Abstract
Physiological stress responses are proposed as a pathway through which stress can “get under the skin” and lead to health problems, specifically obesity. We tested associations of salivary alpha amylase (sAA) diurnal patterns and stress responses with body mass index (BMI) in young, low-income children (51% male; 54% non-Hispanic white). Diurnal saliva samples were collected three times per day across three days for 269 children (M age 50.8 months, SD 6.3). Individual sAA intercept and slope values were calculated using random effect models to represent morning sAA levels and rate of sAA change across the day. A subset of children (n = 195; M age 56.6 months, SD 6.9) participated in a lab-based behavioral stress protocol. Area under the curve increase (AUCI) across four timepoints was calculated to represent increase in sAA output during stress elicitation. Children were weighed and height measured and BMI z-score was calculated. Linear regression was used to evaluate associations of sAA intercept, sAA slope, and sAA AUCI with BMI z-score, controlling for child age, sex, and race/ethnicity; maternal weight status; and family income-to-needs ratio. Diurnal and stress-response sAA patterns were related to child adiposity: for each 1-standard deviation unit (SDU) decrease in morning sAA level, the child’s BMI z-score increased by 0.11 (SE 0.05) SDU’s (p < .04); for each 1-SDU increase in sAA slope across the day, the child’s BMI z-score increased by 0.12 (SE 0.05) SDU’s (p < .03); and for each 1-SDU decrease in sAA AUCI during the stress elicitation, the child’s BMI z-score increased by 0.14 (SE 0.06) SDU’s (p < .03). Blunted stress responses and atypical diurnal patterns of sAA have been found following exposure to chronic life stressors such as poverty. Findings suggest that associations of stress, sAA, and elevated body mass index may develop very early in the lifespan.
Keywords: child; obesity, stress; salivary alpha-amylase (sAA); low-income
1. Introduction
1.1. Salivary Alpha Amylase as a Marker of Sympathetic Nervous System Activity
The autonomic nervous system (ANS) consists of the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS). The functioning of these two complementary systems is measured primarily via indices of cardiovascular physiology (e.g. heart rate, blood pressure) or catecholamines (epinephrine and norepinephrine) (Granger et al., 2007). Salivary alpha amylase (sAA) has been described as a marker of SNS basal activity and stress reactivity (Rohleder et al., 2004; Granger et al., 2007; Nater and Rohleder, 2009), although it is debated as to whether sAA signals pure SNS activity or a combination of SNS and PNS activation (Bosch et al., 2011; Ditzen, Ehlert, and Nater, 2014). It is known that SNS stimulation promotes the secretion of norepinephrine, which in turn increases sAA secretion (Kuebler et al., 2014). The result is a robust diurnal pattern for sAA, defined by a pronounced decline within the first 30 minutes after awakening, steadily rising in the afternoon, and peaking in the late afternoon or evening (Rohleder et al., 2004; Nater et al., 2007). sAA is sensitive to psychological stressors (Nater et al., 2006; Thoma et al., 2012), in general rising in response to stressors (Rohleder et al., 2004).
sAA reactivity in response to stress correlates with stress induced serum catecholamine levels (Ditzen et al., 2014; Thoma et al., 2012) and cardiac physiological changes observed in response to stress (Nater, 2006; El-Sheikh et al., 2008). Associations between basal sAA output and resting SNS activity have been examined less frequently; one recent study of adult males found stronger sAA-SNS associations under challenging compared to resting conditions (Ditzen et al., 2014). Thus, although the specific nature of sAA-SNS associations has yet to be determined, particularly with regard to resting SNS-sAA associations, evidence suggests that sAA may be a reasonable marker of SNS stress reactivity or at least of SNS dominance (Nater and Rohleder, 2009). The methods used to measure SNS functioning directly are typically fairly invasive (e.g., venipuncture, heart rate, skin conductance). As sAA is measured in saliva, it is more easily used in stress research with young children (Hill-Soderlund et al., 2014).
1.2. A Conceptual Model Linking Stress, Behavior, Sympathetic Nervous System Activity, and Adiposity in Children
As has been hypothesized with the hypothalamic-pituitary-adrenal axis (Gunnar and Vazquez, 2001), chronic activation of the SNS in the face of stress has been hypothesized to lead to down-regulation of the system (Lovallo, 2011; Pervanidou and Chrousos, 2012), such that chronic stress exposure is associated with low basal sAA activity and reduced sAA reactivity in response to challenge. Lower basal sAA patterns have been associated with chronic stress exposure in children (Hill-Soderlund et al., 2014; Wolf, Nicholls, and Chen, 2008). Also in children, attenuated sAA reactivity in response to stress has been associated with more disruptive behavior (Granger et al., 2007; Susman et al., 2010; de Vries-Bouw et al., 2012), more anger and impulsivity (Spinrad et al., 2009), and weaker ability to delay gratification (Lisonbee et al., 2010). Both psychosocial stress exposure and these behavioral features have been associated with a higher risk of obesity in children (Lumeng et al., 2003; Seeyave et al., 2009; Lumeng et al., 2013; Leung et al., 2014). We therefore hypothesized that these blunted basal and stress-responsive sAA patterns linked with chronic stress exposure and poor emotional and behavioral regulation among children would also be associated with increased adiposity.
1.3 Assessment of the Evidence Base for the Proposed Conceptual Model
Studies of adults provide support for the proposed conceptual model. Of the few studies that have considered SNS responses to stress in relation to adiposity, lower SNS stress responses assessed using cardiac measures were associated with greater adiposity (Carroll, Phillips, and Der, 2008; Jones et al., 2012). One study assessing SNS reactivity to stress via sAA also found a trend for an association between lower sAA stress response and greater adiposity (Thoma et al., 2012).
Resting SNS activity as measured by cardiac physiology is also related to obesity risk, although evidence for the direction of association is more mixed. For example, cross-sectional studies have reported associations between obesity risk indicators and greater resting SNS activity (Tentolouris, Liatis, and Katsilambros, 2006). However, low resting SNS activity has also been implicated in weight gain (Tataranni et al, 1997) and slower weight loss over time (Astrup et al., 1995). Two studies that examined diurnal sAA and adiposity found lower diurnal sAA associated with higher body mass index (BMI; Nater et al., 2007) or no association (Veen et al., 2012).
