Abstract
Background: Consuming a wider variety of nutrient-dense foods may promote adherence to healthful dietary patterns, leading to improved dietary quality and enhanced metabolic health.
Objective: We used the US Healthy Food Diversity (HFD) index to simultaneously measure dietary variety, quality, and proportionality, hypothesizing a priori that race/ethnicity may moderate associations between diet and health.
Methods: A representative sample of adults (n = 7470) aged 20+ y with two 24-h recalls and complete outcome data from the cross-sectional NHANES 2003–2006 were selected. US HFD values were generated using a previously validated equation with a theoretical range from 0 to nearly 1, with higher scores indicative of more varied diets with a higher proportion of healthful food groups. Metabolic syndrome (MetS) was defined using the most recent harmonized definition. Survey-weighted multivariable linear and logistic regression, adjusted for demographic factors, smoking, energy, screen time, and leisure activity, were used to compute means and ORs (95% CIs).
Results: Adults in the third vs. first US HFD tertile had 21% lower odds of MetS [OR (95% CI): 0.79 (0.64, 0.98)] as well as lower odds of hypertension [0.83 (0.70, 0.995] and elevated waist circumference [0.75 (0.66, 0.86] after multivariable adjustment (P-trend < 0.05). The age- and sex-adjusted odds of low serum HDL cholesterol and impaired fasting plasma glucose (P-trend < 0.05) were lower in the highest vs. lowest US HFD tertile but attenuated with multivariable adjustment (P = 0.06 and 0.22, respectively). Notably, the US HFD index was only protective against adiposity among non-Hispanic white (NHW) and non-Hispanic black (NHB) adults, and MetS associations were driven by NHW adults. No associations were observed among Hispanic adults for any MetS components.
Conclusions: Greater healthful food variety was associated with lower odds of MetS and some MetS components in the total population, NHW adults, and NHB adults. This study provides preliminary evidence that healthful food diversity may protect against MetS and highlights the need for longitudinal and experimental research.
Keywords: dietary variety, dietary diversity, healthy food diversity, healthy variety, metabolic syndrome, health and racial disparities, metabolic syndrome components
See corresponding article on page 555.
Introduction
Cardiovascular disease (CVD)7 is the leading cause of mortality worldwide (1). A key intermediate risk factor for CVD is metabolic syndrome (MetS), which is defined as a clustering of anthropometric and biochemical aberrations including abdominal obesity, impaired fasting glucose, lipid dysregulation, and hypertension (2). Because of the adverse effects of insulin resistance and inflammation on CVD pathophysiology (3–5), adults with MetS are twice as likely to develop CVD within the next 5–10 y compared with those without these perturbations (2, 3).
Although dietary changes can positively modify metabolic markers, it is difficult for most people to develop and sustain healthful dietary patterns (6), particularly given that transformations in food environments have expanded access to inexpensive, energy-dense foods (7–10). As a result, if lifestyle interventions to prevent and manage metabolic risk factors are going to be efficacious, they must consider novel strategies to induce changes in food choice.
Theories of consumer behavior applied to public health may provide promising insights for innovative strategies to promote healthful diets. For example, people exhibit innate preferences for having access to a variety of options across a number of domains, including food choice (11–14). Existing research paradigms have focused on the negative repercussions of dietary variety on energy intake and body weight (15, 16) because variety within energy-dense foods may contribute to overeating and obesity (17, 18). Less attention has been devoted to harnessing people’s innate preference for variety to increase consumption of nutrient-dense foods and displace intake of energy-dense foods (19). Because increasing variety within nutrient-dense foods aligns with consumer preferences, it may be an easier long-term strategy to sustain a healthful diet and to help reduce adiposity and cardio-metabolic health.
For example, greater variety of fruits and vegetables may increase the quantity consumed (20, 21), which in turn has been shown to favorably influence type 2 diabetes (22) and CVD risk (23, 24). Greater total dietary variety has been favorably associated with MetS and its components in non-US populations (25–27). However, research has not adequately explored associations between total dietary variety and metabolic health in the United States, within the context of the food availability and preferences in this country. Moreover, until recently, no dietary variety index has measured variety within all foods while simultaneously considering quality and consumption amounts. Therefore, to more comprehensively evaluate the associations between dietary variety and health, we previously developed the US Healthy Food Diversity (HFD) index to simultaneously measure dietary variety, dietary quality, and proportionality (28).
In the present study, we applied this index to examine the associations between dietary variety, MetS, and its components in a nationally representative, cross-sectional sample of US adults. Because research also suggests that race/ethnicity may moderate the associations between dietary quality and components of MetS potentially because of sociobehavioral, metabolic, or genetic variation (29, 30), a secondary aim was to investigate the associations between dietary variety and MetS stratified by race/ethnicity.
Methods
Population and data collection.
The NHANES is a continuous multistage, national health survey administered by the National Center for Health Statistics (31). Detailed information about the survey, laboratory, and examination procedures has been previously published (31–33). During each 2-y sampling cycle, a representative sample of ∼10,000 noninstitutionalized individuals is interviewed and examined about demographic, dietary, and health-related information (34). In these analyses, we used data from the 2003–2006 NHANES and included adults aged ≥20 y with two 24-h dietary recalls, who were not pregnant or lactating, with self-reported energy intake between 400 and 7000 kilocalories (kcal), and with complete information on the outcome variables of interest (n = 3155 to 7188 depending on the outcome variable given sub-sampling methodology of NHANES). Nearly 90% of participants reported dietary data deemed complete and reliable by NHANES survey staff during the in-person and telephone-administered 24-h recalls (35, 36).
Exposure variable.
The US HFD index was developed based on a validated algorithm (37) that was adapted for a US population and was computed through use of the following equation:
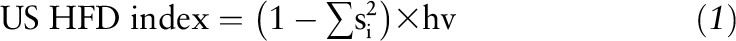 |
where si = share of food item or food group i based on volume in the total diet,  , and hf = health factors of food.
, and hf = health factors of food.
