Abstract
Aims
Ischemic preconditioning (PC) is an adaptive response to transient myocardial ischemia that protects the heart from subsequent ischemia/reperfusion (I/R) injury. However, the mechanisms underlying its cardioprotective effects remain unclear.
Methods and results
Myocardium of adult male C57/BL6 mice, preconditioned by 6 cycles of 4 minutes coronary occlusion and reperfusion, showed nuclear translocation of ATF3, and ATF6 and PERK phosphorylation 30 min after PC. The abundance of ER proteins, ATF3 and ATF4 was increased 24 h after PC; however, there was no evidence of IRE-1 activation in WT or ER-stress activated indicator (ERAI) mice expressing XBP-1-Venus fusion protein. PC-induced nuclear translocation of ATF3 was attenuated in transgenic mice with cardiac-restricted overexpression of inducible ATF6. Ischemic PC increased the abundance of inducible nitric oxide synthase, cyclooxygenase-2, heme oxygenase-1 and aldose reductase to levels similar between WT and ATF3-null hearts; however, the increase in IL-6 and ICAM-1 was exaggerated in ATF3-null hearts. Genetic deletion of ATF3 did not increase infarct size in non-preconditioned hearts but abolished the cardioprotective effects of PC. Larger infarct size in preconditioned ATF3-null hearts was associated with greater neutrophil infiltration in the myocardium, but no ATF3-dependent changes in the total or relative abundance of inflammatory monocytes were observed.
Conclusion
Ischemic PC activates the unfolded protein response (UPR) and the activation of ATF3 by ER stress is essential for the cardioprotective effects of late PC.
Keywords: ATF3, unfolded protein response, ischemic preconditioning, infarct size, inflammatory response
1. Introduction
Although timely reperfusion can salvage the ischemic myocardium, re-introduction of oxygen triggers the production of reactive oxygen species and increases calcium overload leading to additional myocyte injury. Extensive work has uncovered a wide range of adaptive responses that, once activated, could attenuate myocardial injury. One such trigger of adaptive responses is ischemic preconditioning (PC), in which transient episodes of ischemia protect against subsequent ischemic injury. Ischemic PC consists of an initial short-lived (2–3 h) adaptation (early PC) followed by sustained protection that lasts at least 72 h (late PC) [1–3]. Early PC is an immediate response mediated by the activation of several membrane receptors and downstream kinases, whereas late PC involves genetic reprogramming, which culminates in the synthesis of proteins that enhance the resistance of the heart to ischemia. Because late PC lasts longer, it has a higher potential for providing sustained cardioprotection and thus appears to be more clinically relevant. Extensive changes in cell signaling and gene transcription induced during late PC suggest significant reorganization of the myocardial phenotype, indicating that PC is a polygenic response that affects several aspects of signaling, metabolism and gene transcription. Nevertheless, despite extensive investigation, the mechanisms by which late PC protects against ischemic injury remain incompletely understood.
In addition to PC, adaptive responses originating from the ER are also activated by ischemia. The ER is the major subcellular location for folding newly synthesized proteins. Under normal conditions, an estimated 30% of proteins are misfolded during their biogenesis and are therefore degraded [4]. Consequently, even small perturbations in protein folding elicit an unfolded protein response (UPR) to restore physiological function [5–7]. Upon accumulation of misfolded proteins, the ER chaperones such as GRP78 become occupied and release transmembrane ER proteins, IRE-1, PERK and ATF6, which, when activated, regulate multiple pathways affecting cell growth, nutrient availability, ER function, and antioxidant response [6]. The response is primarily adaptive because it is activated to re-establish normal ER function. However, if the perturbation is excessive, the UPR triggers inflammatory signaling to stimulate host-defense responses, and, if homeostasis cannot be established, cell death ensues.
Although both ischemic PC and the UPR have been demonstrated in the heart, it is unclear whether they are linked. The UPR is triggered by several conditions and is highly sensitive to components of ischemia such as hypoxia, glucose deprivation, ATP loss, and depletion of ER calcium [6, 8]; therefore, it may be potentially important in regulating the ischemic sensitivity of the heart [7]. Previous studies have shown that simulated ischemia in neonatal rat cardiomyocytes results in the induction of GRP78, PERK activation, and IRE-1 dependent signaling [9] as well as ATF6-dependent gene transcription [10]. In addition, increased GRP78 levels have been detected in cardiomyocytes in infarcted mouse hearts [11]. Nevertheless, activation of UPR by myocardial ischemia-reperfusion has not been systematically studied and it remains unknown whether these responses are activated by ischemic PC or how do adaptive components of UPR are related to the cardioprotective effects of ischemic PC. The current study was, therefore, designed to investigate the role of ER stress and the UPR in ischemic PC. We sought to determine whether ischemic PC triggers UPR and whether this response affects the cardioprotective mechanisms that are triggered by late PC.
2. Methods
2.1 Mice
Male ATF3-null, ERAI and ATF6-TG and wild-type (WT) mice were bred in-house and maintained on a C57BL/6 background. ATF3-null mice were obtained from Dr. Tsonwin Hai, Ohio State University, Columbus, Ohio, USA. Cardiospecific inducible ATF6-transgenic (TG) mice were a gift from Dr. Chris Glembotski, San Diego State University, San Diego, CA, USA [11]. Activation of the ATF6 transgene was accomplished via 5 daily injections of 40 mg/kg raloxifene (Sigma). ERAI mice were obtained from Riken Corp. Tokyo, Japan and have been described previously [12]. All procedures were approved by the University of Louisville Institutional Animal Care and Use Committee.
2.2 Ischemic PC and MI in mice
The murine model of late PC has been previously described [13, 14]. Ischemic PC was elicited by six cycles of 4 min coronary occlusion/4 min reperfusion (O/R). To prevent hypotension, blood from a donor mouse was given during surgery. Body temperature was measured rectally and maintained at 37°C and heart rate was monitored during the experiment. Myocardial tissue was obtained from the anterior LV walls and snap-frozen in liquid nitrogen for analysis and myocardial infarct size was measured as described before [15].