Fewer studies have been done in children and their results are more conflicting. The only study to examine cardiac reactivity to stress in relation to adiposity was in preschool-aged children, and found a negative association (Alkon et al., 2014). In studies of older children (ages 8–14 years) assessing cardiac physiology, some have found decreased resting SNS activity in association with adiposity (Nagai et al., 2003; Vanderlei et al., 2010; Baum et al., 2013), whereas others have found increased resting SNS activity in association with adiposity (Rabbia et al., 2003; Latchman et al., 2011; Rodríguez-Colón et al., 2011; Tascilar et al., 2011; Soares-Miranda et al., 2011; Altuncu, Baspinar, and Keskin, 2012). Of note, none of these studies examined sAA as potential marker of resting SNS activity or stress response.
1.4 Purpose of the Current Study
To our knowledge there are no studies of how both resting and stress-responsive SNS activity relates to adiposity during early childhood. Further, no study has considered sAA (either diurnal pattern or stress response) in relation to adiposity in young children. Low-income preschool-aged children are at high risk for obesity (Kimbro, Brooks-Gunn, and McLanahan, 2007) and experience high levels of chronic stress (Evans and Kim, 2007). Links between physiological stress pathways and obesity may be a novel point for intervention. Yet, knowledge gaps regarding the nature of such associations must first be addressed. This study therefore sought to examine sAA diurnal pattern and sAA stress response in relation to adiposity among low-income preschool-aged children. Based on the literature showing that low SNS activity, particularly reduced reactivity to stress, is associated with greater obesity risk in adults, and considering the findings on sAA and child behavioral outcomes that have been associated with obesity risk, we hypothesized that lower sAA diurnal output and lower sAA response to stress would each be associated with greater adiposity in young children.
2. Methods
2.1. Study Design and Participants
Participants were children who had attended Head Start, a preschool program free to low-income families in the United States. Families were invited to participate in a study described as seeking to understand whether children with different levels of stress hormones eat differently and included a diurnal saliva sampling protocol. All children in this original study were contacted to participate in a follow-up study, which included a stress-elicitation and associated saliva sampling protocol; children participated in this follow up study on average 5.9 (SD 3.8) months following the initial study. This report therefore includes two overlapping samples of children with complete data for all covariates: those who participated in the diurnal sampling study (n = 269) and the subset of these participants who also participated in the reactivity sampling study (n = 195). Exclusion criteria for both studies were: parent with ≥ 4 year college degree; parent or child not English-speaking; child in foster care, with food allergies, significant medical problems or perinatal complications, or gestational age < 35 weeks. The research was approved by the University of Michigan Institutional Review Board. Written informed consent was provided by the children’s legal guardian and age appropriate assent was obtained from children; families were compensated for their time.
Children in the diurnal sampling study were 50.8 months old on average at the time of diurnal assessment (range: 36.2–62.2; SD = 6.3). The subset of these children who subsequently participated in the reactivity sampling study were on average 56.6 months old at the time of reactivity assessment (SD = 6.9, range: 38.1–78.5; see Table 1). Characteristics of children in the cohort participating in the diurnal assessment, as well as the characteristics of the subset of these children participating in the reactivity assessment, are shown in Table 1. The subset of the diurnal cohort that also participated in the reactivity assessment did not differ from those who did not participate with regard to child sex, child age at the time of participation in the diurnal study, child body mass index z-score at the time of participation in the diurnal study, diurnal sAA measures, maternal weight status, or household income-to-needs ratio; however, the subset of the diurnal cohort that also participated in the reactivity assessment was more likely to be white, non-Hispanic compared to those who did not participate (59% vs. 40%, p = .0038).
Table 1.
Characteristics of the Samples
| Variable | Diurnal Sample (n = 269) |
Reactivity Sample (n = 195) |
|---|---|---|
| Child age (months, M and SD) | 50.81 (6.28) | 56.62 (6.93) |
| Child sex is male, n (%) | 137 (51.0%) | 98 (50.0%) |
| Child race/ethnicity is white, non-Hispanic, n (%) | 144 (53.5%) | 115 (59.0%) |
| Primary caregiver overweight, n (% overweight) | 202 (75.1%) | 153 (78.5%) |
| Income-to-needs Ratio (1.00 = poverty, M (SD)) | 0.96 (0.84) | 0.92 (0.90) |
| Child BMI z-score (M and SD) | 0.69 (0.83) | 0.66 (0.84) |
| Diurnal sAA intercept (M (SD) U/ml) | 26.15 (1.90) | -- |
| Diurnal sAA slope (rate of U/ml change per hour) | 0.06 (0.01) | -- |
| Reactivity sAA AUCI (median (range) U/ml/min) | -- | 17.38 (−2407.20 to 1694.10) |
2.2. Procedure and Measures
2.2.1. Saliva Sampling
Children provided all saliva samples by first rinsing their mouth with water, then chewing on a piece of cotton across all mouth quadrants for 1 minute. Children of this age range were unable to reliably provide the saliva by passive drool sampling. sAA differs based on whether saliva is collected by passive drool or by chewing on cotton, but using a consistent method for all children in the same study is an acceptable approach. Further, stress-induced increases in sAA have been shown to be independent of salivary flow rate (i.e., method of saliva collection) (Rohleder et al., 2006). The primary caregiver reported for the child on the day of each protocol any medication use, illness, unusually good or bad events, exact time of morning awakening (and if it was the usual time), and the last time the child slept or ate prior to the protocol. Children provided diurnal saliva samples 3 times per day (on arrival to preschool, before breakfast, about 8:30am; before lunch, about 11:30am; and at 4:30pm) on 3 consecutive days.
