Abstract
Background
Very-low density-lipoproteins (VLDL) export lipids from liver to peripheral tissues and are the precursors of low-density-lipoproteins. Low levels of hepatic S-adenosylmethionine (SAMe) decrease triglyceride (TG) secretion in VLDL contributing to hepatosteatosis in methionine adenosyltransferase 1A knockout mice but nothing is known about the effect of SAMe over circulating VLDL metabolism.
Objective
We wanted to investigate whether excess SAMe could disrupt VLDL plasma metabolism and unravel the mechanisms involved.
Methods
Glycine N-methyltransferase (GNMT) knockout (KO), GNMT-PLIN2-KO and their respective wild types (WT) were used. A high fat diet (HFD) or a methionine deficient diet (MDD) was administrated to exacerbate or recover VLDL metabolism, respectively. Finally, 33 patients with nonalcoholic fatty-liver disease (NAFLD); 11 with hypertriglyceridemia and 22 with normal lipidemia were used in this study.
Results
We found that excess SAMe increases turnover of hepatic TG stores for secretion in VLDL in GNMT-KO mice, a model of NAFLD with high SAMe levels. The disrupted VLDL assembly resulted in the secretion of enlarged, phosphatidylethanolamine-poor, TG-and apoE-enriched VLDL-particles; special features that lead to increased VLDL clearance and decreased serum TG levels. Re-establishing normal SAMe levels restore VLDL secretion, features and metabolism. In NAFLD patients, serum TG levels are lower when hepatic GNMT-protein expression is decreased.
Conclusion
Excess hepatic SAMe levels disrupt VLDL assembly and features and increase circulating VLDL clearance which will cause increased VLDL-lipid supply to tissues and might contribute to the extrahepatic complications of NAFLD. Electronic word count: 235
Keywords: S-adenosylmethionine, Very-low density-lipoproteins, Glycine N-methyltransferase, nonalcoholic fatty-liver disease
INTRODUCTION
The liver plays a central role in whole body metabolic homeostasis. It can obtain lipids from the circulation, synthesize them and secrete them in lipoproteins into the blood stream. Very-low-density lipoproteins (VLDL) transport triglycerides (TG) from liver to peripheral tissues providing an energy source. Plasma levels of VLDL are defined by the rate of clearance from plasma and the rate of hepatic secretion, so an imbalance between these two processes will lead to dyslipidemia, highly associated with increased risk of cardiovascular disease [1, 2], which is one of the extrahepatic complications of nonalcoholic fatty-liver disease (NAFLD) [3]. Individuals with NAFLD exhibit disrupted VLDL metabolism [4, 5]. In fact, abnormalities in the hepatic uptake of lipoproteins and/or secretion of VLDL could lead to hepatosteatosis [6, 7].
Hepatic VLDL secretion rate is regulated by a variety of factors that must guarantee release of adequate amounts of TG from liver. To ensure VLDL secretion, apolipoprotein (apo) B should be translocated in the lumen of the endoplasmic reticulum (ER) during its translation [8], where it interacts with the microsomal TG transfer protein (MTP), whose lipid transfer activity is one of the major determinants in VLDL secretion [9]. ApoE facilitates apoB maturation and VLDL assembly and secretion [10, 11], and it mediates cellular uptake of several lipoproteins from the circulation [12, 13].
Most of VLDL-TG (60–70%) is derived from intracellular stores [14, 15]; therefore, the mobilization of lipids from the cytosolic lipid droplets towards the ER represents a potentially regulated step in VLDL production and secretion [15, 16].
Low liver S-adenosylmethionine (SAMe) in methionine adenosyltransferase 1A (MAT1A)-KO mice decreases TG secretion in VLDL, which contributes to hepatosteatosis [6]. Glycine N-methyltransferase (GNMT) drives the catabolism of SAMe and its deficiency in mice results in a marked increase in hepatic SAMe content and rapid NAFLD development [17]. SAMe is the methyl donor required for the methylation of phosphatidylethanolamine (PE). In hepatocytes, around 30% of phosphatidylcholine (PC) is synthesized by the sequential methylation of PE, in a reaction catalyzed by the enzyme PE N-methyltranferase (PEMT) [18]. We have found that the flux from PE to PC and its catabolism to diglycerides (DG) and conversion in TG is stimulated in the liver of GNMT-KO mice [19]. There is a requirement of PEMT in liver to ensure normal VLDL secretion [18, 20]. Thus, we have evaluated if high levels of hepatic SAMe will disrupt VLDL assembly and as a consequence VLDL features and plasma metabolism.