2.3 Identification and characterization of inflammatory infiltrates in mouse myocardium
Ischemic tissue or the corresponding regions from sham-operated mice were minced and placed into a cocktail of collagenase I, collagenase XI, DNase I, and hyaluronidase (Sigma-Aldrich) and shaken at 37°C for 1 h [16]. Cells were then triturated through mesh and centrifuged (15 min, 500×g, 4°C). The resulting single-cell suspensions were washed with HBSS with 0.2% BSA and 1% FCS. Cells were stained with fluorescent antibodies consisting of anti-mouse CD45, Gr-1, CD11b, F4/80, and Ly-6C for 20 min at 4°C in phosphate-buffered saline and1% BSA. Unstained and, in some cases, fluorescence minus one (FMO) controls were used to set gates. Data were acquired on a BD LSR II flow cytometer and analyzed using the FlowJo software.
2.4 Western blot analysis
Cytosolic and nuclear extracts were prepared from frozen samples using previously published methods [17, 18]. Western blotting was performed using standard SDS-PAGE as previously described [18, 19]. To ensure equal protein loading the total amounts of proteins transferred from each lane to the PVDF membranes during blotting were stained with Ponceau S. Immunoreactive protein bands were quantified with densitometry and further normalized to the corresponding Ponceau stain signal or α-tubulin by densitometric analysis [18–20] and data are expressed as a percentage of the control group for each experiment. The following primary antibodies were used: an anti–COX-2 monoclonal antibody (Cayman Chemical), anti-iNOS polyclonal antibody (Millipore), ATF3, ATF6, p-PERK, ATF4, SP1, ICAM-1 polyclonal antibodies (Santa Cruz Biotechnologies), HO-1, KDEL antibodies (Assaydesigns), FLAG, GRP78 and PDI antibodies (Cell Signaling). Polyclonal antibodies against recombinant human AR were raised in rabbits [21].
2.5 Measurement of cytokine levels
For cytokine analysis, frozen tissue was homogenized and the supernatant was used for the detection of IL-6, IL-12β and TNF-α using SearchLight Multiplex Immunoassay (Aushon Biosystems). Each sample was normalized to total protein and expressed as picogram cytokine per mg protein.
2.6 Quantitative RT-PCR
RNA was extracted from tissues using the RNeasy mini kit (Qiagen) and RNA concentration was measured using the Nanodrop® 1000A Spectrometer. cDNA was prepared and real-time PCR amplification was performed with SYBR-Green qPCR Master Mix (Qiagen) using a 7900HT Fast Real-Time PCR system (Applied Biosystems). Primers for DNAJB9 were obtained from Integrated DNA technologies: F 5′-TCTCGGATGCCAATAGTCGGA-3′ and R 5′-GACTGTTCAAAAGGACTCCCATT-3′. Relative expression was determined by the 2−ΔΔCT method by internal normalization to HPRT.
2.7 Immunostaining
Tissue samples were placed in 4% (wt/vol) buffered paraformaldehyde overnight and then in 10%, 20%, and 30% sucrose for 8 h each at 4°C. Tissues were embedded in OCT medium (Tissue-Tek) and sectioned at 5μm. Slides were mounted with SlowFade Gold antifade reagent containing DAPI (Molecular Probes) and fluorescence was observed using an EVOS fluorescence microscope (Advanced Microscopy Group) at 20x magnification. The entire ischemic zone of the left ventricle or corresponding portion of control hearts were analyzed every 100μm from proximal to the site of occlusion for ischemic PC to the distal cardiac apex.
2.8 Statistical analysis
Data are mean ± SEM. The results were analyzed either by unpaired Student’s t-test or, when appropriate, by a one-way ANOVA followed by unpaired Student’s t-tests with the Bonferroni correction.
3. Results
3.1 Ischemic PC triggers UPR
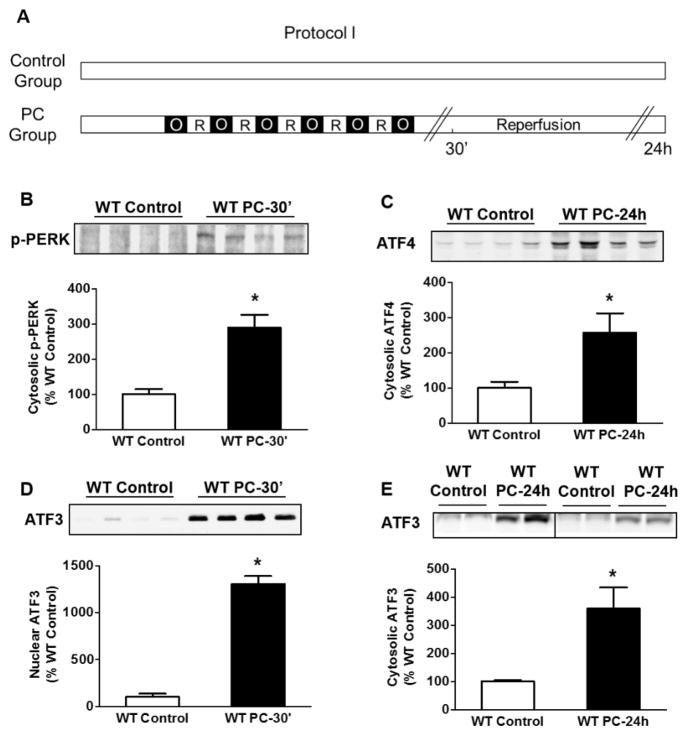
To assess whether brief episodes of PC activate UPR, we subjected adult C57 male mice to 6 cycles of coronary occlusion and reperfusion and measured changes in UPR components 30 min or 24 h after PC in the ischemic zone of the preconditioned hearts (Fig. 1A; Protocol I). As shown in Fig. 1B, ischemic PC led to a significant increase in the phosphorylation of the ER localized type I transmembrane protein PERK. Western blot analysis of the ischemic zone of the preconditioned hearts showed a significant increase in the abundance of ATF4 protein 24 h after ischemic PC (Fig. 1C), indicating activation of PERK-dependent signaling.
Fig. 1.
