Abstract
Survivors of childhood cancer are at risk for treatment-related musculoskeletal late effects. Early detection and orthopedic intervention can help ameliorate musculoskeletal late effects and prevent subsequent complications. This systematic review summarizes the literature describing associations between cancer, its treatment, and musculoskeletal late effects. We searched PubMed and Web of Science for English language articles published between January 1970 and December 2012. The search was limited to investigations with at least 15 participants and conducted at least 2 years after completion of therapy for childhood, adolescent, or young adult cancer. Some late skeletal effects, including low bone mineral density, osteonecrosis, slipped capital femoral epiphyses, oncogenic rickets, and hormone-related growth disturbances have been previously reviewed and were excluded, as were outcomes following amputation and limb-salvage procedures. Of 2347 references identified, 30 met inclusion criteria and were retained. An additional 54 studies that met inclusion criteria were found in reference lists of retained studies. Of 84 studies, 60 focused on associations between radiotherapy, six between chemotherapy, and 18 between surgery and musculoskeletal late effects. We found that younger age, higher radiation dosage, and asymmetric or partial bone radiation volume influences the effects of radiation on the musculoskeletal system. Methotrexate and vincristine are associated with long-term muscular strength and flexibility deficits. Laminectomy and chest wall resection are associated with spinal malalignment, and enucleation is associated with orbital deformities among survivors. Radiotherapy, chemotherapy, and surgery are associated with musculoskeletal late effects independently and additively. Associations are additionally influenced by host and treatment characteristics.
Keywords: craniofacial abnormalities, musculoskeletal diseases, neoplasms, pediatrics, radiation, scoliosis, thoracic wall
INTRODUCTION
The 5-year survival rate among children with malignancies is over 80%. As of January 1, 2010, there were nearly 380,000 survivors of childhood cancer in the United States (1). This growing population is the result of progress in cancer treatment and supportive care. However, survivors remain at risk for significant treatment-related late effects (2, 3), including musculoskeletal complications (4).
Late musculoskeletal effects such as low bone mineral density (BMD) (5), osteonecrosis (6), slipped capital femoral epiphyses (7), oncogenic rickets (8), hormone-related growth disturbances (9), and limb salvage and amputation-related outcomes (10), have been comprehensively reviewed. However, the current state of knowledge about other, equally important, musculoskeletal late effects has not been systematically evaluated. Clinicians providing care to survivors should anticipate musculoskeletal late effects such as muscular atrophy, fibrosis, skeletal hypoplasia, and craniofacial and spinal deformity. Although these late effects influence physical functioning and quality of life of survivors (11–13), they have not been rigorously studied compared to the aforementioned well-reviewed late effects. Early detection and orthopedic intervention can help ameliorate musculoskeletal late effects and prevent subsequent complications.
To aid clinicians in the provision of individualized care through the identification of at-risk survivors and provision of timely intervention, we systematically review and summarize the existing knowledge about musculoskeletal late effects of childhood cancer treatment.
METHODS
Data Sources
We searched the PubMed and Web of Science database (Limits: English, human, dates: 01/01/1970 to 12/31/2012) for clinical trials, observational studies, case series, and reviews using the search terms “musculoskeletal abnormalities”, “adverse effects”, “infant”, “child”, “adolescent”, “adult, young”, “neoplasms”, “survivors”. Studies were selected after reviewing the abstracts for relevance. This search was augmented with selected publications from reference lists of studies retrieved from databases.
Study Selection
The systematic review was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (14). Reports were limited to studies with at least 15 participants that discussed late musculoskeletal effects among childhood cancer survivors evaluated a minimum of 2 years after completion of therapy. Previous literature reviews were excluded. Specific musculoskeletal effects, including low BMD (5), osteonecrosis (6), slipped capital femoral epiphyses (7), limb salvage and amputation related late effects (10), oncogenic rickets (8), and hormone-related growth complications after cranial radiation (9) were excluded because comprehensive reviews have been published. Primary outcomes were defined as the occurrence of musculoskeletal complications at least 2 years after completion of cancer therapy.
Data Extraction and Synthesis
One reviewer (P.L.G.) screened all titles and abstracts for relevance and validity. The methodological quality of the selected studies was independently evaluated by two reviewers (P.L.G. and K.K.N.) (15). Criteria used to determine the quality of the studies included comparability of subjects, clear definition of exposure or intervention, standard outcome measurement and appropriate statistical analysis. Discrepancies in assessment of validity and quality of studies were resolved by discussion between reviewers. We extracted information about sample size, age at diagnosis, type of diagnosis, follow-up period, type and details of treatment and musculoskeletal late effects from the selected studies.
RESULTS
Search Results
Our initial search of PubMed database identified 2347 studies; 30 were retained as relevant. An additional 54 studies that met inclusion criteria were found in reference lists of initially retained studies. No additional studies were identified in the Web of Science database. The 84 studies selected were evaluated according to the predominant anticancer therapy exposure (radiotherapy [n = 60], chemotherapy [n = 6], or surgery [n = 18]) associated with the late musculoskeletal effects (Figure 1).
Figure 1.
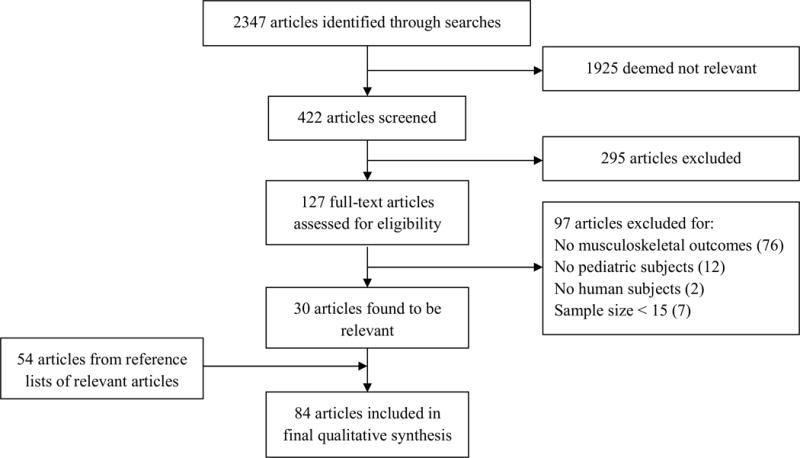
Flow of studies through review.
Study Quality and Risk of Bias
Studies included in this review had clearly defined exposure and outcome measures. Most of the studies were cross-sectional, retrospective in design, from single institutions, and did not mention the eligible source population. Although we excluded studies with fewer than 15 participants, several studies included were conducted among small convenience cohorts because data from larger cohorts documenting musculoskeletal late effects were not available. These study designs may have either excluded patients with the most severe musculoskeletal effects because of death or inability to participate in follow-up testing, or oversampled them because of their concerns regarding late effects. Longitudinal studies included in this review were likely subject to selection bias due to attrition.
Muscular Late Effects of Radiotherapy (Table 1)
Table 1.
Summary of 60 reports of radiation-associated musculoskeletal late effects in long-term survivors of childhood cancer
| First Author (Year) |
Sample Size (n) |
Age at Diagnosis |
Diagnosis | Time since Diagnosis |
Surgery/ HCT |
Chemotherapy Agents |
Radiotherapy (RT) and Dose |
Late Effects | Risk Factors | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscular | Skeletal | Younger age at RT | Growth spurt | RT dosage | RT field size | RT asymmetry | Epiphyses in RT field | Orthovoltage | Accompanied chemo | Accompanied surgery | Longer follow-up | |||||||||||||||||||||
| Atrophy | Fibrosis | Hypoplasia | Myalgia | Scoliosis | Kyphosis | Lordosis | Spinal growth retarded | Vertebral asymmetry | Hypoplasia | LLD | Craniofacial deformity | Orbital deformity | Chest wall deformity | Osteochondroma | ||||||||||||||||||
| Probert(58) 1973 | 22 | < 15 y | HL (n=16) MB (n=2) ALL (n=4) | 9 mo–10.5 y | NA | MOPP (HL) VCR, PRED, MP, MTX, CPM (ALL) | Spinal RT (MeV) > 40 Gy (n=18) < 25 Gy (n=4) | ✓ | ✓ | |||||||||||||||||||||||
| Probert(44) 1975 | 44 plus 15000 controls | < 15 y | HL (n=28) MB (n=3) ALL (n=13) | 3.5 y (> 35 Gy) 1.7 y (< 25 Gy) | NA | MOPP (HL) VCR, PRED, 6-MP, MTX, CPM (ALL) | Spinal RT (MeV) > 35 Gy (n=29) < 25 Gy (n=15) | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||
| Riseborough (23) 1976 | 81 | 0–13 y (mean 3.1 y) | WT | 6.6–21.6 y (mean 11.9 y) | All had nephrectomy | AMD (n=71) | 31 Gy (n=74; OV) 28 Gy (n=7; Co-60) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||
| Oliver(21) 1978 | 21 | 5 mo–9 y | WT | 3.5–35 y | All had nephrectomy | AMD (n=20) | All had RT 20–45 Gy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||
| Heaston(43) 1979 | 25 | 1 mo–9.3 y (mean 3.5 y) | WT | 4–18 y (mean 9.83 y) | All had nephrectomy | All had chemo | 35 Gy (n=20; Co-60) 35 Gy (n=5; OV) | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||
| Jentzsch(26) 1981 | 22 | 4–29 y | ES | > 2 y | NA | Protocol S1–S4 | All had RT mean 50 Gy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||
| Libshitz(119) 1981 | 44 | < 16 y | HL, NHL, ES, RMS | ≥ 3 y | NA | Chemo (n=14) | All had RT 30–55.5 Gy | ✓ | ✓ | ✓ | ||||||||||||||||||||||
| Mayfield(34) 1981 | 74 | 0–10.4 y (mean 1.5 y) | NB | 5–31 y (mean 12.9 y) | Resection (n=6), laminec tomy (n=7) | Resection and chemo (n=6) | 27.5 Gy (n=67; OV) 23.5 Gy (n=1; MeV) 49.9 Gy (n=1;High RT) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||
| Pastore(55) 1982 | 86 | < 1 y | Various cancers | 5–29 y (median 12 y) | NA | Chemo (n=78) | 4.5–40 Gy (n=60; OV) mean 21 Gy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||
| Smith(45) 1982 | 22 | 0.75–9 y (median 2.5 y) | WT | 1–22.5 y (median 10.5 y) | NA | Chemo (n=16) AMD, VCR, ADR, CPM | 22–35 Gy (n=7 for OV & n=15 for MeV) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||
| Guyuron(72) 1983 | 41 | 1–17 y (mean 4.8 y) | Facial tumors | 7–25 y (mean 15.1 y) | NA | NA | Craniofacial RT 4–85.1 Gy | ✓ | ✓ | ✓ | ||||||||||||||||||||||
| Kajanti(27) 1983 | 32 | 0–12.9 y | NB | 3–24.5 y (mean 13.6 y) | Resection (n=29) | Chemo (n=25) CPM and VCR | RT (n=25) MeV (n=19)/X-rays | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||
| Thomas(46) 1983 | 26 | 0–133 mo | WT | 60–226 mo (median153 mo) | Nephrectomy (n=25) | VCR, AMD CPM (n=24) | All had RT 3–44 Gy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||