Details describing the development and validation of the index have been previously published (28). Briefly, individual food files were merged with the MyPyramid Equivalent (MPED) databases 2.0 and 3.0 to disaggregate each individual food into its component parts (38). For example, the MPED database separates whole milk into its proportions of low-fat dairy and solid fat. Next, food shares (si) by volume were calculated by dividing individual food quantities by the total reported intake for each individual. The health value (hv) of the diet was calculated by multiplying each si by predetermined food group health weights and then summing the values. Health weights created for the 26 food groups and subgroups in the MPED database were informed by the 2000-kcal USDA dietary pattern in the 2010 Dietary Guidelines for Americans (DGA) (39). Specifically, the recommended proportions of each of the 26 food groups within the 2000-kcal USDA dietary pattern were used to weight the healthfulness of each food group (Supplemental Table 1). The US HFD index has a theoretical range between 0 and nearly 1, with higher scores reflecting diet patterns with greater variety and a higher proportion of DGA-recommended food groups.
Outcome variables and covariates.
Waist circumference (WC) and blood pressure were measured in the mobile examination center. Waist circumference was measured by trained personnel to the nearest 0.1 cm at the highest point of iliac crest at minimal respiration. After resting quietly in a seated position for 5 min, blood pressure was measured and the mean of up to 4 measurements was used to calculate mean systolic blood pressure (SBP) and diastolic blood pressure (DBP). Serum HDL cholesterol concentrations were assessed after precipitation of other lipoproteins with use of a heparin-manganese chloride mixture. Serum TG concentrations were determined from an enzymatic reaction hydrolyzing TGs to glycerol, and plasma glucose concentrations were assessed with use of an enzymatic reaction with hexokinase. Fasting plasma glucose and serum TG measurements were only available in a subset of participants who were invited to participate in the morning fasting subsample.
We used the most recent harmonized definition of MetS from the International Diabetes Federation, AHA, and other organizations to define the presence of MetS (2), and participants with at least 3 of the 5 risk criteria were identified as having MetS. Risk criteria included the following: 1) elevated WC (≥88 cm in women or ≥102 cm in men); 2) fasting plasma glucose ≥100 mg/dL or treatment of hyperglycemia; 3) SBP ≥130 mm Hg or DBP ≥85 mm Hg or use of antihypertensive medication; 4) serum TGs >150 mg/dL or treatment of dyslipidemia; or 5) serum HDL cholesterol <50 mg/dL in women or <40 mg/dL in men or treatment of dyslipidemia. Information about medication usage, race/ethnicity, physical activity, screen time, smoking status, household income, and educational attainment was self-reported.
Statistical analysis.
Descriptive statistics were generated for the sample across US HFD index tertiles and are presented as either means ± SEs for continuous variables or as percentages for categorical variables. Serum TG values were log-transformed to restore a normal distribution and are presented as back-transformed geometric means with a 95% CI. Individual anthropometric and MetS markers are presented as age- and sex-adjusted mean values. A postcontrast linear trend test with Wald’s F test was used to determine the P for linear trend.
Age- and sex-adjusted and multivariable-adjusted logistic regression analyses were used to generate ORs and 95% CIs to examine the associations between the US HFD index (exposure) and the odds of MetS and its individual components (outcomes). To be consistent with common terminology, we use the term OR to denote “prevalence OR.” We tested for the presence of interactions using an a priori P ≤ 0.05 to determine significance. In exploratory analyses, we stratified by race and present the results with the caveat of reduced power.
The continuous US HFD index was divided into tertiles and race-specific tertiles. The final multivariable regression model adjusted for age, sex, income, education, race, smoking status, minutes of moderate and vigorous physical activity per month, hours per day of screen time (i.e., computer and television use), and energy intake, with “race” excluded when stratifying by such. P for linear trend was determined by examining the median US HFD index value for each tertile and assessing the overall F test for the median US HFD index variable. All analyses were conducted with SAS v. 9.4 (SAS Institute) and used cluster, strata, and 2-d dietary or fasting survey weights per NHANES analytic guidelines to account for the multistage survey design (32).
Associations between the US HFD index and the 3 MetS components collected only on the subsample (fasting plasma glucose, serum TGs, and serum HDL cholesterol) were examined with use of the fasting survey weights as directed by NHANES protocol, which reduced the sample size for those analyses. However, because these weights may exclude individuals who met diagnostic criteria for metabolic dysregulation through medication use, but were not invited for the morning fasting sample, we also examined these associations using a hybrid survey weight (i.e., a combination of the fasting survey weights and 2-d dietary weights). By using a hybrid survey weight, it was possible to retain individuals who met diagnostic criteria based on abnormal laboratory values or through medication use and compute estimates representative of US adults. Both sets of estimates are presented for comparison purposes in the tables.
Results
Population characteristics.
The US HFD index ranged from 0.03 to 0.68 in both sexes, and mean US HFD index values differed by race across tertiles (P-interaction = 0.007). The mean and range of values were 0.37 (0.06–0.68) among non-Hispanic white (NHW) participants, 0.36 (0.12–0.60) among Hispanic participants, and 0.33 (0.03–0.57) among non-Hispanic black (NHB) participants. In the total population, adults in the third vs. first US HFD index tertile were older and more likely to be female, NHW, and have higher educational attainment (P-trend < 0.0001) and household income (P-trend = 0.03) (Table 1). Conversely, the percentage of NHB adults was lower in the third vs. first tertile (P < 0.01), and there was no significant trend among Hispanic adults (P = 0.30). Across increasing tertiles, participants had lower mean values of fasting plasma glucose, SBP, DBP, and WC, and fewer hours/day of television and computer use, as well as lower smoking prevalence (P < 0.05).
TABLE 1.
