wild SH, Byrne CD. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care 2012;35:717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdul-Ghani MA, DeFronzo RA. Plasma glucose concentration and prediction of future risk of type 2 diabetes. Diabetes Care 2009;32: Suppl 2:S194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome. Diabetes Care 2005;28:1769–78. [DOI] [PubMed] [Google Scholar]
- 13.Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 2001;24:683–9. [DOI] [PubMed] [Google Scholar]
- 14.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for water, potassium, sodium, chloride, and sulfate. Washington (DC): National Academy Press, 2004. [Google Scholar]
- 15. Sarno F, Jaime PC, Ferreira SR, Monteiro CA. [Sodium intake and metabolic syndrome: a systematic review]. Arq Bras Endocrinol Metabol 2009;53:608–16 (in Portuguese). [DOI] [PubMed]
- 16.US Department of Health and Human Services [Internet], Office of Disease Prevention and Health Promotion. Healthy People 2020. Washington (DC) [cited 2013 Mar 21]. Available from: http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=21.
- 17.US Department of Agriculture and US Department of Health and Human Services [Internet]. Dietary Guidelines for Americans. 7th ed. Washington (DC): US Government Printing Office; 2010 [cited 2013 Mar 21]. Available from: http://health.gov/dietaryguidelines/dga2010/DietaryGuidelines2010.pdf.
- 18.Higgins JPT, Green S. editors [Internet]. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011 [cited 2014 Jan 8]. Available from: http://www.cochrane-handbook.org/.
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- 20.Luft FC, Fineberg NS, Sloan RS. Estimating dietary sodium intake in individuals receiving a randomly fluctuating intake. Hypertension 1982;4:805–8. [DOI] [PubMed] [Google Scholar]
- 21.Epstein M, Hollenberg NK. Age as a determinant of renal sodium conservation in normal man. J Lab Clin Med 1976;87:411–7. [PubMed] [Google Scholar]
- 22.Strauss MB, Lamdin E, Smith WP, Bleifer DJ. Surfeit and deficit of sodium; a kinetic concept of sodium excretion. AMA Arch Intern Med 1958;102:527–36. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz-Melo D, Coffman TM. A trip to inner space: insights into salt balance from cosmonauts. Cell Metab 2013;17:1–2. [DOI] [PubMed] [Google Scholar]
- 24.Rakova N, Jüttner K, Dahlmann A, Schröder A, Linz P, Kopp C, Rauh M, Goller U, Beck L, Agureev A, et al. Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab 2013;17:125–31. [DOI] [PubMed] [Google Scholar]
- 25.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 26. Mufunda J, Chifamba J, Chitate N, Vengesa PM. Salt sensitivity is not associated with hyperinsulinaemia in a sample of rural black Zimbabweans. Women 1998;42:3–8. [PubMed]
- 27.Sharma AM, Spies KP, Ruland K, Distler A. [Effect of salt-intake on glucose tolerance in salt-sensitive normotensive subjects]. Nieren- und Hochdruckkrankheiten 1991;20:534–6. (in German). [Google Scholar]
- 28.Lind L, Lithell H, Gustafsson IB, Pollare T, Ljunghall S. Metabolic cardiovascular risk factors and sodium sensitivity in hypertensive subjects. Am J Hypertens 1992;5:502–5. [DOI] [PubMed] [Google Scholar]
- 29.Boero R, Pignataro A, Bancale E, Campo A, Morelli E, Nigra M, Novarese M, Possamai D, Prodi E, Quarello F. [Metabolic effects of changes in dietary sodium intake in patients with essential hypertension]. Minerva Urol Nefrol 2000;52:13–6. (in Italian). [PubMed] [Google Scholar]
- 30.Del Río A, Rodríguez-Villamil JL. Metabolic effects of strict salt restriction in essential hypertensive patients. J Intern Med 1993;233:409–14. [DOI] [PubMed] [Google Scholar]
- 31.Dengel DR, Hogikyan RV, Brown MD, Glickman SG, Supiano MA. Insulin sensitivity is associated with blood pressure response to sodium in older hypertensives. Am J Physiol 1998;274:E403–9. [DOI] [PubMed] [Google Scholar]
- 32.Donovan DS, Solomon CG, Seely EW, Williams GH, Simonson DC. Effect of sodium intake on insulin sensitivity. Am J Physiol 1993;264:E730–4. [DOI] [PubMed] [Google Scholar]
- 33.Egan BM, Weder AB, Petrin J, Hoffman RG. Neurohumoral and metabolic effects of short-term dietary NaCl restriction in men. Relationship to salt-sensitivity status. Am J Hypertens 1991;4:416–21. [DOI] [PubMed] [Google Scholar]
- 34.Facchini FS, DoNascimento C, Reaven GM, Yip JW, Ni XP, Humphreys MH. Blood pressure, sodium intake, insulin resistance, and urinary nitrate excretion. Hypertension 1999;33:1008–12. [DOI] [PubMed] [Google Scholar]
- 35.Foo M, Denver AE, Coppack SW, Yudkin JS. Effect of salt-loading on blood pressure, insulin sensitivity and limb blood flow in normal subjects. Clin Sci (Lond) 1998;95:157–64. [PubMed] [Google Scholar]
- 36.Fotherby MD, Potter JF. Metabolic and orthostatic blood pressure responses to a low-sodium diet in elderly hypertensives. J Hum Hypertens 1997;11:361–6. [DOI] [PubMed] [Google Scholar]