| Carli (67) 1986 | 70 | 0–15 y (median 6 y) | RMS | NA | NA | Chemo (n=69) | RT (n=48) 16–84 Gy | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||||
| Fromm (77) 1986 | 20 | 7 mo–13 y (median 6 y) | Soft-tissue sarcoma | 2–10 y (median 5.5 y) | NA | All had Chemo ADR VCR, AMD, CPM | 40–60 Gy (median 50 Gy) | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||
| Heyn (78) 1986 | 50 | 0–20 y | RMS | 3 y | Resection (n=40) | All had Chemo | All had RT 50–60 Gy | ✓ | ✓ | ✓ | ||||||||||||||||||||||
| Brown (70) 1987a | 67 | 0.3–33 y (median 11 y) | ES | NA | Surgery (n=7) & RT (n=24) | VCR, CPM, AMD, DOXO, MELPH, | RT (n=60; MeV) 25–65 Gy | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||
| Shalet (59) 1987 | 79 | 1–15 y | Brain tumors | 16–30 ya | All had surgery | NA | All had RT 27–35 Gy | ✓ | ✓ | |||||||||||||||||||||||
| Dahllöf (120) 1989 | 17 plus 85 controls | 1–12.9 y | Hematolo gical (14) Aplastic (3) | 1.9–5.9 y (median 3.9 y) | All had HCT | High dose CPM 50–60 mg/kg | 10 Gy TBI (n=12) 8 Gy TBI (n=2) 24 Gy CRT (n= 3) | ✓ | ✓ | |||||||||||||||||||||||
| Butler (47) 1990 | 143 | 0.1–12.9 y (mean 8.3 y) | Various cancers | 2–18 y (mean 9.9 y) | Surgery (n=119) | Chemo (n=131) | All had RT 19.5–50 Gy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||
| Larson (33) 1990 | 50 | 2 mo–16 y (median 5 y) | Various cancers | 6–27 y (median 13 y) | NA | Chemo (n=41) | All had RT | ✓ | ✓ | ✓ | ||||||||||||||||||||||
| Sonis (81) 1990 | 97 | < 10 y | ALL | ≥ 5 y | NA | All had chemo | CRT (n=78; MeV) 18 Gy (27) 24 Gy (51) | ✓ | ✓ | ✓ | ||||||||||||||||||||||
| Wallace (42) 1990 | 30 | 0.3–11.3 y (median 2.4 y) | WT | 15.2–39.4 y (median 21 y)a | All had nephrectomy | NA | All had RT 20–30 Gy | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||||
| Silber (62) 1990 | 49 | 0.3–16.7 y (mean 7.3 y) | Various cancers | 3.3–15.4 y (median 8.9 y) | Laminectomy (n=1) | Chemo (n=46) | RT (n=36) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||||
| Evans (24) 1991 | 680 | NA | WT | > 5 y 7416 person-y | NA | AMD, VCR or ADR | RT (n=486) | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||
| Crooks (48) 1991 | 89 | 9 d–13.2 y (median 3.7 y) | ALL, WT, MB, others | 4–23 y (mean 10.8 y) | NA | All ALL patients had intrathecal MTX | All had RT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||
| Rate (49) 1991 | 31 | 0.08–13.4 y (median 4.8 y) | WT | 4–18 y (median13 y) | All had nephrectomy | All had chemo | 28.9 Gy (n=10; OV) 34.5 Gy (n=21; MeV) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||
| Meadows (121) 1992 | 400 | < 2 y (n=93) > 2 y (n=318) | Various cancers | > 5 y | NA | NA | 39 of 93 had RT 71 of 318 had RT | ✓ | ✓ | |||||||||||||||||||||||
| Makipernaa (30) 1993 | 44 | 3 mo–14.7 y (median 2.6 y) | WT, NB and others | 10.8–27.7 y (median 18.9 y) | All had resection | Chemo (n=38) | 13–49 Gy (WT) 17.7–43 Gy (Others) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||
| Mameghan (32) 1993 | 49 | 3–33 y (median 16 y) | ES | 2–24 y (mean 12.3 y) | Amputation/resection(n=5) | Chemo (n=44) | RT (n=41) median 50 Gy | ✓ | ✓ | |||||||||||||||||||||||
| Dahllöf (35) 1994 | 19 plus 19 cont. | 6.2–19.9 y (mean 14.2 y) | ALL, AML Gaucher’s | > 3 y | All had HCT | NA | All had TBI | ✓ | ✓ | ✓ | ||||||||||||||||||||||
| Willman (57) 1994 | 124 | < 16 y (boys) < 14 y (girls) | HL | ≥ 2 y | NA | Chemo (n=77) | All had spinal RT ≥ 33 Gy or < 33 Gy | ✓ | ✓ | ✓ | ||||||||||||||||||||||
| Imhof (82) 1996 | 68 | 0.5–53 mo (mean 11 mo) | RB (52 B/L and 16 U/L) | 95–97 mo | Enucleation (n=43) | NA | RT only (n=77), RT & enucleation (n=43) | ✓ | ✓ | |||||||||||||||||||||||
| Taylor (66) 1997 | 138 | NA | WT | Median 127 m | All had surgery | UKW1 protocol; | Abdominal RT 20–30 Gy | ✓ | ✓ | ✓ | ||||||||||||||||||||||
| Denys (74) 1998 | 26 | 2–18 y (median 13 y) | RMS (6 y), NPC (17 y) | 1–20 y (median 4 y) | NA | All had chemo | 50.2 Gy (RMS) 56.8 Gy (NPC) | ✓ | ✓ | ✓ | ||||||||||||||||||||||
| Hale (50) 1999 | 73 | 0–18.3 y (median 9.2 y) | GCT | 5.1–26.5 y (median 11.3 y) | All had resection | Chemo (n=48) | RT (n=29) 20–30 Gy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||
| Hovi (122) 1999 | 31 | TBI (3 y) Chemo (3 y) | NB poor-risk | TBI (4.7 y) Chemo (7.7 y) | All had HCT | VP-16, MELPH, CP L/CSPL, Thiotepa | 10–12 Gy (n=16; TBI) 4–24 Gy (n=6; chemo) | ✓ | ✓ | |||||||||||||||||||||||
| Paulino (28) 2000a | 42 | 0.5–10.5 y (median 4 y) | WT | 5–25.5 y (median 15 y) | NA | All had chemo AMD, VCR, ADR | All had RT 10–40 Gy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||
| Paulino (75) 2000b | 17 | 2.2–11.6 y (median 5.7 y) | Head and neck RMS | 7.5–33 y (median 20 y) | NA | Chemo (n=15) | All had RT 41.4–65 Gy (MeV) | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||
| Hogeboom (61) 2001 | 2778 | Median 2 y 11 mo | WT (94%) Other (6%) | Median 6 y 2 mo | All had nephrectomy | NWTS-1, NWTS-2 and NWTS-3 | RT (n=1323) 10–40 Gy | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||
| Karsila- Tenovuo (73) 2001 | 40 plus 40 cont. | Median 13.4 y | Craniofacial tumors | Median 6 y | NA | CRT+Chemo (n=18) Chemo only (n=11) | CRT (n=18) 19.5–59.6 Gy | ✓ | ||||||||||||||||||||||||
| Trobs (22) 2001 | 49 | NA | WT | NA | All had nephrectomy | SIOP No.9/GPO SIOP 93-01/GPO | RT (n=48) | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||
| Oberlin (86) 2001 | 306 | < 1–17 y (median 6.8 y) | Orbital RMS | 25–204 mo (median 82 mo) | Resection (n=9) | All had chemo | RT (n=245) 6–64 Gy | ✓ | ||||||||||||||||||||||||
| Paulino (51) 2002 | 53 | 0–1 y | NB | 2–41 y (median 13.1 y) | Resection (n=38) | Chemo (n=22) CPM and/or DOXO | 12–31 Gy (n=20) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||
| Fuchs (123) 2003a | 41 | 5–51 y (mean 16.8 y) | ES | 20–36 y (mean 25 y) | Surgery (n=20) | Chemo (n=32) VCR, CPM or AMD | RT (n=37) | ✓ | ✓ | |||||||||||||||||||||||
| Pinter (36) 2003 | 79 | < 1 y | Solid tumors | 16–25 y (mean 20 y) | Surgery (n=79) | Chemo (n=64) | RT (n=23) always with surgery & chemo | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||
| Merchant (87) 2004 | 15 | 5.1–18.9 y (median 13.3 y) | HL | Median 39.6 mo | NA | Vinblastine, DOXO MTX and PRED | All had asymmetric mantle RT to clavicle | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||
| Paulino (25) 2004 | 15 | 3.5–20 y (median 13 y) | Extremity sarcomas | 6–36 y (median 20 y) | Excision (n=7) | Chemo (n=10) VCR, CPM/DOXO | 45–66 Gy (n=9) 41.4–66.4 Gy (n=6) | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||
| Taitz (88) 2004 | 58 | < 15 y | NB IV & others | ≥ 30 mo | All had HCT | NA | All had TBI | ✓ | ✓ | |||||||||||||||||||||||
| Ness (4) 2005 | 11481 plus3839 siblings | 0–20 y | Various cancers | 8–47 y (median 23 y) | Surgery only (7.7%) | Surgery, Chemo & RT (54%) | RT with or without surgery (12.4%) | ✓ | ✓ | |||||||||||||||||||||||
| Paulino (52) 2005 | 58 | 2 wk–15 y (median 6 y) | NB | 5–46 y (median 10 y) | Laminectomy (n=5) | Chemo (n=33) CPM, DOXO/VCR | 3–39 Gy (n=27; median 20 Gy) | ✓ | ✓ | ✓ | ||||||||||||||||||||||
| Escobar (124) 2006 | 52 | Mean 29.1 ± 31.7 mo | NB stage IV | 12.4 ± 8.3 ya | Surgery (n=51) | CCG protocol (n=50) | RT (n=17) | ✓ | ||||||||||||||||||||||||
| Calaminus(125) 2007 | 36 plus 319 cont. | 1–16 y | Various cancers | 1–10 y (median 5 y) | NA | Intrathecal MTX (n=15) | 12–54 Gy (n=15; median 18 Gy) | ✓ | ||||||||||||||||||||||||
| Mansky (126) 2007 | 32 plus 22 cont. | 7.1–34.2 y (median 15 y) | Sarcoma | 2.9–32.6 y (median 17.3 y) | Surgery (n=19) | DOXO, CPM, Ifos- famide, Etoposide | 3–63 Gy (n=28) | |||||||||||||||||||||||||
| Locoregional functional limitations | ||||||||||||||||||||||||||||||||
| Trahair (53) 2007 | 40 | 0–10.8 y (median 2.7 y) | NB high risk | 0.6–17.8 y (median 4.6 y) | All had HCT | Chemo only conditioning (n=6) | 18 Gy (local) 12 Gy (n=34; TBI) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||
| Hartley (63) 2008 | 61 | 3–13 y (median 7 y) | MB and PNET | 13.8–74.9 mo (median 44 mo) | NA | All had chemo CPM, VCR, CSPL | 36–39.6 Gy (n=26) 23.4 Gy (n=35); CSRT | ✓ | x | ✓ | ||||||||||||||||||||||
| Choi (68) 2010 | 32 orbits | 2–65 mo (median 7 mo) | RB | 55–249 m (median150 mo) | Enucleation (n=14 orbits) | NA | Orbital RT (n=28) 35–54.9 Gy | ✓ | ✓ | |||||||||||||||||||||||
| Van Dijk (29) 2010 | 185 | 0.3–16.5 y (median 3.7 y) | WT | 5–36.7 y (median 18.9 y) | All had nephrectomy | Chemo (n=182) SIOP protocol | RT (n=85) 13–41.1 Gy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||
| Barrena (54) 2011 | 188 | Not reported | NB/GN, PNET/Askin | 2.5 y 3.4 y | Surgical removal | SIOP guidelines | Spinal RT | ✓ | ✓ | ✓ | ||||||||||||||||||||||
| Rombi (127) 2012 | 30 | 1.8–21 y (median 10 y) | ES | Median 38.4 mo | Resection (n=13) | AEWS 0031 and POG protocol | All had proton RT median 54 Gy | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||
= age at study;
✓ = reported late effect or risk factor;
= older age at RT and male sex associated with growth retardation
Abbreviations: AEWS: Trial for Chemotherapy Intensification Through Interval Compression in Ewing’s Sarcoma; ALL: Acute Lymphoblastic Leukemia; ADR: Adriamycin; AMD: Actinomycin-D; B/L: Bilateral; CCG: Children’s Cancer Group; Chemo: Chemotherapy; Co-60: Cobalt-60; cont.: controls; CPL: Carboplatin; CPM: Cyclophosphamide; CML; Chronic Myeloid Leukemia; CRT: Cranial Radiotherapy; CSPL: Cisplatin; CSRT: Craniospinal Radiotherapy; DOXO: doxorubicin; EORTC: European Organization for Research and Treatment of Cancer; ES: Ewing’s Sarcoma; GCT: Germ Cell Tumor; GN: Ganglioneuroma; Gy: Gray; HL: Hodgkin lymphoma; HCT: Hematopoietic Cell Transplant; LLD: limb length discrepancy; mo: months; MB: Medulloblastoma; MELPH: Melphalan; MeV: Megavoltage; MOPP: Mechlorethamine, Vincristine, Procarbazine and Prednisone; MP: Mercaptopurine; MTX: Methotrexate; NA: Not applicable; NB: Neuroblastoma; NHL: non-Hodgkin Lymphoma; NWTS: National Wilms Tumor Study; NPC: Nasopharyngeal carcinoma; OB: Osteoblastoma; OV: Orthovoltage; PNET: Primitive Neuroectodermal Tumor; POG: Pediatric Oncology Group; PRED: Prednisone; RB: Retinoblastoma; RMS: Rhabdomyosarcoma; RT: Radiotherapy; SIOP: International Society of Pediatric Oncology; TBI: Total Body Irradiation; UKW1: United Kingdom Children’s Cancer Study Group First Wilms Tumor Study; U/L: Unilateral; VCR: Vincristine; VP-16: Etoposide; WT: Wilms tumor; y: years.