METHODS
Human samples
This study comprised 33 non-diabetic patients with cholelithiasis and a clinical diagnosis of NAFLD without necroinflammation or fibrosis (Table S1 and S2) and 36 patients with asymptomatic cholelithiasis in whom a liver biopsy was taken during programmed laparoscopic cholecystectomy (Table S1). Inclusion criteria for patients are detailed in supplementary information. The study was performed in agreement with the Declaration of Helsinki and with local and national laws. The Human Ethics Committee of the University Hospital Santa Cristina and the University of Basque Country approved the study procedures and written informed consent was obtained from all patients before inclusion in the study.
Animals
3-month-old male GNMT-KO mice, GNMT-PLIN2-KO mice, PLIN2-KO mice and their WT littermates were produced in the animal facility of CIC bioGUNE. They were maintained on different diets detailed in supplemental information. Animal procedures were approved by the University of the Basque Country and CIC bioGUNE Animal Care and Use Committees.
Preparation of labeled VLDL particles and in vivo clearance
Labeled human VLDL particles were obtained by a method previously described [21, 22] and as detailed in supplemental information.
Quantification of lipids
Lipids were extracted and quantified as described before [23]. TG was quantified using a commercially available kit (A. Menarini diagnostics). PE, PC and DG were separated by thin layer chromatography and quantified as detailed elsewhere [24].
Statistical analysis
Data are represented as means±SEM. Differences between groups were tested using the Student's t-test and two way ANOVA. Significance was defined as p<0.05. The baseline characteristics of the patients studied were compared using the unpaired t test or Mann-Whitney U test. These analyses were performed using SPSS Version 15.0 software and GraphPad Version 5.03. (Additional methods are detailed in Supplemental information)
RESULTS
Deletion of GNMT disrupts VLDL assembly, VLDL features and decreases circulating VLDL levels in serum
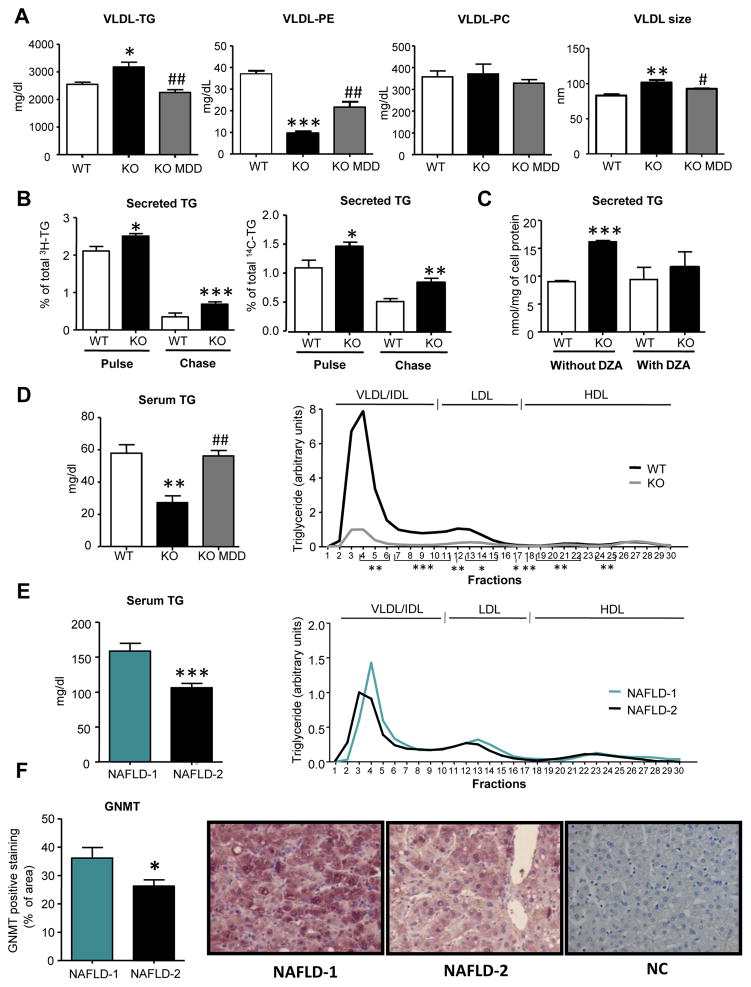
GNMT-KO mice show steatosis and fibrosis with increased serum aminotransferases [17] and no signs of insulin resistance [19]. In GNMT-KO mice VLDL-TG secretion was increased whereas VLDL-PE was decreased as compared to their wild-type (WT) controls (Figure 1A). No changes were observed in VLDL-PC secretion (Figure 1A) while VLDL size was augmented in GNMT-KO mice (Figure 1A). All these compositional and physical VLDL features restored after feeding a methionine deficient diet (MDD) for 3 weeks (Figure 1A). Consistent with the increased VLDL-TG secretion, we found increased turnover of hepatocyte TG lipid stores (Figure 1B) and increased MTP and diacylglycerol acyltransferase (DGAT) activity while no changes in TG lipase activity (Figure S1A). The increased hepatocyte TG secretion was re-established after inhibition of PEMT with 3-deazaadenosine (DZA) (Figure 1C).