Ischemic PC activates PERK. (A) Protocol I for ischemic PC in WT mice. Control mice were sham operated, whereas the preconditioned hearts were subjected to 6 cycles of coronary occlusion (O) followed by reperfusion (R) as shown. Tissue lysates were prepared from the ischemic zone or for control samples from the anterior wall of the non-preconditioned heart. Western blots for (B) phospho-PERK; 30 minutes after PC, (C) cytosolic ATF4; 24 h after PC, (D) nuclear ATF3; 30 minutes after PC and (E) cytosolic ATF3; 24 h after PC. * P<0.05; n=4 mice per group.
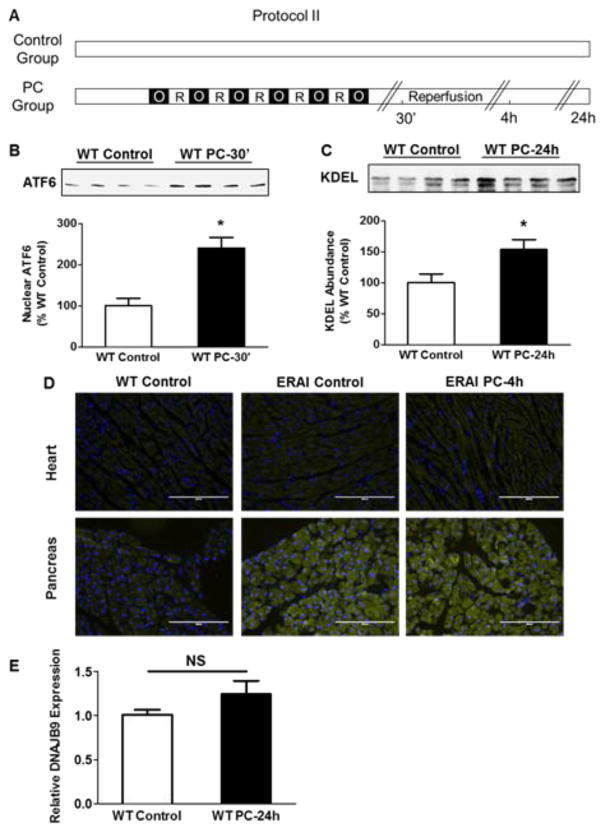
To examine changes in PERK-related signaling, we measured abundance of ATF3 after ischemic PC. These experiments showed a remarkable (10- to 13-fold) increase in ATF3 in nuclear extracts prepared from the ischemic zone 30 min after PC (Fig. 1D), indicating that ischemic PC causes the translocation of ATF3 from cytosol to nucleus. Moreover, 24 h after PC, we observed a consistent increase in cytosolic ATF3, suggesting that in addition to early translocation of ATF3, PC also increases cytosolic ATF3 abundance (Fig. 1E). These observations are consistent with the notion that ischemic PC causes ER stress, which results in the stimulation of the UPR mediator PERK and the activation of PERK-dependent transcription factors – ATF4 and ATF3. In addition to PERK, we found an increase in the nuclear abundance of ATF6 in preconditioned hearts (Fig. 2B), indicating that ischemic PC activates ATF6. In agreement with a role of ATF6 in increasing the transcription of ER resident chaperones [22] we found a consistent increase in abundance of proteins with the ER-specific KDEL localization sequence, 24 h after ischemic PC (Fig. 2C). Taken together, these observations indicate that ischemic PC activates AFT6-dependent UPR, which increases the abundance of ER-resident proteins.
Fig. 2.
Ischemic PC activates UPR adaptive components. (A) Protocol II for ischemic PC in WT and ERAI mice. Control mice were sham operated, whereas the preconditioned hearts were subjected to 6 cycles of coronary occlusion followed by reperfusion as shown. Tissue samples were examined from the ischemic zone or for control samples from the anterior wall of the non-preconditioned heart. Western blots showing nuclear abundance of (B) ATF6; 30 minutes after PC and (C) total cytosolic abundance of proteins containing the KDEL localization sequence; 24 h after PC. (D) Immunofluorescence in WT and ERAI hearts; 4 h after ischemic PC and pancreas. (E) qPCR for spliced XBP-1 gene target DNAJB9; 24 h after PC. * P<0.05; n=4 mice per group.
Accumulation of unfolded proteins in stressed ER also results in the phosphorylation of IRE-1, which in turn cause endonucleolytic cleavage of XBP-1 mRNA [8]. Efforts to measure the phosphorylation status of IRE-1 were unsuccessful; therefore, we used ERAI mice harboring YFP-labeled XBP-1 that is fluorescent only upon XBP-1 splicing [12]. In comparison with WT and control ERAI hearts, preconditioned ERAI hearts show no increase in YFP fluorescence in the ischemic zones, 4 h after PC (Fig. 2D). However, high levels of fluorescence were detected in the pancreas of the unstressed ERAI mice, indicating that in these mice the construct was active and responsive to physiological ER stress. To examine the role of the IRE-1 pathway further, we evaluated changes in the expression of the spliced XBP-1 target gene DNAJB9 [23] by qPCR 24 h after ischemic PC (Fig. 2E); however, no significant changes in its expression were observed. These observations suggest that ischemic PC does not lead to sustained activation of XBP-1.