The underlying pathophysiology linking radiation to muscular late effects is unclear. However, acutely, radiation prevents mitosis of progenitor myosatellite cells (16), disrupts cell membrane permeability and lipid fluidity, and can result in sodium-potassium pump failure at the neuromuscular junction (17). Additionally, in the long-term, post-radiation inflammation, mediated by the transforming growth factor-β family, may prevent muscle growth (18), radiation-induced vascular and parenchymal damage may interfere with muscle nutrition (19), and mantle radiation may result in nemaline myopathy (20), potentially contributing to muscular atrophy (21–26), fibrosis (25–27), and hypoplasia (28).
Younger age and radiation doses ≥ 20 Gy influence the impact of radiation on muscle (28, 29). Thus, muscular late effects are most common among survivors of dysontogenetic tumors such as Wilms tumor (21–24, 29, 30) and neuroblastoma (27, 30) because these tumors typically present in early childhood and require radiation in cases of advanced disease (31). In contrast, while sarcomas (25, 26, 32) present across a wider age spectrum, survivors of these tumors are vulnerable to muscular late effects, not exclusively because of young age at exposure but because high doses of radiation (> 30 Gy) are required to achieve local control if complete surgical resection is not feasible.
All but two studies (24, 29) describing associations between radiotherapy and muscular late effects had small sample sizes (21–23, 25–28, 30, 32–36). Among 185 survivors of Wilms tumor treated at a median age of 3.7 and followed for a median of 18.9 years, Van Dijk et al. (29) reported an increased risk of tissue hypoplasia with increased dose of chest (Odds Ratio [OR]: 1.10 Gy −1; 95% Confidence Interval [CI]: 1.03–1.18) and flank/abdominal (OR: 1.17 Gy −1; 95% CI: 1.10–1.24) radiation. The authors also observed that female sex, younger age at diagnosis, and treatment with anthracyclines increased risk of tissue hypoplasia. A National Wilms Tumor Study (NWTS) analysis (n = 680) reported a higher prevalence of muscular atrophy five years after radiotherapy among survivors of Wilms tumor diagnosed between 1969 and 1979 who received radiation (30.2%) than among those who did not (1%) (24). Post-radiation muscular atrophy is uncommon for survivors of Wilms tumor treated after 1980 because unlike the historical age-adjusted 18–40 Gy radiotherapy, contemporary Wilms tumor patients are treated with 10.8 Gy (31).
Skeletal Late Effects of Radiotherapy (Table 1)
Acutely, radiation damages DNA in osteocytes by causing single or double strand breaks, sugar damage, base damage, by creating protein crosslinks, or by producing free hydroxyl radicals (37, 38). Animal and clinical evidence suggests that radiation may affect bone formation by: 1) arresting chondrogenesis at the epiphyseal growth plate, 2) inducing absorption failure of calcified cartilage and bone at the metaphysis, and 3) altering diaphyseal periosteal activity (39–41). Flat bones grow primarily by membranous ossification may present with diminished or asymmetrical growth after irradiation (Figure 2) (33).
Figure 2.
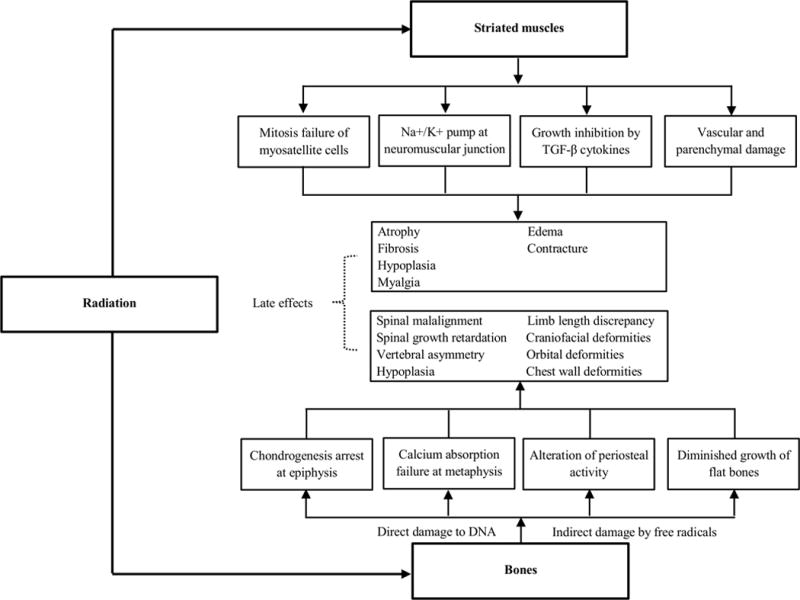
Effect of radiation on striated muscles and bones in childhood cancer patients
Spinal Malalignment
One reported effect of radiotherapy is alteration in axial alignment, often presenting long after treatment as scoliosis or kyphosis (Supplementary figure). In this review, the prevalence of post-radiotherapy scoliosis ranged from 10–80% (42, 43) and kyphosis from 2–48% (23, 43). In a cohort of 123 Wilms tumor survivors followed for a median of 18.9 years, the authors reported a 36.6% prevalence of scoliosis or kyphosis. Most cases (93.3%) were among survivors with a history of radiation (29). An NWTS analysis (n = 680) also reported a higher prevalence of scoliosis or kyphosis among survivors who had received radiation (51.4%) compared to those who had not (4.1%) (24). The evidence for the association between radiotherapy and spinal malalignment is strengthened by other studies (4, 21–23, 27, 28, 30, 34, 36, 42–55).
The effect of radiation on spinal malalignment is influenced by younger age, higher doses, and asymmetric radiation. Differential effects of radiation are reported based on age cutoffs of six months (51), one year (45), five years (23), or six years (44), depending on the population being studied. Similarly, varied thresholds for radiation dose are reported to increase risk for spinal malalignment including, > 20 Gy (34), > 23 Gy (52), > 24 Gy (28), and > 26 Gy (23). Asymmetric radiation increases the risk of spinal deformity in two ways (27, 34, 47). It is possible that radiation impairs the vertebral growth plate more on one side than the other. However, because vertebral bodies are small, it is likely difficult to impair only a portion of a vertebra. Two authors have speculated that spinal deformity is the result of tethering in atrophied paraspinal muscles (23, 34). Butler et al. (47) found that the prevalence of scoliosis after an average follow-up of 9.9 years after diagnosis was higher among survivors of Wilms tumor (63%) and neuroblastoma (83%), who had received asymmetric radiation, than among Hodgkin lymphoma survivors (39%), who received symmetric radiation. High rates of post-irradiation spinal deformity have gradually declined with refinement in radiotherapy techniques for solid tumors and with the use of lower radiation doses (31).
Spinal Growth Retardation
Although growth retardation is reported as an indirect result of growth hormone deficiency after cranial radiation (56), there is also evidence to suggest an association between spine directed radiation and abnormal vertebral growth. Survivors with this impairment are not only shorter in stature but are also cosmetically challenged as their trunk length is not congruent with normal length arms and legs. In a study of 124 Hodgkin lymphoma survivors treated when younger than 16 years of age, those treated before puberty with ≥ 33 Gy radiation to the entire spine and evaluated 2 years later had both standing (7.7%) and sitting height (8.2%) deficits (57). Other studies support the association between spinal radiation and growth retardation (42, 44, 58–61).
Both younger age (42, 58–61) and higher radiation doses (61–63) are associated with spinal growth retardation. Age-related effects of radiotherapy on spinal growth are described with age cutoffs of one year (59), three years (42), six years (58), and before the onset of puberty (60). Hartley et al. (63) found impaired vertebral growth among survivors of medulloblastoma and supratentorial primitive neuroectodermal tumor (median follow-up: 44 months) who received 36–39.6 Gy craniospinal irradiation (n = 26) than among those who received 23.4 Gy (n = 35).
Vertebral Asymmetry
Among cohorts of Wilms tumor survivors treated before 1980, spinal radiotherapy is associated with anterior beaking of vertebrae (23, 43, 45, 46), which results in progressive spinal deformity, typically kyphosis, later in life (64, 65). Riseborough et al. (23) reported beaking of vertebral bodies among 16 of 81 Wilms tumor survivors followed a median of 12 years; 74 had received 31 Gy orthovoltage radiation.
Skeletal Hypoplasia
The inhibitory effect of radiation on osteogenesis commonly manifests as hypoplasia of bones among survivors of childhood cancer (45, 48, 50, 51, 55, 66), reported especially in flat bones (21, 33, 42, 45–47, 50, 66–69). In a study of 89 survivors of various childhood cancers treated at a median age of 3.8 years and followed for an average of 10.8 years, Crooks et al. (48) observed a higher prevalence of skeletal hypoplasia among survivors treated with > 25 Gy than among those treated with ≤ 25 Gy radiation (9/16 vs. 0/8).
Limb Length Discrepancy
Limb length discrepancy may develop in survivors treated with extremity radiation for Ewing sarcoma (25, 26, 32, 70) or abdominal radiation for Wilms tumor when the hemipelvis is included in the radiation field (28, 29, 49). Because the proximal femoral epiphysis accounts for only 30% of growth (71), most of the discrepancy observed among Wilms tumor survivors is likely due to the tilt resulting from iliac wing hypoplasia. Jentzsch et al. (26) observed limb length differences of > 1.5 cm among 9 of 22 lower extremity Ewing sarcoma survivors 2 years after treatment with a median dose of 50 Gy. Among 42 Wilms tumor survivors treated with abdominal radiation of 10–40 Gy (28), five survivors developed limb length differences ranging from 0.8 to 2.5 cm. Although cutoff criteria for defining limb length discrepancy varies across studies, the prevalence is higher among Ewing sarcoma (25, 26, 47) than among Wilms tumor survivors (29, 49). The severity of limb length discrepancy is influenced by younger age at radiation (25, 26). In a study of extremity sarcoma survivors followed for a median of 13 years, those radiated when younger than 10 years had greater limb length discrepancy than those radiated when older than 10 years of age (median: 4.4 vs. 1.2 cm) (25).
