Abstract
The control of sympathetic outflow in the chronic heart failure state (CHF) is markedly abnormal. Patients with heart failure present with increased plasma norepinephrine and increased sympathetic nerve activity. The mechanism for this sympatho-excitation are multiple and varied. Both depression in negative feedback sensory control mechanisms and augmentation of excitatory reflexes contribute to this sympatho-excitation. These include the arterial baroreflex, cardiac reflexes, arterial chemoreflexes and cardiac sympathetic afferent reflexes. In addition, abnormalities in central signaling in autonomic pathways have been implicated in the sympatho-excitatory process in CHF. These mechanisms include increases in central Angiotensin II and the Type 1 receptor, increased in reactive oxygen stress, up regulation in glutamate signaling and NR1 (N-methyl-D-aspartate subtype 1) receptors and others. Exercise training in the CHF state has been shown to reduce sympathetic outflow and result in increased survival and reduced cardiac events. Exercise training has been shown to reduce central Angiotensin II signaling including the Type 1 receptor and reduce oxidative stress by lowering the expression of many of the subunits of NADPH oxidase. In addition, there are profound effects on the central generation of nitric oxide and nitric oxide synthase in sympatho-regulatory areas of the brain. Recent studies have pointed to the balance between Angiotensin Converting Enzyme (ACE) and ACE2, translating into Angiotensin II and Angiotensin 1–7 as important regulators of sympathetic outflow. These enzymes appear to be normalized following exercise training in CHF. Understanding the precise molecular mechanisms by which exercise training is sympatho-inhibitory will uncover new targets for therapy.
Keywords: Sympathetic nerve activity, physical activity, angiotensin II, nitric oxide, oxidative stress
Introduction
The syndrome of chronic heart failure (CHF) impacts every organ system including skeletal muscle both at rest and during exercise (Drexler et al., 1987; Hambrecht et al., 2000; Just, 1991; Magnusson, 1995; Musch et al., 1989; Parmley, 1989; Riley et al., 1990). Further, exercise was initially thought to worsen left ventricular dysfunction in CHF patients (McDonald et al., 1972). One of the most frequent complaints of patients with even mild CHF is the inability to exercise. One would think the mechanism at the root cause of exercise intolerance in CHF is simply a lack of cardiac reserve and an inability to adjust cardiac output to workload. However, because CHF impacts sympathetic outflow, endothelial function, peripheral vascular resistance and skeletal muscle protein synthesis and metabolism, the mechanism of exercise intolerance is multifactorial in this disease state. While the standard of care for CHF in the mid-20th century was bed rest and diuretic and/or cardiac glycoside therapy it has become increasingly accepted that all but the most severe CHF patients can carry out some form of exercise (Downing et al., 2011; McKelvie, 2008). In fact, the American Heart Association has advocated exercise training as a safe form of therapy (Piña et al., 2003). Several clinical studies now show substantial benefits of exercise training in patients with CHF including quality of life, a reduction in hospitalization, cardiac events and survival (Belardinelli et al., 1999; Chicco et al., 2008; Piepoli et al., 2000). The HF-ACTION trial demonstrated a decrease in all-cause mortality and hospitalization in CHF patients who underwent a moderate exercise training regimen (aerobic exercise of either cycling or treadmill walking for 40 minutes at 60% to 70% of heart rate reserve, five times per week) (O’Connor et al., 2009). These benefits are not limited to a single type of exercise modality; resistance training, aerobic exercise, and even calisthenics as tolerated after a cardiac event are all considered to be effective (Piepoli et al., 2000). In fact, a reduction of physical activity in this same CHF patient population may be a contributor to future exercise intolerance and impaired peripheral vascular resistance (Hunt et al., 2005). While there is consensus that exercise training is beneficial in the CHF state, the underlying mechanisms responsible for these effects are not at all clear. Pre-clinical experimental studies have been extremely useful in shedding light on potential pathophysiological mechanisms. This review will focus primarily on the effects of exercise training on sympathetic and cardiovascular reflex function in CHF however, it should be kept in mind that changes in sympathetic outflow is just one mechanism responsible for the beneficial effects of exercise training in CHF.
Sympatho-excitation in CHF
The neural control of cardiovascular function relies on an ancient controller dominated by classical negative feedback servo control systems scattered throughout the cardiovascular system. Normally, just the right amount of sympathetic nerve activity is provided to maintain peripheral vascular resistance and arterial pressure at a set point necessary for adequate tissue perfusion. The sensors are primarily located in the great vessels (baroreceptors), the heart, and in the carotid and aortic bodies (chemoreceptors). A large number of studies, both basic and clinical, have shown marked abnormalities in the ability of these sensors to correctly transmit information concerning arterial pressure, blood volume and oxygen tension (Eckberg et al., 1971; Ellenbogen et al., 1989; La Rovere et al., 2009; Mohanty et al., 1989; Ponikowski et al., 1997; Zucker, 1991) in the setting of CHF. Early work suggested that depression in baroreflex gain mediated sympatho-excitation in patients and animals with CHF (Ferguson et al., 1984; Ferguson et al., 1992; Mancia et al., 1992). Reflexes mediated by sensory endings in the low pressure side of the circulation have also been shown to exhibit reduced gain and contribute to sympatho-excitation by removal of inhibitory restraint (Patel et al., 1996a; Pliquett et al., 2003; Zheng et al., 2006). Further studies also suggested that an increase in chemoreceptor sensitivity in CHF drives sympatho-excitation (Chua et al., 1996; Chua et al., 1997; Chugh et al., 1996; Ponikowski et al., 1997; Schultz et al., 2007; Sun et al., 1999a; Sun et al., 1999b). Finally, excitatory input from so called “cardiac sympathetic afferents” has also been shown to be augmented in the CHF state (Gao et al., 2007a; Gao et al., 2005a; Gao et al., 2004b; Wang et al., 2006; Zhu et al., 2004a; Zhu et al., 2004b; Zhu et al., 2002; Wang and Zucker, 1996). While there is little doubt that these reflexes contribute to sympatho-excitation in CHF the question still remains as to whether these abnormalities are initiating factors or a consequence of the CHF state?