3.2 Activation of ATF3 by ischemic PC
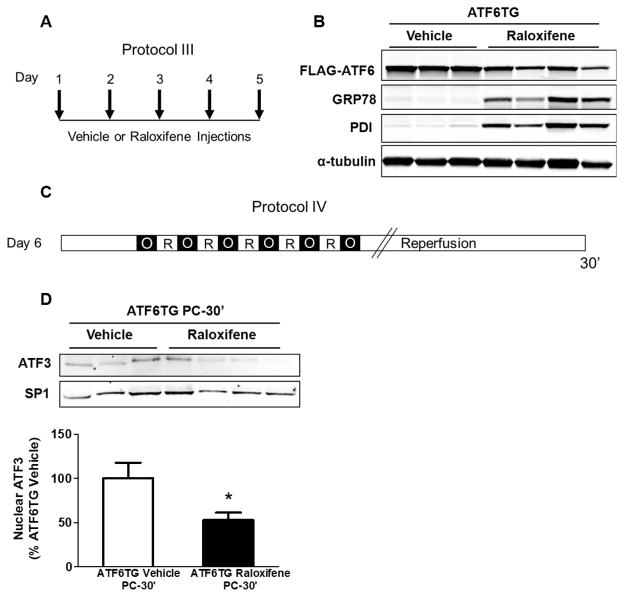
To examine the role of individual UPR components in mediating the effects of ischemic PC, we focused on ATF3 because among the several UPR components activated by ischemic PC, changes in ATF3 were the most robust (vide supra). ATF3 is a member of the ATF/CREB family of transcription factors, and is induced under several stressful conditions [24] including I/R [25]. However, in addition to ER stress, ATF3 could also be activated by cytokines, DNA damage, starvation or proteasome inhibition [26]. Hence to determine whether the activation of ATF3 by PC is due to ER stress, we examined whether preventing ER stress would abolish PC-induced ER stress signaling. To diminish ER stress, we used cardio-specific ATF6-TG mice in which ATF6 is fused with a mutated murine estrogen receptor (MER). As shown in Fig. 3B, treatment with the MER ligand raloxifene for 5 days (Fig. 3A) decreased the abundance of FLAG-ATF6-MER, likely due to proteolytic processing, and an increase in the ATF6 targets such as GRP78, indicating that the activation of ATF6 increases the abundance of ER chaperones. When these mice were subjected to ischemic PC (Fig. 3C), nuclear translocation of ATF3 was significantly reduced in comparison with TG mice treated with vehicle alone (Fig. 3D). These observations suggest that ischemic PC-induced ATF3 activation could be attributed to the induction of ER stress in the preconditioned hearts.
Fig. 3.
Overexpression of ATF6 diminishes ATF3 nuclear translocation by ischemic PC. (A) Protocol III for vehicle or raloxifene injections in ATF6TG mice. Tissue lysates were prepared from the ischemic zone or for control samples from the anterior wall of the non-preconditioned heart. Western blots showing changes in the abundance of (B) FLAG-ATF6 and GRP78; 5 days after raloxifene injections in ATF6-TG mice. (C) Protocol IV for ischemic PC in ATF6TG mice. (D) Nuclear abundance of ATF3 in the ischemic zones of vehicle or raloxifene injected ATF6TG hearts; 30 minutes after PC. SP1 was used as a marker for nuclear fractionation and a loading control. * P<0.05; n=7–8 mice per group.
3.3 ATF3 deletion alters ICAM-1 abundance but not mediators of late PC
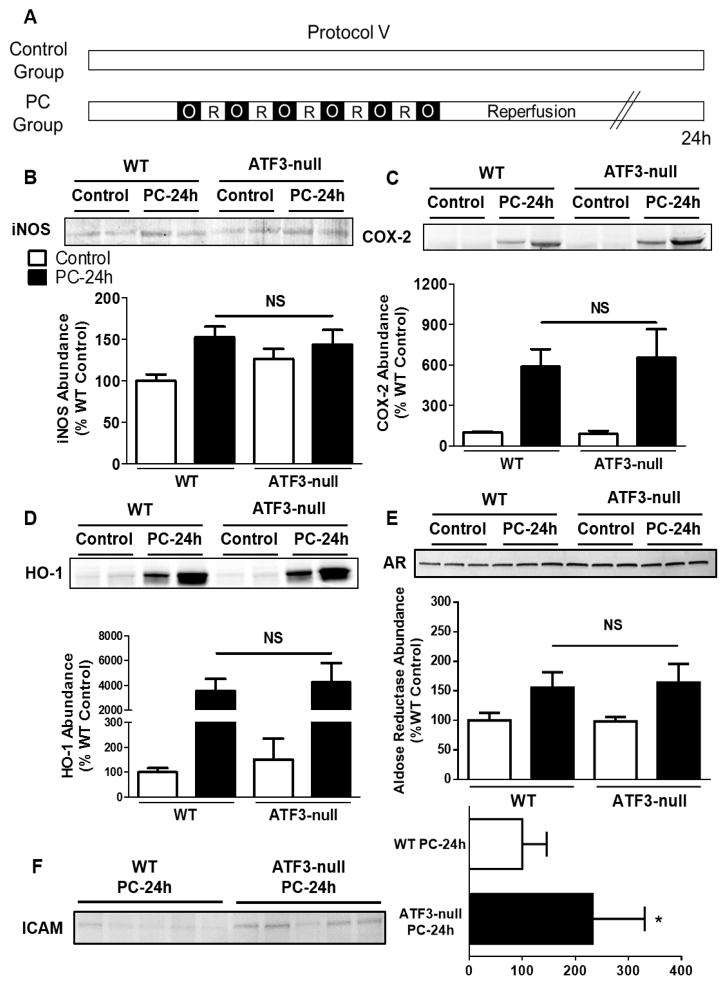
Because of our observation that ATF3 is activated by ischemic PC, we tested whether ATF3 participates in the regulation of major known mediators of late PC. Our laboratory has shown that the effects of late PC are mediated by the induction of the cardioprotective proteins – iNOS, COX-2, HO-1, and AR [2]. To assess whether ATF3 regulates the induction of these proteins, ATF3-null mice were subjected to ischemic PC and changes in the abundance of these proteins were examined 24 h after PC. As reported before, ischemic PC increased the total abundance of COX-2, HO-1, iNOS and AR in the ischemic zone of preconditioned hearts; however, no significant differences were observed between WT and ATF3-null mice (Fig. 4B–4E), suggesting that ATF3 does not regulate the abundance of the major cardioprotective proteins known to be induced by ischemic PC. However, a significant increase in abundance of ICAM-1 was observed in the ATF3-null hearts 24 h after PC in comparison to the WT (Fig. 4F).
Fig. 4.
ATF3 deletion alters ICAM-1 abundance but not the known mediators of late PC. (A) Protocol V for ischemic PC in WT and ATF3-null mice. Tissue lysates were prepared from the ischemic zone or for control samples from the anterior wall of the non-preconditioned heart. Western blots showing the abundance of (B) iNOS, (C) COX-2, (D) HO-1, (E) AR and (F) ICAM-1; 24 h after PC. n=5–8 mice per group.