Descriptive characteristics of 7470 NHANES 2003–2006 participants by tertile of the US HFD index1
| n | Tertile 1: 0.03–0.33 (n = 2490) | Tertile 2: >0.33–0.39 (n = 2490) | Tertile 3: >0.39–0.68 (n = 2490) | β ± SE | P | |
| Age, y | 7470 | 44.6 ± 0.40 | 47.5 ± 0.70 | 50.0 ± 0.70 | — | <0.00012 |
| Female, % | 3684 | 30.3 | 33.3 | 36.3 | — | <0.00012 |
| Race, % | ||||||
| NHW | 4010 | 27.8 | 31.9 | 40.3 | — | <0.00012 |
| NHB | 1544 | 49.0 | 31.9 | 19.2 | — | <0.00012 |
| Hispanic | 1642 | 31.2 | 37.5 | 31.2 | — | 0.302 |
| Some college or more, % | 3582 | 30.7 | 33.0 | 36.3 | — | <0.00012 |
| Current smokers, % | 1652 | 46.1 | 30.4 | 23.5 | — | <0.00012 |
| Household income >$75,000, % | 1563 | 31.7 | 33.0 | 35.3 | — | 0.032 |
| MVPA,3 min/mo | 7470 | 824 ± 52.1 | 906 ± 43.9 | 903 ± 33.7 | — | 0.222 |
| Screen time,4 h/d | 7470 | 3.19 ± 0.06 | 3.02 ± 0.06 | 2.97 ± 0.06 | — | 0.032 |
| MetS markers5 | ||||||
| Fasting plasma glucose, mg/dL | 3179 | 105 ± 0.90 | 104 ± 1.17 | 102 ± 0.92 | −18.3 ± 7.87 | 0.03 |
| Serum HDL cholesterol, mg/dL | 3163 | 53.5 ± 0.42 | 54.4 ± 0.48 | 54.1 ± 0.52 | 3.84 ± 4.02 | 0.35 |
| SBP, mm Hg | 7137 | 126 ± 0.50 | 126 ± 0.62 | 125 ± 0.56 | −9.38 ± 2.64 | 0.001 |
| DBP, mm Hg | 7137 | 91.4 ± 0.73 | 90.6 ± 0.84 | 89.8 ± 0.65 | −7.57 ± 2.81 | 0.01 |
| Serum TGs,6 mg/dL | 3155 | 124 (118, 129) | 124 (119, 129) | 120 (116, 127) | −0.20 ± 0.16 | 0.21 |
| Waist circumference, cm | 7188 | 99.6 ± 0.54 | 98.7 ± 0.39 | 97.0 ± 0.54 | −14.2 ± 3.65 | 0.0005 |
All analyses incorporate appropriate cluster, strata, and survey weights. Categorical variables are presented as percentages and continuous variables are presented as means ± SEs. The theoretical range of the US HFD index is between 0 and nearly 1 with higher values indicative of more healthful, varied dietary patterns. DBP, diastolic blood pressure; HFD, Healthy Food Diversity; MetS, metabolic syndrome; MVPA, moderate-to-vigorous physical activity; NHB, non-Hispanic black; NHW, non-Hispanic white; SBP, systolic blood pressure.
P value for trend.
Among participants who reported engaging in moderate or vigorous leisure activity over the past 30 d, the individual activities they performed, the frequency of those activities, and their duration was queried and summed to generate the number of minutes per month engaged in moderate and vigorous activity.
Hours per day of screen time equals the hours per day of TV and computer use.
Mean values are adjusted for age and sex.
Serum TG values were log transformed for analysis and are presented as adjusted geometric mean for back-transformed values; 95% CIs in parentheses (all such values).
US HFD index and MetS and its components.
The odds of elevated WC in tertile 3 vs. tertile 1 of the US HFD index were at least 25% lower in both the age- and sex-adjusted model and multivariable model (P-trend < 0.0001) (Table 2). The odds of having low serum HDL cholesterol in the third vs. first tertile were also nearly 20% lower in the age- and sex-adjusted model but were attenuated after multivariable adjustment [0.83 (0.68, 1.01), P-trend = 0.06]. The odds of hypertension [0.83 (0.70, 0.995), P-trend < 0.05], particularly systolic hypertension [0.79 (0.65, 0.96), P-trend = 0.02], were lower across US HFD index tertiles in multivariable-adjusted models. Although there was a protective association between US HFD index and the odds of impaired fasting plasma glucose in the age- and sex-adjusted model with use of hybrid survey weights [0.83 (0.68, 1.00), P-trend < 0.05], this association did not retain significance after multivariable adjustment. No associations were observed for elevated serum TGs.
TABLE 2.
Odds of MetS and its components across tertiles of the US HFD index among men and women1
| n | Tertile 1: 0.03–0.33 (n = 2490) | Tertile 2: >0.33–0.39 (n = 2490) | Tertile 3: >0.39–0.68 (n = 2490) | P-trend | |
| Elevated waist circumference | 3984 | ||||
| Age- and sex-adjusted | 1.0 | 0.86 (0.73, 1.01) | 0.74 (0.65, 0.85) | <0.0001 | |
| Multivariable-adjusted2 | 1.0 | 0.86 (0.71, 1.04) | 0.75 (0.66, 0.86) | <0.0001 | |
| Impaired fasting plasma glucose | 1416 | ||||
| Age- and sex-adjusted | 1.0 | 1.00 (0.82, 1.22) | 0.87 (0.70, 1.07) | 0.17 | |
| Age- and sex-adjusted3 | 2003 | 1.0 | 1.02 (0.83, 1.25) | 0.83 (0.68, 1.00) | 0.045 |
| Multivariable-adjusted2 | 1.0 | 1.01 (0.82, 1.23) | 0.90 (0.72, 1.11) | 0.31 | |
| Multivariable-adjusted3 | 1.0 | 1.06 (0.86, 1.30) | 0.89 (0.72, 1.09) | 0.22 | |
| Low serum HDL cholesterol | 2783 | ||||
| Age- and sex-adjusted | 1.0 | 0.91 (0.76, 1.11) | 0.78 (0.64, 0.95) | 0.01 | |
| Multivariable-adjusted2 | 1.0 | 0.97 (0.80, 1.18) | 0.83 (0.68, 1.01) | 0.06 | |
| Hypertension | 4826 | ||||
| Age- and sex-adjusted | 1.0 | 0.89 (0.75, 1.05) | 0.84 (0.71, 0.99) | 0.04 | |
| Multivariable-adjusted2 | 1.0 | 0.88 (0.73, 1.06) | 0.83 (0.70, 0.995) | <0.05 | |
| High SBP | 2573 | ||||
| Age- and sex-adjusted | 1.0 | 0.89 (0.77, 1.04) | 0.74 (0.62, 0.88) | 0.0009 | |
| Multivariable-adjusted2 | 1.0 | 0.92 (0.77, 1.09) | 0.79 (0.65, 0.96) | 0.02 | |
| High DBP | 4319 | ||||
| Age- and sex-adjusted | 1.0 | 0.92 (0.77, 1.10) | 0.87 (0.75, 1.01) | 0.07 | |
| Multivariable-adjusted2 | 1.0 | 0.90 (0.74, 1.10) | 0.86 (0.73, 1.01) | 0.06 | |
| High serum TGs | 1337 | ||||
| Age- and sex-adjusted | 1.0 | 1.08 (0.89, 1.32) | 1.02 (0.79, 1.33) | 0.89 | |
| Age- and sex-adjusted3 | 2036 | 1.0 | 1.06 (0.86, 1.30) | 1.04 (0.81, 1.33) | 0.78 |
| Multivariable-adjusted2 | 1.0 | 1.08 (0.86, 1.35) | 1.01 (0.76, 1.34) | 0.68 | |
| Multivariable-adjusted3 | 1.0 | 1.07 (0.84, 1.35) | 1.03 (0.78, 1.35) | 0.87 |
Values are ORs (95% CIs) unless otherwise indicated. DBP, diastolic blood pressure; HFD, Healthy Food Diversity; MetS, metabolic syndrome; NHB, non-Hispanic black; NHW, non-Hispanic white; SBP, systolic blood pressure.
