Abstract
Background
Primary prevention implantable cardioverter defibrillators (ICDs) reduce all-cause mortality but the benefits are heterogeneous. Current risk stratification based on left ventricular ejection fraction has limited discrimination power. We hypothesize that biomarkers for inflammation, neurohumoral activation and cardiac injury can predict appropriate shocks and all-cause mortality in patients with primary prevention ICDs.
Methods and Results
The Prospective Observational Study of Implantable Cardioverter Defibrillators (PROSe-ICD) enrolled 1,189 patients with systolic heart failure who underwent ICD implantation for primary prevention of sudden cardiac death. The primary endpoint was an ICD shock for adjudicated ventricular tachyarrhythmia. The secondary endpoint was all-cause mortality. After a median follow-up of 4.0 years, 137 subjects experienced an appropriate ICD shock and 343 participants died (incidence rates of 3.2 and 5.8 per 100 person-years, respectively). In multivariable adjusted models, higher interleukin-6 (IL-6) levels increased the risk of appropriate ICD shocks. In contrast, C-reactive protein, IL-6, tumor necrosis factor-α receptor II, pro-brain natriuretic peptide, and cardiac troponin T showed significant linear trends for increased risk of all-cause mortality across quartiles. A score combining these 5 biomarkers identified patients who were much more likely to die than to receive an appropriate shock from the ICD.
Conclusions
An increase in serum biomarkers of inflammation, neurohumoral activation and myocardial injury increased the risk for death but poorly predicted the likelihood of an ICD shock. These findings highlight the potential importance of serum-based biomarkers in identifying patients who are unlikely to benefit from primary prevention ICDs.
Keywords: arrhythmia, sudden cardiac death, inflammation, prevention, implantable cardioverter-defibrillator
Introduction
Implantable cardioverter defibrillators (ICDs) have become the cornerstone to prevent sudden cardiac death (SCD) in patients with systolic heart failure.1,2 However, only a minority of patients with ICDs experience therapy over time with wide variations in all-cause mortality rates among patients eligible for primary prevention ICD implantation.3,4 A significant shortcoming of primary prevention ICD therapy is the inadequacy of clinical selection criteria for patients at greatest risk for arrhythmic SCD. Hence, attention has been focused on identifying novel factors that may better identify those who may benefit the most.5,6
Serum biomarkers of inflammation, neurohumoral activation and myocardial injury have established prognostic utility in various forms of cardiovascular disease.7,8 However, their role in predicting SCD is unclear. We hypothesize that serum markers of inflammation, heart failure status and cardiac injury can predict ICD shocks and mortality in a large, community-based cohort of primary prevention ICD recipients with ischemic and non-ischemic cardiomyopathy.
Methods
Study Design and Clinical Data Collection
The Prospective Observational Study of Implantable Cardioverter-Defibrillators (PROSE-ICD) is a multicenter observational study of patients with systolic heart failure eligible for a primary prevention ICD. Details of the design and baseline characteristics of study participants have been described elsewhere; study participants underwent ICD implantation based on current guidelines.9,10 The study enrolled 1,189 participants. All centers obtained approval from their respective institutional review boards and all patients provided written informed consent.
PROSE-ICD participants were extensively phenotyped as previously described.9 All patients underwent a baseline comprehensive history and cardiovascular examination along with a digitally-recorded resting 12-lead electrocardiogram (ECG), a five minute three lead ECG, an echocardiogram and fasting blood collection. Study participants were evaluated twice a year. ICDs were also interrogated (in person or via remote transmission) to assess arrhythmic events. Patients who were not seen in clinic underwent a telephone interview to update history and medication use.
Serum biomarker analysis
Whole blood samples were collected and were kept at room temperature for one hour prior to centrifugation. Serum was stored at −80°C after quick freezing in liquid nitrogen. All biomarker measurements were made in serum. The inflammatory biomarkers included C-reactive protein (CRP), interleukin-6 (IL-6), IL-10, and tumor necrosis factor α receptor II (TNF-αRII). IL-6 (R&D Systems, Minneapolis MN), IL-10 (R&D Systems) and CRP (ALPCO Diagnostics, Salem NH) were measured with high-sensitivity ELISAs according to manufacturer’s instructions (see Supplemental Material to Methods Section).
Study outcomes
The primary outcome in PROSE-ICD was the occurrence of a first appropriate ICD shock for an adjudicated ventricular tachyarrhythmia. Detailed information from ICDs and patient outcomes were adjudicated as previously described9. For sensitivity analysis, we also examined the association of biomarkers with all-cause mortality after censoring participants at their first appropriate ICD shock.
Statistical Analyses
This was a post-hoc analysis using unpaired t-test, Wilcoxon rank-sum test, and chi-square analyses as appropriate. Two-sided p <0.05 was considered statistically significant. Nominal p-values were presented for analysis of each biomarker without adjustment for multiple comparisons.
Serum biomarkers were log transformed prior to analyses and values expressed as medians. To evaluate the associations between serum biomarkers and endpoint events, we categorized each biomarker into quartiles. For cTnT, cTnI, and myoglobin, since levels were undetectable in >25% of samples, participants were categorized into four groups (group 1, value 0; groups 2–4, tertiles of the non-zero values). We then used separate Cox proportional hazards regressions for each biomarker to estimate the hazard ratios comparing quartiles 2–4 to the first quartile of each biomarker. Cox models were adjusted for age, gender, race, enrollment center, current smoking, BMI, ejection fraction, atrial fibrillation, hypertension, diabetes, chronic kidney disease (CKD, defined as a GFR < 60ml/min/1.73 m2), device type (ICD, CRT-D). Tests for linear trends across quartiles were conducted by including an ordinal variable with the median biomarker level of each quartile in the regression models. A combined score of the association between biomarker levels and study outcomes was created by adding the quartile ranks of all biomarkers that showed significant linear trends with appropriate ICD shocks or mortality. As a sensitivity analysis, death was treated as a competing risk for appropriate shock, and the results were virtually the same (data now shown).