3.4 ATF3 regulates IL-6 production
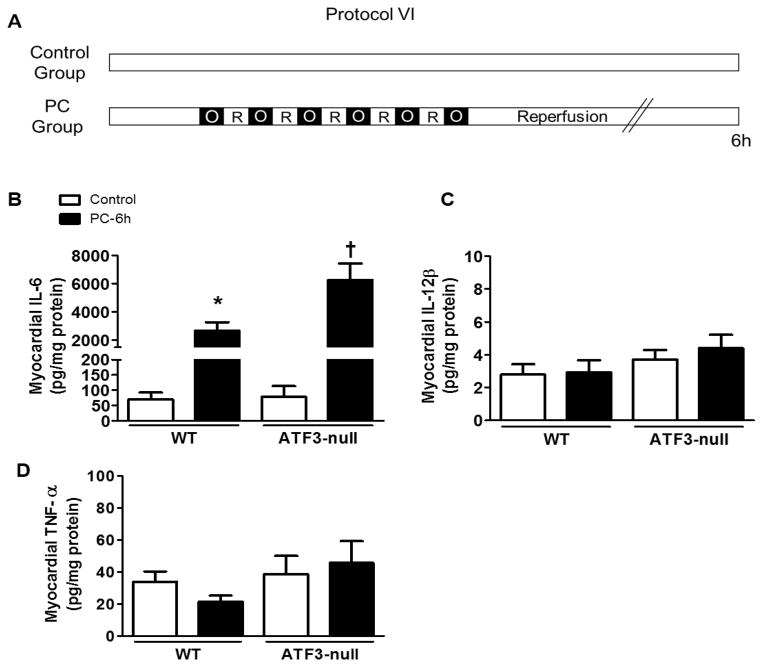
Next, to understand the role of ATF3 in ischemic PC further, we examined changes in cytokine production. In myeloid cells, ATF3 acts as a negative regulator of IL-6 and IL-12β transcription [27]. Although the role of IL-12 in PC remains obscure, IL-6 production is stimulated by ischemic PC [28]. Consistent with these observations, we found a 38-fold increase in IL-6 in the heart 6 h after ischemic PC (Fig. 5B), although the levels of TNF-α and IL-12β were unaffected (Fig. 5C and 5D). The increase in IL-6 was exaggerated in the hearts of ATF3-null mice, whereas the levels of both the TNF-α and IL-12β remained unchanged. These observations suggest that ATF3 negatively regulates the production of IL-6 in the ischemic heart.
Fig. 5.
ATF3 negatively regulates myocardial cytokines stimulated by ischemic PC. (A) Protocol VI for ischemic PC in WT and ATF3-null mice. Tissue lysates were prepared from the ischemic zone or for control samples from the anterior wall of the non-preconditioned heart. SearchLight multiplex immunoassay showing the abundance of myocardial (B) IL-6, (C) IL-12β and (D) TNF-α; 6 hours after PC. * P<0.05 vs. WT Control, † P<0.05 vs. WT PC-6h; n=4–9 mice per group.
3.5 ATF3 mediates the cardioprotective effects of late PC
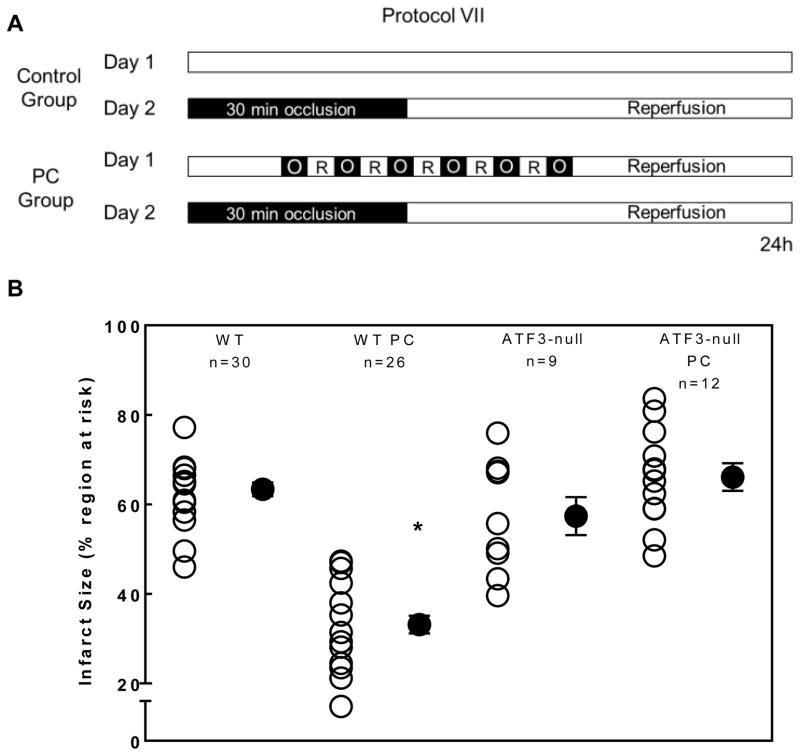
To determine whether ATF3, induced by PC, is functionally important for the cardioprotection conferred by late PC, we examined the effects of deleting ATF3 on the infarct-sparing ability of PC. In control, untreated WT mice that were not preconditioned, a 30-min coronary occlusion followed by 24 h of reperfusion (Fig. 6A) resulted in infarct sizes that averaged 63% of the region at risk (Fig. 6B). In mice subjected to ischemic PC, the infarct size was reduced to 32% of the risk region, indicating a robust late PC effect. To assess the role of ATF3 in late PC, ATF3-null mice were subjected to I/R with and without PC (Fig. 6A). No significant differences were observed between WT and ATF3-null mice with respect to body weight, LV weight or the weight of the risk region. Moreover, when subjected to 30 min of ischemia followed by 24 h of reperfusion, no differences in infarct size were observed between non-preconditioned WT and ATF3-null hearts, indicating that deletion of ATF3 does not affect I/R injury in the absence of PC (Fig. 6B). However, in contrast to WT mice, ischemic PC did not decrease infarct size in ATF3-null mice (Fig. 6B), indicating that ATF3 plays an obligatory role in mediating the infarct-sparing effect of late PC by mediating cardioprotective effects of UPR.