In addition to dysfunction in cardiovascular sensory function there are many alterations in various components in the reflex arcs mediating autonomic outflow in CHF. Central changes in synaptic transmission and membrane sensitivity of pre-sympathetic neurons at several hypothalamic and medullary sites also participate in sympatho-excitation in CHF. Changes in discharge sensitivity of neurons in the rostral ventrolateral medulla (RVLM) and in the paraventricular nucleus (PVN) have been prominent in this regard (Gao et al., 2008; Patel et al., 2000). While it is beyond the scope of this review to detail all of the central changes that take place in CHF some of these changes will be highlighted below because exercise training profoundly influences them.
Does exercise training lower sympathetic outflow in heart failure?
Studies carried out on patients with CHF have shown a reduction in sympathetic outflow following a supervised exercise training regimen (stationary cycling 60 minutes 3 times per week), measured by either direct recording of muscle sympathetic nerve activity (Fraga et al., 2007; Roveda et al., 2003) or urinary norepinephrine excretion (Yousufuddin et al., 2000). Softer indices of sympatho-excitation such as heart rate variability and power spectral analysis have also pointed to a lowering of sympathetic outflow following exercise training in the CHF population (Coats et al., 1992; Colombo et al., 1999; Scalvini et al., 1998). These indices coincide with improvement in baroreflex and chemoreflex sensitivity in CHF (Gao et al., 2007b; Li et al., 2008; Liu et al., 2000; Liu et al., 2002; Negraõ et al., 2008a; Negraõ et al., 2008b). In a recent study by Rengo et al. (Rengo et al., 2014) it was shown that exercise training resulted in a decrease in heart rate, plasma norepinephrine, and brain natriuretic peptide (BNP) while increasing maximal oxygen consumption (MVO2) and ejection fraction slightly. Importantly, these data were prognostic as to outcomes. Those patients with the greatest change in norepinephrine and BNP exhibited significantly better survival profiles. These data support earlier work showing that mortality was reduced in CHF patients that underwent and exercise training program (Belardinelli et al., 1999; Hagerman et al., 2005; Keteyian et al., 2012; O'Connor et al., 2009; Rosenwinkel et al., 2001; Smart et al., 2004). On the other hand, a recent analysis of the HF ACTION database by Ahmad et al. (Ahmad et al., 2014) showed no effect on BNP or cardiac function but an improvement in hospitalizations and survival. In total however, it seems clear that exercise training does indeed impact sympathetic outflow and survival if not cardiac function per se.
What central mechanisms are responsible for sympatho-inhibition following exercise training in CHF?
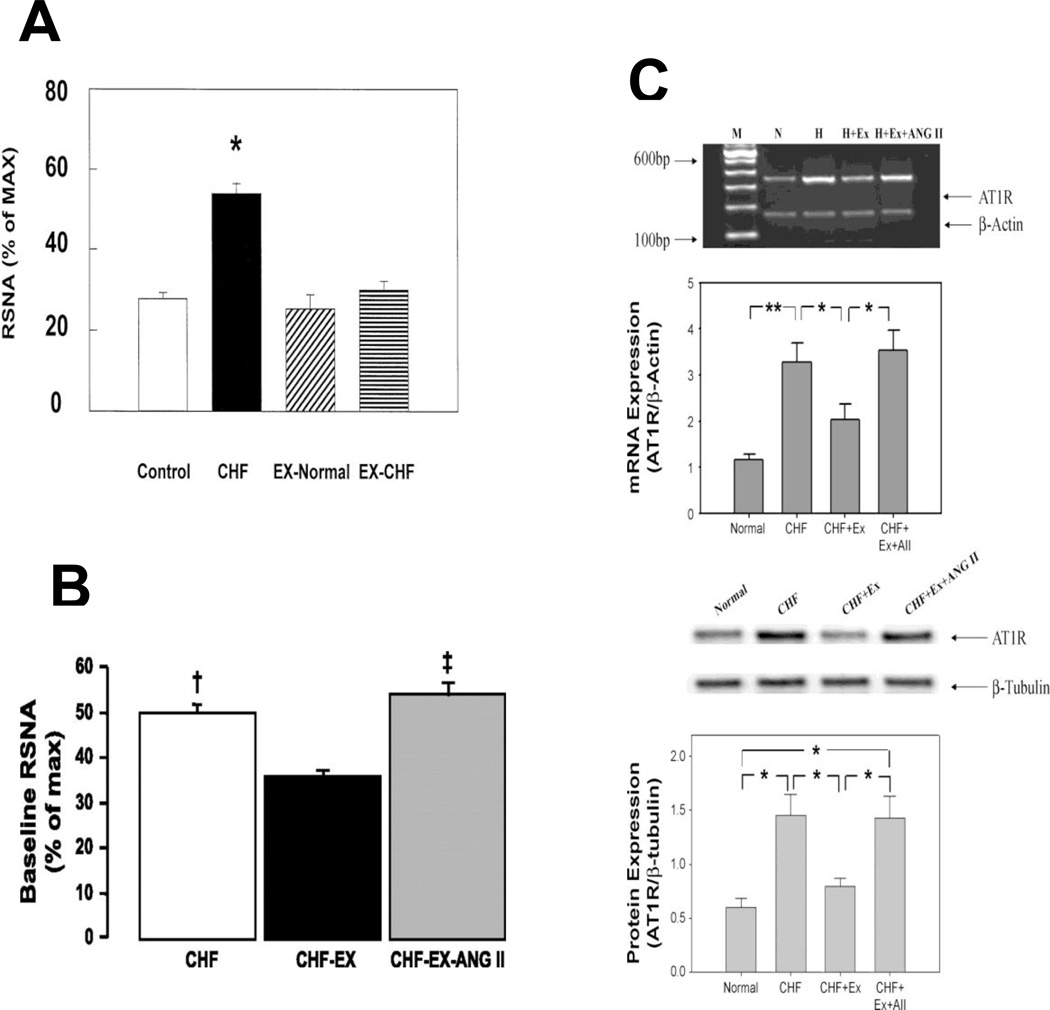
The discharge sensitivity of pre-sympathetic neurons in the RVLM and of sympathetic projecting neurons in the PVN is determined ultimately by activity in membrane ion channel proteins and currents. In the CHF state alterations in several neuronal signaling pathways have been defined that impact channel activity and may be impacted by exercise training. The focus of this work has largely been in three areas; 1. The renin-Angiotensin II (Ang II) system, 2. Reactive oxygen stress (ROS) and 3. Nitric oxide synthase (NOS). In addition, exercise training impacts glutamate signaling in CHF (Kleiber et al., 2008; Llewellyn et al., 2012). Sympatho-excitatory neurons in the RVLM and PVN express Angiotensin II Type 1 receptors (AT1R) (Gao et al., 2005b; Gao et al., 2008; Liu et al., 2000; Wang et al., 2004) that modulate sympathetic discharge when stimulated with Ang II (Gao et al., 2008a). Experiments in various species and models of CHF have shown that AT1R protein and mRNA is increased in CHF in these sympatho-excitatory regions (Gao et al., 2005b). Signaling through the AT1R increases neuronal excitability, in part, by increasing superoxide production thorough activation of NADPH oxidase. Following an exercise training regimen rabbits with CHF exhibit a profound reduction in renal sympathetic nerve activity at rest, and normalization of plasma Ang II (figure 1)(Liu et al., 2000). In addition, exercise trained CHF rabbits exhibited a decrease in AT1R expression in the RVLM (Gao et al., 2004a; Gao et al., 2005b), a decrease in central oxidative stress (Gao et al., 2007b) and an increase in both CuZn and Mn superoxide dismutase (SOD) (Gao et al., 2004a). Importantly, the changes in central AT1R expression, baseline sympathetic nerve activity and the improvement in baroreflex function could be prevented by concomitant systemic infusion of Ang II in order to prevent the normalization of Ang II by exercise training (figure 1)(Mousa et al., 2008). These data fit with the idea that Ang II, derived either from de novo synthesis in the brain or from circulating Ang II that gains access to the central nervous system through the circumventricular organs or in areas with a disrupted blood brain barrier (Biancardi et al., 2014), activates the AT1R pathway. Exercise training, by abrogating AT1R expression and upregulating antioxidant enzymes in the brain reduces this angiotensinergic drive and decreases sympathetic nerve activity (Liu, et al., 2000; Mousa, et al., 2008; Gao, et al. 2007b).