sAA reactivity was captured using the stress-elicitation protocol. Children and their primary caregivers attended a study visit at 1:00pm on one afternoon during which children participated in the protocol and were later weighed and measured. The stress-elicitation protocol has been described in detail elsewhere (Miller et al., 2013). Children were brought to a room separate from the parent and engaged in calming free play with the examiner for 15–20 minutes. The child then participated in a series of tasks with the examiner that were designed to elicit a mild to moderate level of stress, yet mimic the challenges encountered during a child’s daily life (Gunnar, Talge, and Herrera, 2009). Briefly, tasks included an activity in which the child was encouraged to draw a perfect circle for 3.5 minutes and given feedback that the circle was not perfect (Goldsmith and Rothbart, 1996); attempted to solve a puzzle with missing pieces (3 minutes) and one that was too difficult (4 minutes); waited to receive a gift while the gift was being wrapped (1.5 minutes) (McCabe and Brooks-Gunn, 2002); and received a gift that was not what the child had previously selected (Cole, 1986). When the wrong gift was received, the examiner waited 30 seconds, then “realized” she had made a mistake and brought the correct gift for the child. After this time, the child engaged in free play with the examiner or watched a calm, age appropriate children’s movie for 40 additional minutes to allow for the saliva sampling in intervals timed to allow for sAA to rise in response to the stress elicitation. Tasks were all delivered in standardized fashion by trained examiners.
Following the notion that sAA peaks about 10 minutes following the onset of a stressor, (Granger et al., 2007), saliva was sampled at five points during the stress-elicitation protocol: (1) 20 minutes after room entry, to reflect sAA prior to beginning the study session; (2) after 30 minutes of free play to reflect the baseline prior to beginning the challenge tasks; (3) at 10 minutes after receipt of the gift; (4) at 20 minutes after receipt of the gift; and (5) at 40 minutes after receipt of the gift. The second sample was used to estimate baseline sAA level prior to the stressor onset to avoid assessing an anticipatory stress response. The 3rd, 4th, and 5th samples were used to estimate sAA response to stress.
2.2.2. sAA Assays
Following collection, saliva samples were stored at −20° C until assayed in duplicate using an alpha amylase kinetic reaction assay kit (Catalog No. 1-1902, 96-Well Kit, Salimetrics LLC, PA, USA). On the day of the assay, the sample was thawed completely, vortexed, centrifuged at 3000 rpm for 15 minutes, separated from debris and submitted to the steps for alpha amylase activity detection following manufacturer’s instructions. The kinetic reaction assay uses a chromagenic substrate, 2-chloro-pnitrophenol linked with maltotriose. The enzymatic action of alpha-amylase on this substrate yields 2-chloro-p-nitrophenol, which is spectrophotometrically measured 2 minutes after the start of the reaction at 405 nm wavelength using a calibrated plate reader. The amount of alpha amylase activity present in the sample is directly proportional to the increase in absorbance detected at 405 nm. Results are computed in units per milliliter (U/ml) of alpha amylase. High, medium and low salivary alpha amylase controls were included in each assay. The intra-assay variation computed for the mean of 20 replicate tests included in 10 separate assays was less than 6.5%. The inter-assay variation computed for the mean of average duplicates for 10 separate assay runs was less than 4.8%. The sensitivity is governed by the lower change in absorbance reading for each assay, which was 0.01 units. Samples below this value were reassayed using a dilution to achieve a higher concentration of the sample and thus, a greater absorbance reading. We report sAA in enzyme units per milliliter (U/ml).
2.2.3. Anthropometry
The child was weighed at the time of the diurnal assessment as well as at the time of the reactivity assessment. The primary caregiver was weighed at the time of the diurnal assessment. Individuals were weighed and measured without shoes or heavy clothing by trained staff using standard protocols on a +/− 0.1kg calibrated scale (Detecto Physician’s Scale Model DR550) and a +/− 0.1 cm calibrated stadiometer (Seca 217/213).
2.3. Statistical Analysis and Variable Creation
Data analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC). Outliers as described below were excluded from the analysis. A log transformation was implemented to capture the log-linear pattern of the diurnal sAA rhythm and ensure normality for the residuals.
2.3.1. sAA Variables
For both the diurnal and reactivity assessments, sAA values were excluded if the value was > 3 SD’s from the mean. For the diurnal assessment, sAA values were also excluded if they were > 2 SD’s from the mean and either did not fit the child’s diurnal pattern and/or there was an unusual circumstance (i.e., child was reported “getting sick”). Of a total of 2839 diurnal and reactivity samples, 76 (2.7%) were excluded for these reasons. To be included in the diurnal analysis, a child needed to have at least 5 valid sAA values over ≥ 2 days; the number of valid diurnal sAA values per child was mean 8.5 (SD 1.7). To be included in the reactivity analysis, a child needed to have all sAA measures from the stress elicitation from the 2nd sample (the calming baseline) to the 5th final sample (40 minutes post-stressor).
The diurnal sAA pattern was characterized quantitatively in the following manner to generate variables for use as predictors in our models. sAA follows a known diurnal pattern, such that sAA decreases initially after morning awakening, reaching a nadir within about 30 minutes and after that rises gradually over the course of the day (Nater et al., 2007). Thus, using the log transformed sAA values as the outcome and the time (since awakening) at which sAA sampling occurred as the independent variable, the diurnal sAA pattern would be linear on time in a log-scale (for time > 60 minutes). Such a linear trajectory can then be captured by two parameters, intercept and slope. We used hierarchical linear models (HLM) using random parameters to capture individual diurnal sAA curves for each participant. The HLM approach is a powerful modeling technique for estimating individual trajectories, provided that trajectories have a known parametric form (e.g. linear, log-linear, quadratic) (Hruschka, Kohrt, and Worthman, 2005). This approach is also powerful because it accounts for the time differential in the measurement of sAA in a direct way using the parametric function of the diurnal sAA pattern. The random intercept is an estimate of the expected sAA level at 60 minutes after awakening for a given individual, and the random slope is the expected rate of increase in sAA after 60 minutes post-awakening. Thus, both the random intercept and the random slope capture the diurnal sAA patterns of an individual.