Figure 1. Deletion of GNMT disrupts VLDL features and serum VLDL clearance.
Wild type (WT), GNMT-KO (KO) and GNMT-KO mice fed a MDD (KO MDD) were fasted 2. (A) Before 1 g/kg poloxamer (P-407) injection and 6 hours later, VLDL were isolated from serum and characterized for triglyceride (TG), phosphatidylethanolamine (PE) and phosphatidylcholine (PC) content and VLDL size. (B) Hepatocytes were incubated with 0.4 mM [3H]oleic acid (5 μCi/dish) and [14C]glycerol (0.5 μCi/dish) (pulse). The percentage of the total [3H]-TG and [14C]-TG secreted to the media after both the pulse (4 hours) and chase periods (4 hours) was calculated. (C) Hepatocytes were incubated with 3-deazaadenosine (DZA, 10 μM) for 4 hours and the secreted TG to the medium was determined. (D) TG levels in serum and in lipoprotein sub-fractions were measured in mice fed a control diet. (E) Serum samples from NAFLD patients were obtained after a 12 h overnight fast. Serum samples from NAFLD-1 and NAFLD-2 patients were pooled and TG content in serum lipoprotein subfractions were quantified. (F) Liver samples from patients with NAFLD (n=17) (6 from NAFLD-1 and 11 from NAFLD-2) were obtained by liver biopsy GNMT immunohistochemistry. Magnification 40x. Quantitative assessments of IHC staining were performed using FRIDA image analysis software. Expressed in % of positive staining per area. A negative control (NC) was performed by omission of primary antibody. Values are mean±SEM of 5–6 animals per group and of the previously indicated patients. Statistical differences between GNMT-KO and WT mice or NAFLD-1 and NAFLD-2 patients are denoted by *p<0.05; **p<0.01; ***p<0.001 (Student’s t test); between GNMT-KO and GNMT-KO fed a MDD are denoted by # p<0.05; ## p<0.01 (Student’s t test).
One of the factors that define the levels of circulating TG in blood is the hepatic secretion rate. To our surprise, GNMT deletion in mice resulted in a decrease of serum TG (Figure 1D) due to most of all VLDL subclasses and some of LDL (Figure 1D). The decrease in VLDL and LDL was also evident when apoB levels were analyzed in serum (Figure S1B). Re-establishing SAMe hepatic levels feeding the MDD restored serum TG and apoB levels in GNMT-KO mice (Figure 1D and Figure S1B).