Figure 1.
Renal sympathetic nerve activity (A and B) and AT1 receptor expression (C) in the RVLM from rabbits with CHF that underwent an exercise training regimen or were sedentary. (A. from Liu, J.L. et al. 2000; B and C from Mousa, T.M. et al. 2008, with persmission.)
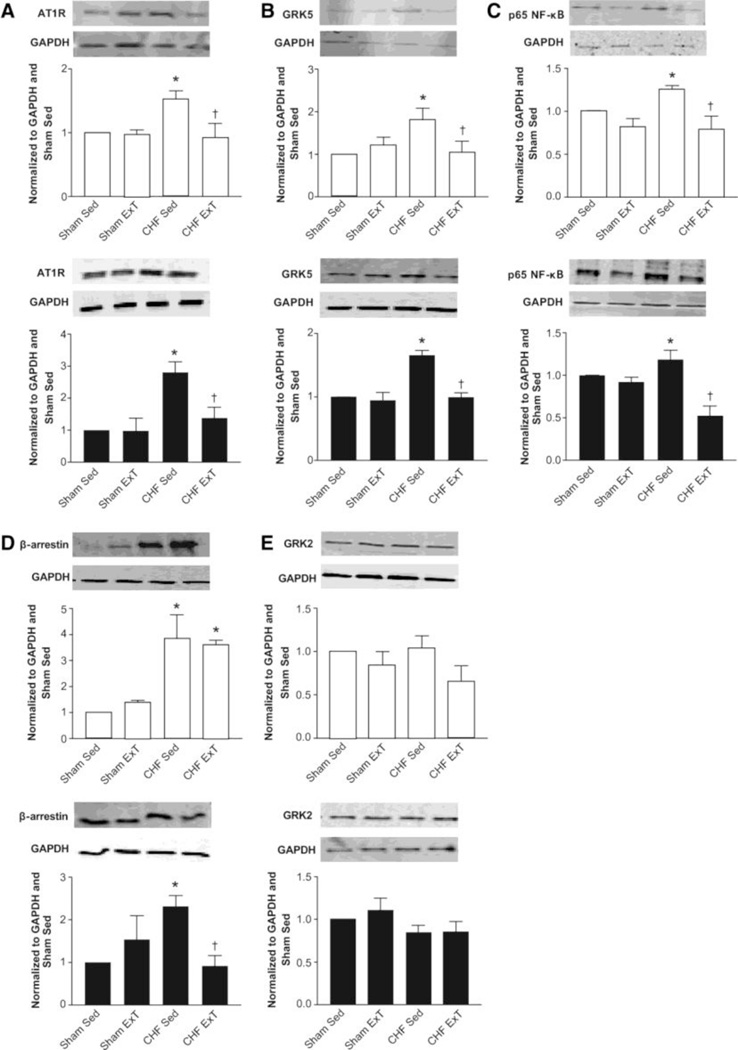
The regulation of AT1R expression in neurons in the heart failure state and following exercise training is complex but appears to involve an NFkB (nuclear factor kappa B) – dependent cascade at the DNA level. NFkB is a protein dimer that is a transcription factor for a number of other pro-inflammatory cytokines and stress response genes (Kumar et al., J Mol Med (2004) 82:434–448). It is not clear if exercise training alters this pathway but data from our laboratory indicates that NFkB is reduced following exercise training in animals with CHF (figure 2)(Haack et al., 2012). In addition, the regulation of AT1R turnover may be affected by changes in the G-protein coupled receptor, GRK5 (Haack et al., 2012). Importantly, exercise training in heart failure also reduces cytokine levels, a major source of NFkB activation (Conraads et al., 2002; Gielen et al., 2003; LeMaitre et al., 2004). A study by Nunes and others demonstrated that aerobic exercise in CHF rats decreased plasma levels of the inflammatory cytokines interleukin (IL)-6 and tumor necrosis factor alpha (TNF-α) (Nunes et al., 2013). The reduction in plasma IL-6 and TNF-α following exercise training was also seen in CHF patients (Smart and Steele, 2011).
Figure 2.