The reactivity sAA pattern was characterized quantitatively in the following manner. Area under the curve increase (AUCI) was calculated using standard methods described elsewhere (Pruessner et al., 2003). AUCI reflected the child’s increase in sAA output during the stress elicitation from the 2nd sample (the calming baseline) to the 5th and final sample (40 minutes post-stressor) and is typically used in this manner as an indicator of the dynamics of the stress response (Pruessner et al., 2003). AUCI units were standardized for analyses to have a mean of 0 and standard deviation (SD) of 1.
The child taking a medication known or hypothesized to affect sAA, the child having experienced an unusual circumstance that day (i.e., an unusually good or bad day), or the child being ill (i.e., with a cold or flu-like illness), exact time of morning awakening and if it was the usual time, and the time the child last ate prior to the protocol were not associated with sAA diurnal intercept or slope or reactivity AUCI and therefore were not considered further in analyses.
2.3.2. Body Mass Index
BMI’s were calculated from measured weights and heights as weight in kilograms divided by height in meters, squared. Primary caregivers’ BMI’s were categorized as overweight (BMI ≥ 25) versus not. Because the normal distribution of children’s BMI’s differs based on age and sex, children’s BMI’s were converted to z-scores based on the US Centers for Disease Control (CDC) reference growth curves. Therefore, the child BMI outcome in all analyses is presented as BMI z-score (BMIz) such that a BMIz of 0.0 represents the 50th percentile, −1.0 represents one standard deviation unit below the reference population mean, and +1.0 represents one standard deviation above the reference population mean. Per CDC guidelines children were also categorized as obese (BMI ≥ 95th percentile for age and sex), overweight (BMI < 95th percentile and ≥ 85th percentile for age and sex) or non-overweight (BMI < 85th percentile for age and sex). We excluded from this analysis children with a BMI <5th percentile or ≥ 99th percentile as extreme obesity and underweight are associated with differential patterns of relevant stress physiology indicators (e.g., cortisol (Kumari et al., 2010)).
2.3.3. Analysis Plan
We conducted descriptive statistics to assess central tendency and examined correlations between diurnal and reactivity sAA. Diurnal sAA intercept and slope were highly intercorrelated (r = −.89) so we chose to run separate models to examine them as predictors of adiposity. We therefore performed three multiple linear regression analyses to examine the main effect of (1) diurnal sAA intercept, (2) diurnal sAA slope, and (3) reactivity AUCI in association with child BMIz, controlling for child age, child sex, child race/ethnicity (categorized for this analysis as non-Hispanic white vs. not), maternal weight status (overweight versus not), and household income-to-needs ratio (annual gross family income divided by the poverty threshold for a family of the same size). We also conducted three ordinal logistic regression analyses to examine each sAA variable in association with child weight status (obese, overweight, or non-overweight) as the outcome. We used an alpha level of 0.05 (two-tailed) to determine statistical significance.
3. Results
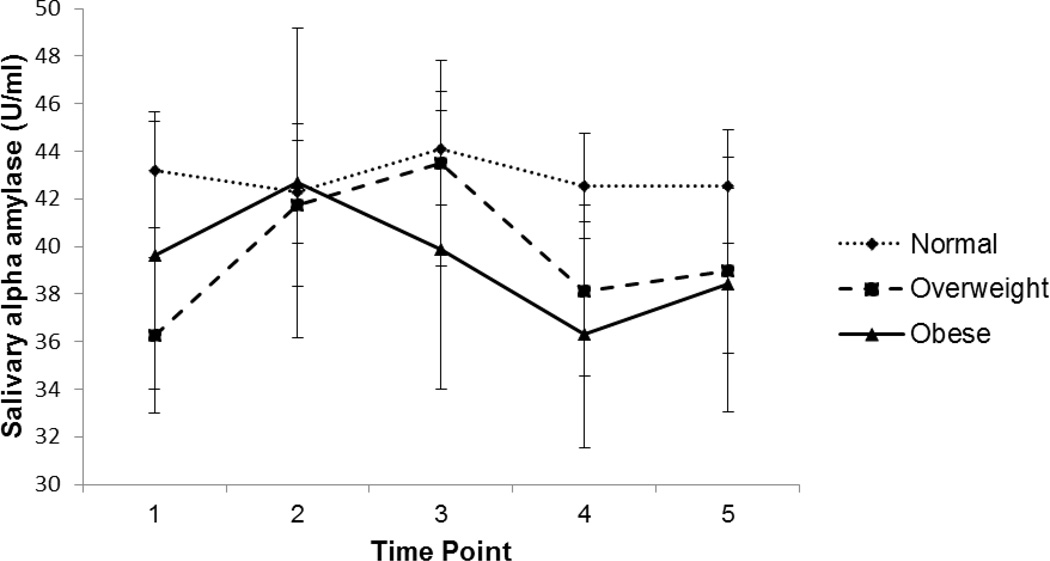
Mean diurnal sAA morning level (i.e., “intercept”) was 26.15 U/ml (SD = 1.90) and mean diurnal sAA slope was 0.06 (SD = 0.01), indicating that the general diurnal pattern was for children to show lower morning sAA levels that increased over the course of the day. Median reactivity sAA AUCI was 17.38 U/ml/min (range: −2407.20 to 1694.10), indicating a slight increase from baseline overall. Figure 1 illustrates sAA responses across the protocol (stratified by weight status).
