Abstract
Synchronous bilateral disease occurs in approximately 5% of children with Wilms tumor (WT), and is independently associated with an increased risk of renal insufficiency. Nephron-sparing surgery (NSS) allows preservation of renal mass and improved renal function. Published oncologic and functional outcomes with NSS to date are generally good, likely reflecting proper patient selection and excellent surgical technique during tumor excision, as well as appropriate use of upfront and adjuvant therapies. Here we highlight important issues regarding the use of NSS in children with bilateral Wilms tumor (BWT).
Introduction
Wilms tumor is the most common renal tumor in children, affecting approximately 650 children in the United States each year. Of these, approximately 5% will have synchronous bilateral disease1 and 2–3% will develop a contralateral metachronous tumor.2 Bilateral Wilms tumor (BWT) differs from unilateral WT (UWT) in that it is more commonly associated with genetic syndromes which often include intrinsic renal disease, and tends to present earlier.1 Historically, the most significant morbidity associated with BWT has been renal insufficiency most often due to resection of a large portion of the renal parenchyma.3 End stage renal disease (ESRD) adversely affects not only the overall health of a patient but also the quality of life, with these effects being most pronounced in young children. Thus, there is a clear theoretical benefit to any procedure that preserves native renal function for a prolonged period of time. In recent years, nephron-sparing surgery (NSS) has been become the standard of care for the management of most adult renal tumors, with excellent oncologic control and functional outcomes. Owing to the comparative rarity of BWT, NSS is less commonly used in pediatric renal tumors, but has been shown to provide excellent oncologic control and to preserve renal function. This article will provide an overview of the role of NSS in BWT.
Rationale for NSS
Unlike unilateral disease, BWT is more commonly seen in the setting of syndromes associated with potential renal dysfunction (e.g. Denys-Drash), and usually occurs at an earlier age than tumors limited to one kidney.1 Children with bilateral disease also have an increased risk of tumor multifocality, as well as of tumor recurrence.4 Together, these factors underscore the importance of renal parenchymal preservation in the population with BWT. Although renal failure is treatable with dialysis as well as with renal transplantation, both of these measures have deleterious effects on quality of life. Children on dialysis may have nutritional deficiencies, growth limitation, and increased infection risk, in addition to a high mortality rate, particularly in younger children,5 while those undergoing renal transplantation adopt the attendant risks of renal allograft implantation and systemic immunosuppression. In children, up to 50% of renal allografts will fail by 10 years following transplantation.6 Thus, facilitating nephron preservation in order to prevent or delay renal transplantation is very beneficial for growing children.
BWT is associated with a 10% rate of end-stage renal disease, compared with only 0.7% in children with UWT.7 Again, this high rate of renal insufficiency in children treated for BWT in large part reflects inclusion of anephric patients, but has declined recently as NSS is increasingly employed. In adults, preservation of 25–33% of a solitary renal unit is adequate to preserve sufficient renal function in order to avoid dialysis.8 Renal insufficiency rates are substantially higher in patients with metachronous WT compared with synchronous BWT (19.3% vs 4%), perhaps reflecting the relative increase in nephron preservation with bilateral NSS in synchronous cases compared with unilateral nephrectomy and contralateral NSS in metachronous cases.9
Evolving Experience with NSS
Early reports documenting some success with NSS for BWT were published 15 years ago.10 In Japan, a 15-year series found that bilateral NSS for synchronous BWT could be successfully accomplished in only 36% of children, even after upfront chemotherapy,11 and, consequently, 40% of children with BWT developed renal insufficiency. A 21-year series of BWT in Italy over a similar time period reported on 48 patients undergoing bilateral NSS; this represented 53.3% of children with BWT. The overall survival rate was 80% and event-free survival was 66.5% at 5 years. The standard chemotherapy regimen in this group was 4 weeks of vincristine and actinomycin, with adriamycin added only when either metastatic disease or venous tumor thrombi were noted radiographically.12
St Jude Children’s Research Hospital reported on 12 patients with BWT, 10 of whom underwent bilateral NSS following upfront three-agent chemotherapy (vincristine, doxorubicin, and actinomycin D [VAD]). At four years of follow up, two patients had died of disease (after developing diffuse anaplasia, despite the initial histology being favorable) and two had recurrent tumor, with one patient developing renal insufficiency.13
More recent studies have reported similar success with nephron-sparing surgery. One study from the Children’s Hospital of Philadelphia documented 12 consecutive patients undergoing NSS for either synchronous (75%) or metachronous (25%) BWT. The authors reported no intraoperative or perioperative complications, no recurrences at nearly 3 years of follow-up, and no elevations in serum creatinine levels. Notably, three patients (25%) received adjuvant radiotherapy, although the specific indications were not delineated.14
It is evident that published studies are typically small series from single institutions, reflecting the relative rarity of BWT and perhaps the continued underutilization of NSS. The current Children’s Oncology group (COG) protocol, AREN0534, is a multicenter, protocol-driven study in which NSS is encouraged and which may be less susceptible to the biases inherent to small, single-institution case series.