Western blot data from rostral ventrolateral medulla and paraventricular nucleus in rats with heart failure and following exercise training. Exercise training reduces AT1R, GRK5, NFkB and β-arrestin in heart failure. (from Haack, KK. et al., 2012; with permission)
Current evidence suggests that nitric oxide (NO) is an important sympatho-inhibitory molecule in the medulla and hypothalamus (Ma et al., 1999; Wang et al., 2013; Zheng et al., 2011a; Zhu et al., 2004c). It has been known for some time that NO is reduced in the medulla and hypothalamus of animals with CHF (Patel et al., 1996; Zhang et al., 2001; Zhang et al., 1998). This is due, in part, to a reduction in NO synthase (NOS) (mostly neuronal NOS, nNOS) protein and mRNA and, in part, to a reduction in the bioavailability of NO due to increased superoxide production (Campese et al., 2004; Chan et al., 2012; Zanzinger et al., 2000). Exercise training has clearly been shown to increase nNOS in the kidney (Ito et al., 2013), vasculature (Kuru et al., 2009; Mayhan et al., 2011), skeletal muscle (Kingwell, 2000), in the carotid body (Li et al., 2008) and in the brain (Zheng et al., 2005). The sympatho-inhibitory effects of NO in animals with CHF are increased following upregulation of nNOS in the PVN or after exercise training. Another potential mechanism by which upregulation of nNOS and formation of NO may be beneficial in the heart failure state is by inhibition of glutamate signaling. In a study by Zheng et al. (Zheng et al., 2011a)) adenoviral gene transfer of the PVN with nNOS in rats with CHF inhibited the sympatho-excitatory response to the neurotransmitter N-methyl D aspartate (NMDA) and reduced NMDA receptor (NR1) expression. Furthermore, Kleiber et al. showed that exercise training in the CHF state reduced the response to NMDA in the PVN (Kleiber et al., 2008).
Another potential mechanism that can influence the sympatho-inhibitory action of NO is the balance between Angiotensin Converting Enzyme (ACE) and Angiotensin Converting Enzyme 2 (ACE2) in the central nervous system of animals with CHF. These two enzymes regulate the amount of pro-AT1R (Ang II and superoxide production) vs pro-mas receptor signaling (Ang 1–7 and NO production). In rabbits with pacing – induced CHF we showed increased ACE protein and decreased ACE2 protein in the rostral ventrolateral medulla (RVLM) and PVN (Kar et al., 2010). Importantly, those CHF rabbits that underwent an exercise training regimen exhibited normal levels of both proteins. Zheng et al. (Zheng et al., 2011b) showed that adenoviral overexpression of ACE2 in the PVN of rats with CHF increased NOS synthesis and reversed the abnormal hemodynamic and sympathetic responses to PVN L-NMMA (L-N-monomethyl arginine, a nonselective inhibitor of all NOS isoforms) microinjection, thereby mimicking the effects of exercise training. Additional data supporting an important role of Ang 1–7/Ang II balance in the CHF state comes from the use of transgenic mice that overexpress human ACE2 selectively in central neurons (Feng et al., 2009). When these mice develop CHF (infarction model) they exhibit lower renal sympathetic nerve activity and improved baroreflex function (Xiao et al., 2011). A similar effect is observed in rabbits with CHF in response if intracerebroventricular infusion of Ang 1–7 (Kar et al., 2011). Future studies are needed to examine the relative abundance of these pathways following exercise training in CHF patients.
Summary
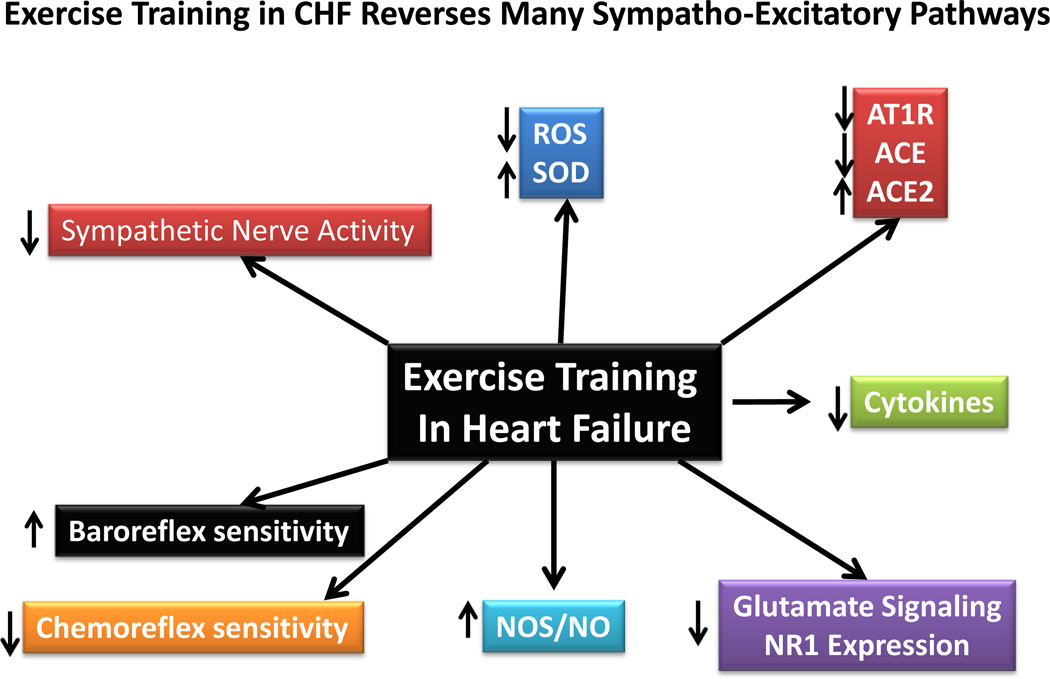
Taken together, there is a wealth of information strongly suggesting that the benefits of exercise training are multiple in the CHF state. Every organ system is positively impacted by exercise training as is negatively impacted by heart failure. Those stimuli that have been shown to increase the discharge sensitivity of pre-sympathetic neurons in CHF are significantly ameliorated following an exercise training regimen. These include Ang II, ROS, glutamate, NOS/NO, ACE and ACE2 and antioxidant enzymes such as SOD. There are many other substances that have not been discussed in this short review (e.g. endothelin-1, vasopressin, etc.), all of which have been, or are targets for therapy in the CHF state. Figure 3 provides a summary of the beneficial effects of exercise training on sympathetic outflow and the neurohumoral mediators affected. Clearly the mechanisms by which exercise training operates to reduce sympatho-excitation in diseases such as heart failure and hypertension is complex and further research will be necessary to understand exactly how this paradigm translated to normalization of pre-sympathetic neuronal function.
Figure 3.