To examine whether the biomarker score improves prediction for mortality and shock beyond conventional clinical variables, we compared the performance of a basic model (a model with all clinical variables associated with either all-cause mortality or appropriate shock with a p-value <0.1 in the univariate analysis) with a model incorporating both the clinical variables and the serum biomarker risk score. Model discrimination was determined by c-statistics, and risk reclassification was assessed using both net reclassification improvement (NRI) and integrated discrimination improvement (IDI), all accounted for censoring.11–12 In describing the NRI, 5-year mortality and shock risk was categorized into three groups (<15%, 15–50%, and >50% for mortality, and <10%, 10–20% and >20% for appropriate shock) based upon the observed distribution of risk in the cohort. Confidence intervals for these statistics were obtained using boot-strapping. We used the added variable version of Gronnesby and Borgan goodness-of-fit test for Cox regression, and a p-value of >0.05 from the goodness of fit test indicates that predicted risk of the model is similar to the observed risk. All analyses were performed using STATA version 12 (StataCorp LP, College Station, TX).
Results
The average age (SD) of study participants at baseline was 60.6 (12.7) years and detailed information regarding their clinical characteristics are noted in Table 1. Most participants received a single chamber ICD (55.1%) with 17.7% receiving dual chamber systems and 27.2% CRT devices. The average lowest cut off zone for tachycardia therapy was programmed to 185.2 beats per minute (bpm) (14.6).
Table 1.
Baseline characteristics of participants, by appropriate ICD shock
| Characteristic | Total (n = 1189) |
No appropriate ICD shock (n=1052) |
Appropriate ICD shock (n = 137) |
p-value |
|---|---|---|---|---|
| Age (year) | 60.6 (12.7) | 60.7 (12.8) | 59.9 (11.6) | 0.46 |
| Sex | 0.01 | |||
| Male | 867 (72.9) | 755 (71.8) | 112 (81.8) | |
| Female | 322 (27.1) | 297 (28.2) | 25 (18.2) | |
| Race | 0.008 | |||
| White | 679 (57.1) | 584 (55.5) | 95 (69.3) | |
| Black | 477 (40.1) | 437 (41.5) | 40 (29.2) | |
| Other | 33 (2.8) | 31 (2.9) | 2 (1.5) | |
| Smoking | 0.02 | |||
| Never | 398 (33.5) | 367 (34.9) | 31 (22.6) | |
| Former | 541 (45.5) | 469 (44.6) | 72 (52.6) | |
| Current | 250 (21.0) | 216 (20.5) | 34 (24.8) | |
| Body mass index (kg/m2) | 29.8 (6.5) | 29.6 (6.6) | 30.8 (6.2) | 0.05 |
| Ejection fraction (%) | 22.3 (7.4) | 22.4 (7.4) | 21.6 (7.5) | 0.27 |
| Heart rate (beats/min) | 76.4 (17.1) | 76.9 (17.3) | 72.3 (14.4) | 0.004 |
| QTc (ms) | 459.8 (43.4) | 459.7 (43.8) | 460.8 (40.0) | 0.77 |
| QRS (ms) | 118.2 (30.2) | 117.9 (30.3) | 120.5 (28.8) | 0.36 |
| NHYA class | 0.54 | |||
| Class I | 196 (16.5) | 168 (16.0) | 28 (20.4) | |
| Class II | 524 (44.1) | 467 (44.4) | 57 (41.6) | |
| Class III | 464 (39.0) | 413 (39.3) | 51 (37.2) | |
| Class IV | 5 (0.4) | 4 (0.4) | 1 (0.7) | |
| Cardiomyopathy | 0.71 | |||
| Non-ischemic | 547 (46.0) | 486 (46.2) | 61 (44.5) | |
| Ischemic | 642 (54.0) | 566 (53.8) | 76 (55.5) | |
| Atrial fibrillation | 312 (26.2) | 280 (26.6) | 32 (23.4) | 0.42 |
| Diabetes | 414 (34.8) | 369 (35.1) | 45 (32.8) | 0.61 |
| Hypertension | 747 (62.8) | 674 (64.1) | 73 (53.3) | 0.01 |
| Chronic kidney disease | 360 (30.3) | 331 (31.5) | 29 (21.2) | 0.03 |
| Medications | ||||
| ASA | 781 (65.7) | 689 (65.5) | 92 (67.2) | 0.70 |
| ACE-I/ARB | 850 (71.5) | 748 (71.1) | 102 (74.5) | 0.41 |
| Beta blocker | 1061 (89.2) | 943 (89.6) | 118 (86.1) | 0.21 |
| Diuretics | 857 (72.1) | 757 (72.0) | 100 (73.0) | 0.80 |
| Aldosterone antagonist | 302 (25.4) | 267 (25.4) | 35 (25.5) | 0.97 |
| Device type | 0.74 | |||
| Single | 655 (55.1) | 574 (54.6) | 81 (59.1) | |
| BiV (no atrial lead) | 26 (2.2) | 24 (2.3) | 2 (1.5) | |
| Dual | 211 (17.7) | 189 (18.0) | 22 (16.1) | |
| Dual/BiV | 297 (25.0) | 265 (25.2) | 32 (23.4) | |
| Lowest rate of cutoff (beats/min) | 185.2 (14.6) | 185.5 (14.7) | 182.9 (13.4) | 0.05 |
| ATP used | 694 (58.4) | 627 (59.6) | 67 (48.9) | 0.02 |
Values are number (%), or mean (SD)
After a median follow-up of 4.0 years, 137 subjects experienced an appropriate ICD shock and 343 participants died (incidence rates of 3.2 and 5.8 per 100 person-years, respectively). The majority of participants who died did not experience an appropriate ICD shock (294 out of 343, 85.7%). Patients who experienced an appropriate ICD shock during follow-up were more likely to be male, Caucasian, current or former smokers, and to have higher BMI, lower resting heart rate, and less hypertensive and less likely to have CKD (Table 1). Patients who died during follow-up were more often male, current or former smokers, with NYHA Class III symptoms, longer QTc intervals and QRS durations, ischemic cardiomyopathy, atrial fibrillation, diabetes, hypertension, CKD, lower BMI, lower ejection fraction, and lower ICD therapy cutoff rates (Table 2). Participants who died during follow-up were more frequently taking diuretics, ASA and ACE-I/ARBs compared to those who survived.