In patients with NAFLD (n=33) serum TG levels range from 54 to 317 mg/dl showing a high heterogeneity among subjects (Table S1). In order to investigate whether altered SAMe metabolism could be linked with increased VLDL clearance in patients with NAFLD, we classified the individuals into two groups depending on serum TG levels (Table S2). In a first group (NAFLD-1) we introduced those subjects with serum TG levels higher than the mean of TG in NAFLD patients (130.1 mg/dl) (n=11) and in a second group (NAFLD-2) those with TG levels below 130.1 mg/dl (n=22). We quantified TG levels in serum and analyzed TG distribution in lipoproteins in serum in the two groups (Figure 1E) and found that the decreased TG levels corresponded to most all VLDL subclasses and some of LDL (Figure 1E). We also found that In NAFLD-2 patients there is a shift to the left of the maximum peak of VLDL population indicating that VLDL particles are enlarged (Figure 1E). High hepatic SAMe levels in GNMT-KO mice are linked with enlarged VLDL particles (Figure 1A) while decreased hepatic SAMe with smaller VLDL particle size [6], so we hypothesized that SAMe content could also be increased in NAFLD-2 patients as a consequence of decreased GNMT expression. It has recently been described [25, 26] that the expression of GNMT and MAT1A genes is decreased in patients with severe NAFLD. Thus, we measured GNMT protein levels in 17 NAFLD liver samples (6 from NAFLD-1 and 11 from NAFLD-2) and observed that in NAFLD-2 liver samples GNMT protein levels were lower than in livers from NAFLD-1 patients (Figure 1F). When compared with normal livers (NL), GNMT protein expression was not changed in livers from NAFLD-1 subjects while in livers from NAFLD-2 the decrease was evident (Figure S2A). Hepatic PE content was decreased in NAFLD-2 patients making the PC to PE ratio higher (Figure S2B) which suggest increased PEMT flux and VLDL secretion. Thus, in NAFLD-2 patients the decreased TG levels in serum could be due to increased VLDL clearance as a consequence of increased hepatic SAMe levels.
A high fat diet (HFD) induces VLDL clearance and TG storage in liver of GNMT-KO mice
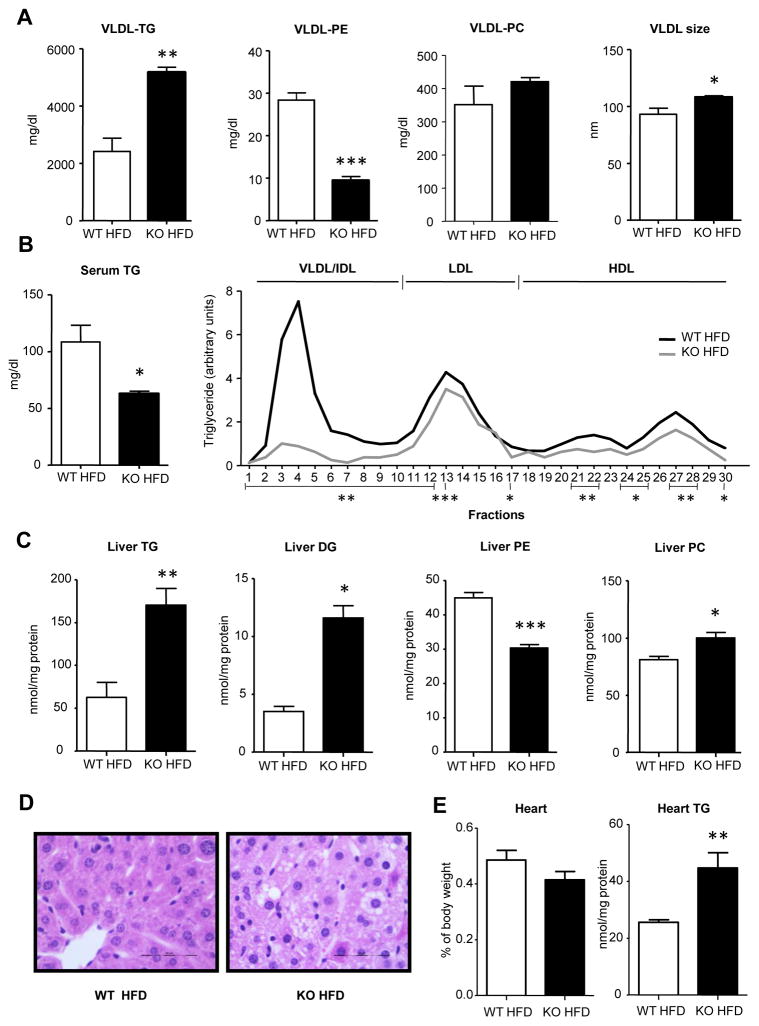