A schematic overview of some of the factors impacted by exercise training in the heart failure state. Arrows denote the direction of the changes.
Highlights.
Although initially considered deleterious, exercise training in chronic heart failure (CHF) patients improves quality of life outcomes and decreases all-cause mortality.
A hallmark of chronic heart failure is an increase in sympathetic drive; this is markedly reduced in both animal models and patients with CHF. In addition, exercise training restores baroreflex sensitivity and decreases chemoreflex sensitivity in CHF.
Potential mechanisms underlying this improvement in autonomic imbalance following exercise training include: a reduction in reactive oxygen species and a concomitant increase in nitric oxide signaling, a reduction in Angiotensin II type 1 receptor signaling and a restoration of the imbalance of Angiotensin converting enzyme (ACE) and ACE2 expression, a decrease in circulating pro-inflammatory cytokines like NFkB, TNF-α, and IL-6, and a decrease in N-methyl D-aspartate (NMDA) receptor expression and signaling.
Acknowledgements
Some of the work shown in this review was supported by a grant from the National Institutes of Health, Heart, Lung and Blood Institute (PO1 HL62222). Karla Haack was supported by a post-doctoral fellowship (F32 HL116172).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad T, Fiuzat M, Mark DB, Neely B, Neely M, Kraus WE, Kitzman DW, Whellan DJ, Donahue M, Zannad F, Pina IL, Adams K, O'Connor CM, Felker GM. The effects of exercise on cardiovascular biomarkers in patients with chronic heart failure. American heart journal. 2014;167:193 e191–202 e191. doi: 10.1016/j.ahj.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure - Effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension. 2014;63:572–579. doi: 10.1161/HYPERTENSIONAHA.113.01743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol. 2004;287:H695–H703. doi: 10.1152/ajpheart.00619.2003. [DOI] [PubMed] [Google Scholar]
- Chan SH, Chan JY. Brain stem oxidative stress and its associated signaling in the regulation of sympathetic vasomotor tone. J. Appl. Physiol. 2012;113:1921–1928. doi: 10.1152/japplphysiol.00610.2012. [DOI] [PubMed] [Google Scholar]
- Chicco AJ, McCune SA, Emter CA, Sparagna GC, Rees ML, Bolden DA, Marshall KD, Murphy RC, Moore RL. Low-intensity exercise training delays heart failure and improves survival in female hypertensive heart failure rats. Hypertension. 2008;51:1096–1102. doi: 10.1161/HYPERTENSIONAHA.107.107078. [DOI] [PubMed] [Google Scholar]
- Chua TP, Clark AL, Amadi AA, Coats AJS. Relation between chemosensitivity and the ventilatory response to exercise in chronic heart failure. Journal for the American College of Cardiology. 1996;27:650–657. doi: 10.1016/0735-1097(95)00523-4. [DOI] [PubMed] [Google Scholar]
- Chua TP, Coats AJS. The role of peripheral chemoreflex in chronic congestive heart failure. Congestive Heart Failure. 1997;3:22–28. [Google Scholar]
- Chugh SS, Chua TP, Coats AJS. Peripheral chemoreflex in chronic heart failure: friend and foe. American Heart Journal. 1996;132:900–904. doi: 10.1016/s0002-8703(96)90333-6. [DOI] [PubMed] [Google Scholar]
- Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, Solda PL, Davey P, Ormerod O, Forfar C. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85:2119–2131. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- Colombo R, Mazzuero G, Spinatonda G, Lanfranchi P, Giannuzzi P, Ponikowski P, Coats AJS, Minuco G. Comparison between spectral analysis and the phenylephrine method for the assessment of baroreflex sensitivity in chronic heart failure. Clic. Sci. 1999;97:503–507. [PubMed] [Google Scholar]
- Conraads VM, Beckers P, Bosmans J, De Clerck LS, Stevens WJ, Vrints CJ, Brutsaert DL. Combined endurance/resistance training reduces plasma TNF-alpha receptor levels in patients with chronic heart failure and coronary artery disease. Eur Heart J. 2002;23:1854–1860. doi: 10.1053/euhj.2002.3239. [DOI] [PubMed] [Google Scholar]
- Downing J, Balady GJ. The role of exercise training in heart failure. Journal of the American College of Cardiology. 2011;58:561–569. doi: 10.1016/j.jacc.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Drexler H, Faude F, Hoing S, Just H. Blood flow distribution within skeletal muscle during exercise in the presence of chronic heart failure:effect of milrinone. Circulation. 1987;76(6):1344–1352. doi: 10.1161/01.cir.76.6.1344. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. New Engl. J. Med. 1971;285:877–883. doi: 10.1056/NEJM197110142851602. [DOI] [PubMed] [Google Scholar]
- Ellenbogen KA, Mohanty PK, Szentpetery S, Thames MD. Arterial baroreflex abnormalities in heart failure. Reversal after orthotopic cardiac transplantation. Circulation. 1989;79:51–58. doi: 10.1161/01.cir.79.1.51. [DOI] [PubMed] [Google Scholar]
- Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, Speth RC, Sigmund CD, Lazartigues E. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106:373–382. doi: 10.1161/CIRCRESAHA.109.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DW, Abboud FM, Mark AL. Selective impariment of baroreflex mediated vasoconstrictor responses in patients with ventricular dysfunction. Circulation. 1984;69:451–460. doi: 10.1161/01.cir.69.3.451. [DOI] [PubMed] [Google Scholar]
- Ferguson DW, Berg WJ, Roach PJ, Oren RM, Mark AL, Kempf JS. Effects of heart failure on baroreflex control of sympathetic neural activity. American Journal of Cardiology. 1992;69:523–531. doi: 10.1016/0002-9149(92)90998-e. [DOI] [PubMed] [Google Scholar]
- Fraga R, Franco FG, Roveda F, de Matos LN, Braga AM, Rondon MU, Rotta DR, Brum PC, Barretto AC, Middlekauff HR, Negrao CE. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail. 2007;9:630–636. doi: 10.1016/j.ejheart.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gao L, Pan YX, Wang WZ, Li YL, Schultz HD, Zucker IH, Wang W. Cardiac sympathetic afferent stimulation augments the arterial chemoreceptor reflex in anesthetized rats. J. Appl. Physiol. 2007a;102:37–43. doi: 10.1152/japplphysiol.00681.2006. [DOI] [PubMed] [Google Scholar]