Table 2.
Baseline characteristics of participants, by all-cause mortality
| Characteristic | Alive (n=846) |
Dead (n = 343) |
p-value |
|---|---|---|---|
| Age (year) | 58.8 (12.4) | 65.2 (12.2) | <0.001 |
| Sex | 0.003 | ||
| Male | 596 (70.4) | 271 (79.0) | |
| Female | 250 (29.6) | 72 (21.0) | |
| Race | 0.28 | ||
| White | 475 (56.1) | 204 (59.5) | |
| Black | 344 (40.7) | 133 (38.8) | |
| Other | 27 (3.2) | 6 (1.7) | |
| Smoking | 0.02 | ||
| Never | 303 (35.8) | 95 (27.7) | |
| Former | 374 (44.2) | 167 (48.7) | |
| Current | 169 (20.0) | 81 (23.6) | |
| Body mass index (kg/m2) | 30.1 (6.8) | 29.0 (5.9) | 0.009 |
| Ejection fraction (%) | 22.7 (7.3) | 21.2 (7.5) | 0.001 |
| Heart rate (beats/min) | 76.1 (16.9) | 77.3 (17.4) | 0.29 |
| QTc (ms) | 457.3 (42.0) | 466.0 (46.1) | 0.002 |
| QRS (ms) | 117.1 (29.8) | 120.9 (31.0) | 0.05 |
| NHYA class | <0.001 | ||
| Class I | 166 (19.6) | 30 (8.7) | |
| Class II | 391 (46.2) | 133 (38.8) | |
| Class III | 285 (33.7) | 179 (52.2) | |
| Class IV | 4 (0.5) | 1 (0.3) | |
| Cardiomyopathy | <0.001 | ||
| Non-ischemic | 417 (49.3) | 130 (37.9) | |
| Ischemic | 429 (50.7) | 213 (62.1) | |
| Atrial fibrillation | 198 (23.4) | 114 (33.2) | <0.001 |
| Diabetes | 255 (30.1) | 159 (46.4) | <0.001 |
| Hypertension | 501 (59.2) | 246 (71.7) | <0.001 |
| Chronic kidney disease | 198 (23.4) | 162 (47.2) | <0.001 |
| Medications | |||
| ASA | 536 (63.4) | 245 (71.4) | 0.008 |
| ACE-I/ARB | 589 (69.6) | 261 (76.1) | 0.03 |
| Beta blocker | 762 (90.1) | 299 (87.2) | 0.14 |
| Diuretics | 582 (68.8) | 275 (80.2) | <0.001 |
| Aldosterone antagonist | 214 (25.3) | 88 (25.7) | 0.90 |
| Device type | 0.15 | ||
| Single | 482 (57.0) | 173 (50.4) | |
| BiV (no atrial lead) | 16 (1.9) | 10 (2.9) | |
| Dual | 141 (16.7) | 70 (20.4) | |
| Dual/BiV | 207 (24.5) | 90 (26.2) | |
| Lowest rate of cutoff (beats/min) | 187.0 (14.3) | 180.8 (14.4) | <0.001 |
| ATP used | 510 (60.3) | 184 (53.6) | 0.11 |
Values are number (%), or mean (SD)
Number of events (eg ICD shock and all-cause mortality) by quartile levels of biomarkers are noted in Table 3. Median (Q1 to Q3) levels of study biomarkers are shown in Supplemental Table 1. CRP and IL-6, cTnT and IL-6, and cTnT and cTnI showed pairwise Spearman correlation coefficients >0.4 (Supplemental Table 2). In multivariable adjusted models, the only biomarker showing a significant linear trend across quartiles with increased risk of appropriate ICD shock was IL-6 (Figure 1). The hazard ratio (95% CI) for appropriate ICD shocks comparing the highest to the lowest quartiles of IL-6 was 2.23 (1.20 to 4.14). When IL-6 was introduced as a log-transformed continuous variable in Cox models, the hazard ratio for appropriate ICD shock comparing the 80th to the 20th percentile of the IL-6 distribution was 1.25 (0.98 to 1.60) (Supplemental Table 3).
Table 3.