NSS has also been used with success in patients with Denys-Drash, Beckwith-Wiedemann, and other associated syndromes. Since WT-associated syndromes confer a significantly higher risk of metachronous WT and renal insufficiency (up to 80% in children with Denys-Drash and 50% with WAGR),9 use of NSS may promote preservation of renal parenchyma in this population. In one Canadian study, syndromic BWT occurred in 13/85 (19%) of patients with BWT; 5 of these patients underwent unilateral NSS, with one having stage 3 disease. The authors reported no evidence of disease recurrence and no elevations of serum creatinine; one patient had hypertension following NSS.15
Patient Selection
To date, NSS has been primarily utilized in patients with BWT, whether synchronous or metachronous. However, many of the studies addressing the radiographic and pathologic criteria that might be employed to preoperatively assess feasibility of NSS in a given renal unit have been performed in patients with UWT. Although some of the data from these studies have raised the question of whether some patients with UWT might be appropriate candidates for NSS, the standard of care for UWT remains ipsilateral nephrectomy, and the epidemiologic differences between patients with UWT and BWT should be kept in mind when interpreting these findings.
Ferrer and colleagues16 considered children younger than two years, with tumors weighing less than 550 grams and negative lymph nodes, and retrospectively reviewed preoperative imaging studies to assess whether tumors would be considered amenable to NSS based on tumor size, weight, location (including proximity to vascular structures), and approximate volume of nephrons spared. Overall, although the study population reflected the very-low-risk population of children with WT as identified by COG (i.e. the population that is typically watched conservatively following surgery rather than given adjuvant chemotherapy), only 8% of tumors in this series were felt to be amenable to NSS. The most common radiographic finding that was felt to preclude partial nephrectomy was proximity of the tumor to the renal vessels. Only five children were felt to be candidates for partial nephrectomy; by radiographic assessment, NSS would be expected to spare at least half of the renal volume in these patients.
Radiographic imaging remains less than optimally sensitive for preoperatively identifying patients with tumors amenable to NSS. Giel et al reported that a large proportion of BWT’s that were characterized as unresectable based on radiographic appearance were in fact found to be amenable to NSS at the time of surgery.13 More recently, the RENAL nephrometry score has successfully used imaging characteristics of renal tumors to assess resectability with NSS in adult renal cancers, but it appears less predictive of complexity for WT.17 This may reflect limitations of the scoring system to capture the complexities of this tumor type. Even MRI, which is considered the gold standard for imaging of WT, has imperfect specificity: lesions in 6% (2/30) of patients were incorrectly characterized as WT; on pathologic evaluation, the lesions were identified as cystic renal dysplasia, nephrogenic rest, and chronic pyelonephritis.18 Overall, physicians and surgeons treating children with suspected WT are cautioned to employ clinical judgment rather than relying solely on radiographic findings for treatment planning.
In Ferrer’s study, retrospective review of the nephrectomy specimens from the five children felt to be possible NSS candidates showed nephrogenic rests in 80%. While nephrogenic rests are common in children with WT, this finding underscores the importance of intraoperative US use and consideration of multifocal disease when employing NSS in patients with WT. Cost’s group19 reviewed 78 nephrectomy specimens (pre-chemotherapy) for unilateral WT. In this series, nearly 90% of tumors were unifocal, and nearly half were associated with loss of no more than two-thirds of renal volume. Overall, approximately a quarter of children could be considered optimal candidates for renal preservation procedures. The authors did note that it is possible that expansion of these criteria might allow more children to be considered for NSS in the setting of UWT. Additionally, given the exquisite sensitivity of most WT to chemotherapy, this study likely underestimates the proportion of tumors amenable to renal preservation techniques, given existing protocols for BWT. Cost’s findings underscore the importance of intraoperative evaluation for suitability for NSS, as approximately three times as many patients were found to have tumors amenable to NSS compared to Ferrer’s study. Cost’s group felt that the most important intraoperative finding predicting successful NSS was the finding of a clear demarcation between the tumor and the adjacent renal parenchyma, rather than neoplastic infiltration into the surrounding tissue.