Craniofacial Deformities
Craniofacial deformities are reported after radiotherapy for head and neck tumors (72–74) including retinoblastoma (68) and rhabdomyosarcoma (69, 75–80). In a recent study, all of 25 retinoblastoma survivors treated with 35–54.9 Gy and followed for 5 to 21 years presented with midfacial hypoplasia (68). Another study reported facial deformity in 73% of survivors of head and neck rhabdomyosarcoma exposed to 41.4–65.0 Gy and followed for a median of 20 years (75).
The impact of radiation on craniofacial structure is age (73, 74, 77, 81) and dose dependent (72, 81). Younger age at diagnosis (2–9 vs. 11–13 years) was associated with a higher prevalence of craniofacial deformities (16/16 vs. 0/4) in survivors of head and neck sarcoma (77). Craniofacial deformity assessed using cephalometric analysis of lateral radiographs was present among 18 of 20 ALL survivors treated when younger than 5 years of age with 24 Gy but not among those treated when older than 5 years of age or those treated with < 24 Gy cranial irradiation (81).
Orbital Deformities
Several observational studies (68, 75, 78, 82–86) have reported associations between radiotherapy and orbital hypoplasia. This association is also influenced by younger age at radiation (82). Imhof et al. (82) examined 68 retinoblastoma survivors (120 affected orbits) diagnosed at a mean age of 11 months, treated with 42 Gy radiation, and followed for 96 months. Among 16 unilateral retinoblastoma survivors treated with radiotherapy alone, the mean orbital width, height, and edge-tragus distance were shorter in irradiated orbits when compared to contralateral non-irradiated orbits. Mean orbital widths, heights, and edge-tragus distances of irradiated orbits improved with increasing age at radiation (82).
Chest Wall Deformities
Although usually reported as a late effect of surgery, chest wall deformities may result from radiotherapy (22, 47, 55, 66, 87). These deformities are not only important cosmetically but also impact risk for scoliosis and restrictive lung disease. Among 143 survivors treated with 19.5–50 Gy spinal and/or extremity radiation at a mean age of 8 years and followed for an average of 10 years, Butler et al. (47) observed chest and rib deformities in 36%. A higher prevalence of chest and rib deformities (63%) was observed in a subsample of 30 Wilms tumor survivors following asymmetric radiation (median dose 20 Gy) that included caudal ribs (47). Among 15 early-stage Hodgkin lymphoma survivors treated with 15 Gy asymmetric mantle radiation at a median age of 13 years (median follow-up of 39.6 months), Merchant et al. (87) observed impaired growth in fully versus partially irradiated clavicles (1.3 ± 1.1 vs. 1.8 ± 1.2 cm).
Osteochondroma
Osteochondromas are observed after exposure to TBI (53, 88) or local radiotherapy (23, 24, 28, 43, 45, 46, 51, 55), with a suggested higher prevalence reported following TBI. Assessments of TBI-exposed survivors reported osteochondroma among 9% (88) to 42% (53). Evans et al. (24) noted osteochondroma in 3.5% Wilms tumor survivors treated with local radiation, and Pastore et al. (55) observed osteochondroma in 8% survivors treated with an average of 21 Gy radiation. Although most osteochondromas do not require any treatment, some may require intervention if the tumor becomes abnormally large or symptomatic (89) or undergoes malignant transformation to secondary chondrosarcomas (90).
Muscular Late Effects of Chemotherapy (Table 2)
Table 2.
Summary of 6 reports of chemotherapy-associated musculoskeletal late effects in long-term survivors of childhood cancer
| First Author (Year) |
Sample Size (n) |
Age at Diagnosis |
Diagnosis | Time since Diagnosis |
Surgery | Chemotherapy Agents |
Radiotherapy (RT) and Dose |
Late Effects | Risk Factors | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loss of muscle strength | Spinal growth retardation | Nephrotoxicity | Rickets | Younger age at treatment | Accompanied RT | Vincristine & Methotrexate | Ifosfamide | L-asparaginase | ||||||||
| Olshan (96) 1992 | 38 | 1.3–11.3 y (mean 6.8 y) | MB | 4 consecutive years follow-up | All had surgery | Chemo (n=23), VCR, lomustine and CSPL | CSRT 38.1 Gy and 36.3 Gy with chemo | ✓ | ✓ | |||||||
| Hovi (91) 1993 | 43 plus 69 controls | 2–14 y (mean 7 y) | ALL | 1–19 y (mean 8 y) | NA | All had Scandinavian protocol | 24 Gy (n=32; CRT) 10–12 Gy (n=6; TBI) | ✓ | ✓ | ✓ | ||||||
| Loebstein (97) 1999 | 174 | 0.4–21.2 y (median 8 y) | Various cancers | 5 y | NA | All had IF and Mesna CSPL (n=123) | NA | ✓ | ✓ | ✓ | ✓ | |||||
| Chow (60) 2007 | 2434 plus 3009 siblings | 0–18 y | ALL | ≥ 5 y | NA | All had chemo | RT (65.1%) CRT and CSRT | ✓ | ✓ | |||||||
| Hartman (93) 2008 | 92 plus 155 cont. | Mean 4.3 ± 2.0 y | ALL, WT, NHL, others | 1–7.5 y (mean 3.3 y) | NA | Dutch Childhood Oncology Protocol | RT (n=9) | ✓ | ||||||||
| Ness (92) 2012 | 415 | 0.2–18.8 y (median 4.8 y) | ALL | 13.7–46.5 y (median 29.9 y) | NA | VCR (n=415) MTX (n=385) | CRT (n=305) | ✓ | ✓ | |||||||
✓ = reported late effect or risk factor
Abbreviations: ALL: Acute Lymphoblastic Leukemia; ADR: Adriamycin; CRT: Cranial Radiotherapy; CSPL: Cisplatin; Gy: Gray; IF: Ifosfamide; MB: Medulloblastoma; MTX: Methotrexate; NA: Not applicable; NHL: non-Hodgkin Lymphoma; TBI: Total Body Irradiation; VCR: Vincristine; WT: Wilms tumor; y: years.
Recent studies have reported muscular late effects of chemotherapeutic agents including L-asparaginase, methotrexate, and vincristine that commonly present as loss of muscular strength (91–93) and/or flexibility (92). The inhibitory effect of L-asparaginase on protein synthesis (94) and the neurotoxicity (95) of methotrexate and vincristine may be responsible for these long-term muscular late-effects. In a study of 43 female survivors of ALL (mean follow-up: 8 years), Hovi et al. (91), even after accounting for physical activity levels, reported an association between L-asparaginase exposure and muscle weakness (effect sizes: 0.83–1.01). A more recent study of 415 survivors of ALL (median follow-up: 29.9 years) observed that survivors treated with cumulative dose of intrathecal methotrexate ≥ 215 mg/m2 were at a higher risk for impaired strength, walking efficiency, and ankle range of motion than those not treated with methotrexate (92). Restricted ankle range of motion was also associated with a cumulative dose of vincristine ≥ 39 mg/m2 (92).
Skeletal Late Effects of Chemotherapy (Table 2)
The skeletal late effects of chemotherapy have been observed as a result of additive effect with radiotherapy (96) or as an independent effect due to renal injury (97). Medulloblastoma survivors treated with both chemotherapy and craniospinal irradiation had lower growth velocities than those who received craniospinal irradiation alone (SDS: −3.57 vs. −0.88) when assessed for four consecutive years after treatment (96). However, the mechanism of this additive effect is unclear. Ifosfamide induces proximal tubular injury to the kidneys, presenting as loss of glucose, proteins, phosphate and bicarbonates (98), and is associated with rickets after an average of 5 years follow-up (97).
Skeletal Late Effects of Surgery (Table 3)
Table 3.
Summary of 18 reports of musculoskeletal late effects associated with surgery in long-term survivors of childhood cancer
| First Author (Year) |
Sample Size (n) |
Age at Diagnosis |
Diagnosis | Time since Diagnosis |
Surgery | Chemotherapy Agents |
Radiotherapy (RT) and Dose |
Late Effects | Risk Factors | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scoliosis | Kyphosis | Paraparesis/motor deficit | Tibial torsion/contractures | Orbital growth retardation | Orbital asymmetry | Orbital implant migration | Younger age at treatment | Laminectomy | Thoracotomy | Number of ribs resected | No orbital implant | Small orbital implant | Accompanied RT | Follow-up time | ||||||||
| Osborne (109) 1974 | 65 | 0–13+ y | NA | 1–11+ y | Enucleation (31 with implant and 34 without implant) | NA | NA | ✓ | ✓ | ✓ | ✓ | |||||||||||
| King (103) 1975 | 16 | 0–15 y | NB intraspinal | ≥ 2 y | Resection; complete (n=11), partial (n=5) | Chemo (n=3) CPM | All had RT | ✓ | ✓ | ✓ | ||||||||||||
| Ameniya (83) 1977 | 17 | Mean 1.6 y | RB U/L | Mean 8.8 y | Enucleation | NA | RT (n=12) | ✓ | ✓ | |||||||||||||
| Kumar (107) 1977 | 22 | 3.5–25 y (median 10 y) | ES, RMS, NB, OS. | 7–175 mo | Resection (n=11) | Chemo (n=21) | 23.0–56.1 Gy (n=17; Co-60) | ✓ | ✓ | |||||||||||||
| Kennedy (110) 1992 | 42 | 0–15 y (median 4 y) | Tumors & trauma | 22 mo–40 y (mean 11.25 y) | Enucleation (29 with implant and 13 without) | NA | NA | ✓ | ✓ | ✓ | ||||||||||||
| Plantaz (104) 1996 | 42 | 1 d–14 y (median 8 mo) | NB intraspinal | 0–66 mo | Resection (n=40) Neurosurgery (n=17) | Chemo (n=32) | RT (n=3) | ✓ | ✓ | ✓ | ||||||||||||
| Kaste (85) 1997 | 54 (82 orbits) | 0–6.9 y (median 13 mo) | RB | 4.9–25.8 y (median 7.5 y) | Enucleation (n=51) with RT (n=26) | NA | 22.5–44 Gy (n=29) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Hoover (105) 1999 | 26 | 4 d–6.5 y (median 0.9 y) | NB intraspinal | 2–29 y (median 10.1 y) | Laminectomy (n=15) | Chemo (n=17) | RT (n=4) | ✓ | ✓ | ✓ | ✓ | |||||||||||
| De Bernardi (100) 2001 | 54 | 0–15 y (median 16 mo) | NB intraspinal | 4–209 mo (median 139 mo) | Laminectomy (n=32) | Chemo (n=33) | RT (n=11) | ✓ | ✓ | ✓ | ||||||||||||
| Katzenstein (106) 2001 | 73 | 0–13 y 2m (median 10 mo) | NB Intraspinal | 0–10 y | Laminectomy (n=27) Resection (n=31) | Chemo (n=66) | RT (n=8) | ✓ | ✓ | |||||||||||||
| Nahum (112) 2001 | 21 (28 orbits) | Median 24 mo | RB | Median 12 y | Enucleation (n=21) | Chemo (n=8) | RT (n=21) | ✓ | ✓ | ✓ | ||||||||||||
| Peylan-Ramu (84) 2001 | 28 (45 orbits plus 45 control orbits) | Mean 13.3 mo | RB | 7.2–246 mo (median 50 mo) | Enucleation only (n=9) Enucleation with RT (10) | Chemo (n=17) | RT only (n=26) 44–54 Gy | ✓ | ✓ | |||||||||||||
| de Jonge (99) 2005 | 76 | 2 mo–16 y (mean 4 y 7 mo) | 9 mo–20.2 y (mean 6 y 7 mo) | Laminectomy (n=45) or Laminoplasty (n=10) | NA | 35–45 Gy (n=43) | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Lyle (111) 2007 | 33 | 1–82 mo (mean 21.4 mo) | RB 33 U/L | 31–251 mo (mean 141.2 mo) | Enucleation with implants | NA | NA | ✓ | ||||||||||||||
| Laverdiere (101) 2009 | 954 plus 3899 siblings | 0–20.7 y (median 0.9 y) | NB | 5.7–45.2 ya (median 23.3 y) | All except 16 had surgery | Chemo (n=484) | RT (n=400) | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Angelini (108) 2011 | 98 | 0–150 mo (median 8 mo) | NB | 2–23 y (median 7.3 y) | Neurosurgery (n=46) | Chemo (n=89) | RT (n=16) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Lin (114) 2011 | 18 | Mean 29 ± 23 mo | RB | Mean 49 ± 31 mo | Enucleation with implants | NA | NA | ✓ | ✓ | |||||||||||||
| Shildkrot (113) 2011 | 133 (135 orbits) | 0.2–9 y (median 2.2 y) | RB (128) others (6) | 0.1–9.3 y (median 3.6 y) | All had Enucleation and implants | Chemo (69) Chemo only (49) Chemo+ RT (20) | RT (n=21) | ✓ | ✓ | ✓ | ||||||||||||
= age at study; ✓ = reported late effect or risk factor
ADR: Adriamycin; AMD: Actinomycin-D; CPM: Cyclophosphamide; Co-60: Cobalt-60; CRT: Cranial Radiotherapy; ES: Ewing’s Sarcoma; Gy: Gray; mo: months; NB: Neuroblastoma; OS: Osteosarcoma; NA: Not applicable; POG: Pediatric Oncology Group; RMS: Rhabdomyosarcoma; U/L: Unilateral; VCR: Vincristine; y: year
Spinal Malalignment
Surgeries such as laminectomy for any type of spinal tumor (99), neuroblastoma (52, 100–102) or intraspinal neuroblastoma (103–106), and chest wall resection (107) are associated with spinal malalignment. A study of 954 neuroblastoma survivors treated primarily with surgery reported a 20-year cumulative incidence of 5.8% for scoliosis requiring surgical correction (101). The authors also found that neuroblastoma survivors were 27 times (95% CI: 13.6–53.4) more likely to develop severe scoliosis than their siblings. Laminectomy (Relative Risk [RR]: 11.0; 95% CI: 5.8–21.1), spinal radiotherapy (RR: 5.6; 95% CI: 1.7–18.4) and thoracotomy (RR: 3.1; 95% CI: 1.6–6.1) were independently associated with severe scoliosis (101). In a study of 26 intraspinal neuroblastoma survivors followed for a median of 10 years, Hoover et al. (105) reported scoliosis among 66.7% of those treated with, compared to 36.4% among those treated without, laminectomy.