Number of events and incidence rates (number of events/total person-years at risk) by levels of biomarkers.
| Biomarkers | Appropriate ICD shock
|
All-cause mortality
|
||||
|---|---|---|---|---|---|---|
| Number of events | Person-years | Incidence rate (%) | Number of events | Person-years | Incidence rate (%) | |
|
|
|
|||||
| hs-CRP (μg/mL) | ||||||
| 1: ≤1.6 | 27 | 1143.7 | 2.4 | 54 | 1495.1 | 3.6 |
| 2: 1.7–4.4 | 36 | 1076.3 | 3.3 | 60 | 1430.5 | 4.2 |
| 3: 4.5–10.6 | 36 | 955.2 | 3.8 | 85 | 1344.6 | 6.3 |
| 4: >10.6 | 31 | 860.4 | 3.6 | 115 | 1297.1 | 8.8 |
| IL-6 (pg/mL) | ||||||
| 1: ≤1.1 | 18 | 1020.6 | 1.8 | 34 | 1342.5 | 2.5 |
| 2: 1.2–2.0 | 35 | 1064.9 | 3.3 | 56 | 1473.5 | 3.8 |
| 3: 2.1–4.3 | 35 | 1048.6 | 3.3 | 93 | 1423.7 | 6.5 |
| 4: >4.3 | 42 | 905.2 | 4.6 | 131 | 1332 | 9.8 |
| IL-10 (pg/mL) | ||||||
| 1: ≤0.9 | 31 | 1157.1 | 2.7 | 69 | 1607.1 | 4.3 |
| 2: 1.0–1.4 | 41 | 984.2 | 4.2 | 71 | 1343.6 | 5.3 |
| 3: 1.5–2.8 | 29 | 899.6 | 3.2 | 88 | 1263.3 | 6.9 |
| 4: >2.8 | 28 | 993.8 | 2.8 | 86 | 1348.2 | 6.4 |
| TNF-α rec II (pg/mL) | ||||||
| 1: ≤2318.8 | 36 | 1133.3 | 3.2 | 48 | 1589.6 | 3.0 |
| 2: 2318.9–3160.4 | 33 | 1099.5 | 3.0 | 58 | 1491.4 | 3.9 |
| 3: 3160.5–4754.1 | 36 | 1014 | 3.6 | 86 | 1386 | 6.1 |
| 4: >4754.1 | 25 | 792.5 | 3.2 | 122 | 1104.7 | 11.0 |
| Pro-BNP (ng/mL) | ||||||
| 1: ≤1.7 | 22 | 1048.1 | 2.1 | 30 | 1413.8 | 2.1 |
| 2: 1.8–2.6 | 40 | 1122.2 | 3.6 | 58 | 1546.1 | 3.8 |
| 3: 2.7–4.1 | 41 | 1075.3 | 3.8 | 79 | 1455.2 | 5.4 |
| 4: >4.1 | 25 | 772.8 | 3.2 | 146 | 1127.2 | 12.9 |
| cTnT (ng/mL) | ||||||
| 1:00 | 43 | 1918.3 | 2.2 | 75 | 2469 | 3.0 |
| 2: 0.001–0.017 | 37 | 793.8 | 4.7 | 53 | 1199.6 | 4.4 |
| 3: 0.018–0.050 | 23 | 741 | 3.1 | 83 | 1019.3 | 8.0 |
| 4: >0.050 | 26 | 577.7 | 4.5 | 103 | 869.9 | 11.8 |
| cTnI (ng/mL) | ||||||
| 1:00 | 35 | 1248.5 | 2.8 | 72 | 1623.7 | 4.4 |
| 2: 0.001–0.015 | 26 | 921.9 | 2.8 | 67 | 1256.1 | 5.3 |
| 3: 0.016–0.057 | 30 | 899.4 | 3.3 | 77 | 1286.1 | 6.0 |
| 4: >0.057 | 34 | 865.2 | 3.9 | 89 | 1246.9 | 7.1 |
| CK-MB (ng/mL) | ||||||
| 1: ≤1.7 | 26 | 991.8 | 2.6 | 62 | 1365.9 | 4.5 |
| 2: 1.8–2.5 | 30 | 968.4 | 3.1 | 77 | 1328.5 | 5.8 |
| 3: 2.6–4.1 | 32 | 1000.9 | 3.2 | 75 | 1388.6 | 5.4 |
| 4: >4.1 | 37 | 973.9 | 3.8 | 91 | 1329.8 | 6.8 |
| Myoglobin (ng/mL) | ||||||
| 1:00 | 22 | 1070.7 | 2.1 | 87 | 1625.1 | 5.3 |
| 2: 0.1–21.3 | 31 | 876 | 3.5 | 46 | 1163.1 | 4.0 |
| 3: 21.4–25.2 | 29 | 803.8 | 3.6 | 74 | 1067.4 | 6.9 |
| 4: >25.2 | 32 | 835.6 | 3.8 | 77 | 1109.4 | 6.9 |
Figure 1.
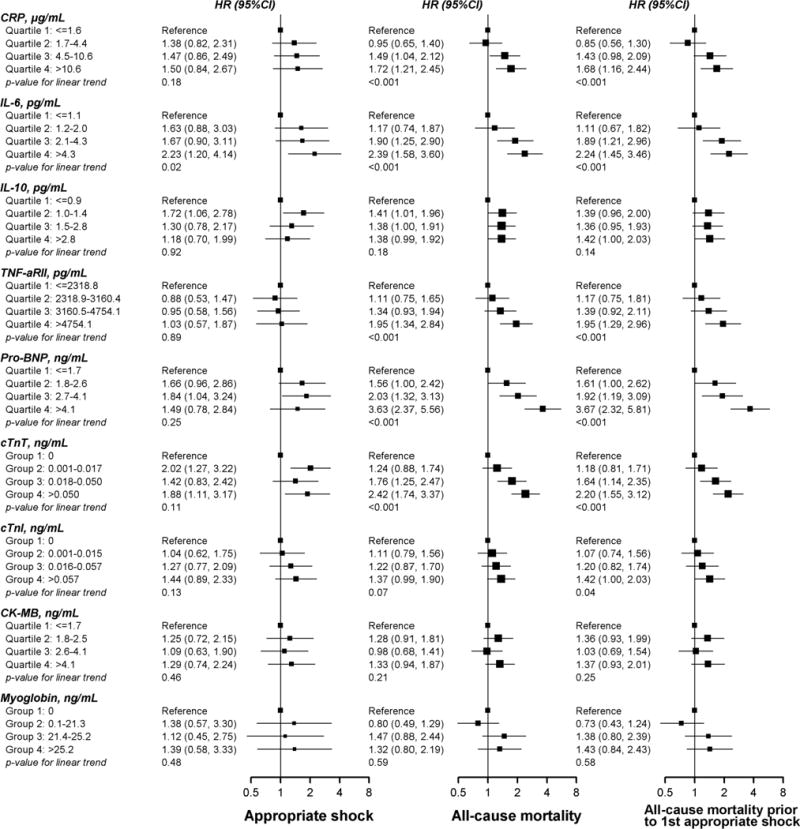
Multivariable adjusted hazard ratios (HR) for appropriate ICD shocks (left), all-cause mortality (center) and all-cause mortality censored at the first appropriate ICD shock (right) by quartiles of biomarkers of inflammation, neurohumoral activation and myocardial injury. Hazard ratios were adjusted for age, sex, race, study center, smoking status, body mass index, NYHA class, atrial fibrillation, diabetes, hypertension, chronic kidney disease, and CRT device.