Multidisciplinary Treatment
Upfront Chemotherapy
Children with bilateral tumors and planned NSS who are enrolled on the COG study receive upfront chemotherapy, without biopsy, with vincristine, adriamycin, and dactinomycin for two cycles (6 weeks); if there is poor tumor response by imaging (<50% volume reduction), then surgery (biopsy, or when possible, complete resection) is undertaken, while patients with radiographically evident tumor shrinkage usually receive 6 more weeks of chemotherapy prior to surgical excision. There appears to be no clinical benefit associated with extending the duration of upfront chemotherapy beyond 12 weeks.20 Risks of prolonged chemotherapy administration include those associated with the chemotherapy itself (e.g. myelosuppression, secondary malignancies, fertility issues) and agent-specific toxicities (e.g. cardiac toxicity with doxorubicin). Patients enrolled on the SIOP renal tumor protocol, regardless of whether the disease was bilateral or unilateral, received only vincristine and dactinomycin in the neoadjuvant setting. Patients enrolled in either protocol had adjuvant chemotherapy adjusted based on the histologic findings at the time of tumor resection.
Surgical Technique
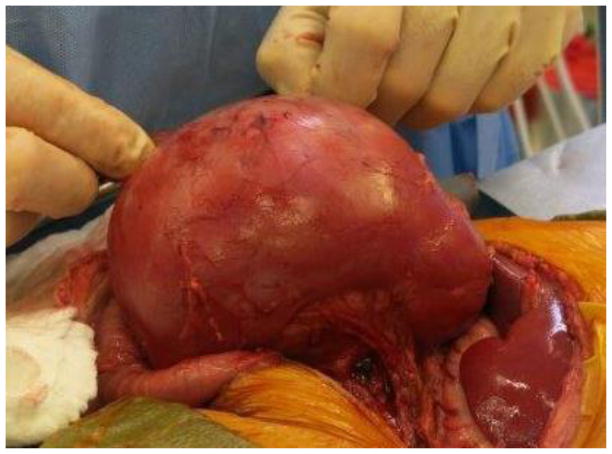
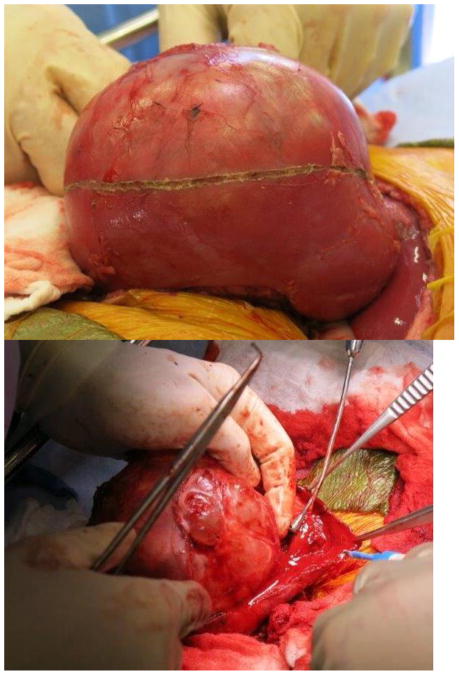
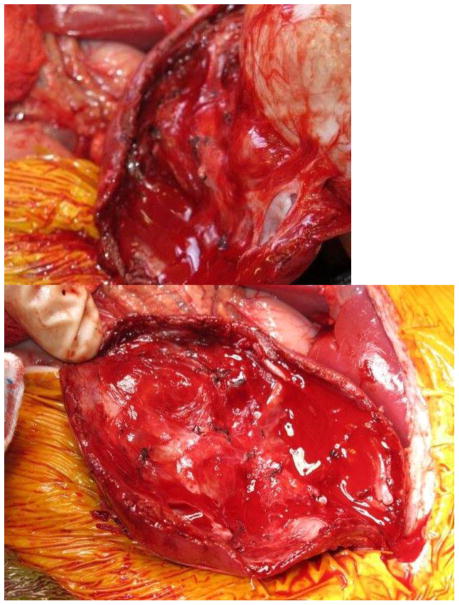
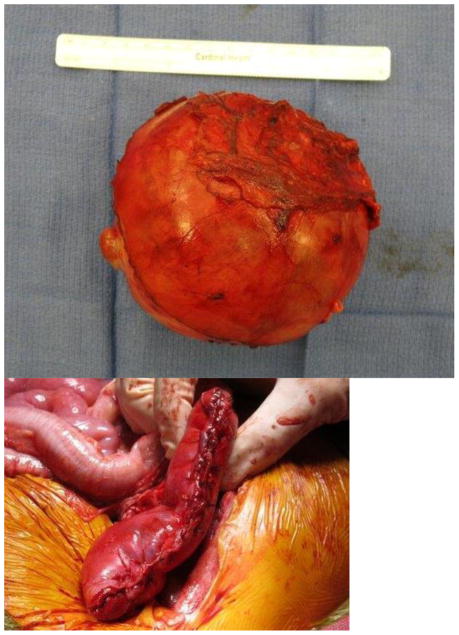
Current Children’s Oncology Group and International Society of Pediatric oncology (SIOP) protocols mandate removal of the entire tumor as well as lymph node sampling. Owing to the size of unilateral tumors (and the fact that only the SIOP, not COG, protocols mandate upfront chemotherapy), ipsilateral nephrectomy is usually performed, and thus NSS is reserved for BWT. Upfront chemotherapy usually results in significant tumor shrinkage, and most surgeons report that that it is technically easier to dissect the tumor free from the surrounding normal renal parenchyma. The selected approach must allow exposure of the retroperitoneum to facilitate tumor excision and lymph node sampling. We utilize a transperitoneal approach via a chevron incision. The bowel is reflected away, exposing each renal unit and associated tumor(s), sequentially. The renal vessels are identified and isolated, and can be manually compressed during the conduct of the NSS. The kidney surface is