Figure 1.
sAA reactivity among overweight, obese, and non-overweight children (raw sAA values; n=195)
Unadjusted linear regression models predicting child BMIz indicated that diurnal sAA intercept (β = −0.09 (SE 0.05), p < .09) was marginally associated with child BMIz. Diurnal sAA slope (β = 0.11 (SE 0.05), p < .04) and reactivity sAA AUCI (β = −0.13 (SE 0.05), p < .04) were significantly associated with child BMIz. Results for the linear regression models adjusted for covariates are presented in Table 2. Diurnal sAA intercept was independently associated with child BMIz (β = −0.11 (SE 0.05), p < .04, Table 2). Diurnal sAA slope was also associated with child BMIz (β = 0.12 (SE 0.05), p < .03, Table 2). In these diurnal models, being female was also associated with higher child BMIz and higher income-to-needs ratio was associated with marginally lower child BMIz (see Table 2). In the adjusted linear regression model for reactivity sAA, AUCI was independently associated with child BMIz (β = −0.14 (SE 0.06), p < .03, Table 2). No covariates were associated with child BMIz in the reactivity model. We repeated these analyses using two alternate approaches to calculate sAA reactivity: we computed maximum increase (from timepoint 2 calming baseline to the highest of the first two post-stress samples), and a modified AUCI based on data within 30 minutes of stressor (as opposed to all samples collected). The results were essentially unchanged (β = −0.15 (SE 0.06), p < .03 for maximum increase; β = −0.14 (SE 0.06), p < .03 for modified AUCI)).
Table 2.
Adjusted parameter estimates for models predicting child BMIz from diurnal and reactivity sAA
| Predictors | Beta (95% CI) |
|---|---|
| Diurnal sAA intercept | −0.11 (−0.21 – −0.01)* |
| Child age | 0.00 (−0.02 – 0.01)) |
| Child sex (female vs. male) | 0.24 (0.04 – 0.44)* |
| Child race/ethnicity (minority vs. white, non-Hispanic) | 0.07 (−0.13 – 0.27) |
| Mother overweight (overweight vs. not overweight) | 0.11 (−0.12 – 0.34) |
| Income-to-needs Ratio | −0.12 (−0.24 – 0.002)* |
| Diurnal sAA slope | 0.12 (0.02 – 0.21)* |
| Child age | 0.00 (−0.02 – 0.01) |
| Child sex (female vs. male) | 0.24 (0.04 – 0.44)* |
| Child race/ethnicity (minority vs. white, non-Hispanic) | 0.07 (−0.13 – 0.27) |
| Mother overweight (overweight vs. not overweight) | 0.12 (−0.11 – 0.35) |
| Income-to-needs Ratio | −0.11 (−0.23 – 0.01)† |
| Reactivity sAA AUCI | −0.14 (−0.27 – −0.02)* |
| Child age | −0.01 (−0.03 – 0.01) |
| Child sex (female vs. male) | 0.09 (−0.15 – 0.33) |
| Child race/ethnicity (minority vs. white, non-Hispanic) | −0.01 (−0.26 – 0.23) |
| Mother overweight (overweight vs. not overweight) | 0.20 (−0.10 – 0.49) |
| Income-to-needs Ratio | −0.05 (−0.19 – 0.08) |
Note.
p<.10.
p<.05.
Diurnal n = 269, reactivity n = 195.
In the unadjusted ordinal logistic regression models predicting child weight status, neither diurnal sAA intercept (β = −0.15 (SE 0.13), p =. 23) nor diurnal sAA slope (β = 0.17 (SE 0.13), p = .15) were significant predictors of child weight status. There was a trend for sAA reactivity AUCI to be associated with child weight status (β = −0.30 (SE 0.16), p = .06), where lower AUCI was marginally associated with having higher weight status. Figure 1 presents sAA reactivity patterns by weight status (12.8 % of the children in the sample were obese, 21.0% overweight, and 66.2% non-overweight). The adjusted ordinal logistic regression models predicting child weight status showed the same sAA reactivity pattern.
4. Discussion
We found that lower sAA morning level (the diurnal intercept) and more rapid increase in sAA over the course of the day (the diurnal slope) were each associated with a higher BMI z-score. Being female was associated with higher BMIz in these models. Lower sAA reactivity to a stressor was associated with a higher BMI z-score. These results provide support for our conceptual model that stress physiology reflective of chronic stress exposure is associated with greater adiposity among low-income, preschool aged children. Diurnal sAA patterns were not related to child weight status, and sAA reactivity was only marginally associated. The results suggest that the nature of the association between sAA and child weight was linear.
Our findings are consistent with the prior literature showing low SNS cardiac reactivity to a stressor among obese adults (Carroll et al., 2008; Jones et al., 2012). Results are also consistent with some studies of school-age children that showed reduced SNS activity associated with obesity (Nagai et al., 2003; Vanderlei et al., 2010; Baum et al., 2013), but inconsistent with other studies that have found increased SNS activity associated with obesity (Latchman et al., 2011; Rodríguez-Colón et al., 2011; Soares-Miranda et al., 2011; Tascilar et al., 2011; Altuncu et al., 2012). Importantly, most of these studies did not assess reactivity to a stressor, but rather resting, ambulatory, or overnight cardiac autonomic modulation, and were also conducted with older children than in the current report. The single study involving preschool-age children also found that obesity was associated with blunted SNS activity over time (Alkon et al., 2014).
Regarding sAA specifically, one prior study of adults found lower diurnal sAA output associated with higher BMI (Nater et al., 2007), whereas the other study found no association (Veen et al., 2012). The single study that examined sAA reactivity to a stressor and concurrent BMI in adults found a trend consistent with our findings (Thoma et al., 2012). Our study is the first to evaluate the association between either diurnal sAA or sAA reactivity to a stressor and adiposity in children. A low morning sAA value, more rapid increase across the day, and a blunted response to stress may be important in early obesity risk.
There are several potential mechanisms for observed associations. As others with similar findings of blunted stress responses across both the hypothalamic-pituitary-adrenal axis and sympathetic nervous systems have also speculated (Lovallo, 2011; Jones et al., 2012; Pervanidou and Chrousos, 2012), we hypothesize that attenuated stress reactivity may reflect a failing stress response as a consequence of down-regulation of stress receptors responsible for stress activation following chronic stress exposure. Growing up in poverty can expose children to chronic stressors that can affect biological stress responses (Evans and Kim, 2007; Hill-Soderlund et al., 2014), which may be the case for some children in our sample. If the stress response system becomes blunted due to repeated stimulation, the system in question may be limited in its ability to cope adaptively with stress when needed. Thus, an individual with such a profile may engage in maladaptive or unhealthy strategies to manage stress (e.g., comfort food consumption, drug use, impulsive or risky behaviors), which may lead to poor health outcomes, including obesity (Lovallo, 2011). Our finding that lower morning sAA and more rapid increase across the day were associated with higher BMIz could also be cautiously interpreted as consistent with this perspective. The few extant studies of diurnal sAA and stress in children (Wolf et al., 2008; Hill-Soderlund et al., 2014) suggest that lower sAA levels are associated with chronic stress. Careful longitudinal and additional mechanistic work is critical in order to test this hypothesis further and to disentangle these associations.