Adjusted for age, sex, race (NHW, NHB, Hispanic, other), education (less than college vs. some college or more), smoking, income (<$75,000 vs. >$75,000), hours of screen time per day, minutes of moderate-to-vigorous activity per month, and energy intake. All analyses incorporate appropriate cluster, strata, and survey weights.
If participants met criteria for impaired fasting plasma glucose, abnormal serum TGs, or MetS through medication usage and did not participate in the morning fasting sample, we created a hybrid weight using 2-d dietary weights for participants with medication data and fasting weights for participants in the morning subsample to provide estimates representative of US adults.
US HFD index, MetS, and its components: race/ethnicity variations.
The odds of elevated WC were lower among both NHW and NHB adults in the third vs. first tertile after multivariable adjustment (P-trend < 0.05) (Table 3). Among NHW participants, adults in the third vs. first tertile of the index had 24% lower odds of having low serum HDL cholesterol and 27% lower odds of elevated SBP [0.73 (0.54, 0.97)]. Among NHB participants, adults in the third vs. first tertile had lower odds of hypertension (P-trend = 0.008) and elevated DBP [0.60 (0.45, 0.80)] after multivariable adjustment. No associations were observed among Hispanic adults between the US HFD index and any MetS components.
TABLE 3.
Odds of MetS components across tertiles of the US HFD index among adults stratified by race1
| NHW (n = 4010) |
Hispanic (n = 1642) |
NHB (n = 1544) |
||||||||||
| Tertile 1 | Tertile 2 | Tertile 3 | P-trend | Tertile 1 | Tertile 2 | Tertile 3 | P-trend | Tertile 1 | Tertile 2 | Tertile 3 | P-trend | |
| Elevated waist circumference | ||||||||||||
| Age- and sex-adjusted | 1.0 | 0.80 (0.66, 0.96) | 0.73 (0.61, 0.87) | 0.0003 | 1.0 | 0.96 (0.59, 1.56) | 0.70 (0.41, 1.18) | 0.19 | 1.0 | 0.95 (0.67, 1.35) | 0.71 (0.52, 0.97) | 0.04 |
| Multivariable-adjusted2 | 1.0 | 0.80 (0.66, 0.98) | 0.74 (0.63, 0.87) | 0.0002 | 1.0 | 1.08 (0.63, 1.87) | 0.83 (0.49, 1.38) | 0.48 | 1.0 | 0.90 (0.64, 1.26) | 0.62 (0.45, 0.87) | 0.007 |
| Impaired fasting plasma glucose | ||||||||||||
| Age- and sex-adjusted | 1.0 | 0.94 (0.68, 1.29) | 0.85 (0.66, 1.10) | 0.21 | 1.0 | 1.38 (0.995, 1.91) | 1.15 (0.73, 1.82) | 0.50 | 1.0 | 0.80 (0.52,1.23) | 0.85 (0.45,1.64) | 0.62 |
| Age- and sex-adjusted3 | 1.0 | 0.97 (0.70, 1.33) | 0.84 (0.67, 1.06) | 0.15 | 1.0 | 1.32 (0.95, 1.84) | 1.06 (0.66, 1.70) | 0.74 | 1.0 | 0.76 (0.53, 1.09) | 0.82 (0.45, 1.48) | 0.50 |
| Multivariable-adjusted2 | 1.0 | 0.99 (0.72, 1.37) | 0.88 (0.69, 1.14) | 0.34 | 1.0 | 1.37 (0.99, 1.91) | 1.15 (0.72, 1.83) | 0.51 | 1.0 | 0.82 (0.53, 1.25) | 0.87 (0.45,1.70) | 0.69 |
| Multivariable-adjusted3 | 1.0 | 1.05 (0.76, 1.44) | 0.91 (0.72, 1.14) | 0.42 | 1.0 | 1.32 (0.95, 1.82) | 1.10 (0.68, 1.78) | 0.64 | 1.0 | 0.78 (0.55, 1.13) | 0.86 (0.47, 1.60) | 0.64 |
| Low serum HDL cholesterol | ||||||||||||
| Age- and sex-adjusted | 1.0 | 0.77 (0.62, 0.95) | 0.70 (0.54, 0.90) | 0.006 | 1.0 | 0.92 (0.64, 1.33) | 0.78 (0.51,1.20) | 0.26 | 1.0 | 1.08 (0.74, 1.57) | 0.86 (0.65, 1.15) | 0.32 |
| Multivariable-adjusted2 | 1.0 | 0.81 (0.65, 1.02) | 0.76 (0.59, 0.98) | 0.04 | 1.0 | 0.94 (0.67, 1.32) | 0.79 (0.52, 1.19) | 0.26 | 1.0 | 1.21 (0.83,1.77) | 0.97 (0.70, 1.35) | 0.89 |