Follow-up time (52, 99) and concomitant radiotherapy (107, 108) influence the effects of surgery on spinal malalignment. In a study of neuroblastoma survivors, Paulino et al. (52) observed that those treated with laminectomy developed scoliosis after a median of 23 months, and those treated with radiotherapy developed scoliosis after a median of 68.5 months. Among 98 neuroblastoma survivors with symptomatic epidural compression, the prevalence of spinal deformity among those who had chemotherapy, radiotherapy and surgery was 62.5% compared to those who had chemotherapy and surgery (30%) or surgery alone (50%) (108).
Orbital Deformities
Orbital hypoplasia (84, 85, 109–112) and orbital asymmetry (84, 85, 112) occur in retinoblastoma survivors after enucleation. Among 65 retinoblastoma survivors treated with unilateral enucleation, Osborne et al. (109) observed a higher mean percent difference in orbital volume (enucleated – contralateral) among those enucleated at 0–2 years (10.4%) and 3–12 years (9.6%) than those enucleated when older than 13 years of age (3.6%). In a cohort of 54 retinoblastoma survivors treated at a median age of 13 months and followed for a median of 7.5 years, Kaste et al. (85) observed that orbital volume of survivors treated with enucleation alone were smaller than the contralateral orbits (median volume difference = 1.5 cm3; p = 0.01)
There is consistent evidence suggesting that concomitant radiation (83, 112, 113), younger age at enucleation (110, 114), and use of appropriate orbital implants (85, 109–111) impact the effect of enucleation on orbital deformities. Ameniya et al. (83) reported greater differences in size between enucleated and contralateral orbits after an average of 8.8 years of follow-up among 12 retinoblastoma survivors treated with both radiation and enucleation compared to 5 survivors treated with enucleation alone. Another study of 18 unilateral retinoblastoma survivors noted that children younger than 1 year of age at enucleation had higher mean orbital volume differences compared to those treated when older than 1 year of age (1.8 ± 1.5 vs. 0.6 ± 0.6 cm3) (114). Kaste et al. (85) also observed that survivors treated with smaller orbital implants (12–14 mm) had higher prevalence (70%) of orbits with smaller volume than survivors treated with larger orbital implants (16 mm: 60% and 18–22 mm: 50%).
CONCLUSION AND FUTURE DIRECTION
This systematic review summarizes the evidence that describes treatment-related musculoskeletal late effects in childhood cancer survivors. It includes information about both well-established musculoskeletal toxicities and novel musculoskeletal toxicities identified in recent studies. This information provides a foundation for providers who care for these children. Providers can identify children most at risk for adverse musculoskeletal outcomes so that they can target surveillance for outcomes amenable to early intervention (e.g. limb length differences, muscle weakness), or counsel those children and families who will require intervention when they reach skeletal maturity (e.g. mid-facial hypoplasia).
There has been a gradual refinement in therapeutic approaches to reduce late effects (31, 115) and several studies included in this review were instrumental in this process. Identification of adverse musculoskeletal effects after asymmetric and high-dose radiation among children treated for Wilms tumor led to the use of symmetric administration and lowering of radiation dose and restriction of radiation to only those with advanced stage tumor (31). Despite the gradual refinement in therapeutic approaches, many contemporary protocols include the same chemotherapeutic agents used in the past and may also include high-dose radiotherapy for advanced stage disease and radiotherapy for younger patients (31).
Very few studies have evaluated the musculoskeletal late effects of contemporary protocols and most of the studies in our review were conducted in small convenience cohorts. Therefore, continued work is needed to document musculoskeletal outcomes among those treated with newer radiotherapy delivery methods (116), personalized chemotherapy (117), and modern surgical management (118) so that risk factors can be updated for survivors treated on contemporary protocols. Continued observational research will help clinicians identify survivors at high risk of developing musculoskeletal late effects with improved precision and perhaps facilitate the design of interventions structured to ameliorate musculoskeletal late effects.
Acknowledgments
Dr. Prasad L. Gawade: Dr. Gawade searched, selected and reviewed the articles, drafted the initial manuscript and approved the final manuscript as submitted.
Dr. Kirsten K. Ness: Dr. Ness conceptualized the systematic review, searched, selected and reviewed the articles, revised the manuscript and approved the final manuscript as submitted.
Dr. Melissa M. Hudson: Dr. Hudson reviewed and revised the manuscript and approved the final manuscript as submitted.
Dr. Sue. C. Kaste: Dr. Kaste reviewed and revised the manuscript and approved the final manuscript as submitted.
Dr. Joseph P. Neglia: Dr. Neglia reviewed and revised the manuscript and approved the final manuscript as submitted.
Dr. Karen Wasilewski-Masker: Dr. Wasilewski-Masker reviewed and revised the manuscript and approved the final manuscript as submitted.
Dr. Louis S. Constine: Dr. Constine reviewed and revised the manuscript and approved the final manuscript as submitted.
Dr. Leslie L. Robison: Dr. Robison reviewed and revised the manuscript and approved the final manuscript as submitted.
Funding source: This work was supported by a Cancer Center Support (CORE) grant CA 21765 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC).
Abbreviations
- ALL
Acute lymphoblastic leukemia
- BMD
Bone mineral density
- CI
Confidence interval
- DNA
Deoxyribonucleic acid
- Gy
Gray
- HCT
Hematopoietic cell transplant
- NHL
non-Hodgkin lymphoma
- NWTS
National Wilms Tumor Study
- RR
Relative risk
- SDS
Standard deviation score
- TBI
Total body irradiation
Footnotes
Financial disclosure statement: The authors have no financial interest to declare.