scored and the tumor then carefully separated from the kidney (Figure 1). Any entry into the collecting system is oversewn with fine absorbable suture. Prior to closing the collecting system, antegrade placement of a ureteral stent may be performed if the closure is complex and the risk of leak felt to be high. Specific bleeding sites on the cut surface of the parenchyma are oversewn with absorbable suture and the exposed renal parenchyma may be coagulated with an argon beam to further ensure hemostasis. The defect in the renal parenchyma is closed by folding over the cut edges of the kidney, often over thrombin-soaked gel foam, with the reniform shape of the kidney maintained with interrupted horizontal mattress sutures placed through the capsule on each side. Pledgets and bolsters are typically not necessary. Occasionally, tachosil is placed over the suture line to prevent against leak.21 Lymph node sampling is performed by removing lymph nodes from the peri-aortic (left side) and peri-caval (right side) regions. Attempts should be made to spare the adrenal gland unless this would compromise oncologic control or other aspects of surgical quality. A Foley catheter is left in place and percutaneous penrose drains are placed in the flank if entry to the collecting system is noted and there is concern for the potential of a urine leak. The Foley catheter is left in place until the penrose drains have minimal output for at least 24 hours. Drain output is then monitored after the Foley catheter is removed; the catheter is replaced if the drain output increases, otherwise the Penrose drains are then removed. Stents are removed cystoscopically generally at the completion of therapy.
Figure 1. Nephron-sparing surgery.
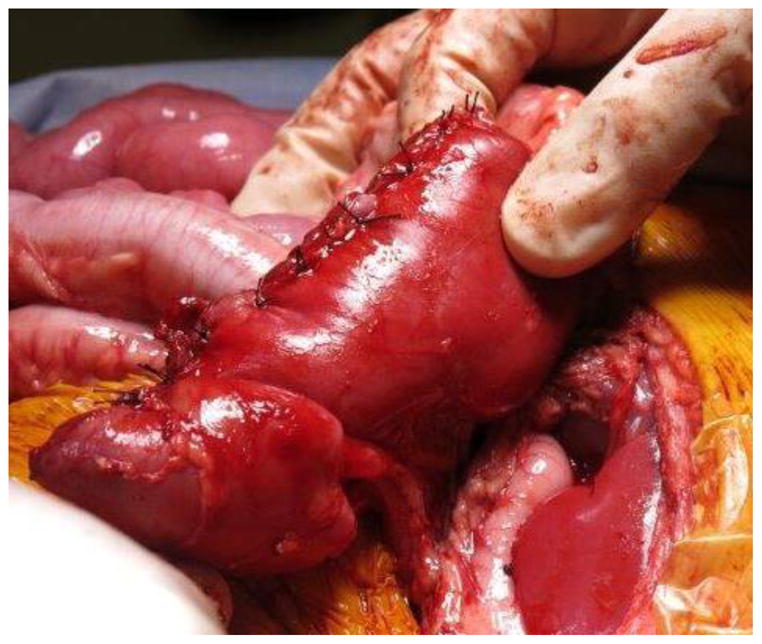
A. Kidney and tumor completely mobilized. B. Scoring of the capsule at the tumor-kidney interface. C. Dissection in the plane between tumor and kidney. D. An opening in the collecting system that requires closure with running monocryl suture. E. Cut surface of the remaining normal kidney parenchyma after ensuring hemostasis and closure of the collecting system. F. The resected specimen. G. Longitudinal folding over of the remaining normal kidney with reniform contour maintained with silk mattress sutures. H. View of the residual normal kidney at completion of nephron-sparing procedure.