In contrast to appropriate ICD shocks, CRP, IL-6, TNF-αRII, pro-BNP, and cTnT showed significant linear trends for increased risk of mortality across quartiles (Figure 1). The hazard ratios for all-cause mortality comparing the highest to the lowest quartiles of CRP, IL-6, TNF-αRII, pro-BNP, and cTnT were 1.72 (1.21 to 2.45), 2.39 (1.58 to 3.60), 1.95 (1.34 to 2.84), 3.63 (2.37 to 5.56), and 2.42 (1.74 to 3.37), respectively. The associations with all-cause mortality were similar when these analyses were repeated after censoring participants at their first appropriate ICD shock.
In order to understand the combined impact of biomarker levels on risk, we created a cumulative score by adding the quartile rank for the 5 biomarkers that showed a significant trend with all-cause mortality. The median score was 12 and ranged from 5 to 20. The proportion of participants with scores 5 to 9, 10 to 14, and 15 to 20 were 27.2, 38.1, and 27.2%, respectively. The rates of appropriate ICD shocks for patients with scores 5 to 9, 10 to 14, and 15 to 20 were 2.4, 3.2, and 4.1 per 100 person-years, respectively (Figure 2). The corresponding rates for all-cause mortality were 1.8, 4.4, and 12.8 per 100 person-years, respectively.
Figure 2.
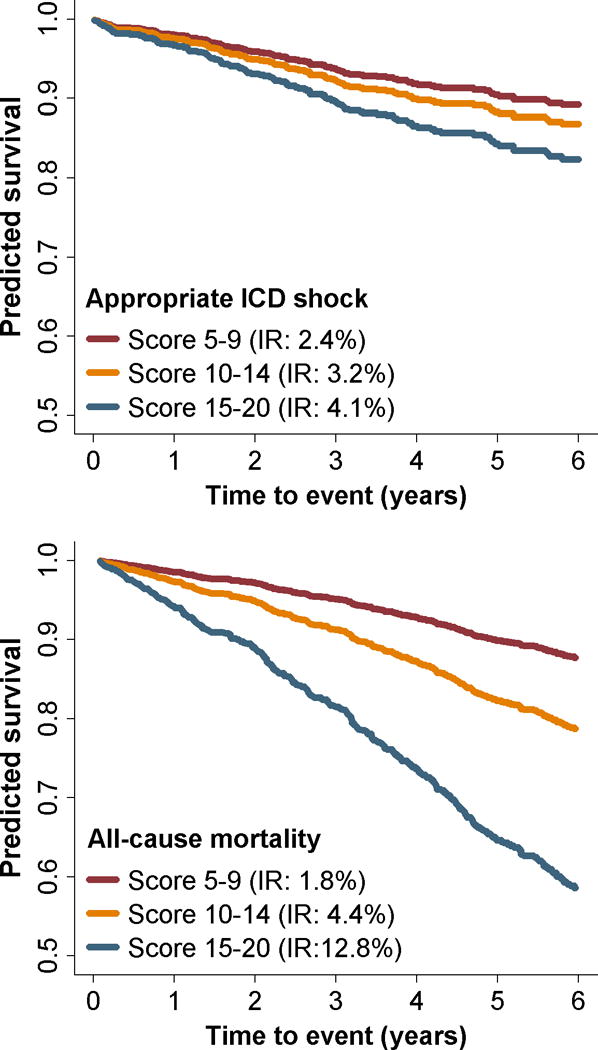
Probability of survival free from appropriate ICD shocks (top) and all-cause mortality (bottom) as a function of a combined biomarker score. The combined score was created by adding the quartile rank for CRP, IL-6, TNF-αRII, pro-BNP, and cTnT. The median score was 12 (range 5 to 20). The proportion of participants with scores 5 to 9, 10 to 14, and 15 to 20 were 27.2, 38.1, and 27.2%, respectively.
To better understand the added potency of the serum biomarker risk score on discrimination for all-cause mortality, we incorporated the biomarker risk score into a set of clinical variables associated with an increased risk for death. By adding the biomarker score to the clinical variables, the c-statistics improved from 0.74 to 0.78 (difference in c-statistics = 0.04, 95% CI 0.02 to 0.06), with an NRI of 15.7% (8.5% to 22.3%) and the p-value of the goodness of fit tests of 0.29 (Table 4). With respect to appropriate ICD shocks, adding the biomarker risk score resulted in only minor improvement in the prediction for appropriate shocks with c-statistics from 0.67 to 0.69 (difference in c-statistics = 0.02, 95% CI −0.008 to 0.04).
Table 4.