Conflict of interest statement: The authors have no conflicts of interest to declare.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute; 2013. [May 15, 2013]; based on November 2012 SEER data submission posted to the SEER web site April 3]. Available from: http://seer.cancer.gov/csr/1975_2010/ [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic Health Conditions in Adult Survivors of Childhood Cancer. New Engl J Med. 2006;355(15):1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MM, Mertens AC, Yasui Y, et al. Health Status of Adult Long-term Survivors of Childhood Cancer. JAMA. 2003;290(12):1583–92. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 4.Ness KK, Mertens AC, Hudson MM, et al. Limitations on Physical Performance and Daily Activities among Long-Term Survivors of Childhood Cancer. Ann Intern Med. 2005;143(9):639–47. doi: 10.7326/0003-4819-143-9-200511010-00007. [DOI] [PubMed] [Google Scholar]
- 5.Wasilewski-Masker K, Kaste SC, Hudson MM, et al. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics. 2008;121(3):e705–13. doi: 10.1542/peds.2007-1396. Epub 2008/03/04. [DOI] [PubMed] [Google Scholar]
- 6.Kaste SC, Karimova EJ, Neel MD. Osteonecrosis in Children After Therapy for Malignancy. AJR Am J Roentgenol. 2011;196(5):1011–8. doi: 10.2214/AJR.10.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu SC, Tsai CC, Huang CH. Atypical slipped capital femoral epiphysis after radiotherapy and chemotherapy. Clin Orthop Relat Res. 2004(426):212–8. doi: 10.1097/01.blo.0000136655.51838.84. Epub 2004/09/04. [DOI] [PubMed] [Google Scholar]
- 8.Lee DY, Choi IH, Lee CK, Chung CY, Cho KH. Acquired vitamin D-resistant rickets caused by aggressive osteoblastoma in the pelvis: a case report with ten years’ follow-up and review of the literature. J Pediatr Orthop. 1994;14(6):793–8. doi: 10.1097/01241398-199414060-00020. Epub 1994/11/01. [DOI] [PubMed] [Google Scholar]
- 9.Chemaitilly W, Sklar CA. Endocrine complications in long-term survivors of childhood cancers. Endocr-Relat Cancer. 2010;17(3):R141–59. doi: 10.1677/ERC-10-0002. Epub 2010/05/11. [DOI] [PubMed] [Google Scholar]
- 10.Nagarajan R, Neglia JP, Clohisy DR, Robison LL. Limb salvage and amputation in survivors of pediatric lower-extremity bone tumors: what are the long-term implications? J Clin Oncol. 2002;20(22):4493–501. doi: 10.1200/JCO.2002.09.006. Epub 2002/11/15. [DOI] [PubMed] [Google Scholar]
- 11.Kinahan KE, Sharp LK, Seidel K, et al. Scarring, Disfigurement, and Quality of Life in Long-Term Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2012;30(20):2466–74. doi: 10.1200/JCO.2011.39.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Punyko JA, Gurney JG, Scott Baker K, et al. Physical impairment and social adaptation in adult survivors of childhood and adolescent rhabdomyosarcoma: A report from the Childhood Cancer Survivors Study. Psycho-oncology. 2007;16(1):26–37. doi: 10.1002/pon.1072. Epub 2006/08/22. [DOI] [PubMed] [Google Scholar]
- 13.Nagarajan R, Clohisy DR, Neglia JP, et al. Function and quality-of-life of survivors of pelvic and lower extremity osteosarcoma and Ewing’s sarcoma: the Childhood Cancer Survivor Study. Br J Cancer. 2004;91(11):1858–65. doi: 10.1038/sj.bjc.6602220. Epub 2004/11/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLos Med. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West S, King V, Carey TS, et al. Systems to rate the strength of scientific evidence. Evid Rep Technol Assess (Summ) 2002(47):1–11. Epub 2002/05/01. [PMC free article] [PubMed] [Google Scholar]
- 16.Caiozzo VJ, Giedzinski E, Baker M, et al. The radiosensitivity of satellite cells: cell cycle regulation, apoptosis and oxidative stress. Radiat Res. 2010;174(5):582–9. doi: 10.1667/RR2190.1. Epub 2010/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leyko W, Bartosz G. Membrane effects of ionizing radiation and hyperthermia. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;49(5):743–70. doi: 10.1080/09553008514552971. Epub 1986/05/01. [DOI] [PubMed] [Google Scholar]
- 18.Anscher MS, Jirtle RL. Role of transforming growth factor-β and hepatocyte growth factor in late normal tissue effects of radiation. Radiat Oncol Invest. 1993;1(6):305–13. [Google Scholar]
- 19.Powers BE, Gillette EL, Gillette SL, LeCouteur RA, Withrow SJ. Muscle injury following experimental intraoperative irradiation. Int J Radiat Oncol Biol Phys. 1991;20(3):463–71. doi: 10.1016/0360-3016(91)90058-c. Epub 1991/03/01. [DOI] [PubMed] [Google Scholar]
- 20.Portlock CS, Boland P, Hays AP, Antonescu CR, Rosenblum MK. Nemaline myopathy: a possible late complication of Hodgkin’s disease therapy. Hum Pathol. 2003;34(8):816–8. doi: 10.1016/s0046-8177(03)00242-9. [DOI] [PubMed] [Google Scholar]
- 21.Oliver JH, Gluck G, Gledhill RB, Chevalier L. Musculoskeletal deformities following treatment of Wilms’ tumour. Can Med Assoc J. 1978;119(5):459–64. Epub 1978/09/09. [PMC free article] [PubMed] [Google Scholar]
- 22.Trobs RB, Hansel M, Friedrich T, Bennek J. A 23-year experience with malignant renal tumors in infancy and childhood. Eur J Pediatr Surg. 2001;11(2):92–8. doi: 10.1055/s-2001-13793. Epub 2001/05/24. [DOI] [PubMed] [Google Scholar]
- 23.Riseborough EJ, Grabias SL, Burton RI, Jaffe N. Skeletal alterations following irradiation for Wilms’ tumor: with particular reference to scoliosis and kyphosis. J Bone Joint Surg Am. 1976;58(4):526–36. Epub 1976/06/01. [PubMed] [Google Scholar]
- 24.Evans AE, Norkool P, Evans I, Breslow N, D’Angio GJ. Late effects of treatment for Wilms’ tumor. A report from the National Wilms’ Tumor Study Group. Cancer. 1991;67(2):331–6. doi: 10.1002/1097-0142(19910115)67:2<331::aid-cncr2820670202>3.0.co;2-7. Epub 1991/01/15. [DOI] [PubMed] [Google Scholar]
- 25.Paulino AC. Late effects of radiotherapy for pediatric extremity sarcomas. Int J Radiat Oncol Biol Phys. 2004;60(1):265–74. doi: 10.1016/j.ijrobp.2004.02.001. Epub 2004/09/01. [DOI] [PubMed] [Google Scholar]
- 26.Jentzsch K, Binder H, Cramer H, et al. Leg function after radiotherapy for Ewing’s sarcoma. Cancer. 1981;47(6):1267–78. doi: 10.1002/1097-0142(19810315)47:6<1267::aid-cncr2820470607>3.0.co;2-#. Epub 1981/03/15. [DOI] [PubMed] [Google Scholar]
- 27.Kajanti M. Neuroblastoma in 88 children. Clinical features, prognostic factors, results and late effects of therapy. Ann Clin Res. 1983;15(Suppl 39):1–68. Epub 1983/01/01. [PubMed] [Google Scholar]
- 28.Paulino AC, Wen BC, Brown CK, et al. Late effects in children treated with radiation therapy for Wilms’ tumor. Int J Radiat Oncol Biol Phys. 2000;46(5):1239–46. doi: 10.1016/s0360-3016(99)00534-9. Epub 2000/03/22. [DOI] [PubMed] [Google Scholar]
- 29.van Dijk IW, Oldenburger F, Cardous-Ubbink MC, et al. Evaluation of late adverse events in long-term wilms’ tumor survivors. Int J Radiat Oncol Biol Phys. 2010;78(2):370–8. doi: 10.1016/j.ijrobp.2009.08.016. Epub 2010/02/09. [DOI] [PubMed] [Google Scholar]
- 30.Makipernaa A, Heikkila JT, Merikanto J, Marttinen E, Siimes MA. Spinal deformity induced by radiotherapy for solid tumours in childhood: a long-term follow up study. Eur J Pediatr. 1993;152(3):197–200. doi: 10.1007/BF01956143. Epub 1993/03/01. [DOI] [PubMed] [Google Scholar]
- 31.Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013 Feb 15; doi: 10.1002/pbc.24487. [Epub ahead of print]. Epub 2013/02/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mameghan H, Fisher RJ, O’Gorman-Hughes D, et al. Ewing’s sarcoma: long-term follow-up in 49 patients treated from 1967 to 1989. Int J Radiat Oncol Biol Phys. 1993;25(3):431–8. doi: 10.1016/0360-3016(93)90064-3. Epub 1993/02/15. [DOI] [PubMed] [Google Scholar]
- 33.Larson DL, Kroll S, Jaffe N, Serure A, Goepfert H. Long-term effects of radiotherapy in childhood and adolescence. Am J Surg. 1990;160(4):348–51. doi: 10.1016/s0002-9610(05)80540-9. Epub 1990/10/01. [DOI] [PubMed] [Google Scholar]
- 34.Mayfield JK, Riseborough EJ, Jaffe N, Nehme ME. Spinal deformity in children treated for neuroblastoma. J Bone Joint Surg Am. 1981;63(2):183–93. Epub 1981/02/01. [PubMed] [Google Scholar]
- 35.Dahllof G, Krekmanova L, Kopp S, et al. Craniomandibular dysfunction in children treated with total-body irradiation and bone marrow transplantation. Acta Odontol Scand. 1994;52(2):99–105. doi: 10.3109/00016359409029062. Epub 1994/04/01. [DOI] [PubMed] [Google Scholar]
- 36.Pinter AB, Hock A, Kajtar P, Dober I. Long-term follow-up of cancer in neonates and infants: a national survey of 142 patients. Pediatr Surg Int. 2003;19(4):233–9. doi: 10.1007/s00383-002-0760-0. [DOI] [PubMed] [Google Scholar]
- 37.Baverstock KF, Will S. Evidence for the dominance of direct excitation of DNA in the formation of strand breaks in cells following irradiation. Int J Radiat Biol. 1989;55(4):563–8. doi: 10.1080/09553008914550611. Epub 1989/04/01. [DOI] [PubMed] [Google Scholar]
- 38.Powell S, McMillan TJ. DNA damage and repair following treatment with ionizing radiation. Radiother Oncol. 1990;19(2):95–108. doi: 10.1016/0167-8140(90)90123-e. Epub 1990/10/01. [DOI] [PubMed] [Google Scholar]
- 39.De Smet AA, Kuhns LR, Fayos JV, Holt JF. Effects of radiation therapy on growing long bones. AJR Am J Roentgenol. 1976;127(6):935–9. doi: 10.2214/ajr.127.6.935. Epub 1976/12/01. [DOI] [PubMed] [Google Scholar]
- 40.Eifel PJ, Donaldson SS, Thomas PR. Response of growing bone to irradiation: a proposed late effects scoring system. Int J Radiat Oncol Biol Phys. 1995;31(5):1301–7. doi: 10.1016/0360-3016(94)00420-P. Epub 1995/03/30. [DOI] [PubMed] [Google Scholar]
- 41.Bluemke DA, Fishman EK, Scott WW., Jr Skeletal complications of radiation therapy. Radiographics. 1994;14(1):111–21. doi: 10.1148/radiographics.14.1.8128043. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 42.Wallace WH, Shalet SM, Morris-Jones PH, Swindell R, Gattamaneni HR. Effect of abdominal irradiation on growth in boys treated for a Wilms’ tumor. Med Pediatr Oncol. 1990;18(6):441–6. doi: 10.1002/mpo.2950180602. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 43.Heaston D, Libshitz H, Chan R. Skeletal effects of megavoltage irradiation in survivors of Wilms’ tumor. AJR Am J Roentgenol. 1979;133(3):389–95. doi: 10.2214/ajr.133.3.389. [DOI] [PubMed] [Google Scholar]
- 44.Probert JC, Parker BR. The effects of radiation therapy on bone growth. Radiology. 1975;114(1):155–62. doi: 10.1148/114.1.155. Epub 1975/01/01. [DOI] [PubMed] [Google Scholar]