Fig. 6.
Inhibition of UPR and deletion of ATF3 abolish cardioprotection due to late PC. (A) Protocol VII for infarct size assessment with or without ischemic PC in WT and ATF3-null mice and (B) Quantification of infarct size as a percentage of area at risk in WT and ATF3-null mice subjected to infarction with or without ischemic PC. * P<0.05 vs. WT; n=9–30 mice per group.
3.6 ATF3 deletion alters cellular infiltrates in the ischemic myocardium
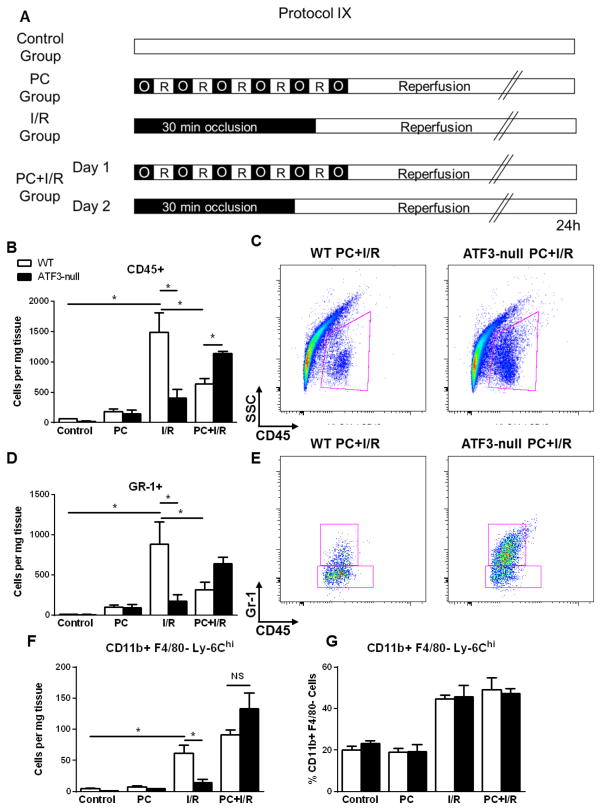
In addition to its role in mediating UPR, ATF3 has also been shown to regulate inflammatory responses [26, 27] which could affect tissue injury. Therefore, we measured the infiltration of inflammatory cells in the heart by flow cytometry. Without I/R, PC by itself led to a modest, but non-significant increase in inflammatory (CD45+ and GR-1+) cells after 24 h of reperfusion; however, when hearts were subjected to I/R alone, a significant increase in both cell populations was observed (Fig. 7B and 7D). This increase in inflammatory cells was, however, attenuated in ATF3-null hearts in comparison with WT hearts. Ischemic PC prior to I/R significantly reduced the infiltration of CD45+ and GR-1+ cells in the myocardium as compared with I/R alone. Deletion of ATF3 did not affect cell infiltration by PC alone, but led to a greater increase in total cell infiltrate and GR-1+ cells after PC+I/R as compared with WT. Moreover, deletion of ATF3 did not increase the total tissue abundance of inflammatory (CD11b+/F480−/Ly6Chi) monocytes (Fig. 7F) nor did it change the monocyte population relative to the total CD45+ cells (Fig. 7G). Interestingly, ATF3-null hearts demonstrated less inflammatory infiltration than WT after I/R alone. Collectively, these results suggest that deletion of ATF3 does not affect monocyte and neutrophil infiltration in hearts subjected to PC alone, but it increases the abundance of inflammatory cells and neutrophils in the hearts subjected to I/R after ischemic PC.
Fig. 7.
ATF3 deletion increases cellular infiltrates in preconditioned hearts subjected to ischemia/reperfusion. (A) Protocol IX for sham operation, ischemic PC, I/R or ischemic PC+I/R in WT and ATF3-null mice. Cellular infiltrates were prepared from the ischemic zone or control samples from the anterior wall of non-ischemic hearts. Quantification of cells per milligram ventricular tissue for (B) CD45+, (D) Gr-1+ and (F) CD11b+/F4/80-/Ly-6Chi as measured by flow cytometry. Representative dot plots of WT and ATF3-null hearts subjected to PC+I/R and gated for (C) SSC and CD45 and (E) Gr-1 and CD45. (G) Relative percentage of Ly-6Chi cells within total CD11b+/F4/80−. * P<0.05 as indicated; n=3–4 mice per group.
4. Discussion
The results of this study show that ischemic PC triggers the early phase of the UPR in the heart, which is characterized by an increase in PERK phosphorylation and nuclear translocation of the transcription factors, ATF6 and ATF3. These changes are followed by an increase in the myocardial levels of ATF4 and ATF3 and the up-regulation of ER-resident proteins, 24 h after PC. The full development of the response coincides with an increase in the resistance of the heart to sustained I/R injury. Our experiments also indicate that ATF3, activated by ER stress, is an obligatory mediator of the infarct-sparing effect of late PC. Collectively, these findings reveal a previously undiscovered link between ischemic PC and UPR and identify ATF3 as an important, novel regulator of myocardial sensitivity to ischemia/reperfusion. Extensive investigations into the mechanisms of late PC have led to the identification of a range of adaptive responses [2, 29]. It is currently believed that ischemic PC is initiated by triggers generated during the PC stimulus, such as adenosine, NO, cytokines and reactive oxygen species. These triggers activate non-redundant signal transduction pathways which in turn activate transcription factors to enhance myocardial resistance to ischemia. The “early mediators” of ischemic PC upregulate “distal mediators” such as iNOS, HO-1, COX-2 and AR, which protect the heart by acting upon the “end-effectors” or “targets”, which remain mostly unknown. Our present observations showing that ischemic PC results in the activation of UPR-dependent “early” (p-PERK, ATF3, ATF6) and distal” (ATF4, ATF3, and ER-resident proteins) mediators add a new facet to our understanding of the highly polygenic nature of late PC and suggest that the PC response may be more complex than currently believed.