Predicted 5-year mortality using clinical variables with and without cytokine score
| Clinical variables + Cytokine score | ||||
|---|---|---|---|---|
| <15% | 15–50% | >50% | Total | |
|
|
||||
| Patients with events | ||||
| Clinical variables only * | ||||
| <15% | 23 | 13 | 0 | 36 |
| 15–50% | 16 | 126 | 34 | 176 |
| >50% | 0 | 9 | 54 | 63 |
| Total | 39 | 148 | 88 | 275 |
| Patients without events | ||||
| Clinical variables only * | ||||
| <15% | 297 | 43 | 0 | 340 |
| 15–50% | 100 | 220 | 17 | 337 |
| >50% | 0 | 15 | 20 | 35 |
| Total | 397 | 278 | 37 | 712 |
|
| ||||
| Net reclassification improvement (NRI): 15.7% (8.5% to 22.3%) Integrated discrimination improvement (IDI): 0.06 (0.04 to 0.07) |
||||
Clinical variables including age, sex, race, smoking, body mass index, ejection fraction, heart rate, QTc, QRS, NYHA class III/IV, ischemic cardiomyopathy, atrial fibrillation, diabetes, hypertension, chronic kidney disease, ASA, ACE-I/ARB, diuretics, lowest rate of cutoff, and ATP used.
Given the recent studies demonstrating improvements in mortality and patient outcomes when ICDs are programmed with higher rate cutoffs, we performed a secondary analysis dividing the cohort into two groups, those with a rate cutoff <200 bpm (n=766) and those ≥200bpm (n=221). When comparing the baseline characteristics between these two groups, patients with a lower cutoff rate were more likely to be older (average age 62.8 vs. 52.4), male (74.6% vs. 64.1%), smoker (68.9% vs. 58.5%), have ischemic cardiomyopathy (58.0% vs. 38.2%), diabetes (36.5% vs. 27.6%), hypertension (66.2% vs. 49.2%), and CKD (31.7% vs. 22.4%). Both the clinical score and the serum biomarker risk score performed better in patients with device cutoff ≥200 bpm, but the incremental improvement of the serum biomarker risk score (when added to the clinical variables only) in risk prediction was similar among the two groups (Supplemental Table 4).
Discussion
In this large cohort study of stable systolic heart failure patients who were candidates for primary prevention ICD implantation, serum biomarkers did poorly in predicting the likelihood of an appropriate ICD shock (primary endpoint). However, they did identify patients at increased risk of dying (secondary endpoint) without experiencing an appropriate ICD shock. Interestingly, IL-6 showed a significant trend predicting the occurrence of both appropriate ICD shocks and all-cause mortality, thus making it potentially less useful in identifying patients likely to experience an appropriate ICD shock. By utilizing a composite score of these biomarkers, we were able to identify patients who were much more likely to die than to benefit from an appropriate ICD shock for a ventricular tachyarrhythmia. Furthermore, adding biomarker data to conventional clinical risk factors improved discrimination for all-cause mortality. Hence, our results suggest that inflammation, neurohumoral modulation, and cardiac injury assess complementary pathophysiological mechanisms that promote the progression of heart failure and ultimately increase the likelihood of death but they are poor predictors of ventricular arrhythmias that are effectively treated by ICD shocks. This profile of serum biomarkers allows for prospective identification of a subgroup of patients with a higher risk of death relative to their risk for an appropriate ICD shock.
The role of inflammation and neurohumoral activation in cardiovascular disease is well known and most strongly linked to atherosclerosis and heart failure. These studies have also provided insight into the predictive power of these biomarkers in the development of atrial and ventricular arrhythmias.13–15 While these findings may apply to previously healthy subjects, studies in patients with coronary disease or heart failure have been less definitive.16–19 In fact, the CAMI-GUIDE study demonstrated marginal predictive power of elevated CRP levels for heart failure mortality, but not for arrhythmic SCD.19
The role of inflammation in predicting appropriate ICD therapy has also been inconsistent. While some studies have demonstrated that elevated IL-6 and CRP levels were associated with ICD shocks,20,21 others have not.22 This inconsistency is most likely explained by the small size and heterogeneity of the cohorts studied. Our findings demonstrate that CRP and other markers of systemic inflammation are inadequate predictors of the development of ICD shocks but can identify individuals who are at increased risk for non-arrhythmic modes of death. This latter point is particularly important given the controversy regarding the benefits of primary prevention ICDs and the need to develop more refined risk-stratification metrics.
The associations between pro-BNP levels, ICD shocks and all-cause mortality, were similar to the relationship for markers of inflammation. Pro-BNP failed to predict the development of ICD shocks but did predict death after ICD implantation. Earlier studies suggested a strong link between BNP levels and ICD events,23 but our findings are aligned with more recent studies which do not.19,24 From the perspective of overall mortality, our findings support an earlier study demonstrating that elevated levels of BNP are associated with an increased risk for death despite patients appearing clinically euvolemic.25 Hence, obtaining information on BNP may provide additional discriminatory power in those at highest risk for death despite primary prevention ICD implantation.
Biomarkers for subclinical cardiac injury have long been recognized to predict major cardiovascular events in patients with cardiomyopathy. A number of these clinical studies occurred in cohorts where medical therapy for heart failure was not optimal by contemporary standards. Hence, the prognostic utility of cardiac injury markers in well-treated heart failure patients remains unclear. Our studies demonstrate that markers for cardiac injury remain an important predictor of death after ICD implantation. The mechanisms remain unclear but prior studies have suggested that they may promote inflammation.26
The limited sensitivity and specificity of using the EF as the primary means for SCD risk stratification has resulted in many individuals receiving ICDs deriving little benefit and also failing to identify individuals with relatively preserved function but remain at high SCD risk.27 In fact, the greatest number of SCD events occur in the general population without known heart disease,28 thus the overall impact of the ICD on the population burden of SCD is relatively small. Hence, many have sought to develop a more personalized approach whereby the risk of an individual for SCD is not based on population-based observations but rather unique features of the particular individual.29 Our findings add to these efforts by focusing on the biology of heart failure and shedding more light on potential mechanistic relationships between inflammation, neurohumoral activation, ongoing myocardial injury and death. It is difficult to determine which of the three mechanisms predominates but our composite biomarker scoring schema suggests that they are in fact complementary to each other in predicting patient outcomes.