Laparoscopic partial nephrectomy for WT has been reported,22–23 although there is at least one report of extensive peritoneal involvement with tumor following minimally invasive NSS.24 At present, an open surgical approach remains the standard of care for NSS in patients with BWT.
Adjuvant Radiotherapy
Adjuvant radiotherapy is reserved for those patients with residual tumor (tumor spill or positive margins), those with anaplastic histology, and those with positive lymph nodes. The duration and location of radiotherapy is based on the indication for treatment (e.g. patients with positive margins are generally treated with flank radiotherapy, while those with extensive tumor spill receive whole-abdomen radiation). There is an ongoing effort to limit the radiation duration, field, and strength by using conformal radiation.
Oncologic Outcome
Oncologic control with NSS for BWT is generally good, with 5-year event-free survival rates exceeding 90% in most cases. Some published reports of early experience with NSS for BWT did note lower event-free survival rates (80–83%), which may reflect factors such as learning curves and patient selection.11,13 Recent clinical success has been excellent in unilateral WT treated with NSS as well; one Italian study reported no deaths and no recurrences in stage I WT patients undergoing NSS after 6 years of follow-up, although the patients in this series were treated with upfront chemotherapy on the SIOP protocol.25 These excellent results likely reflect the combined influences of upfront chemotherapy, proper surgical technique, and judicious use of adjuvant radiotherapy.
Intraoperatively, the importance of a meticulous dissection with careful attention to complete tumor resection cannot be overstated; the operating surgeon must identify all tumors, excise all lesions with a rim of surrounding normal renal parenchyma, where feasible, sample lymph nodes, and avoid tumor spill. Enucleation of lesions may be performed in order to preserve renal function, and often is not associated with a positive margin since Wilms tumors typically grow within a surrounding fibrous capsule.13 In a SEER review of over 1800 patients with WT, only 111 underwent NSS (74 for unilateral disease and 37 for bilateral disease). NSS was not predictive of overall survival (predictive parameters included age, race, stage, and nodal status), but NSS was associated with a higher proportion of patients with unknown nodal status.26 Thus, it is crucial that adequate nodal sampling be performed at the time of NSS to ensure adequate staging.
The SIOP 2001 protocol considers NSS an acceptable option for non-central unilateral tumors in which complete resection of tumor burden can be anticipated. Patients received either 4 (localized) or 6 (metastatic) weeks of upfront chemotherapy before undergoing resection. 3% of patients underwent NSS (in the case of children with metastatic disease, fewer than 1% underwent NSS). Patients undergoing NSS had significantly smaller tumors. Two-thirds of NSS with stage III disease had positive margins, which the authors postulated may have been secondary to the choice of NSS rather than total nephrectomy. Rates of tumor rupture and postoperative complications were similar in patients undergoing total nephrectomy and NSS (though urinary complications were not recorded separately). Recurrence rate in NSS patients was one-third that of the total nephrectomy group (4% vs 13%), and 5-year event-free and overall survival rates were higher in NSS patients, although this may reflect the predominance of localized disease in this population. The authors also noted that usage of NSS was lower than expected, perhaps reflecting caution with the implementation of a relatively novel technique27; thus, the findings of this study may not be widely generalizable to future patients undergoing NSS.