- Gao L, Schultz HD, Patel KP, Zucker IH, Wang W. Augmented input from cardiac sympathetic afferents inhibits baroreflex in rats with heart failure. Hypertension. 2005a;45:1173–1181. doi: 10.1161/01.HYP.0000168056.66981.c2. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res. 2004a;95:937–944. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Li YL, Schultz HD, Liu DM, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: Roles for AT 1 receptor upregulation and NAD(P)H oxidase in RVLM. American Journal of Physiology: Heart and Circulatory Physiology. 2005b;288:H2271–H2279. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation. 2007b;115:3095–3102. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin receptor expression and function in the RVLM: Potential mechanism for sympathetic overactivity in heart failure. Hypertension. 2008;52:708–714. doi: 10.1161/HYPERTENSIONAHA.108.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Zhu Z, Zucker IH, Wang W. Cardiac sympathetic afferent stimulation impairs baroreflex control of renal sympathetic nerve activity in rats. American Journal of Physiology: Heart and Circulatory Physiology. 2004b;286:H1706–H1711. doi: 10.1152/ajpheart.01097.2003. [DOI] [PubMed] [Google Scholar]
- Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll. Cardiol. 2003;42:861–868. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- Haack KK, Engler CW, Papoutsi E, Pipinos II, Patel KP, Zucker IH. Parallel changes in neuronal AT1R and GRK5 expression following exercise training in heart failure. Hypertension. 2012;60:354–361. doi: 10.1161/HYPERTENSIONAHA.112.195693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman I, Tyni-Lenn R, Gordon A. Outcome of exercise training on the long-term burden of hospitalisation in patients with chronic heart failure. A restrospective study. International Journal of Cardiology. 2005;98:487–491. doi: 10.1016/j.ijcard.2003.10.063. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, Schoene N, Schuler G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure. JAMA. 2000;283:3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- Ito D, Ito O, Mori N, Cao P, Suda C, Muroya Y, Hao K, Shimokawa H, Kohzuki M. Exercise training upregulates nitric oxide synthases in the kidney of rats with chronic heart failure. Clin Exp Pharmacol Physiol. 2013;40:617–625. doi: 10.1111/1440-1681.12130. [DOI] [PubMed] [Google Scholar]
- Just H. Peripheral adaptations in congestive heart failure: A review. American Journal of Medicine. 1991;90(Suppl. 5B):5B23S–25B26S. doi: 10.1016/0002-9343(91)90269-4. [DOI] [PubMed] [Google Scholar]
- Kar S, Gao L, Belatti DA, Curry PL, Zucker IH. Central Angiotensin (1–7) enhances baroreflex gain in conscious rabbits with heart failure. Hypertension. 2011;58:627–634. doi: 10.1161/HYPERTENSIONAHA.111.177600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar S, Gao L, Zucker IH. Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J. Appl. Physiol. 2010;108:923–932. doi: 10.1152/japplphysiol.00840.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keteyian SJ, Leifer ES, Houston-Miller N, Kraus WE, Brawner CA, O'Connor CM, Whellan DJ, Cooper LS, Fleg JL, Kitzman DW, Cohen-Solal A, Blumenthal JA, Rendall DS, Pina IL. Relation between volume of exercise and clinical outcomes in patients with heart failure. Journal of the American College of Cardiology. 2012;60:1899–1905. doi: 10.1016/j.jacc.2012.08.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingwell BA. Nitric oxide-mediated metabolic regulation during exercise: effects of training in health and cardiovascular disease. FASEB J. 2000;14:1685–1696. doi: 10.1096/fj.99-0896rev. [DOI] [PubMed] [Google Scholar]
- Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am. J. Physiol Regul. Integr. Comp Physiol. 2008;294:R1863–R1872. doi: 10.1152/ajpregu.00757.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Takada, Y.,Boriek AM, Aggarwal BB. Nuclear factor-kB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- Kuru O, Senturk UK, Kocer G, Ozdem S, Baskurt OK, Cetin A, Yesilkaya A, Gunduz F. Effect of exercise training on resistance arteries in rats with chronic NOS inhibition. J. Appl. Physiol. 2009;107:896–902. doi: 10.1152/japplphysiol.91180.2008. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Maestri R, Robbi E, Caporotondi A, Guazzotti G, Sleight P, Febo O. Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. Journal of the American College of Cardiology. 2009;53:193–199. doi: 10.1016/j.jacc.2008.09.034. [DOI] [PubMed] [Google Scholar]
- LeMaitre JP, Harris S, Fox KA, Denvir M. Change in circulating cytokines after 2 forms of exercise training in chronic stable heart failure. Am Heart J. 2004;147:100–105. doi: 10.1016/j.ahj.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Li YL, Ding Y, Agnew C, Schultz HD. Exercise training improves peripheral chemoreflex function in heart failure rabbits. J Appl Physiol. 2008;105:782–790. doi: 10.1152/japplphysiol.90533.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: A role for angiotensin II. Circulation. 2000;102:1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- Liu JL, Kulakofsky J, Zucker IH. Exercise training enhances baroreflex control of heart rate by a vagal mechanism in rabbits with heart failure. J. Appl. Physiol. 2002;92:2403–2408. doi: 10.1152/japplphysiol.00039.2002. [DOI] [PubMed] [Google Scholar]
- Llewellyn T, Zheng H, Liu X, Xu B, Patel KP. Median preoptic nucleus and subfornical organ drive renal sympathetic nerve activity via a glutamatergic mechanism within the paraventricular nucleus. American journal of physiology. Regulatory, integrative and comparative physiology. 2012;302:R424–R432. doi: 10.1152/ajpregu.00403.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Zucker IH, Wang W. Reduced NO enhances the central gain of cardiac sympathetic afferent reflex in dogs with heart failure. Am. J. Physiol. 1999;276:H19–H26. doi: 10.1152/ajpheart.1999.276.1.H19. [DOI] [PubMed] [Google Scholar]