There are a number of limitations that need to be considered. First, we did not have a comparison cohort with heart failure who did not undergo ICD implantation. This was not a clinical trial and randomizing patients for ICD therapy in patients who fulfilled current guidelines would be unethical. We attempted to account for this by censoring patients who experienced appropriate ICD shocks prior to death for the all-cause mortality analysis. Despite this, we cannot exclude the possibility that the ICD prevented bradycardia-induced SCD since all devices provided pacing support. However, prior studies suggest that this mode of death is uncommon.30 Second, this was a post-hoc analysis and no analysis was performed on temporal changes in biomarker levels especially immediately prior to an endpoint event. Hence, these results alone do not resolve the inadequacy of ICD patient selection but may provide preliminary data in guiding the design of future prospective studies. Third, we do not have detailed information on what proportion of patients were “at target” with their heart failure medications or more detailed information on the detection duration parameters for ICD therapy delivery. Fourth, we excluded ATP therapy from the primary endpoint in order to identify the best surrogate for SCD and because of prior reports highlighting the prognostic importance of ICD shocks on mortality outcomes.31 Fifth, the number of participants with appropriate ICD shocks was only 137, which limited our ability to identify modest predictors of risk. Lastly, our observations on the relationship between biomarkers and appropriate ICD shocks and death may not be applicable in all populations at risk of SCD including those with preserved left ventricular function. Despite this, we believe our findings remain relevant to the majority of ICD recipients given that most have systolic dysfunction and improvements on currently available risk prediction models are urgently needed to refine application of this therapy to those who would benefit the most.
Using a limited set of serum biomarkers of inflammation, neurohumoral activation and myocardial injury obtained at the time enrollment, we identified patients who were likely to die after primary prevention ICD implantation without receiving ICD shocks for ventricular tachyarrhythmias. These findings may provide more specific criteria to identify those who are most likely to benefit from a primary prevention ICD but will need to be further validated in large prospective clinical studies.
Supplementary Material
Acknowledgments
Funding Sources: The Donald W. Reynolds Foundation funded the initial design of the study and patient enrollment. Patient follow-up, data collection and analyses were supported by NIH R01 HL091062 (GFT) and NIH R01 HL103946 (AC).
Footnotes
Clinical Trial Registration – clinicaltrials.gov; Unique Identifier: NCT00733590.
Journal Subject Codes: [121] Primary prevention, [22] Ablation/ICD/surgery, [106] Electrophysiology, [5] Arrhythmias, clinical electrophysiology, drugs
Conflict of Interest Disclosures: Dr. A Cheng received honoraria from Boston Scientific, Medtronic and St. Jude Medical. Dr. D. Dalal’s contributions to the study pre-dated his current employment with Novartis. Dr. Z. Eldadah received an honorarium from St. Jude Medical. Dr. K. Ellenbogen received honoraria from Medtronic, Boston Scientific, Biotronik, served as a consultant for Medtronic, Boston Scientific, St. Jude Medical and received fellowship support from Medtronic and Boston Scientific. Dr. D. Spragg received honoraria from Biotronik and Medtronic. All other authors have no relevant disclosures to report.
References
- 1.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, Multicenter Automatic Defibrillator Implantation Trial II Investigators Prophylactic implantation of a defibrillator in patients with myocardial Infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH, Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators Amiodarone or an Implantable Cardioverter-Defibrillator for Congestive Heart Failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 3.Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T, Talajic M, Wilber DJ, Fishbein DP, Packer DL, Mark DB, Lee KL, Bardy GH. Prognostic Importance of Defibrillator Shocks in Patients with Heart Failure. N Engl J Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buxton AE. Implantable cardioverter-defibrillators for primary prevention of sudden death: the quest to identify patients most likely to benefit. J Am Coll Cardiol. 2012;60:1656–1658. doi: 10.1016/j.jacc.2012.06.041. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML, MADIT-II Investigators Risk stratification for primary prevention implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–296. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 6.Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2012;60:1647–1655. doi: 10.1016/j.jacc.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emerging Risk Factors Collaboration. C-reactive protein, fibrinogen and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beatty AL, Ku IA, Christenson RH, DeFilippi CR, Schiller NB, Wholley MA. High-sensitivity cardiac troponin T levels and secondary events in outpatients with coronary heart disease from the Heart and Soul Study. JAMA Intern Med. 2013;173:763–769. doi: 10.1001/jamainternmed.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng A, Dalal D, Butcher B, Norgard S, Zhang Y, Dickfeld T, Eldadah ZA, Ellenbogen KA, Guallar E, Tomaselli GF. Prospective observational study of implantable cardioverter-defibrillators in primary prevention of sudden cardiac death: study design and cohort description. J Am Heart Assoc. 2013;2:e000083. doi: 10.1161/JAHA.112.000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:e1–e62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 11.