| Hypertension | ||||||||||||
| Age- and sex-adjusted | 1.0 | 0.97 (0.81, 1.16) | 0.91 (0.72, 1.16) | 0.46 | 1.0 | 0.77 (0.56, 1.06) | 0.99 (0.71, 1.38) | 0.88 | 1.0 | 0.88 (0.60,1.29) | 0.69 (0.49, 0.96) | 0.03 |
| Multivariable-adjusted2 | 1.0 | 0.94 (0.77, 1.14) | 0.87 (0.67, 1.14) | 0.31 | 1.0 | 0.75 (0.54, 1.06) | 0.97 (0.68,1.39) | 0.82 | 1.0 | 0.85 (0.56, 1.30) | 0.65 (0.47, 0.89) | 0.008 |
| High SBP | ||||||||||||
| Age- and sex-adjusted | 1.0 | 0.75 (0.60, 0.93) | 0.70 (0.53, 0.93) | 0.01 | 1.0 | 0.96 (0.62, 1.51) | 0.92 (0.62, 1.35) | 0.66 | 1.0 | 0.87 (0.65, 1.18) | 0.82 (0.57, 1.18) | 0.28 |
| Multivariable-adjusted2 | 1.0 | 0.76 (0.59, 0.97) | 0.73 (0.54, 0.97) | 0.03 | 1.0 | 0.81 (0.50, 1.30) | 0.80 (0.53, 1.20) | 0.28 | 1.0 | 0.88 (0.63, 1.22) | 0.81 (0.55, 1.19) | 0.28 |
| High DBP | ||||||||||||
| Age- and sex-adjusted | 1.0 | 0.99 (0.81, 1.21) | 0.92 (0.73, 1.16) | 0.48 | 1.0 | 0.77 (0.56, 1.06) | 0.99 (0.71, 1.38) | 0.40 | 1.0 | 0.76 (0.55, 1.06) | 0.65 (0.49, 0.86) | 0.003 |
| Multivariable-adjusted2 | 1.0 | 0.96 (0.76, 1.19) | 0.88 (0.68, 1.14) | 0.34 | 1.0 | 0.73 (0.50, 1.05) | 0.86 (0.62. 1.21) | 0.37 | 1.0 | 0.73 (0.50, 1.05) | 0.60 (0.45, 0.80) | 0.0005 |
| High serum TGs | ||||||||||||
| Age- and sex-adjusted | 1.0 | 1.01 (0.80, 1.26) | 0.89 (0.66, 1.20) | 0.44 | 1.0 | 1.26 (0.82, 1.93) | 1.11 (0.66, 1.86) | 0.67 | 1.0 | 1.02 (0.59, 1.76) | 1.01 (0.61, 1.69) | 0.96 |
| Age- and sex-adjusted3 | 1.0 | 1.03 (0.81, 1.31) | 0.92 (0.70, 1.22) | 0.58 | 1.0 | 1.28 (0.83, 1.99) | 1.14 (0.67, 1.94) | 0.61 | 1.0 | 0.86 (0.55, 1.34) | 1.01 (0.63, 1.62) | 0.97 |
| Multivariable-adjusted2 | 1.0 | 1.03 (0.81, 1.31) | 0.92 (0.67, 1.27) | 0.62 | 1.0 | 1.30 (0.86, 1.99) | 1.22 (0.69, 2.15) | 0.48 | 1.0 | 1.17 (0.65, 2.11) | 1.15 (0.65, 2.04) | 0.63 |
| Multivariable-adjusted3 | 1.0 | 1.06 (0.82, 1.37) | 0.96 (0.71, 1.31) | 0.80 | 1.0 | 1.31 (0.85, 2.00) | 1.25 (0.70, 2.21) | 0.44 | 1.0 | 0.95 (0.59, 1.54) | 1.11 (0.68, 1.81) | 0.67 |
Values are ORs (95% CIs) unless otherwise indicated. DBP, diastolic blood pressure; HFD, Healthy Food Diversity; MetS, metabolic syndrome; NHB, non-Hispanic black; NHW, non-Hispanic white; SBP, systolic blood pressure.
Adjusted for age, sex, education (less than college vs. some college or more), smoking, income (<$75,000 vs. >$75,000), hours of screen time per day, minutes of moderate-to-vigorous activity per month, and energy intake. All analyses incorporate appropriate cluster, strata, and survey weights.
If participants met criteria for impaired fasting plasma glucose, abnormal serum TGs, or MetS through medication usage and did not participate in the morning fasting sample, we created a hybrid weight using 2-d dietary weights for participants with medication data and fasting weights for participants in the morning subsample to provide estimates representative of US adults.
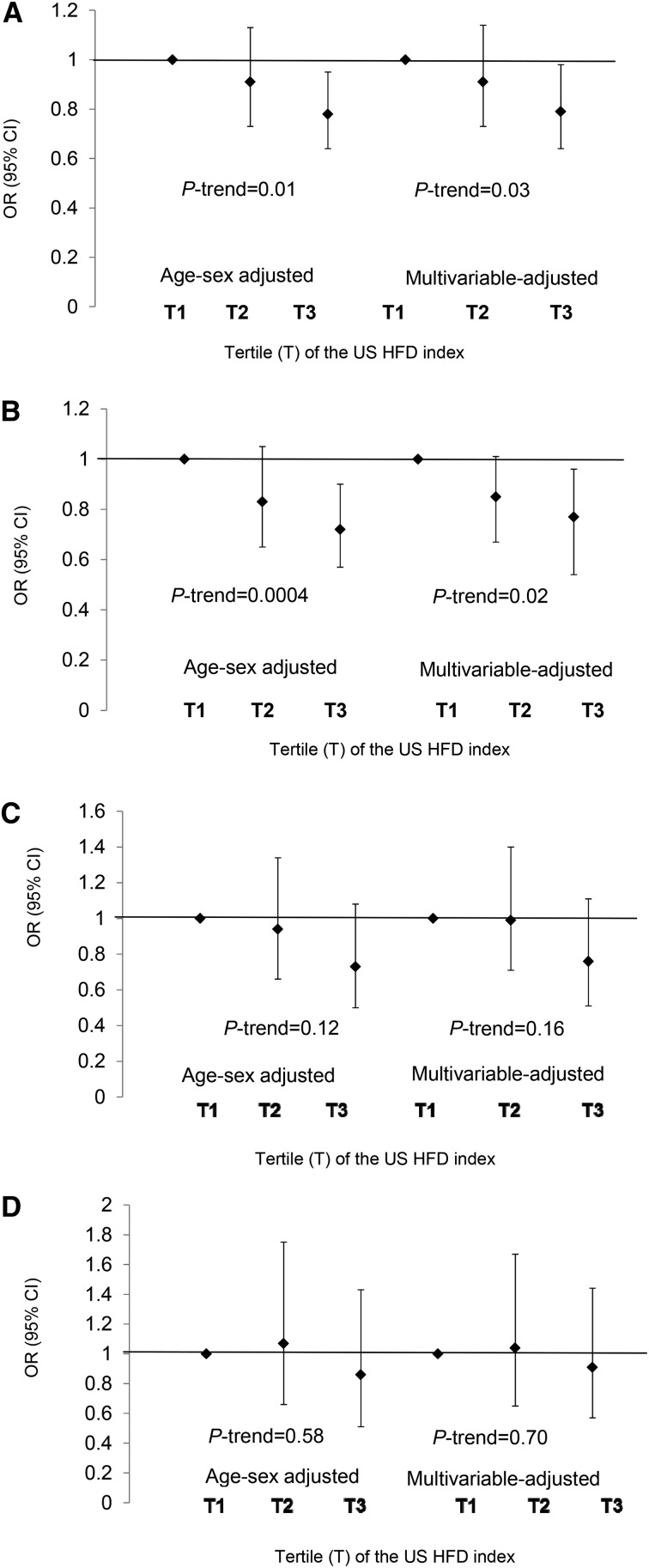
In the total population, MetS odds were 20% lower in tertile 3 vs. tertile 1 in both the age- and sex-adjusted model [0.78 (0.64, 0.95)] and the multivariable-adjusted model [0.79 (0.64, 0.98), P-trend = 0.03] (Figure 1). Among NHW participants, adults in the third vs. first tertile of the index had 23% lower odds of MetS [0.77 (0.61, 0.96)]. No associations were observed between the US HFD index and MetS among Hispanic or NHB adults.