- 45.Smith R, Davidson JK, Flatman GE. Skeletal effects of orthovoltage and megavoltage therapy following treatment of nephroblastoma. Clin Radiol. 1982;33(6):601–13. doi: 10.1016/s0009-9260(82)80380-2. Epub 1982/11/01. [DOI] [PubMed] [Google Scholar]
- 46.Thomas PR, Griffith KD, Fineberg BB, Perez CA, Land VJ. Late effects of treatment for Wilms’ tumor. Int J Radiat Oncol Biol Phys. 1983;9(5):651–7. doi: 10.1016/0360-3016(83)90230-4. Epub 1983/05/01. [DOI] [PubMed] [Google Scholar]
- 47.Butler MS, Robertson WW, Jr, Rate W, D’Angio GJ, Drummond DS. Skeletal sequelae of radiation therapy for malignant childhood tumors. Clin Orthop Relat Res. 1990(251):235–40. Epub 1990/02/01. [PubMed] [Google Scholar]
- 48.Crooks GM, Baron-Hay GS, Byrne GC, et al. Late effects of childhood malignancies seen in Western Australia. Am J Pediatr Hematol Oncol. 1991;13(4):442–9. doi: 10.1097/00043426-199124000-00009. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 49.Rate WR, Butler MS, Robertson WW, Jr, D’Angio GJ. Late orthopedic effects in children with Wilms’ tumor treated with abdominal irradiation. Med Pediatr Oncol. 1991;19(4):265–8. doi: 10.1002/mpo.2950190410. Epub 1991/01/11. [DOI] [PubMed] [Google Scholar]
- 50.Hale GA, Marina NM, Jones-Wallace D, et al. Late effects of treatment for germ cell tumors during childhood and adolescence. J Pediatr Hematol Oncol. 1999;21(2):115–22. doi: 10.1097/00043426-199903000-00007. Epub 1999/04/17. [DOI] [PubMed] [Google Scholar]
- 51.Paulino AC, Mayr NA, Simon JH, Buatti JM. Locoregional control in infants with neuroblastoma: role of radiation therapy and late toxicity. Int J Radiat Oncol Biol Phys. 2002;52(4):1025–31. doi: 10.1016/s0360-3016(01)02713-4. Epub 2002/04/18. [DOI] [PubMed] [Google Scholar]
- 52.Paulino AC, Fowler BZ. Risk factors for scoliosis in children with neuroblastoma. Int J Radiat Oncol Biol Phys. 2005;61(3):865–9. doi: 10.1016/j.ijrobp.2004.07.719. [DOI] [PubMed] [Google Scholar]
- 53.Trahair TN, Vowels MR, Johnston K, et al. Long-term outcomes in children with high-risk neuroblastoma treated with autologous stem cell transplantation. Bone Marrow Transplant. 2007;40(8):741–6. doi: 10.1038/sj.bmt.1705809. Epub 2007/08/29. [DOI] [PubMed] [Google Scholar]
- 54.Barrena S, Miguel M, de la Torre CA, et al. Late Surgery for Spinal Deformities in Children Previously Treated for Neural Tumors. Eur J Pediatr Surg. 2011;21(1):54–7. doi: 10.1055/s-0030-1267968. [DOI] [PubMed] [Google Scholar]
- 55.Pastore G, Antonelli R, Fine W, Li FP, Sallan SE. Late effects of treatment of cancer in infancy. Med Pediatr Oncol. 1982;10(4):369–75. doi: 10.1002/mpo.2950100407. Epub 1982/01/01. [DOI] [PubMed] [Google Scholar]
- 56.Gurney JG, Ness KK, Stovall M, et al. Final height and body mass index among adult survivors of childhood brain cancer: childhood cancer survivor study. J Clin Endocrinol Metab. 2003;88(10):4731–9. doi: 10.1210/jc.2003-030784. Epub 2003/10/15. [DOI] [PubMed] [Google Scholar]
- 57.Willman KY, Cox RS, Donaldson SS. Radiation induced height impairment in pediatric Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 1994;28(1):85–92. doi: 10.1016/0360-3016(94)90144-9. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 58.Probert JC, Parker BR, Kaplan HS. Growth retardation in children after megavoltage irradiation of the spine. Cancer. 1973;32(3):634–9. doi: 10.1002/1097-0142(197309)32:3<634::aid-cncr2820320316>3.0.co;2-a. Epub 1973/09/01. [DOI] [PubMed] [Google Scholar]
- 59.Shalet SM, Gibson B, Swindell R, Pearson D. Effect of spinal irradiation on growth. Arch Dis Child. 1987;62(5):461–4. doi: 10.1136/adc.62.5.461. Epub 1987/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chow EJ, Friedman DL, Yasui Y, et al. Decreased Adult Height in Survivors of Childhood Acute Lymphoblastic Leukemia: A Report from the Childhood Cancer Survivor Study. J Pediatr. 2007;150(4):370–5. doi: 10.1016/j.jpeds.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hogeboom CJ, Grosser SC, Guthrie KA, et al. Stature loss following treatment for Wilms tumor. Med Pediatr Oncol. 2001;36(2):295–304. doi: 10.1002/1096-911X(20010201)36:2<295::AID-MPO1068>3.0.CO;2-Y. Epub 2001/07/17. [DOI] [PubMed] [Google Scholar]
- 62.Silber JH, Littman PS, Meadows AT. Stature loss following skeletal irradiation for childhood cancer. J Clin Oncol. 1990;8(2):304–12. doi: 10.1200/JCO.1990.8.2.304. Epub 1990/02/01. [DOI] [PubMed] [Google Scholar]
- 63.Hartley KA, Li C, Laningham FH, et al. Vertebral body growth after craniospinal irradiation. Int J Radiat Oncol Biol Phys. 2008;70(5):1343–9. doi: 10.1016/j.ijrobp.2007.08.085. Epub 2008/01/01. [DOI] [PubMed] [Google Scholar]
- 64.Field RE, Buchanan JA, Copplemans MG, Aichroth PM. Bone-marrow transplantation in Hurler’s syndrome. Effect on skeletal development. J Bone Joint Surg Br. 1994;76(6):975–81. Epub 1994/11/01. [PubMed] [Google Scholar]
- 65.Tandon V, Williamson JB, Cowie RA, Wraith JE. Spinal problems in mucopolysaccharidosis I (Hurler syndrome) J Bone Joint Surg Br. 1996;78(6):938–44. doi: 10.1302/0301-620x78b6.1279. Epub 1996/11/01. [DOI] [PubMed] [Google Scholar]
- 66.Taylor RE. Morbidity from abdominal radiotherapy in the First United Kingdom Children’s Cancer Study Group Wilms’ Tumour Study. United Kingdom Children’s Cancer Study Group. Clin Oncol (R Coll Radiol) 1997;9(6):381–4. doi: 10.1016/s0936-6555(97)80131-8. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 67.Carli M, De Bernardi B, Castello M, et al. Long-term results in childhood rhabdomyosarcoma: a retrospective study in Italy. Pediatr Hematol Oncol. 1986;3(4):371–8. doi: 10.3109/08880018609031241. Epub 1986/01/01. [DOI] [PubMed] [Google Scholar]
- 68.Choi SY, Kim MS, Yoo S, et al. Long term follow-up results of external beam radiotherapy as primary treatment for retinoblastoma. J Korean Med Sci. 2010;25(4):546–51. doi: 10.3346/jkms.2010.25.4.546. Epub 2010/04/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaste SC, Hopkins KP. Micrognathia after radiation therapy for childhood facial tumors. Report of two cases with long-term follow-up. Oral Surg Oral Med Oral Pathol. 1994;77(1):95–9. doi: 10.1016/s0030-4220(06)80115-5. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 70.Brown AP, Fixsen JA, Plowman PN. Local control of Ewing’s sarcoma: an analysis of 67 patients. Br J Radiol. 1987;60(711):261–8. doi: 10.1259/0007-1285-60-711-261. Epub 1987/03/01. [DOI] [PubMed] [Google Scholar]
- 71.Digby KH. The Measurement of Diaphysial Growth in Proximal and Distal Directions. J Anat Physiol. 1916;50(Pt 2):187–8. Epub 1916/01/01. [PMC free article] [PubMed] [Google Scholar]
- 72.Guyuron B, Dagys AP, Munro IR, Ross RB. Effect of irradiation on facial growth: a 7- to 25-year follow-up. Ann Plast Surg. 1983;11(5):423–7. doi: 10.1097/00000637-198311000-00010. Epub 1983/11/01. [DOI] [PubMed] [Google Scholar]
- 73.Karsila-Tenovuo S, Jahnukainen K, Peltomaki T, et al. Disturbances in craniofacial morphology in children treated for solid tumors. Oral Oncol. 2001;37(7):586–92. doi: 10.1016/s1368-8375(01)00002-1. Epub 2001/09/21. [DOI] [PubMed] [Google Scholar]
- 74.Denys D, Kaste SC, Kun LE, et al. The effects of radiation on craniofacial skeletal growth: a quantitative study. Int J Pediatr Otorhinolaryngol. 1998;45(1):7–13. doi: 10.1016/s0165-5876(98)00028-7. Epub 1998/11/06. [DOI] [PubMed] [Google Scholar]
- 75.Paulino AC, Simon JH, Zhen W, Wen BC. Long-term effects in children treated with radiotherapy for head and neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2000;48(5):1489–95. doi: 10.1016/s0360-3016(00)00799-9. Epub 2000/12/21. [DOI] [PubMed] [Google Scholar]
- 76.Jaffe N, Toth BB, Hoar RE, et al. Dental and maxillofacial abnormalities in long-term survivors of childhood cancer: effects of treatment with chemotherapy and radiation to the head and neck. Pediatrics. 1984;73(6):816–23. Epub 1984/06/01. [PubMed] [Google Scholar]
- 77.Fromm M, Littman P, Raney RB, et al. Late effects after treatment of twenty children with soft tissue sarcomas of the head and neck. Experience at a single institution with a review of the literature. Cancer. 1986;57(10):2070–6. doi: 10.1002/1097-0142(19860515)57:10<2070::aid-cncr2820571032>3.0.co;2-g. Epub 1986/05/15. [DOI] [PubMed] [Google Scholar]
- 78.Heyn R, Ragab A, Raney RB, Jr, et al. Late effects of therapy in orbital rhabdomyosarcoma in children. A report from the Intergroup Rhabdomyosarcoma Study. Cancer. 1986;57(9):1738–43. doi: 10.1002/1097-0142(19860501)57:9<1738::aid-cncr2820570905>3.0.co;2-3. Epub 1986/05/01. [DOI] [PubMed] [Google Scholar]
- 79.Berkowitz RJ, Neuman P, Spalding P, et al. Developmental orofacial deficits associated with multimodal cancer therapy: case report. Pediatr Dent. 1989;11(3):227–31. Epub 1989/09/01. [PubMed] [Google Scholar]
- 80.Moller P, Perrier M. Dento-maxillofacial sequelae in a child treated for a rhabdomyosarcoma in the head and neck. A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86(3):297–303. doi: 10.1016/s1079-2104(98)90175-5. Epub 1998/10/13. [DOI] [PubMed] [Google Scholar]
- 81.Sonis AL, Tarbell N, Valachovic RW, et al. Dentofacial development in long-term survivors of acute lymphoblastic leukemia. A comparison of three treatment modalities. Cancer. 1990;66(12):2645–52. doi: 10.1002/1097-0142(19901215)66:12<2645::aid-cncr2820661230>3.0.co;2-s. Epub 1990/12/15. [DOI] [PubMed] [Google Scholar]
- 82.Imhof SM, Mourits MP, Hofman P, et al. Quantification of orbital and mid-facial growth retardation after megavoltage external beam irradiation in children with retinoblastoma. Ophthalmology. 1996;103(2):263–8. doi: 10.1016/s0161-6420(96)30706-9. Epub 1996/02/01. [DOI] [PubMed] [Google Scholar]
- 83.Ameniya T, Matsumura M, Hirose Y. Effects of radiation after enucleation without implantation on orbital development of patients with retinoblastoma. Ophthalmologica. 1977;174(3):137–44. doi: 10.1159/000308591. Epub 1977/01/01. [DOI] [PubMed] [Google Scholar]
- 84.Peylan-Ramu N, Bin-Nun A, Skleir-Levy M, et al. Orbital growth retardation in retinoblastoma survivors: work in progress. Med Pediatr Oncol. 2001;37(5):465–70. doi: 10.1002/mpo.1231. Epub 2001/12/18. [DOI] [PubMed] [Google Scholar]