- 37.Grey A, Braatvedt G, Holdaway I. Moderate dietary salt restriction does not alter insulin resistance or serum lipids in normal men. Am J Hypertens 1996;9:317–22. [DOI] [PubMed] [Google Scholar]
- 38.Iwaoka T, Umeda T, Inoue J, Naomi S, Sasaki M, Fujimoto Y, Gui C, Ideguchi Y, Sato T. Dietary NaCl restriction deteriorates oral glucose tolerance in hypertensive patients with impairment of glucose tolerance. Am J Hypertens 1994;7:460–3. [DOI] [PubMed] [Google Scholar]
- 39.Inoue J, Cappuccio FP, Sagnella GA, Markandu ND, Folkerd EJ, Sampson B, Miller MA, Blackwood AM, MacGregor GA. Glucose load and renal sodium handling in mild essential hypertension on different sodium intakes. J Hum Hypertens 1996;10:523–9. [PubMed] [Google Scholar]
- 40.Meland E, Aamland A. Salt restriction among hypertensive patients: modest blood pressure effect and no adverse effects. Scand J Prim Health Care 2009;27:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meland E, Laerum E, Aakvaag A, Ulvik RJ, Hostmark AT. Salt restriction: effects on lipids and insulin production in hypertensive patients. Scand J Clin Lab Invest 1997;57:501–5. [DOI] [PubMed] [Google Scholar]
- 42.Raji A, Williams GH, Jeunemaitre X, Hopkins PN, Hunt SC, Hollenberg NK, Seely EW. Insulin resistance in hypertensives: effect of salt sensitivity, renin status and sodium. J Hypertens 2001;19:99–105. [DOI] [PubMed] [Google Scholar]
- 43.Ruppert M, Diehl J, Kolloch R, Overlack A, Kraft K, Göbel B, Hittel N, Stumpe KO. Short-term dietary sodium restriction increases serum lipids and insulin in salt-sensitive and salt-resistant normotensive adults. Klin Wochenschr 1991;69(Suppl 25):51–7. [PubMed] [Google Scholar]
- 44.Schorr U, Distler A, Sharma AM. Effect of sodium chloride- and sodium bicarbonate-rich mineral water on blood pressure and metabolic parameters in elderly normotensive individuals: a randomized double-blind crossover trial. J Hypertens 1996;14:131–5. [PubMed] [Google Scholar]
- 45.Suzuki M, Kimura Y, Tsushima M, Harano Y. Association of insulin resistance with salt sensitivity and nocturnal fall of blood pressure. Hypertension 2000;35:864–8. [DOI] [PubMed] [Google Scholar]
- 46.Dengel DR, Mayuga RS, Kairis GM, Goldberg AP, Weir MR. Effect of dietary sodium on insulin sensitivity in older, obese, sedentary hypertensives. Am J Hypertens 1997;10:964–70. [DOI] [PubMed] [Google Scholar]
- 47.Dziwura J, Bínczak-Kuleta A, Miazgowski T, Ziemak J, Widecka K. The associations between G972R polymorphism of the IRS-1 gene, insulin resistance, salt sensitivity and non-dipper hypertension. Hypertens Res 2011;34:1082–6. [DOI] [PubMed] [Google Scholar]
- 48.Egan BM, Stepniakowski K. Effects of enalapril on the hyperinsulinemic response to severe salt restriction in obese young men with mild systemic hypertension. Am J Cardiol 1993;72:53–7. [DOI] [PubMed] [Google Scholar]
- 49.Egan BM, Stepniakowski K, Nazzaro P. Insulin levels are similar in obese salt-sensitive and salt-resistant hypertensive subjects. Hypertension 1994;23(1 Suppl);I1–7. [DOI] [PubMed] [Google Scholar]
- 50.Fuenmayor N, Moreira E, Cubeddu LX. Salt sensitivity is associated with insulin resistance in essential hypertension. Am J Hypertens 1998;11:397–402. [DOI] [PubMed] [Google Scholar]
- 51.Lima NK, Tozetto DJ, Lima LG, Nobre F, Moriguti JC, Ferriolli E, Foss MC. Salt and insulin sensitivity after short and prolonged high salt intake in elderly subjects. Braz J Med Biol Res 2009;42:738–43. [DOI] [PubMed] [Google Scholar]
- 52.Nakandakare ER, Charf AM, Santos FC, Nunes VS, Ortega K, Lottenberg AM, Mion D, Jr, Nakano T, Nakajima K, D'Amico EA, et al. Dietary salt restriction increases plasma lipoprotein and inflammatory marker concentrations in hypertensive patients. Atherosclerosis 2008;200:410–6. [DOI] [PubMed] [Google Scholar]
- 53.Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, Martin M. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med 1989;321:580–5. [DOI] [PubMed] [Google Scholar]
- 54.Melander O, Groop L, Hulthén UL. Effect of salt on insulin sensitivity differs according to gender and degree of salt sensitivity. Hypertension 2000;35:827–31. [DOI] [PubMed] [Google Scholar]
- 55.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991;133:144–53. [DOI] [PubMed] [Google Scholar]
- 56.Institute of Medicine Committee on Strategies to Reduce Sodium Intake. Henney JE, Taylor CL, Boon CS, eds. Strategies to reduce sodium intake in the United States. Washington (DC): National Academies Press; 2010. [PubMed]
- 57.Lim TO, Bakri R, Morad Z, Hamid MA. Bimodality in blood glucose distribution: is it universal? Diabetes Care 2002;25:2212–7. [DOI] [PubMed] [Google Scholar]
- 58.Menke A, Rust KF, Savage PJ, Cowie CC. Hemoglobin A1c, fasting plasma glucose, and 2-hour plasma glucose distributions in U.S. population subgroups: NHANES 2005–2010. Ann Epidemiol 2014;24:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010;362:590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.