We have observed that excess SAMe in liver increases VLDL-TG secretion as a consequence of increased PEMT flux, increased MTP activity, and increased turnover of TG lipid stores. High levels of hepatic SAMe also transforms VLDL features and increases VLDL clearance from blood (Figure 2). We wondered whether challenging with a HFD for six weeks could affect differently WT and GNMT-KO mice exacerbating VLDL secretion and clearance in GNMT-KO mice. The metabolic characterization of mice fed a HFD showed that in GNMT-KO mice the food intake was slightly decreased as compared to their WT (Figure S3A). The body weight and the % of WAT did not change (Figure S3B and S3C), while the % of liver weight was increased (Figure S3D) as when fed a control diet (CD) [19]. GNMT-KO mice fed a HFD did not exhibit insulin resistance (Figure S3E). The HFD induced VLDL-TG secretion only in GNMT-KO mice if compared with animals fed the CD (Figure 3A and Figure 1A). The HFD did not alter VLDL-PE or VLDL-PC secretion nor VLDL size since the observed changes when compared to their controls (Figure 3A) were of the same magnitude as when fed a CD (Figure 1A). Even though the HFD increased VLDL-TG secretion, serum TG levels were still half of the WT mice values indicating higher VLDL clearance in GNMT-KO mice fed the HFD than the CD (Figure 3B). The low TG levels in serum were also due to the decrease in VLDL and some LDL sub-fractions (Figure 3B).
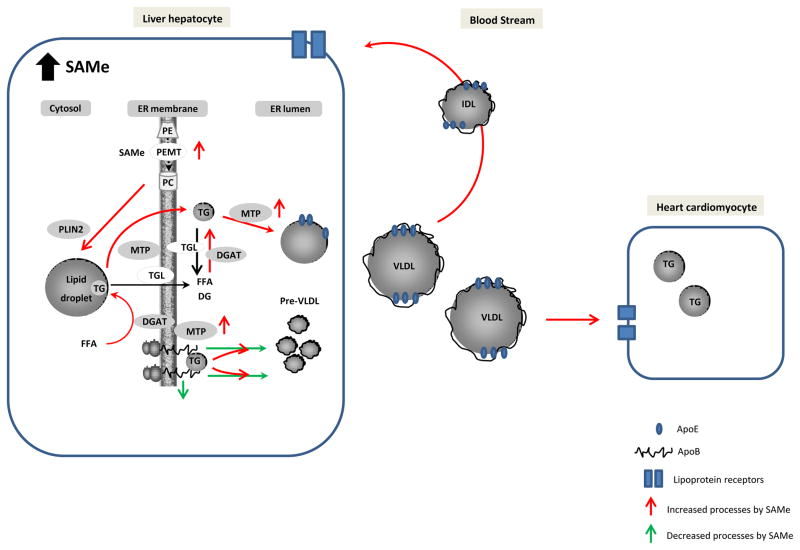
Figure 2. Proposed model of disrupted VLDL assembly, secretion and blood stream metabolism in GNMT-KO mice.
Excess levels of SAMe induce the secretion of less number of enlarged, PE-poor and TG -enriched VLDL particles. ApoB availability is decreased in liver due to lower mRNA synthesis and stability. There is an increased hepatic TG secretion mainly due to stimulated PEMT flux, which will increase TG storage in lipid droplets. The rise in mobilization of TG from the cytoplasmic lipid droplets storage will enhance the availability of TG to be channelled towards VLDL secretion. MTP activity and DGAT activity will promote this process. VLDL particles will be rapidly metabolized from the blood stream due to the specific VLDL features leading to increased lipid supply in tissues such as liver and heart.
Abbreviations: Apo, apolipoprotein; DGAT, acyl-CoA:diacylglycerol acyltransferase; ER, endoplasmic reticulum; MTP, microsomal triglyceride transfer protein; PE, phosphatidylethanolamine; PEMT, phosphatidylethanolamine N-methyltransferase; PLIN2, perilipin 2; SAMe, S-adenosylmethionine; TG, triglyceride; TGL, TG lipase; VLDL, very-low-density lipoprotein.
Figure 3. A HFD induces VLDL clearance and liver TG storage in GNMT-KO mice.