FIGURE 1.
Odds of MetS across US HFD index tertiles by race/ethnicity. All adults: n per tertile = 1497, 1516, 1507 (A); NHW adults: n per tertile = 857, 803, 814 (B); NHB adults: n per tertile = 298, 294, 303 (C); and Hispanic adults: n per tertile = 323, 347, 323 (D). Analyses were adjusted for age, sex, race (NHW, NHB, Hispanic, unless stratified by such), education (less than college vs. some college or more), smoking, income (<$75,000 vs. >$75,000), hours of screen time per day, minutes of moderate-to-vigorous activity per month, and energy intake. All analyses incorporate appropriate cluster, strata, and survey weights. If participants met criteria for impaired fasting plasma glucose, abnormal serum TGs, or MetS through medication usage and did not participate in the morning fasting sample, we created a hybrid weight using 2-d dietary weights for participants with medication data and fasting weights for participants in the morning subsample to provide estimates representative of US adults. Age- and sex-adjusted and multivariable-adjusted analyses were also conducted with use of NHANES recommended survey weights, and results were similar between the 2 analyses. HFD, Healthy Food Diversity; MetS, metabolic syndrome; NHB, non-Hispanic black; NHW, non-Hispanic white.
Discussion
The objective of this study was to examine the associations between healthful dietary variety and MetS and its components in a representative national sample of US adults. The multidimensional US HFD index used in this study uniquely captures dietary variety while simultaneously considering the healthfulness of each food and its consumption amount. Overall, this study supports the premise that greater variety within DGA-recommended food groups favorably influences MetS and some of its individual components. Higher US HFD index values representing more varied, healthful diets were associated with significantly lower odds of elevated WC, hypertension, and MetS in multivariable-adjusted models in the total population. The odds of impaired fasting plasma glucose and low serum HDL cholesterol were also significantly lower after age and sex adjustment, although the results attenuated with further adjustment.
Favorable associations between other dietary variety measures and MetS and its components have been reported in diverse populations (22, 25–27), although some studies conducted on fruit and vegetables did not detect a benefit of variety apart from quantity (23, 24). Baik et al. (26) reported a similar strength of association between the top quintile of a healthy high-variety diet score and MetS (RR = 0.76) as we report in our study, although their score was created with use of factor analysis. Our US HFD index was developed with use of validated algorithms and included several DGA-recommended foods; this standardization allows for it to be easily applied in other populations for better comparison between studies. Azadbakht et al. (25) found that higher dietary diversity scores among a representative sample of Tehranian adults were associated with nearly 30% lower odds of MetS and some MetS components. The authors speculated that higher intakes of fruits, vegetables, and calcium among those with higher diversity scores partly explained the observed protective effects. Intake of low-fat dairy (an important food source of calcium), fruits and vegetables, and whole grains was also higher among adults with higher US HFD index scores, suggesting that the underlying mechanisms that confer protection may be similar between the 2 studies (28).
Calcium helps modulate blood pressure by regulating the sympathetic nervous system, reducing vessel constriction, and influencing sodium excretion (40). The matrix of nutrients associated with high fruit and vegetable intake also assists with blood pressure regulation and potentially other metabolic markers (41, 42). Plant-based fibers, phytochemicals, and antioxidants found in whole grains, fruits, and vegetables help regulate blood glucose by reducing oxidative stress and insulin resistance (43, 44). These food groups are also lower in energy density and glycemic index, which may favorably influence satiety (45, 46) and contribute to the protective results we observed for excess adiposity (47) and fasting plasma glucose (48). In sensitivity analyses, adding BMI to multivariable models attenuated associations between greater healthful food variety and MetS components, further supporting the role of adiposity as a mediating factor.
Our analyses revealed that there may be differences by race/ethnicity that warrant investigation in future research. Notably, the US HFD index was protective against excess adiposity only among NHW and NHB adults, and the associations for MetS appeared to be driven by NHW adults. Although the absence of significant protection in NHB adults and Hispanics could be related to reduced statistical power, protective associations for hypertension were observed among the smaller subset of NHB adults, suggesting that genetic and/or sociobehavioral factors may also contribute to this discrepancy (30, 49). Observing stronger protective associations between diet quality and hypertension among NHB adults is consistent with previous literature demonstrating that blood pressure among NHB individuals is more responsive to the protective effects of reduced salt, high potassium, and “Dietary Approaches to Stop Hypertension Trial”–style dietary patterns (49). Because protective US HFD index scores can be achieved by implementing a variety of healthful dietary patterns, it is possible that differential food selection contributed to the observed pattern of results. Salt intake is also not captured directly with the US HFD index, and diet patterns high in plant-based foods may still be high in sodium, potentially attenuating blood pressure associations across all races.
Importantly, no protective associations between higher US HFD index scores and components of MetS were observed among Hispanic adults. Although this finding is consistent with a study among Guatemalan young adults (50), there may be heterogeneity introduced by specific Hispanic ethnicity that may contribute to variability in diet and lifestyle behaviors associated with the etiology of MetS. Factors including length of residence in the United States, varying cultural food traditions, healthcare service utilization, and acculturation may markedly affect diet quality (51). In an exploratory model (data not shown), incorporating the number of years living in the United States as a covariate strengthened protective associations between the US HFD index, MetS, and its components among Hispanic adults. However, the associations remained nonsignificant potentially because of the large amount of missing data in this variable. We also explored other variables associated with acculturation, however, none of these are considered reliable measures of this construct (52) and did not substantively improve model fit. Future research is needed to explore how acculturation, ethnicity, sociobehavioral factors, and genetic variation modify associations between dietary patterns and metabolic health outcomes (29, 30).