Critics of the routine use of NSS for WT have expressed concerns that the technical challenges of the surgery may increase the risk of positive margins and tumor spill, and note that residual tumor may be overlooked in preserved renal parenchyma. Although patients with positive margins are routinely treated with flank radiotherapy, there are limited published data on the long-term effects of positive margins with respect to oncologic control and renal functional outcomes. In one study, the positive margin rate after NSS was 23.8%, and patients with residual tumor received flank irradiation. Interestingly, locoregional recurrence rates were higher (though not significantly so) in patients with negative margins compared with those with positive margins; this likely reflected the benefit of adjuvant radiotherapy in the latter group.28
Although tumor recurrence is an undesirable outcome, redo NSS can be successfully performed without compromising oncologic or renal functional outcomes. In one series, 22% of patients undergoing NSS (after upfront chemotherapy) underwent repeat NSS for recurrent disease, with 5% of patients undergoing a third surgery. Two of the eight redo NSS patients died of disease, both of whom had initial favorable histology but blastemal-predominant histology with subsequent anaplasia after recurrence (despite aggressive chemotherapy regimens). The remaining six patients are alive without evidence of disease 4.5 years after initial redo NSS; renal functional parameters (e.g. serum creatinine) are normal in these patients, although two have hypertension controlled with single-agent oral medications. Despite the excellent outcomes, surgeons should be aware that redo NSS was associated with a higher risk of positive margins compared with initial NSS (37.5% versus 12.5%), likely reflecting the increased technical difficulties associated with redo surgery. The two patients requiring pharmacological treatment for hypertension also received flank radiotherapy, and so nephron volume may not be the sole factor influencing blood pressure status.29
Functional Outcome
Renal Function
Published reports of renal function following NSS typically assess serum creatinine, glomerular filtration rate (GFR), hypertension, and/or proteinuria; the absence of universal operational definitions for renal insufficiency following NSS may limit the generalizability of findings from one institution to another. Equally challenging is the fact that tumor resection may be associated with an improvement in (not just stability of) some of these parameters. Cost and colleagues reported their findings in a multicenter international study comparing children with UWT treated with either NSS or radical nephrectomy. They found that smaller tumors were associated with stable or increased GFR, while larger tumors were associated with declining GFR. Patients presenting with worse preoperative chronic kidney disease (CKD stage II or greater) appeared to derive the greatest benefit from NSS, since there was a significant decrease in the proportion of patients with higher stage CKD after NSS, as well as an increase in estimated GFR in patients with higher grade CKD following NSS (but not radical nephrectomy).30 Although the majority of published studies assessing renal functional outcomes following NSS for WT report universally normal serum creatinine levels in patients who are not dialysis-dependent, many of these studies are small and from a single institution, and so Cost’s findings should raise awareness that assessment of renal functional parameters must be considered in light of the patient’s baseline renal function as well as the measurement techniques employed. Dialysis dependence has been reported in patients following NSS, almost always in the setting of WT-associated syndromes conferring an increased risk for renal insufficiency or the need for resection of a large proportion of kidney mass.31
Hypertension
Just as with renal functional outcomes, assessment of hypertension in patients undergoing NSS for WT is made challenging by the lack of a single standardized operational definition of this condition in all publications. A recent manuscript from Germany describes a lower proportion of patients developing hypertension after bilateral NSS compared with unilateral nephrectomy and contralateral NSS, with equivalent relapse-free and overall survival rates. Although this was a small study, it suggests that the development of hypertension is by no means universal following NSS.32
The factors leading to hypertension following NSS remain poorly defined. Abdominal and flank radiation have long been thought to increase the risk of high blood pressure, but one study found that the prevalence of hypertension was similar in patients who were radiation-treated compared with those who were radiation-naïve (35% overall, of whom all but one patient required pharmacologic management).28 An earlier report on a smaller cohort of patients in this same series noted that the prevalence of hypertension after NSS decreased slightly (from 58.8% preoperatively to 41.2% postoperatively).31 These findings suggest that hypertension is likely multifactorial and may reflect tumor activity as well as tumor location and postoperative nephron preservation; a specific patient’s hypertensive status may also change over time.
Proteinuria
Proteinuria is less commonly reported as an outcome than is renal function or hypertension. In studies where the prevalence of proteinuria is reported, a minority of patients appear to be affected. In one study, 15% of patients (three patients total) developed proteinuria (mild in one case and moderate in two), and in another, none of the patients who were not dialysis-dependent had proteinuria.31 More recently, nephrologists have suggested that microalbuminuria is a more sensitive predictor of long-term renal insufficiency,33 but to date we are unaware of any publications reporting on this parameter.
Surgical Complications
Because the current published studies on NSS for BWT are small, few report intraoperative, perioperative, or long-term complications; additionally, the small number of patients undergoing NSS for BWT has impeded identification of possible factors predisposing to complications. Some series report no complications associated with NSS; which may reflect technical excellence but should always be interpreted in light of a small sample size.34 The recent SIOP 2001 reports suggested that while complication rates were higher with NSS, the difference was not statistically significant.27 The St Jude group reported early postoperative complications including urine leak requiring collecting system drainage in three patients, pyelonephritis in one patient, and residual tumor requiring additional surgery in two patients; as a result of the last complication, the authors now employ routine intraoperative ultrasound during all partial nephrectomies to identify neoplastic lesions that may not be readily visually evident. Long-term complications included local tumor recurrence in two patients, intestinal obstruction in two patients, ureteropelvic junction obstruction in one patient, and renal failure in one patient.13
Conclusion
NSS is a safe and effective technique to achieve oncologic control and maintain renal function in children with BWT, and should be considered in the majority of patients with this condition. Published data are promising; however, these outstanding outcomes rely heavily on proper patient selection, excellent operative technique, and administration of appropriate upfront and adjuvant therapies.