Given that prior work has linked higher sAA (Harthoorn and Dransfield, 2008) and SNS activity (Bray, 1991) with satiety, it is possible that the pathway may also operate directly through lower sAA being associated with a greater sense of hunger and lesser sense of satiety, ultimately leading to excessive consumption (perhaps particularly during times of stress) and weight gain. sAA measured after a meal was positively associated with ratings of satiety, and inversely with hunger (Harthoorn and Dransfield, 2008). In addition, lower SNS activity is associated with lower resting metabolic rate (Tentolouris et al., 2006), which could also contribute to additional weight gain. It is also possible that food intake and SNS activity are associated only because they are regulated by anatomically related brain regions (Tataranni et al., 1997).
Our study has several strengths. Our sample was young, racially/ethnically diverse, high-risk, and had relatively high levels of adiposity compared to population references. We also studied both diurnal pattern and reactivity to a stressor. One limitation of our study is that we did not have measures of cardiac physiology, and that sAA may not be a precise measure of SNS activity. Few studies have explored relationships between sAA and cardiac physiology in children and this is an important area for future work, particularly given debate about whether sAA represents activation of both sympathetic and parasympathetic systems (Bosch et al., 2011). Our sample was also limited to low-income preschool-aged children who had been enrolled in Head Start, which limits generalizability to other developmental periods and other socioeconomic circumstances. Given that chronic stressors, including poverty, have been associated with low diurnal sAA levels (Wolf, 2008; Rohleder et al., 2009; Hill-Soderlund et al., 2014), it is important to investigate sAA-BMI associations in less-stressed populations. Children in our study did not show a strong response to the stressor as is typical of young children this age (Gunnar et al., 2009), and we only obtained measures of diurnal sAA and sAA reactivity to a stressor on single occasions, which limits our ability to draw conclusions about individual variability in response to different stressors, stability of associations with adiposity over time, or bidirectional associations. Longitudinal studies are needed to better understand the evolution of these associations and possible bidirectional relationships. The possibility that obesity itself inhibits the SNS and sAA response to stress requires investigation, as the implications of SNS “hypo-responsivity” to a stressor for health and wellbeing, particularly for very young children, are unknown. Finally, BMI is limited as a measure of adiposity. Although our field-based data collection protocols precluded us from gathering additional measures (e.g., body fat percentage), incorporating such indicators in future work will be important in order to specify the mechanisms of association between SNS activity and adiposity.
Our findings provide further impetus to examine associations of sAA and child weight more systematically at earlier ages, as well as in low-income populations such as ours, who may experience more early life stress than their peers (Evans and Kim, 2007). It is possible that for some populations, chronic stress influences obesity risk very early in the lifespan and it is vital to understand how biological stress responses may contribute.
Highlights.
Salivary alpha-amylase predicted body mass index z-score in young children.
Diurnal and stress-responsive salivary alpha-amylase patterns were measured.
Lower morning alpha-amylase and faster daily increase predicted greater adiposity.
Blunted response to a behavioral stressor was associated with greater adiposity.
Acknowledgements
Funding for this research was provided by the National Institutes of Health, grant numbers NIH 1RC1DK086376, NIH 1R21DK090718 and the American Heart Association Midwest Affiliate Grant-in-Aid grant number 10GRNT4460043
These sponsors of the research had no involvement in the study design, collection, analysis, or interpretation of the data, or the writing of the report or decision to submit the manuscript for publication. The Corresponding Author had full access to all of the data in the study and had the final responsibility for the decision to submit for publication.
Abbreviations
- BMI
body mass index
- AUCI
area under the curve increase
- sAA
salivary alpha-amylase
- SNS
sympathetic nervous system
- SDU
standard deviation unit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest to disclose.
Conflict of Interest Statement:
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Contributions of Authors:
Alison L. Miller, PhD designed the study, obtained funding, guided analyses, interpreted results, drafted and edited the manuscript, and approved the manuscript as written.
Julie Sturza, MPH conducted analyses, edited the manuscript, and approved the manuscript as written.
Katherine Rosenblum, PhD interpreted results, edited the manuscript, and approved the manuscript as written.
Delia M. Vazquez, MD helped design the study, interpreted results, edited the manuscript, and approved the manuscript as written.
Niko Kaciroti, PhD helped design the study, guided analyses, interpreted results, edited the manuscript, and approved the manuscript as written.
Julie C. Lumeng, MD designed the study, obtained funding, guided analyses, interpreted results, edited the manuscript, and approved the manuscript as written.