Wild type mice fed a high fat diet (WT HFD) and GNMT-KO mice fed a HFD (KO HFD) were fasted 2 hours. (A) Before 1 g/kg poloxamer (P-407) injection and 6 hours later, VLDL were isolated from serum and characterized for triglyceride (TG), phosphatidylethanolamine (PE) and phosphatidylcholine (PC) content and VLDL size. (B) TG levels in serum and in lipoprotein sub-fractions were measured. (C) Liver triglycerides (TG), diglycerides (DG), phosphatidylethanolamine (PE) and phosphatidylcholine (PC) levels from WT and KO mice fed the HFD were quantified. (D) Representative liver hematoxylin and eosin. (E) Percentage of heart weight. Heart TG levels were quantified after lipid extraction Values are mean±SEM of 4–5 animals per group. Statistical differences between KO and WT mice are denoted by *p<0.05; **p<0.01; ***p<0.001 (Student’s t test)
In a condition in which VLDL clearance is increased, VLDL lipid supply to tissues will also be increased. After feeding the HFD the liver TG levels were 2.75 fold increased as compared to their WTs (Figure 3C and 3D), whereas when fed a CD the increase of TG in liver was of a 75% [19]. The altered hepatic DG, PE and PC content in GNMT-KO mice fed the HFD maintained as when fed the CD [19]. When fed the HFD the TG storage in heart was twice the WT value (Figure 3E) whereas when fed a CD it was not increase (data not shown).
The HFD did not modify mRNA levels of proteins involved in lipid synthesis and NADPH production in GNMT-KO mice if compared to their controls (Figure S3F). Serum ketone bodies and fatty acids (Figure S3G) were not changed, and the hepatic CD36 protein content was not increased (Figure S3H).
Specific VLDL features in GNMT-KO mice are linked with increased VLDL clearance
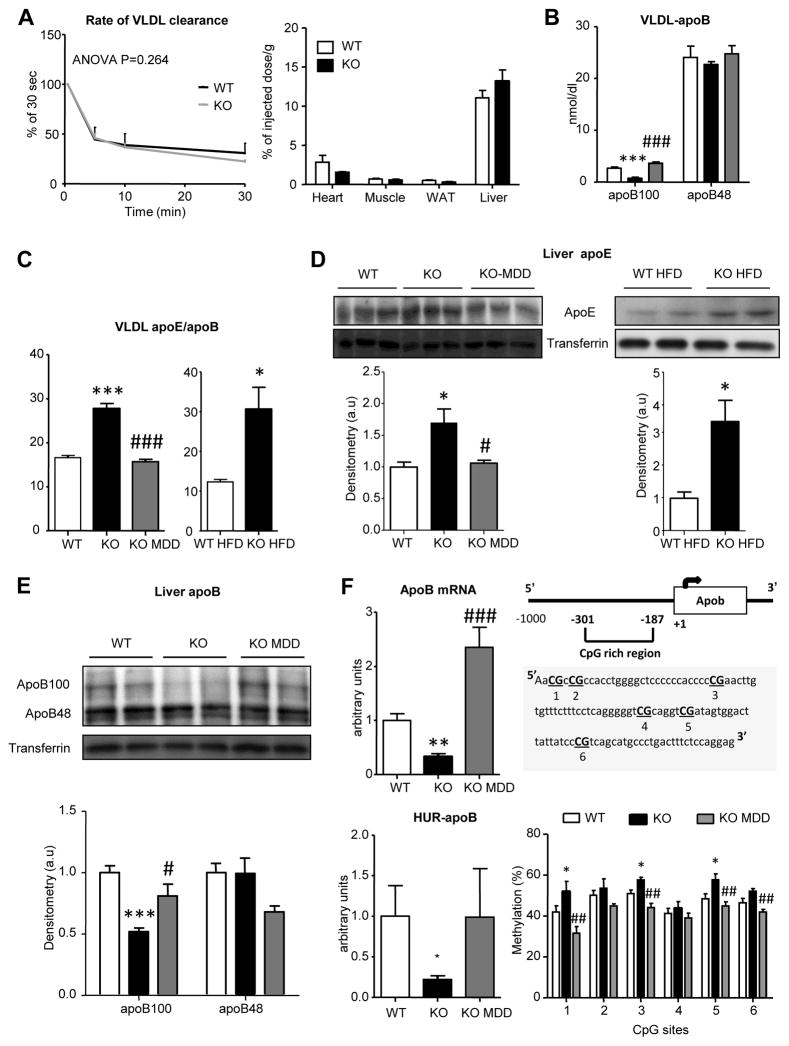
To define if the increased VLDL clearance depended on GNMT-KO mice VLDL specific features, human VLDL particles were labeled and administered intravenously to GNMT-KO and WT mice. We found that the kinetic of clearance of labeled VLDL was similar in WT and GNMT-KO mice (Figure 4A), and that the % of injected dose per g of tissue after 30 minutes of injection was not altered in GNMT-KO mice (Figure 4A), verifying that the increased clearance depended only on GNMT-KO mice VLDL specific features.