The results of several previous studies support the view that the ER is an important target of ischemic injury. Induction of ischemia in cerebral [30], renal [31], hepatic [32] and myocardial [7] tissue causes ER stress and results in the robust activation of UPR mediators. Studies with isolated cardiomyocytes have shown that simulated ischemia or hypoxia activates the three major branches of the UPR characterized by the induction of ATF6, induction and splicing of XBP-1 and activation of PERK-dependent genes ATF4 and GADD34 [9]. Also, the induction of the ER chaperones GRP78 or GRP94 has been reported in mouse hearts subjected to I/R ex vivo [11] and in the peri-infarct zone of the heart in vivo [11] suggesting that myocardial ischemia triggers the activation of adaptive responses to increase the protein folding capacity of the ER. In agreement with these reports, our data show an increase in ER proteins and activation of ATF3 and ATF4 in the preconditioned heart. However, we found no evidence for the activation of the IRE-1-XBP-1 pathway. Activation of IRE-1 is considered to be a defining feature of UPR; however, it has been noted that several conditions lead to partial activation of the UPR [6]. Moreover, there is functional redundancy between the IRE1 and the ATF6 [8] signaling pathways and therefore activation of the ATF6 pathway alone may be sufficient to enhance the protein-folding capacity of the heart following ischemic PC. This view is supported by our current data showing that ischemic PC led to an increase in the abundance of ER-resident proteins.
We found robust activation of ATF3 in the preconditioned heart. Although several types of stress can lead to ATF3 activation, attenuation of ATF3 activation in ATF6-TG hearts suggests that ATF3 activation in the preconditioned heart is due to the induction of ER stress. Moreover, because the ATF6 gene was driven by the cardiac myocyte-specific α-myosin heavy chain promoter, it appears that the ATF3 activation is localized to cardiac myocytes and not to other cell types present in the heart. The functional significance of this activation event was evident from the results of our experiments showing that even though deletion of ATF3 did not affect infarct size in non-preconditioned hearts, it completely abrogated the cardioprotective effects of PC. These observations suggest that induction of ER stress leading to ATF3 activation is an obligatory event in the manifestation of the infarct-sparing effects of late PC. However, deletion of ATF3 did not affect the induction of several of the previously identified mediators of ischemic PC such as iNOS, COX-2, HO-1 and AR, although late PC was abolished in ATF3-null hearts despite the induction of these cardioprotective proteins. These observations underscore the polygenic nature of late PC and suggest that simultaneous activation of multiple signaling pathways is required for preconditioning and that deletion of even one of these mediators results in loss of cardioprotection.
Despite the demonstration of an essential role of ATF3 in mediating the beneficial effects of late PC, the mechanism by which ATF3 confers cardioprotection remains unknown. ATF3 is a member of the CREB family of stress-inducible transcription factors. It has been found to regulate inflammatory responses as part of a negative feedback loop [27] and to act as a negative regulatory transcription factor in the TLR pathway. Along with Rel, ATF3 binds to the regulatory regions of IL-6 and IL-12β, thereby regulating the extent and the duration of production of these cytokines. In accordance with this role, it has been shown that deletion of ATF3 increases IL-12β and IL-6 production in TLR-stimulated macrophages [33] and that ATF3 regulates IL-6 production during heat shock [34] or infection [35]. Our observation that deletion of ATF3 increases IL-6 production and the expression of ICAM-1 in the heart is consistent with its role in regulating the inflammatory response both during PC and subsequent I/R injury. Consistent with the increase in IL-6 and ICAM-1, we found increased accumulation of inflammatory cells such as neutrophils in ATF3-null hearts following PC+I/R. This result suggests that deletion of ATF3 increases the inflammatory response in the preconditioned heart. This is in contrast to the non-preconditioned hearts, where deletion of ATF3 prevented the increase in inflammatory cells. Because deletion of ATF3 did not reduce infarct size in non-preconditioned heart, it would suggest that the decreased inflammatory response in ATF3 null hearts, by itself is not sufficient to attenuate myocardial injury. However, in the preconditioned heart, deletion of ATF3 increased infract size and the inflammatory cell infiltrate, indicating that changes in the inflammatory cells infiltration may be more important in the preconditioned than the non-preconditioned heart. Moreover, it is also plausible that the increase in the inflammatory response in preconditioned ATF3-null hearts is secondary to increased injury. However, the increase in IL-6 and ICAM was observed in the preconditioned heart, before the index ischemia; therefore, it remains likely that the exaggerated inflammatory response precedes I/R injury and that increased recruitment of inflammatory cells abrogated PC despite the upregulation of the cardioprotective protein mediators of PC. It is also likely that the protective effects of ATF3 are due to its influence on other target genes, which were not examined in this study. Further experiments are required to evaluate more completely how ER stress and ATF3 activation regulate I/R injury and long-term remodeling of the heart after infarction.
5. Conclusions
In summary, the results of this study show that the cardioprotective actions of late PC are due in part to the induction of ER stress, resulting in the activation of adaptive UPR signaling. Our results show that deletion of ATF3 abolishes the cardioprotective effects of ischemic PC and that these effects are mediated in part by the activation of ATF3, which functions as a repressor of IL-6 and ICAM-1 in the preconditioned myocardium. These findings expand our current understanding of ischemic PC; they also reveal an inextricable link between the adaptive responses that are triggered by ATF3, ER stress and ischemic PC; and suggest that stimulation of UPR signaling may be a therapeutically gainful strategy for preventing myocardial ischemic injury.
Highlights.
Ischemic preconditioning activates UPR signaling including ATF3.
Overexpression of ATF6 diminishes PC-induced ATF3 nuclear translocation.
ATF3 deletion exaggerates IL-6 and ICAM-1 abundance during late phase preconditioning.
Deletion of ATF3 abolishes cardioprotection by ischemic preconditioning.
ATF3 deletion causes greater inflammatory infiltration in preconditioned hearts following I/R.
Acknowledgments
We thank Susan Dougherty, Lu-Ping Guo, Rachel Keith, Donald Mosley, Emily Steinmetz, David Young, Xiaoping Zhu and Junjie Du for their technical assistance.