- 85.Kaste SC, Chen G, Fontanesi J, Crom DB, Pratt CB. Orbital development in long-term survivors of retinoblastoma. J Clin Oncol. 1997;15(3):1183–9. doi: 10.1200/JCO.1997.15.3.1183. Epub 1997/03/01. [DOI] [PubMed] [Google Scholar]
- 86.Oberlin O, Rey A, Anderson J, et al. Treatment of orbital rhabdomyosarcoma: survival and late effects of treatment–results of an international workshop. J Clin Oncol. 2001;19(1):197–204. doi: 10.1200/JCO.2001.19.1.197. Epub 2001/01/03. [DOI] [PubMed] [Google Scholar]
- 87.Merchant TE, Nguyen L, Nguyen D, et al. Differential attenuation of clavicle growth after asymmetric mantle radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(2):556–61. doi: 10.1016/j.ijrobp.2003.11.005. Epub 2004/05/18. [DOI] [PubMed] [Google Scholar]
- 88.Taitz J, Cohn RJ, White L, Russell SJ, Vowels MR. Osteochondroma after total body irradiation: an age-related complication. Pediatr Blood Cancer. 2004;42(3):225–9. doi: 10.1002/pbc.10426. Epub 2004/01/31. [DOI] [PubMed] [Google Scholar]
- 89.Mavrogenis AF, Papagelopoulos PJ, Soucacos PN. Skeletal osteochondromas revisited. Orthopedics. 2008;31(10) Epub 2009/02/20. [PubMed] [Google Scholar]
- 90.Lin PP, Moussallem CD, Deavers MT. Secondary chondrosarcoma. J Am Acad Orthop Surg. 2010;18(10):608–15. doi: 10.5435/00124635-201010000-00004. Epub 2010/10/05. [DOI] [PubMed] [Google Scholar]
- 91.Hovi L, Era P, Rautonen J, Siimes MA. Impaired muscle strength in female adolescents and young adults surviving leukemia in childhood. Cancer. 1993;72(1):276–81. doi: 10.1002/1097-0142(19930701)72:1<276::aid-cncr2820720148>3.0.co;2-2. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 92.Ness KK, Hudson MM, Pui CH, et al. Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: associations with physical performance and chemotherapy doses. Cancer. 2012;118(3):828–38. doi: 10.1002/cncr.26337. Epub 2011/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hartman A, van den Bos C, Stijnen T, Pieters R. Decrease in peripheral muscle strength and ankle dorsiflexion as long-term side effects of treatment for childhood cancer. Pediatr Blood Cancer. 2008;50(4):833–7. doi: 10.1002/pbc.21325. [DOI] [PubMed] [Google Scholar]
- 94.Villa P, Corada M, Bartosek I. L-asparaginase effects on inhibition of protein synthesis and lowering of the glutamine content in cultured rat hepatocytes. Toxicol Lett. 1986;32(3):235–41. doi: 10.1016/0378-4274(86)90113-x. Epub 1986/09/01. [DOI] [PubMed] [Google Scholar]
- 95.Harila-Saari AH, Vainionpaa LK, Kovala TT, Tolonen EU, Lanning BM. Nerve lesions after therapy for childhood acute lymphoblastic leukemia. Cancer. 1998;82(1):200–7. doi: 10.1002/(sici)1097-0142(19980101)82:1<200::aid-cncr25>3.0.co;2-5. Epub 1998/01/15. [DOI] [PubMed] [Google Scholar]
- 96.Olshan JS, Gubernick J, Packer RJ, et al. The effects of adjuvant chemotherapy on growth in children with medulloblastoma. Cancer. 1992;70(7):2013–7. doi: 10.1002/1097-0142(19921001)70:7<2013::aid-cncr2820700734>3.0.co;2-j. Epub 1992/10/11. [DOI] [PubMed] [Google Scholar]
- 97.Loebstein R, Atanackovic G, Bishai R, et al. Risk factors for long-term outcome of ifosfamide-induced nephrotoxicity in children. J Clin Pharmacol. 1999;39(5):454–61. [PubMed] [Google Scholar]
- 98.Goren MP, Wright RK, Horowitz ME, Pratt CB. Ifosfamide-induced subclinical tubular nephrotoxicity despite mesna. Cancer Treat Rep. 1987;71(2):127–30. Epub 1987/02/01. [PubMed] [Google Scholar]
- 99.de Jonge T, Slullitel H, Dubousset J, et al. Late-onset spinal deformities in children treated by laminectomy and radiation therapy for malignant tumours. Eur Spine J. 2005;14(8):765–71. doi: 10.1007/s00586-004-0778-1. Epub 2005/07/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Bernardi B, Pianca C, Pistamiglio P, et al. Neuroblastoma with symptomatic spinal cord compression at diagnosis: treatment and results with 76 cases. J Clin Oncol. 2001;19(1):183–90. doi: 10.1200/JCO.2001.19.1.183. Epub 2001/01/03. [DOI] [PubMed] [Google Scholar]
- 101.Laverdiere C, Liu Q, Yasui Y, et al. Long-term outcomes in survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(16):1131–40. doi: 10.1093/jnci/djp230. Epub 2009/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laverdière C, Cheung N-KV, Kushner BH, et al. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr Blood Cancer. 2005;45(3):324–32. doi: 10.1002/pbc.20331. [DOI] [PubMed] [Google Scholar]
- 103.King D, Goodman J, Hawk T, Boles ET, Jr, Sayers MP. Dumbbell Neuroblastomas in Children. Arch Surg. 1975;110(8):888–91. doi: 10.1001/archsurg.1975.01360140032006. [DOI] [PubMed] [Google Scholar]
- 104.Plantaz D, Rubie H, Michon J, et al. The treatment of neuroblastoma with intraspinal extension with chemotherapy followed by surgical removal of residual disease. A prospective study of 42 patients–results of the NBL 90 Study of the French Society of Pediatric Oncology. Cancer. 1996;78(2):311–9. doi: 10.1002/(SICI)1097-0142(19960715)78:2<311::AID-CNCR19>3.0.CO;2-Z. Epub 1996/07/15. [DOI] [PubMed] [Google Scholar]
- 105.Hoover M, Bowman LC, Crawford SE, et al. Long-term outcome of patients with intraspinal neuroblastoma. Med Pediatr Oncol. 1999;32(5):353–9. doi: 10.1002/(sici)1096-911x(199905)32:5<353::aid-mpo8>3.0.co;2-2. Epub 1999/04/29. [DOI] [PubMed] [Google Scholar]
- 106.Katzenstein HM, Kent PM, London WB, Cohn SL. Treatment and outcome of 83 children with intraspinal neuroblastoma: the Pediatric Oncology Group experience. J Clin Oncol. 2001;19(4):1047–55. doi: 10.1200/JCO.2001.19.4.1047. Epub 2001/02/22. [DOI] [PubMed] [Google Scholar]
- 107.Kumar AP, Green AL, Smith JW, Pratt CB. Combined therapy for malignant tumors of the chest wall in children. J Pediatr Surg. 1977;12(6):991–9. doi: 10.1016/0022-3468(77)90611-x. Epub 1977/12/01. [DOI] [PubMed] [Google Scholar]
- 108.Angelini P, Plantaz D, De Bernardi B, et al. Late sequelae of symptomatic epidural compression in children with localized neuroblastoma. Pediatr Blood Cancer. 2011;57(3):473–80. doi: 10.1002/pbc.23037. [DOI] [PubMed] [Google Scholar]
- 109.Osborne D, Hadden OB, Deeming LW. Orbital growth after childhood enucleation. Am J Ophthalmol. 1974;77(5):756–9. doi: 10.1016/0002-9394(74)90545-5. Epub 1974/05/01. [DOI] [PubMed] [Google Scholar]
- 110.Kennedy RE. The effect of early enucleation on the orbit in animals and humans. Adv Ophthalmic Plast Reconstr Surg. 1992;9:1–39. Epub 1992/01/01. [PubMed] [Google Scholar]
- 111.Lyle CE, Wilson MW, Li CS, Kaste SC. Comparison of orbital volumes in enucleated patients with unilateral retinoblastoma: hydroxyapatite implants versus silicone implants. Ophthal Plast Reconstr Surg. 2007;23(5):393–6. doi: 10.1097/IOP.0b013e3181462ca8. Epub 2007/09/21. [DOI] [PubMed] [Google Scholar]
- 112.Nahum MP, Gdal-On M, Kuten A, et al. Long-term follow-up of children with retinoblastoma. Pediatr Hematol Oncol. 2001;18(3):173–9. doi: 10.1080/08880010151114769. Epub 2001/04/11. [DOI] [PubMed] [Google Scholar]
- 113.Shildkrot Y, Kirzhner M, Haik BG, et al. The effect of cancer therapies on pediatric anophthalmic sockets. Ophthalmology. 2011;118(12):2480–6. doi: 10.1016/j.ophtha.2011.05.024. Epub 2011/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin HY, Liao SL. Orbital development in survivors of retinoblastoma treated by enucleation with hydroxyapatite implant. Br J Ophthalmol. 2011;95(5):630–3. doi: 10.1136/bjo.2009.175778. Epub 2010/09/21. [DOI] [PubMed] [Google Scholar]
- 115.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: Opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58(3):334–43. doi: 10.1002/pbc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kun LE. In: General Principles of Radiation Oncology. 6. Pizzo PA, Poplack DG, editors. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2011. pp. 406–25. [Google Scholar]
- 117.Pui CH, Mullighan CG, Evans WE, Relling MV. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood. 2012;120(6):1165–74. doi: 10.1182/blood-2012-05-378943. Epub 2012/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shamberger RC. Cooperative group trials in pediatric oncology: The surgeon’s role. J Pediatr Surg. 2013;48(1):1–13. doi: 10.1016/j.jpedsurg.2012.10.068. Epub 2013/01/22. [DOI] [PubMed] [Google Scholar]
- 119.Libshitz HI, Edeiken BS. Radiotherapy changes of the pediatric hip. AJR Am J Roentgenol. 1981;137(3):585–8. doi: 10.2214/ajr.137.3.585. Epub 1981/09/01. [DOI] [PubMed] [Google Scholar]
- 120.Dahllöf G, Forsberg C-M, Ringdén O, et al. Facial growth and morphology in long-term survivors after bone marrow transplantation. Eur J Orthod. 1989;11(4):332–40. doi: 10.1093/oxfordjournals.ejo.a036004. [DOI] [PubMed] [Google Scholar]
- 121.Meadows AT, Gallagher JA, Bunin GR. Late effects of early childhood cancer therapy. Br J Cancer Suppl. 1992;18:S92–5. Epub 1992/08/01. [PMC free article] [PubMed] [Google Scholar]
- 122.Hovi L, Saarinen-Pihkala UM, Vettenranta K, Lipsanen M, Tapanainen P. Growth in children with poor-risk neuroblastoma after regimens with or without total body irradiation in preparation for autologous bone marrow transplantation. Bone Marrow Transplant. 1999;24(10):1131–6. doi: 10.1038/sj.bmt.1702021. Epub 1999/12/01. [DOI] [PubMed] [Google Scholar]
- 123.Fuchs B, Valenzuela RG, Inwards C, Sim FH, Rock MG. Complications in long-term survivors of Ewing sarcoma. Cancer. 2003;98(12):2687–92. doi: 10.1002/cncr.11891. Epub 2003/12/12. [DOI] [PubMed] [Google Scholar]
- 124.Escobar MA, Grosfeld JL, Powell RL, et al. Long-term outcomes in patients with stage IV neuroblastoma. J Pediatr Surg. 2006;41(2):377–81. doi: 10.1016/j.jpedsurg.2005.11.032. Epub 2006/02/17. [DOI] [PubMed] [Google Scholar]
- 125.Calaminus G, Weinspach S, Teske C, Göbel U. Quality of Survival in Children and Adolescents After Treatment for Childhood Cancer: The Influence of Reported Late Effects on Health Related Quality of Life. Klin Padiatr. 2007;219(03):152,7. doi: 10.1055/s-2007-973846. [DOI] [PubMed] [Google Scholar]
- 126.Mansky P, Arai A, Stratton P, et al. Treatment late effects in long-term survivors of pediatric sarcoma. Pediatr Blood Cancer. 2007;48(2):192–9. doi: 10.1002/pbc.20871. [DOI] [PubMed] [Google Scholar]
- 127.Rombi B, DeLaney TF, MacDonald SM, et al. Proton radiotherapy for pediatric Ewing’s sarcoma: initial clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82(3):1142–8. doi: 10.1016/j.ijrobp.2011.03.038. Epub 2011/08/23. [DOI] [PubMed] [Google Scholar]