References
- 1.Breslow N, Olshan A, Beckwith JB, et al. Epidemiology of Wilms tumor. Med Pediatr Oncol. 1993;21:172–81. doi: 10.1002/mpo.2950210305. [DOI] [PubMed] [Google Scholar]
- 2.Moorman-Voestermans CGM, Aronson DC, et al. Is partial nephrectomy appropriate treatment for unilateral Wilms’ tumor? J Pediatr Surg. 1998;33:165–70. doi: 10.1016/s0022-3468(98)90425-0. [DOI] [PubMed] [Google Scholar]
- 3.Ritchey ML, Green DM, Thomas PR, et al. Renal failure in Wilms’ tumor patients: A report from the National Wilms’ Tumor Study Group. Med Pediatr Oncol. 1996;26:75–80. doi: 10.1002/(SICI)1096-911X(199602)26:2<75::AID-MPO1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Breslow NE, Beckwith JB. Epidemiological features of Wilms’ tumor: results of the National Wilms’ Tumor Study. J Natl Cancer Inst. 1982;68:429–36. [PubMed] [Google Scholar]
- 5.Warady BA, Neu AM, Schaefer F. Optimal care of the infant, child, and adolescent on dialysis: 2014 update. Am J Kidney Dis. 2014;64:128–42. doi: 10.1053/j.ajkd.2014.01.430. [DOI] [PubMed] [Google Scholar]
- 6.Dharnidharka VR, Fiorina P, Harmon WE. Kidney transplantation in children. N Engl J Med. 2014;371:549–58. doi: 10.1056/NEJMra1314376. [DOI] [PubMed] [Google Scholar]
- 7.Breslow NE, Collins AJ, Ritchey ML, et al. End stage renal disease in patients with Wilms tumor: results from the National Wilms Tumor Study Group and the United States Renal Data System. J Urol. 2005;174:1972–5. doi: 10.1097/01.ju.0000176800.00994.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166:6–18. [PubMed] [Google Scholar]
- 9.Lange J, Peterson SM, Takashima JR, et al. Risk factors for end stage renal disease in non-WT1-syndromic Wilms tumor. J Urol. 2011;186:378–86. doi: 10.1016/j.juro.2011.03.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs J, Wünsch L, Flemming P, et al. Nephron-sparing surgery in synchronous bilateral Wilms’ tumors. J Pediatr Surg. 1999;34:1505–9. doi: 10.1016/s0022-3468(99)90113-6. [DOI] [PubMed] [Google Scholar]
- 11.Oue T, Koshinaga T, Okita H, et al. Bilateral Wilms tumors treated according to the Japan Wilms Tumor Study Group protocol. Pediatr Blood Cancer. 2014;61:1184–9. doi: 10.1002/pbc.24979. [DOI] [PubMed] [Google Scholar]
- 12.Indolfi P, Jenkner A, Terenziani M, et al. for the AIEOP Wilms Tumor Working Group. Synchronous bilateral Wilms tumor: a report from the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) Cancer. 2013;15; 119:1586–92. doi: 10.1002/cncr.27897. [DOI] [PubMed] [Google Scholar]
- 13.Davidoff AM, Giel DW, Jones DP, et al. The feasibility and outcome of nephron-sparing surgery for children with bilateral Wilms tumor. The St Jude Children’s Research Hospital experience: 1999–2006. Cancer. 2008;112:2060–70. doi: 10.1002/cncr.23406. [DOI] [PubMed] [Google Scholar]
- 14.Sulkowski J, Kolon T, Mattei P. Nephron-sparing partial nephrectomy for bilateral Wilms’ tumor. J Pediatr Surg. 2012;47:1234–8. doi: 10.1016/j.jpedsurg.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Romão RL, Pippi Salle JL, Shuman C, et al. Nephron sparing surgery for unilateral Wilms tumor in children with predisposing syndromes: single center experience over 10 years. J Urol. 2012;188:1493–8. doi: 10.1016/j.juro.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Ferrer FA, Rosen N, Herbst K, et al. Image based feasibility of renal sparing surgery for very low risk unilateral Wilms tumors: a report from the Children’s Oncology Group. J Urol. 2013;190:1846–51. doi: 10.1016/j.juro.2013.05.060. [DOI] [PubMed] [Google Scholar]
- 17.Cost NG, DeFoor WR, Jr, Crotty EJ, Geller JI. The initial experience with RENAL Nephrometry in children, adolescents, and young adults with renal tumors. Pediatr Blood Cancer. 2014;61:1434–9. doi: 10.1002/pbc.25027. [DOI] [PubMed] [Google Scholar]
- 18.Cox SG, Kilborn T, Pillay K, et al. Magnetic resonance imaging versus histopathology in Wilms tumor and nephroblastomatosis: 3 examples of noncorrelation. J Pediatr Hematol Oncol. 2014;36:e81–4. doi: 10.1097/MPH.0b013e318290c60d. [DOI] [PubMed] [Google Scholar]