- Magnusson G. Factors Limiting Exercise Capacity in Heart Failure: Effects of Local Muscle Training Karolinska Institute, Stockholm, Sweden. 1995:1–44. [Google Scholar]
- Mancia G, Seravalle G, Giannattasio C, Bossi M, Preti L, Cattaneo BM, Grassi G. Reflex cardiovascular control in congestive heart failure. American Journal of Cardiology. 1992;69:17G–23G. doi: 10.1016/0002-9149(92)91251-x. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Arrick DM, Patel KP, Sun H. Exercise training normalizes impaired NOS-dependent responses of cerebral arterioles in type 1 diabetic rats. Am. J. Physiol Heart Circ. Physiol. 2011;300:H1013–H1020. doi: 10.1152/ajpheart.00873.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CD, Burch GE, Walsh JJ. Prolonged bed rest in the tratment of idiopathic cardiomyopathy. Am. J. Med. 1972;52:41–50. doi: 10.1016/0002-9343(72)90006-x. [DOI] [PubMed] [Google Scholar]
- McKelvie RS. Exercise training in patients with heart failure: clinical outcomes, safety, and indications. Heart Fail. Rev. 2008;13:3–11. doi: 10.1007/s10741-007-9052-z. [DOI] [PubMed] [Google Scholar]
- Mohanty PK, Arrowood JA, Ellenbogen KA, Thames MD. Neurohumoral and hemodynamic effects of lower body negative pressure in patients with congestive heart failure. Am. Heart J. 1989;118:78–85. doi: 10.1016/0002-8703(89)90075-6. [DOI] [PubMed] [Google Scholar]
- Mousa TM, Liu D, Cornish KG, Zucker IH. Exercise training enhances baroreflex sensitivity by an angiotensin II-dependent mechanism in chronic heart failure. J. Appl. Physiol. 2008;104:616–624. doi: 10.1152/japplphysiol.00601.2007. [DOI] [PubMed] [Google Scholar]
- Musch TI, Moore RL, Smaldone PG, Riedy M, Zelis R. Cardiac adaptations to endurance training in rats with a chronic myocardial infarction. J. Appl. Physiol. 1989;66(2):712–719. doi: 10.1152/jappl.1989.66.2.712. [DOI] [PubMed] [Google Scholar]
- Negraõ CE, Middlekauff HR. Adaptations in autonomic function during exercise training in heart failure. Heart Fail. Rev. 2008a;13:51–60. doi: 10.1007/s10741-007-9057-7. [DOI] [PubMed] [Google Scholar]
- Negraõ CE, Middlekauff HR. Exercise training in heart failure: reduction in angiotensin II, sympathetic nerve activity, and baroreflex control. J. Appl. Physiol. 2008b;104:577–578. doi: 10.1152/japplphysiol.01368.2007. [DOI] [PubMed] [Google Scholar]
- Nunes RB, Alves JP, Kessler LP, Dal Lago P. Aerobic exercise improves the inflammatory profile correlated with cardiac remodeling and function in chronic heart failure rats. Clinics (Sao Paulo) 2013;68:876–882. doi: 10.6061/clinics/2013(06)24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley WW. Pathophysiology and current therapy of congestive heart failure. Journal of the American College of Cardiology. 1989;13:771–785. doi: 10.1016/0735-1097(89)90215-5. [DOI] [PubMed] [Google Scholar]
- Patel KP, Zhang K, Kenney MJ, Weiss M, Mayhan WG. Neuronal expression of Fos protein in the hypothalamus of rats with heart failure. Brain Research. 2000;865:27–34. doi: 10.1016/s0006-8993(00)02186-7. [DOI] [PubMed] [Google Scholar]
- Patel KP, Zhang K, Zucker IH, Krukoff TL. Decreased gene expression of neuronal nitric oxide synthase in hypothalamus and brainstem of rats in heart failure. Brain Research. 1996a;734:109–115. [PubMed] [Google Scholar]
- Patel KP, Zhang PL, Carmines PK. Neural influences on renal responses to acute volume expansion in rats with heart failure. American Journal of Physiology: Heart and Circulatory Physiology. 1996b;271:H1441–H1448. doi: 10.1152/ajpheart.1996.271.4.H1441. [DOI] [PubMed] [Google Scholar]
- Piepoli MF, Capucci A. Exercise training in heart failure: effect on morbidity and mortality. International Journal of Cardiology. 2000;73:3–6. doi: 10.1016/s0167-5273(99)00220-x. [DOI] [PubMed] [Google Scholar]
- Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- Pliquett RU, Cornish KG, Patel KP, Schultz HD, Peuler JD, Zucker IH. Amelioration of depressed cardiopulmonary reflex control of sympathetic nerve activity by short-term exercise training in male rabbits with heart failure. J. Appl. Physiol. 2003;95:1883–1888. doi: 10.1152/japplphysiol.00486.2003. [DOI] [PubMed] [Google Scholar]
- Ponikowski P, Chua TP, Pepoli M, Ondusova d, Webb-Peplie K, Harrington D, Anker SD, Volterrani M, Colombo R, Mazzuero G, Giordano A, Coats AJS. Augmented peripheral chemosensitiviy as a potential input to baroreflex impairment and autonomic imbalance in chronic heart failure. Circulation. 1997;96:2586–2594. doi: 10.1161/01.cir.96.8.2586. [DOI] [PubMed] [Google Scholar]
- Rengo G, Pagano G, Parisi V, Femminella GD, de Lucia C, Liccardo D, Cannavo A, Zincarelli C, Komici K, Paolillo S, Fusco F, Koch WJ, Perrone Filardi P, Ferrara N, Leosco D. Changes of plasma norepinephrine and serum N-terminal pro-brain natriuretic peptide after exercise training predict survival in patients with heart failure. International journal of cardiology. 2014;171:384–389. doi: 10.1016/j.ijcard.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Riley M, Elborn JS, Bell N, Stanford CF, Nicholls DP. Substrate utilization during exercise in chronic heart failure. Clin. Sci. 1990;79:89–95. doi: 10.1042/cs0790089. [DOI] [PubMed] [Google Scholar]
- Rosenwinkel ET, Bloomfield DM, Arwady MA, Goldsmith RL. Exercise and autonomic function in health and cardiovascular disease. Cardiol Clin. 2001;19:369–387. doi: 10.1016/s0733-8651(05)70223-x. [DOI] [PubMed] [Google Scholar]
- Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrao CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll. Cardiol. 2003;42:854–860. doi: 10.1016/s0735-1097(03)00831-3. [DOI] [PubMed] [Google Scholar]