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, Wildevuur CR, Eijsman L, Trouwborst A, Hack CE. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–3548. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 14.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, Van Wagoner DR. C-reactive protein elevation in patient with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 15.Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective Study of C-Reactive Protein, Homocysteine and Plasma Lipid Levels as Predictors of Sudden Cardiac Death. Circulation. 2002;105:2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 16.Lamblin N, Mouquet F, Hennache B, Dagorn J, Susen S, Bauters C, de Groote P. High-sensitivity C-reactive protein: potential adjunct for risk stratification in patients with stable congestive heart failure. Eur Heart J. 2005;26:2245–2250. doi: 10.1093/eurheartj/ehi501. [DOI] [PubMed] [Google Scholar]
- 17.Ketch TR, Turner SJ, Sacrinty MT, Lingle KC, Applegate RJ, Kutcher MA, Sane DC. Derived fibrinogen compared with C-reactive protein and brain natriuretic peptide for predicting events after myocardial infarction and coronary stenting. Am Heart J. 2008;156:234–240. doi: 10.1016/j.ahj.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Empana JP, Jouven X, Canoui-Poitrine F, Luc G, Tafflet M, Haas B, Arveiler D, Ferrieres J, Ruidavets JB, Montaye M, Yarnell J, Morange P, Kee F, Evans A, Amouyel P, Ducimetiere P. C-Reactive Protein, Interleukin 6, Fibrinogen and Risk of Sudden Death in European Middle-Aged Men: The PRIME Study. Arterioscler Thromb Vasc Biol. 2010;30:2047–2052. doi: 10.1161/ATVBAHA.110.208785. [DOI] [PubMed] [Google Scholar]
- 19.Biasucci LM, Bellocci F, Landolina M, Rordorf R, Vado A, Menardi E, Giubilato G, Orazi S, Sassara M, Castro A, Massa R, Kheir A, Zaccone G, Klersy C, Accardi F, Crea F. Risk stratification of ischaemic patients with implantable cardioverter defibrillators by C-reactive protein and a multi-markers strategy: results of the CAMI-GUIDE study. Eur Heart J. 2012;33:1344–1350. doi: 10.1093/eurheartj/ehr487. [DOI] [PubMed] [Google Scholar]
- 20.Streitner F, Kuschyk J, Veltmann C, Brueckmann M, Streitner I, Brade J, Neumaier M, Bertsch T, Schumacher B, Borggrefe M, Wolpert C. Prospective study of interleukin-6 and the risk of malignant ventricular tachyarrhythmia in ICD-recipients—A pilot study. Cytokine. 2007;40:30–34. doi: 10.1016/j.cyto.2007.07.187. [DOI] [PubMed] [Google Scholar]
- 21.Theuns DA, Smith T, Szili-Torok T, Muskens-Heemskerk A, Janse P, Jordaens L. Prognostic role of high-sensitivity C-reactive protein and B-type natriuretic peptide in implantable cardioverter-defibrillator patients. PACE. 2012;35:275–282. doi: 10.1111/j.1540-8159.2011.03289.x. [DOI] [PubMed] [Google Scholar]
- 22.Flevari P, Theodorakis G, Leftheriotis D, Kroupis C, Kolokathis F, Dima K, Anastasiou-Nana M, Kremastinos D. Serum markers of deranged myocardial collagen turnover: their relation to malignant ventricular arrhythmias in cardioverter-defibrillator recipients with heart failure. Am Heart J. 2012;164:530–537. doi: 10.1016/j.ahj.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Scott PA, Barry J, Roberts PR, Morgan JM. Brain natriuretic peptide for the prediction of sudden cardiac death and ventricular arrhythmias: a meta-analysis. Eur J Heart Fail. 2009;11:958–966. doi: 10.1093/eurjhf/hfp123. [DOI] [PubMed] [Google Scholar]
- 24.Battipaglia I, Barone L, Mariani L, Infusino F, Remoli R, Careri G, Pinnacchio G, Tarzia P, Lanza GA, Crea F. Relationship between cardiac autonomic function and sustained ventricular tachyarrhythmias in patients with an implantable cardioverter defibrillators. Europace. 2010;12:1725–1731. doi: 10.1093/europace/euq408. [DOI] [PubMed] [Google Scholar]
- 25.Kara K, Mahabadi AA, Berg MH, Lehmann N, Möhlenkamp S, Kälsch H, Bauer M, Moebus S, Dragano N, Jöckel KH, Neumann T, Erbel R. Predicting risk of coronary events and all-cause mortality: role of B-type natriuretic peptide above traditional risk factors and coronary artery calcium scoring in the general population: the Heinz Nixdorf Recall Study. Eur J Prev Cardiol. 2014;21:1171–1179. doi: 10.1177/2047487313490256. [DOI] [PubMed] [Google Scholar]
- 26.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 27.Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, Josephson ME, Lehmann MH, Prystowsky EN, MUSTT Investigators Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1150–1157. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 28.Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–1052. doi: 10.1161/CIRCULATIONAHA.111.023846. [DOI] [PubMed] [Google Scholar]
- 29.van Rees JB, Borleffs CJ, van Welsenes GH, van der Velde ET, Bax JJ, van Erven L, Putter H, van der Bom JG, Schalij MJ. Clinical prediction model for death prior to appropriate therapy in primary prevention implantable cardioverter defibrillator patients with ischaemic heart disease: the FADES risk score. Heart. 2012;98:872–877. doi: 10.1136/heartjnl-2011-300632. [DOI] [PubMed] [Google Scholar]
- 30.Packer DL, Prutkin JM, Hellkamp AS, Mitchell LB, Bernstein RC, Wood F, Boehmer JP, Carlson MD, Frantz RP, McNulty SE, Rogers JG, Anderson J, Johnson GW, Walsh MN, Poole JE, Mark DB, Lee KL, Bardy GH. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation. 2009;120:2170–2176. doi: 10.1161/CIRCULATIONAHA.109.853689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweeney MO, Sherfesee L, DeGroot PJ, Wathen MS, Wilkoff BL. Differences in effects of electrical therapy type for ventricular arrhythmias on mortality in implantable cardioverter-defibrillator patients. Heart Rhythm. 2010;7:353–360. doi: 10.1016/j.hrthm.2009.11.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