Sources of Funding
This study was supported by grants from the National Institute of Health HL55477, HL59378, HL65660, HL78825, and GM103492.
Glossary
- AR
aldose reductase
- ATF
activating transcription factor
- COX-2
cyclooxygenase 2
- ERAI
endoplasmic reticulum stress activated indicator
- HO-1
heme oxygenase 1
- MI
myocardial infarction
- iNOS
inducible nitric oxide synthase
- IRE-1
inositol-requiring kinase
- I/R
ischemia/reperfusion
- MER
murine estrogen receptor
- PDI
protein disulfide isomerase
- PERK
protein kinase-like endoplasmic reticulum kinase
- PC
preconditioning
- SP1
specificity protein 1
- UPR
unfolded protein response
- XBP-1
X-box binding protein 1
Footnotes
Disclosures
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–83. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 3.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–9. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 4.Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–4. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 5.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–49. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 6.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–64. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groenendyk J, Sreenivasaiah PK, Kim do H, Agellon LB, Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ Res. 2010;107:1185–97. doi: 10.1161/CIRCRESAHA.110.227033. [DOI] [PubMed] [Google Scholar]
- 8.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 9.Szegezdi E, Duffy A, O’Mahoney ME, Logue SE, Mylotte LA, O’Brien T, et al. ER stress contributes to ischemia-induced cardiomyocyte apoptosis. Biochem Biophys Res Commun. 2006;349:1406–11. doi: 10.1016/j.bbrc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Doroudgar S, Thuerauf DJ, Marcinko MC, Belmont PJ, Glembotski CC. Ischemia activates the ATF6 branch of the endoplasmic reticulum stress response. J Biol Chem. 2009;284:29735–45. doi: 10.1074/jbc.M109.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, et al. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98:1186–93. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- 12.Iwawaki T, Akai R, Kohno K, Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat Med. 2004;10:98–102. doi: 10.1038/nm970. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci U S A. 1999;96:11507–12. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275:H1375–87. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Y, Flaherty MP, Wu WJ, Tan W, Zhu X, Li Q, et al. Genetic background, gender, age, body temperature, and arterial blood pH have a major impact on myocardial infarct size in the mouse and need to be carefully measured and/or taken into account: results of a comprehensive analysis of determinants of infarct size in 1,074 mice. Basic Res Cardiol. 2012;107:288. doi: 10.1007/s00395-012-0288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. The Journal of experimental medicine. 2012;209:123–37. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, et al. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci U S A. 2000;97:10197–202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci U S A. 2001;98:9050–5. doi: 10.1073/pnas.161283798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO, et al. Role of the protein kinase C-epsilon-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation. 2005;112:1971–8. doi: 10.1161/CIRCULATIONAHA.105.561522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xuan YT, Guo Y, Zhu Y, Han H, Langenbach R, Dawn B, et al. Mechanism of cyclooxygenase-2 upregulation in late preconditioning. J Mol Cell Cardiol. 2003;35:525–37. doi: 10.1016/s0022-2828(03)00076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruef J, Liu SQ, Bode C, Tocchi M, Srivastava S, Runge MS, et al. Involvement of aldose reductase in vascular smooth muscle cell growth and lesion formation after arterial injury. Arterioscler Thromb Vasc Biol. 2000;20:1745–52. doi: 10.1161/01.atv.20.7.1745. [DOI] [PubMed] [Google Scholar]
- 22.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–99. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–59. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, et al. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol. 2004;24:1365–77. doi: 10.1128/MCB.24.3.1365-1377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin T, Sandhu G, Wolfgang CD, Burrier A, Webb RL, Rigel DF, et al. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. J Biol Chem. 1997;272:19943–50. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- 26.Hai T, Wolford CC, Chang YS. ATF3, a hub of the cellular adaptive-response network, in the pathogenesis of diseases: is modulation of inflammation a unifying component? Gene Expr. 2010;15:1–11. doi: 10.3727/105221610x12819686555015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilchrist M, Thorsson V, Li B, Rust AG, Korb M, Roach JC, et al. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature. 2006;441:173–8. doi: 10.1038/nature04768. [DOI] [PubMed] [Google Scholar]
- 28.Dawn B, Xuan YT, Guo Y, Rezazadeh A, Stein AB, Hunt G, et al. IL-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovasc Res. 2004;64:61–71. doi: 10.1016/j.cardiores.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hausenloy DJ, Yellon DM. The second window of preconditioning (SWOP) where are we now? Cardiovasc Drugs Ther. 2010;24:235–54. doi: 10.1007/s10557-010-6237-9. [DOI] [PubMed] [Google Scholar]
- 30.DeGracia DJ, Montie HL. Cerebral ischemia and the unfolded protein response. J Neurochem. 2004;91:1–8. doi: 10.1111/j.1471-4159.2004.02703.x. [DOI] [PubMed] [Google Scholar]
- 31.Bailly-Maitre B, Fondevila C, Kaldas F, Droin N, Luciano F, Ricci JE, et al. Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103:2809–14. doi: 10.1073/pnas.0506854103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53:1752–63. doi: 10.1002/hep.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitmore MM, Iparraguirre A, Kubelka L, Weninger W, Hai T, Williams BR. Negative regulation of TLR-signaling pathways by activating transcription factor-3. J Immunol. 2007;179:3622–30. doi: 10.4049/jimmunol.179.6.3622. [DOI] [PubMed] [Google Scholar]
- 34.Takii R, Inouye S, Fujimoto M, Nakamura T, Shinkawa T, Prakasam R, et al. Heat shock transcription factor 1 inhibits expression of IL-6 through activating transcription factor 3. J Immunol. 2010;184:1041–8. doi: 10.4049/jimmunol.0902579. [DOI] [PubMed] [Google Scholar]
- 35.Hoetzenecker W, Echtenacher B, Guenova E, Hoetzenecker K, Woelbing F, Bruck J, et al. ROS-induced ATF3 causes susceptibility to secondary infections during sepsis-associated immunosuppression. Nat Med. 2012;18:128–34. doi: 10.1038/nm.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]