One limitation of this study was its cross-sectional design, which prevents temporal inference between dietary intake and metabolic health. Reduced statistical power also influenced these analyses, particularly for fasting plasma glucose, serum TGs, and MetS where reliance on fasting weights reduced the sample size by more than half. When we used hybrid weights that allowed for inclusion of individuals who met the criteria for abnormalities in these categories through medication usage, some results approached or attained significance, although these analyses remained underpowered relative to some of the other individual components. Thus, the results presented likely underestimate the beneficial effects of greater healthful food variety on MetS and its components. Similarly, confounding introduced by the inter-relations between the individual components of MetS may have further attenuated the associations between the US HFD index and MetS. Finally, the possibility of residual confounding cannot be eliminated because higher US HFD index scores were correlated with more healthful lifestyle characteristics, which may have been imperfectly captured in these analyses.
Our study had a number of strengths worthy of mention. We used a population-based, representative sample of US adults, making these results generalizable to noninstitutionalized US adults. Additionally, by using all available data through creation of hybrid weights, we reduced the bias associated with eliminating individuals from our analysis who met diagnostic criteria through medication use rather than fasting criteria. Future research using NHANES data could take advantage of all available data by using hybrid weights. Because dietary data were collected with use of 24-h dietary recalls rather than FFQs, we were able to explore diverse cultural food patterns that were not constrained by pre-established food lists. Anthropometric and biochemical data were all collected by trained interviewers rather than self-reported, reducing the amount of measurement error in these variables and increasing our confidence in the observed associations.
More importantly, our study considered whether a wider assortment of healthful foods potentially improves acceptance of more healthful dietary patterns that protect against chronic disease. This application of the US HFD index provides preliminary evidence that dietary interventions that promote greater variety within DGA-recommended food groups may support metabolic health in a more sustainable manner. Future research should consider whether increasing the variety of healthful foods available improves diet quality, and more broadly, whether alternative strategies that modify our local food environments can enhance cardiometabolic health. Experimental studies are needed to examine whether aligning food choice with innate consumer preferences for variety can be favorably used to influence diet quality and health.
Supplementary Material
Acknowledgments
We thank Juan R Albertorio, Freid Virginia, and Julia Holmes from the National Center for Health Statistics for their guidance creating hybrid sampling weights. MV conceptualized, designed, and analyzed all data and was the lead author of the paper; NP critically reviewed the manuscript for important intellectual content; and JM designed the analyses along with MV, supervised the research at all stages, and critically reviewed the manuscript for important intellectual content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CVD, cardiovascular disease; DBP, diastolic blood pressure; DGA, Dietary Guidelines for Americans; HFD, Healthy Food Diversity; MetS, metabolic syndrome; MPED, MyPyramid Equivalent; NHB, non-Hispanic black; NHW, non-Hispanic white; SBP, systolic blood pressure; WC, waist circumference.
References
- 1.Ma X, Zhu S. Metabolic syndrome in the prevention of cardiovascular diseases and diabetes still a matter of debate? Eur J Clin Nutr 2013;67:518–21. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Alberti K, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2010;375:181–3. [DOI] [PubMed] [Google Scholar]
- 4.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–28. [DOI] [PubMed] [Google Scholar]
- 5.Marcial JM, Altieri PI, Banchs H, Escobales N, Crespo M. Metabolic syndrome among Puerto Ricans and other Hispanic populations. P R Health Sci J 2011;30:145–51. [PubMed] [Google Scholar]
- 6.Fappa E, Yannakoulia M, Pitsavos C, Skoumas I, Valourdou S, Stefanadis C. Lifestyle intervention in the management of metabolic syndrome: could we improve adherence issues? Nutrition 2008;24:286–91. [DOI] [PubMed] [Google Scholar]
- 7.Putnam J, Allshouse J. Food consumption, prices, and expenditures. Statistical Bulletin 965. Washington, DC: US Department of Agriculture, Economic Research Service; 1999.
- 8.Remick AK, Polivy J, Pliner P. Internal and external moderators of the effect of variety on food intake. Psychol Bull 2009;135:434–51. [DOI] [PubMed]
- 9.Jekanowski MD, Binkley JK. Food purchase diversity across U.S. markets. Agribusiness 2000;16:417–33. [Google Scholar]
- 10.Moss M. Salt sugar fat: how the food giants hooked us. New York: Random House; 2013.