- 19.Cost NG, Lubahn JD, Granberg CF, et al. Pathological review of Wilms tumor nephrectomy specimens and potential implications for nephron sparing surgery in Wilms tumor. J Urol. 2012;188:1506–10. doi: 10.1016/j.juro.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton TE, Ritchey ML, Haase GM, et al. The management of synchronous bilateral Wilms’ tumor: a report from the National Wilms Tumor Study Group. Ann Surg. 2011;253:1004–10. doi: 10.1097/SLA.0b013e31821266a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mele E, Ceccanti S, Schiavetti A, et al. The use of Tachosil as hemostatic sealant in nephron sparing surgery for Wilms tumor: preliminary observations. J Pediatr Surg. 2013;48:689–94. doi: 10.1016/j.jpedsurg.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Piché N, Barrieras D. Minimally invasive nephron-sparing surgery for unilateral Wilms tumor. J Pediatr Surg. 2012;47:e1–4. doi: 10.1016/j.jpedsurg.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Rauth TP, Slone J, Crane G, et al. Laparoscopic nephron-sparing resection of synchronous Wilms tumors in a case of hyperplastic perilobar nephroblastomatosis. J Pediatr Surg. 2011;46:983–8. doi: 10.1016/j.jpedsurg.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chui CH, Lee AC. Peritoneal metastases after laparoscopic nephron-sparing surgery for localized Wilms tumor. J Pediatr Surg. 2011;46:e19–21. doi: 10.1016/j.jpedsurg.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Zani A, Schiavetti A, Gambino M, et al. Long-term outcome of nephron sparing surgery and simple nephrectomy for unilateral localized Wilms tumor. J Urol. 2005;173:946–8. doi: 10.1097/01.ju.0000152580.90861.d3. [DOI] [PubMed] [Google Scholar]
- 26.Wang HH, Abern MR, Cost NG, et al. Use of nephron sparing surgery and impact on survival in children with Wilms Tumor: a SEER analysis. J Urol. 2014 doi: 10.1016/j.juro.2014.04.003. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilde JC, Aronson DC, Sznajder B, et al. Nephron sparing surgery (NSS) for unilateral Wilms tumor (UWT): the SIOP 2001 experience. Pediatr Blood Cancer. 2014 doi: 10.1002/pbc.25185. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 28.Kieran K, Williams MA, Dome JS, et al. Margin status and tumor recurrence after nephron-sparing surgery for bilateral Wilms tumor. J Pediatr Surg. 2013;48:1481–5. doi: 10.1016/j.jpedsurg.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 29.Kieran K, Williams MA, McGregor LM, et al. Repeat nephron-sparing surgery for children with bilateral Wilms tumor. J Pediatr Surg. 2014;49:149–53. doi: 10.1016/j.jpedsurg.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 30.Cost NG, Sawicz-Birkowska K, Kajbafzadeh AM, et al. A comparison of renal function outcomes after nephron-sparing surgery and radical nephrectomy for nonsyndromic unilateral Wilms tumor. Urology. 2014;83:1388–93. doi: 10.1016/j.urology.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 31.Giel DW, Williams MA, Jones DP, et al. Renal function outcomes in patients treated with nephron sparing surgery for bilateral Wilms tumor. J Urol. 2007;178:1786–9. doi: 10.1016/j.juro.2007.03.183. [DOI] [PubMed] [Google Scholar]
- 32.Hubertus J, Günther B, Becker K, et al. Development of hypertension is less frequent after bilateral nephron-sparing surgery for bilateral Wilms tumors in a long-term survey. J Urol. 2014 doi: 10.1016/j.juro.2014.07.116. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 33.Shirzai A, Yildiz N, Biyikli N, et al. Is microalbuminuria a risk factor for hypertension in children with solitary kidney? Pediatr Nephrol. 2014;29:283–8. doi: 10.1007/s00467-013-2641-2. [DOI] [PubMed] [Google Scholar]
- 34.Sulkowski J, Kolon T, Mattei P. Nephron-sparing partial nephrectomy for bilateral Wilms’ tumor. J Pediatr Surg. 2012;47:1234–8. doi: 10.1016/j.jpedsurg.2012.03.032. [DOI] [PubMed] [Google Scholar]