- Scalvini S, Volterrani M, Zanelli E, Pagani M, Mazzuero G, Coats AJ, Giordano A. Is heart rate variability a reliable method to assess autonomic modulation in left ventricular dysfunction and heart failure? Assessment of autonomic modulation with heart rate variability. International Journal of Cardiology. 1998;67:9–17. doi: 10.1016/s0167-5273(98)00252-6. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Li YL. Carotid body function in heart failure. Respir. Physiol Neurobiol. 2007;157:171–185. doi: 10.1016/j.resp.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Marwick TH. Exercise training for patients with heart failure: A systematic review of factors that improve mortality and morbidity. American Journal of Medicine. 2004;116:693–706. doi: 10.1016/j.amjmed.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Smart NA, Steele M. The effect of physical training on systemic proinflammatory cytokine expression in heart failure patients: a systematic review. Congest Heart Fail. 2011;17:110–114. doi: 10.1111/j.1751-7133.2011.00217.x. [DOI] [PubMed] [Google Scholar]
- Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J. Appl. Physiol. 1999a;86(4):1264–1272. doi: 10.1152/jappl.1999.86.4.1264. [DOI] [PubMed] [Google Scholar]
- Sun SY, Zucker IH, Wang W, Schultz HD. Enhanced activity of carotid body chemoreceptors in rabbits with heart failure: role of nitric oxide. Journal of Applied Physiology. 1999b;86(4):1273–1282. doi: 10.1152/jappl.1999.86.4.1273. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Zhang F, Zhang Y, Gao XY, Wang W, Zhu GQ. AT 1 receptor in paraventricular nucleus mediates the enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Autonomic Neuroscience-Basic & Clinical. 2004;121:56–63. doi: 10.1016/j.autneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Wang W, Zucker IH. Cardiac sympathetic afferent reflex in dogs with congestive heart failure. Am J Physiol. 1996;271:R751–R756. doi: 10.1152/ajpregu.1996.271.3.R751. [DOI] [PubMed] [Google Scholar]
- Wang WZ, Gao L, Pan YX, Zucker IH, Wang W. Differential effects of cardiac sympathetic afferent stimulation on neurons in the nucleus tractus solitarius. Neurosci Lett. 2006;409:146–150. doi: 10.1016/j.neulet.2006.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Golledge J. Neuronal nitric oxide synthase and sympathetic nerve activity in neurovascular and metabolic systems. Curr. Neurovasc. Res. 2013;10:81–89. doi: 10.2174/156720213804805963. [DOI] [PubMed] [Google Scholar]
- Xiao L, Gao L, Lazartigues E, Zucker IH. Brain-selective overexpression of Angiotensin-converting enzyme 2 attenuates sympathetic nerve activity and enhances baroreflex function in chronic heart failure. Hypertension. 2011;58:1057–1065. doi: 10.1161/HYPERTENSIONAHA.111.176636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousufuddin M, Shamim W, Chambers JS, Henein M, Amin FR, Anker SD, Kemp M, Hooper J, Coats AJS. Superiority of endothelin-1 over norepinephrine in exercise-induced alterations of the conduit artery tone of the non-exercised arm in patients with chronic heart failure. International Journal of Cardiology. 2000;73:15–25. doi: 10.1016/s0167-5273(99)00200-4. [DOI] [PubMed] [Google Scholar]
- Zanzinger J, Czachurski J. Chronic oxidative stress in the RVLM modulates sympathetic control of circulation in pigs. Pflugers Archiv European Journal of Physiology. 2000;439:489–494. doi: 10.1007/s004249900204. [DOI] [PubMed] [Google Scholar]
- Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. American Journal of Physiology: Heart and Circulatory Physiology. 2001;281:H995–H1004. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zucker IH, Patel KP. Altered number of diaphorase (NOS) positive neurons in the hypothalamus of rats with heart failure. Brain Research. 1998;786:219–225. doi: 10.1016/s0006-8993(97)01449-2. [DOI] [PubMed] [Google Scholar]
- Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. American Journal of Physiology: Heart and Circulatory Physiology. 2005;288:H2332–H2341. doi: 10.1152/ajpheart.00473.2004. [DOI] [PubMed] [Google Scholar]
- Zheng H, Li YF, Zucker IH, Patel KP. Exercise training improves renal excretory responses to acute volume expansion in rats with heart failure. American Journal of Physiology: Renal Physiology. 2006;291:F1148–F1156. doi: 10.1152/ajprenal.00501.2005. [DOI] [PubMed] [Google Scholar]
- Zheng H, Liu X, Li Y, Sharma NM, Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension. 2011a;58:966–973. doi: 10.1161/HYPERTENSIONAHA.111.176222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Liu X, Patel KP. Angiotensin-converting enzyme 2 overexpression improves central nitric oxide-mediated sympathetic outflow in chronic heart failure. Am. J. Physiol Heart Circ. Physiol. 2011b;301:H2402–H2412. doi: 10.1152/ajpheart.00330.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu GQ, Gao L, Li YF, Patel KP, Zucker IH, Wang W. AT 1 receptor mRNA antisense normalizes enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. American Journal of Physiology: Heart and Circulatory Physiology. 2004a;287:H1828–H1835. doi: 10.1152/ajpheart.01245.2003. [DOI] [PubMed] [Google Scholar]
- Zhu GQ, Gao L, Patel KP, Zucker IH, Wang W. ANG II in the paraventricular nucleus potentiates the cardiac sympathetic afferent reflex in rats with heart failure. Journal of Applied Physiology. 2004b;97:1746–1754. doi: 10.1152/japplphysiol.00573.2004. [DOI] [PubMed] [Google Scholar]
- Zhu GQ, Gao XY, Zhang F, Wang W. Reduced nitric oxide in the rostral ventrolateral medulla enhances cardiac sympathetic afferent reflex in rats with chronic heart failure. Sheng Li Xue. Bao. 2004c;56:47–53. [PubMed] [Google Scholar]
- Zhu GQ, Patel KP, Zucker IH, Wang W. Microinjection of ANG II into the paraventricular nucleus enhances cardiac sympathetic afferent reflex in rats. American Journal of Physiology: Heart and Circulatory Physiology. 2002;282:H2039–H2045. doi: 10.1152/ajpheart.00854.2001. [DOI] [PubMed] [Google Scholar]
- Zucker IH. Cardiac and baroreflex control of the circulation in heart failure. In: Lewis BS, Kimichi A, editors. Heart Failure Mechanisms and Management. Berlin, Heidelberg: Springer-Verlag; 1991. pp. 45–55. [Google Scholar]