- 11.Leotti LA, Iyengar SS, Ochsner KN. Born to choose: the origins and value of the need for control. Trends Cogn Sci 2010;14:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki S. Effects of number of alternatives on choice in humans. Behav Processes 1997;39:205–14. [DOI] [PubMed] [Google Scholar]
- 13.Bown NJ, Read D, Summers B. The lure of choice. J Behav Decis Making 2003;16:297–308. [Google Scholar]
- 14.Suzuki S. Choice between single-response and multichoice tasks in humans. Psychol Rec 2000;50:105–15. [Google Scholar]
- 15.Raynor HA. Can limiting dietary variety assist with reducing energy intake and weight loss? Physiol Behav 2012;106:356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raynor HA, Niemeier HM, Wing RR. Effect of limiting snack food variety on long-term sensory-specific satiety and monotony during obesity treatment. Eat Behav 2006;7:1–14. [DOI] [PubMed] [Google Scholar]
- 17.Raynor HA, Epstein LH. Dietary variety, energy regulation, and obesity. Psychol Bull 2001;127:325–41. [DOI] [PubMed] [Google Scholar]
- 18.Vadiveloo M, Dixon LB, Parekh N. Associations between dietary variety and measures of body adiposity: a systematic review of epidemiological studies. Br J Nutr 2013;109:1557–72. [DOI] [PubMed] [Google Scholar]
- 19.McCrory MA, Burke A, Roberts SB. Dietary (sensory) variety and energy balance. Physiol Behav 2012;107:576–83. [DOI] [PubMed] [Google Scholar]
- 20.Epstein LH, Temple JL, Roemmich JN, Bouton ME. Habituation as a determinant of human food intake. Psychol Rev 2009;116:384–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raynor HA, Osterholt KM. Greater variety of fruit served in a four-course snack increases fruit consumption. Appetite 2012;59:662–7. [DOI] [PubMed] [Google Scholar]
- 22.Cooper AJ, Sharp SJ, Lentjes MA, Luben RN, Khaw KT, Wareham NJ, Forouhi NG. A prospective study of the association between quantity and variety of fruit and vegetable intake and incident type 2 diabetes. Diabetes Care 2012;35:1293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oude Griep LM, Verschuren WM, Kromhout D, Ocke MC, Geleijnse JM. Variety in fruit and vegetable consumption and 10-year incidence of CHD and stroke. Public Health Nutr 2012;15:2280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhupathiraju SN, Wedick NM, Pan A, Manson JE, Rexrode KM, Willett WC, Rimm EB, Hu FB. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am J Clin Nutr 2013;98:1514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azadbakht L, Mirmiran P, Azizi F. Dietary diversity score is favorably associated with the metabolic syndrome in Tehranian adults. Int J Obes (Lond) 2005;29:1361–7. [DOI] [PubMed] [Google Scholar]
- 26.Baik I, Lee M, Jun NR, Lee JY, Shin C. A healthy dietary pattern consisting of a variety of food choices is inversely associated with the development of metabolic syndrome. Nutr Res Pract 2013;7:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Oliveira EP, McLellan KC, Vaz de Arruda Silveira L, Burini RC. Dietary factors associated with metabolic syndrome in Brazilian adults. Nutr J 2012;11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vadiveloo M, Dixon LB, Mijanovich T, Elbel B, Parekh N. Development and evaluation of the US Healthy Food Diversity index. Br J Nutr 2014;112:1562–74. [DOI] [PubMed]
- 29.Zamora D, Gordon-Larsen P, He K, Jacobs DR, Jr, Shikany JM, Popkin BM. Are the 2005 Dietary Guidelines for Americans associated with reduced risk of type 2 diabetes and cardiometabolic risk factors? Twenty-year findings from the CARDIA Study. Diabetes Care 2011;34:1183–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diaz VA, Mainous AG, 3rd, Koopman RJ, Carek PJ, Geesey ME. Race and diet in the overweight: association with cardiovascular risk in a nationally representative sample. Nutrition 2005;21:718–25. [DOI] [PubMed] [Google Scholar]
- 31.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey protocol (2012). [cited 2014 Sep 15]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 32.CDC, National Center for Health Statistics. Analytic and reporting guidelines: the National Health and Nutrition Examination Survey (NHANES). Atlanta, GA: CDC, National Center for Health Statistics; 2006.
- 33.Dwyer J, Picciano MF, Raiten DJ. Collection of food and dietary supplement intake data: what we eat in America—NHANES. J Nutr 2003;133:590S–600S. [DOI] [PubMed] [Google Scholar]
- 34.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National Health and Nutrition Examination Survey: plan and operations, 1999–2010. Vital Health Stat 1 2013;56:1–37. [PubMed]
- 35.CDC, National Center for Health Statistics. 2005–2006 Data documentation codebook and frequencies: dietary interview (2008). [cited 2014 Sep 15]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2005–2006/DRXDOC_D.htm.
- 36.CDC, National Center for Health Statistics. 2003–2004 Data documentation codebook and frequencies: dietary interview—individual foods (2006). [cited 2014 Sep 15]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2003–2004/DR1IFF_C.htm.
- 37.Drescher LS, Thiele S, Mensink GB. A new index to measure healthy food diversity better reflects a healthy diet than traditional measures. J Nutr 2007;137:647–51. [DOI] [PubMed]
- 38.Bowman SA, Friday JE. A. M. MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004 [Online]. Beltsville, MD: Food Surveys Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, USDA; 2008. [cited 2014 Dec 17]. Available from: http://www.ars.usda.gov/ba/bhnrc/fsrg.
- 39. USDA and US Department of Health and Human Services. Dietary guidelines for Americans, 2010. 7th ed. Washington, DC: US Government Printing Office; December 2010. [DOI] [PMC free article] [PubMed]
- 40.Kotchen TA, Kotchen JM. Nutrition, diet, and blood pressure. In: Shils M, et al., editors. Modern nutrition in health & disease. 9th ed. Philadelphia: Lippincott, Williams and Wilkins; 1999. p. 1217–28.
- 41.Conlin PR, Chow D, Miller ER, Svetkey LP, Lin P-H, Harsha DW, Moore TJ, Sacks FM, Appel LJ. The effect of dietary patterns on blood pressure control in hypertensive patients: results from the dietary approaches to stop hypertension (DASH) trial. Am J Hypertens 2000;13:949–55. [DOI] [PubMed] [Google Scholar]
- 42.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 1997;277:1624–32. [DOI] [PubMed] [Google Scholar]
- 43.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi T, Azizi F. Beneficial effects of a dietary approaches to stop hypertension eating plan on features of the metabolic syndrome. Diabetes Care 2005;28:2823–31. [DOI] [PubMed] [Google Scholar]
- 44.Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension 2003;41:422–30. [DOI] [PubMed] [Google Scholar]
- 45.Roberts SB. High-glycemic index foods, hunger, and obesity: is there a connection? Nutr Rev 2000;58:163–9. [DOI] [PubMed] [Google Scholar]
- 46.Rebello CJ, Liu AG, Greenway FL, Dhurandhar NV. Dietary strategies to increase satiety. Adv Food Nutr Res 2013;69:105–82. [DOI] [PubMed] [Google Scholar]
- 47.Rolls BJ. Dietary strategies for weight management. Nestle Nutr Inst Workshop Ser 2012;73:37–48. [DOI] [PubMed]
- 48.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2003;26:2261–7. [DOI] [PubMed] [Google Scholar]
- 49.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006;47:296–308. [DOI] [PubMed] [Google Scholar]
- 50.Gregory CO, McCullough ML, Ramirez-Zea M, Stein AD. Diet scores and cardio-metabolic risk factors among Guatemalan young adults. Br J Nutr 2009;101:1805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindberg NM, Stevens VJ, Halperin RO. Weight-loss interventions for Hispanic populations: the role of culture. J Obes 2013;2013:542736. [DOI] [PMC free article] [PubMed]
- 52.Chakraborty BM, Chakraborty R. Concept, measurement and use of acculturation in health and disease risk studies. Coll Antropol 2010;34:1179–91. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.