REFERENCES
- Alkon A, Harley KG, Neilands TB, Tambellini K, Lustig RH, Boyce WT, Eskenazi B. Latino children's body mass index at 2–3.5 years predicts sympathetic nervous system activity at 5 years. Child Obes. 2014;10(3):214–224. doi: 10.1089/chi.2013.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altuncu ME, Baspinar O, Keskin M. The use of short-term analysis of heart rate variability to assess autonomic function in obese children and its relationship with metabolic syndrome. Cardiol J. 2012;19(5):501–506. doi: 10.5603/cj.2012.0091. [DOI] [PubMed] [Google Scholar]
- Astrup A, Buemann B, Gluud C, Bennett P, Tjur T, Christensen N. Prognostic markers for diet-induced weight loss in obese women. Int J Obes Relat Metab Disord. 1995;19:275–278. [PubMed] [Google Scholar]
- Baum P, Petroff D, Classen J, Kiess W, Bluher S. Dysfunction of autonomic nervous system in childhood obesity: a cross-sectional study. PLoS ONE. 2013;8(1):24. doi: 10.1371/journal.pone.0054546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch JA, Veerman ECI, de Geus EJ, Proctor GB. α-Amylase as a reliable and convenient measure of sympathetic activity: don’t start salivating just yet! Psychoneuroendocrinology. 2011;36(4):449–453. doi: 10.1016/j.psyneuen.2010.12.019. doi: http://dx.doi.org/10.1016/j.psyneuen.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Bray GA. Reciprocal relation between the sympathetic nervous system and food intake. Brain Research Bulletin. 1991;27(3–4):517–520. doi: 10.1016/0361-9230(91)90152-a. doi: http://dx.doi.org/10.1016/0361-9230(91)90152-A. [DOI] [PubMed] [Google Scholar]
- Carroll D, Phillips AC, Der G. Body mass index, abdominal adiposity, obesity, and cardiovascular reactions to psychological stress in a large community sample. Psychosomatic Medicine. 2008;70(6):653–660. doi: 10.1097/PSY.0b013e31817b9382. [DOI] [PubMed] [Google Scholar]
- Cole PM. Children's spontaneous control of facial expression. Child Development. 1986:1309. [Google Scholar]
- de Vries-Bouw M, Jansen L, Vermeiren R, Doreleijers T, Van de Ven P, Popma A. Concurrent attenuated reactivity of alpha-amylase and cortisol is related to disruptive behavior in male adolescents. Hormones and Behavior. 2012;62(1):77–85. doi: 10.1016/j.yhbeh.2012.05.002. doi: http://dx.doi.org/10.1016/j.yhbeh.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Ehlert U, Nater UM. Associations between salivary alpha-amylase and catecholamines: A multilevel modeling approach. Biological Psychology. 2014;103:15–18. doi: 10.1016/j.biopsycho.2014.08.001. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Mize J. Cortisol and children's adjustment: the moderating role of sympathetic nervous system activity. J Abnorm Child Psychol. 2008;36(4):601–611. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and health: cumulative risk exposure and stress dysregulation. Psychol Sci. 2007;18(11):953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Goldsmith H, Rothbart M. The Laboratory Temperament Assessment Battery (LabTAB): Locomotor, Version 3 (Technical Manual) Madison, WI: Department of Psychology, University of Wisconsin; 1996. [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary alphaamylase in biobehavioral research: recent developments and applications. Ann NY Acad Sci. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34(7):953–967. doi: 10.1016/j.psyneuen.2009.02.010. doi: http://dx.doi.org/10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13(03):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Harthoorn LF, Dransfield E. Periprandial changes of the sympathetic-parasympathetic balance related to perceived satiety in humans. Eur J Appl Physiol. 2008;102:601–608. doi: 10.1007/s00421-007-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Soderlund AL, Holochwost SJ, Willoughby MT, Granger DA, Gariépy J-L, Mills-Koonce WR, Cox MJ. Developmental course of salivary alpha-amylase and cortisol from 12 to 36 months: Relations with early poverty and later behavior problems. Psychoneuroendocrinology. 2014 doi: 10.1016/j.psyneuen.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, Worthman CM. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30(7):698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Jones A, McMillan MR, Jones RW, Kowalik GT, Steeden JA, Deanfield JE, Preussner JC, Taylor AM, Muthurangu V. Adiposity is associated with blunted cardiovascular, neuroendocrine and cognitive responses to acute mental stress. PLoS ONE. 2012;7(6):e39143. doi: 10.1371/journal.pone.0039143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbro RT, Brooks-Gunn J, McLanahan S. Racial and ethnic differentials in overweight and obesity among 3-year-old children. American Journal of Public Health. 2007;97(2):298–305. doi: 10.2105/AJPH.2005.080812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler U, von Känel R, Heimgartner N, Zuccarella-Hackl C, Stirnimann G, Ehlert U, Wirtz PH. Norepinephrine infusion with and without alpha-adrenergic blockade by phentolamine increases salivary alpha amylase in healthy men. Psychoneuroendocrinology. 2014;49:290–298. doi: 10.1016/j.psyneuen.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Kumari M, Chandola T, Brunner E, Kivimaki M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2010;95(9):4415–4423. doi: 10.1210/jc.2009-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman PL, Mathur M, Bartels MN, Axtell RS, De Meersman RE. Impaired autonomic function in normotensive obese children. Clin Auton Res. 2011;21(5):319–323. doi: 10.1007/s10286-011-0116-8. [DOI] [PubMed] [Google Scholar]
- Leung CY, Lumeng JC, Kaciroti NA, Chen YP, Rosenblum K, Miller AL. Surgency and negative affectivity, but not effortful control, are uniquely associated with obesogenic eating behaviors among low-income preschoolers. Appetite. 2014;78:139–146. doi: 10.1016/j.appet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisonbee JA, Pendry P, Mize J, Gwynn EP. Hypothalamic–pituitary–adrenal and sympathetic nervous system activity and children's behavioral regulation. Mind, Brain, and Education. 2010;4(4):171–181. [Google Scholar]
- Lovallo WR. Do low levels of stress reactivity signal poor states of health? Biological Psychology. 2011;86(2):121–128. doi: 10.1016/j.biopsycho.2010.01.006. doi: http://dx.doi.org/10.1016/j.biopsycho.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng JC, Gannon K, Cabral HJ, Frank DA, Zuckerman B. Association between clinically meaningful behavior problems and overweight in children. Pediatrics. 2003;112(5):1138–1145. doi: 10.1542/peds.112.5.1138. [DOI] [PubMed] [Google Scholar]
- Lumeng JC, Wendorf K, Pesch MH, Appugliese DP, Kaciroti N, Corwyn RF, Bradley RH. Overweight adolescents and life events in childhood. Pediatrics. 2013;132(6):2013–1111. doi: 10.1542/peds.2013-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe L, Brooks-Gunn J. Unpublished manual for live gift wrap protocol. 2002 [Google Scholar]
- Miller AL, Clifford C, Sturza J, Rosenblum K, Vazquez DM, Kaciroti N, Lumeng JC. Blunted cortisol response to stress is associated with higher body mass index in low-income preschool-aged children. Psychoneuroendocrinology. 2013;38(11):2611–2617. doi: 10.1016/j.psyneuen.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai N, Matsumoto T, Kita H, Moritani T. Autonomic nervous system activity and the state and development of obesity in Japanese school children. Obes Res. 2003;11(1):25–32. doi: 10.1038/oby.2003.6. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, Ehlert U. Stress-induced changes in human salivary alpha-amylase activity - associations with adrenergic activity. Psychoneuroendocrinology. 2006;31:49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32(4):392–401. doi: 10.1016/j.psyneuen.2007.02.007. doi: http://dx.doi.org/10.1016/j.psyneuen.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Pervanidou P, Chrousos GP. Metabolic consequences of stress during childhood and adolescence. Metabolism. 2012;61(5):611–619. doi: 10.1016/j.metabol.2011.10.005. doi: http://dx.doi.org/10.1016/j.metabol.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Pruessner J, Kirschbaum C, Meinlschmid G, Hellhammer D. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinol. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rabbia F, Silke B, Conterno A, Grosso T, De Vito B, Rabbone I, Chiandussi L, Veglio F. Assessment of cardiac autonomic modulation during adolescent obesity. Obes Res. 2003;11(4):541–548. doi: 10.1038/oby.2003.76. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Colón SM, Bixler EO, Li X, Vgontzas AN, Liao D. Obesity is associated with impaired cardiac autonomic modulation in children. International Journal of Pediatric Obesity. 2011;6(2):128–134. doi: 10.3109/17477166.2010.490265. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Marin TJ, Ma R, Miller GE. Biologic cost of caring for a cancer patient: Dysregulation of pro- and anti-inflammatory signaling pathways. J Clin Oncol. 2009;27(18):2909–2915. doi: 10.1200/JCO.2008.18.7435. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Nater UM, Wolf JM, Ehlert U, Kirschbaum C. Psychosocial stress-induced activation of salivary alpha-amylase: An indicator of sympathetic activity? Annals of the New York Academy of Sciences. 2004;1032(1):258–263. doi: 10.1196/annals.1314.033. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Wolf JM, Maldonado EF, Kirschbaum C. The psychosocial stress-induced increase in salivary alpha-amylase is independent of saliva flow rate. Psychophysiology. 2006;43(6):645–652. doi: 10.1111/j.1469-8986.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- Seeyave DM, Coleman S, Appugliese D, Corwyn RF, Bradley RH, Davidson NS, Kaciroti N, Lumeng JC. Ability to delay gratification at age 4 years and risk of overweight at age 11 years. Arch Pediatr Adolesc Med. 2009;163(4):303–308. doi: 10.1001/archpediatrics.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Miranda L, Alves AJ, Vale S, Aires L, Santos R, Oliveira J, Mota J. Central fat influences cardiac autonomic function in obese and overweight girls. Pediatr Cardiol. 2011;32(7):924–928. doi: 10.1007/s00246-011-0015-8. [DOI] [PubMed] [Google Scholar]
- Spinrad TL, Eisenberg N, Granger DA, Eggum ND, Sallquist J, Haugen RG, Kupfer A, Hofer C. Individual differences in preschoolers' salivary cortisol and alpha-amylase reactivity: Relations to temperament and maladjustment. Hormones and Behavior. 2009;56(1):133–139. doi: 10.1016/j.yhbeh.2009.03.020. doi: http://dx.doi.org/10.1016/j.yhbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Granger DA, Blades KT, Randazzo W, Heaton JA, Dorn LD. Cortisol and alpha amylase reactivity and timing of puberty: Vulnerabilities for antisocial behaviour in young adolescents. Psychoneuroendocrinology. 2010;35(4):557–569. doi: 10.1016/j.psyneuen.2009.09.004. doi: http://dx.doi.org/10.1016/j.psyneuen.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tascilar ME, Yokusoglu M, Boyraz M, Baysan O, Koz C, Dundaroz R. Cardiac autonomic functions in obese children. J Clin Res Pediatr Endocrinol. 2011;3(2):60–64. doi: 10.4274/jcrpe.v3i2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni PA, Young JB, Bogardus C, Ravussin E. A low sympathoadrenal activity is associated with body weight gain and development of central adiposity in Pima Indian men. Obes Res. 1997;5(4):341–347. doi: 10.1002/j.1550-8528.1997.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Tentolouris N, Liatis S, Katsilambros N. Sympathetic system activity in obesity and metabolic syndrome. Annals of the New York Academy of Sciences. 2006;1083(1):129–152. doi: 10.1196/annals.1367.010. [DOI] [PubMed] [Google Scholar]
- Thoma MV, Kirschbaum C, Wolf JM, Rohleder N. Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biological Psychology. 2012;91(3):342–348. doi: 10.1016/j.biopsycho.2012.07.008. doi: http://dx.doi.org/10.1016/j.biopsycho.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Vanderlei LC, Pastre CM, Junior IF, de Godoy MF. Fractal correlation of heart rate variability in obese children. Auton Neurosci. 2010;155(1–2):125–129. doi: 10.1016/j.autneu.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Veen G, Giltay EJ, Vreeburg SA, Licht CMM, Cobbaert CM, Zitman FG, Penninx BWJH. Determinants of salivary evening alpha-amylase in a large sample free of psychopathology. International Journal of Psychophysiology. 2012;84(1):33–38. doi: 10.1016/j.ijpsycho.2012.01.005. doi: http://dx.doi.org/10.1016/j.ijpsycho.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Nicholls E, Chen E. Chronic stress, salivary cortisol, and alpha-amylase in children with asthma and healthy children. Biological Psychology. 2008;78:20–28. doi: 10.1016/j.biopsycho.2007.12.004. [DOI] [PubMed] [Google Scholar]