Figure 4. Specific VLDL features in GNMT-KO mice are linked with increased VLDL clearance.
Wild type (WT), GNMT-KO (KO), GNMT-KO mice fed a MDD (KO MDD), wild type mice fed a high fat diet (WT HFD) and GNMT-KO mice fed a HFD (KO HFD) were fasted 2 hours. (A) VLDL clearance rate was measured after intravenous administration of radiolabeled VLDL particles. The percentage of injected radiolabel dose per gram of tissue was calculated. Before 1 g/kg poloxamer (P-407) injection and 6 hours later, VLDL were isolated from serum and characterized for (B) apoB and apoE content. (C) The ratio apoE/apoB was calculated. Liver apoE (D) and apoB (E) content in liver from mice was assessed by immunoblotting using transferrin as loading control. (F) Quantitative RT-PCR analysis of apoB mRNA. Binding of apoB mRNA to HuR was evaluated in livers by immunoprecipitation followed by quantitative real-time PCR. The % of methylation of CpG sites in apoB promoter was assessed by bisulfite pyrosequencing. Values are mean±SEM of 4–6 animals per group. Statistical differences between GNMT-KO and WT mice are denoted by *p<0.05; **p<0.01; ***p<0.001 (Student’s t test) and by two-way ANOVA; between GNMT-KO and GNMT-KO fed a MDD are denoted by # p<0.05; ## p<0.01; ### p<0.001 (Student’s t test).
To go further in the characterization of GNMT-KO mice VLDL features we analyzed VLDL-apoB and –apoE secretion. In GNMT-KO mice the secretion of VLDL-apoB100 was decreased while no changes were observed in those VLDL bearing apoB48 as compared to their WT (Figure 4B). Each VLDL particle contains a single copy of apoB but several copies of apoE. The results showed that in GNMT-KO mice VLDL-apoE secretion was increased as compared to WT mice (Figure S4A), an increase even higher when fed the HFD (Figure S4A). This made each secreted VLDL particle to be apoE enriched (Figure 4C). We observed that the altered VLDL-apoE (Figure S4A) and VLDL-apoB100 secretion (Figure 4B) responded to changes in the hepatic content of these proteins and restored after re-establishing hepatic SAMe levels (Figure 4D and 4E). It has been suggested that lysosomal enzymes may degrade part of apoE [27], so we investigated the effect of NH4Cl/leupeptine inhibitors. The treatment did not induce changes in GNMT-KO or WT hepatocytes apoE levels (Figure S4B). In addition, we observed that in GNMT-KO mice there is an accumulation of ubiquitinated proteins (Figure S4C) and since part of apoE is degraded through proteasoma in HepG2 cells [28], we wondered whether the increased apoE content could be due to decreased proteasomal degradation. Inhibition of proteasome using MG132 did not increase apoE levels in WT hepatocytes (Figure S4C) nor VLDL-apoE secretion (data not shown) and decreased apoE content in GNMT-KO mice hepatocytes (Figure S4C) and secretion in VLDL (data not shown).
Increased apoE levels in liver were not linked with changes in apoE mRNA (Figure S4D). However, the decreased hepatic apoB100 content driven by the excess hepatic SAMe as shown after feeding the MDD (Figure 4E) was coupled to lower levels of apoB mRNA (Figure 4F), in which binding to the mRNA stabilizing HUR protein was reduced (Figure 4F). Knowing that global DNA methylation is increased in GNMT-KO mice [17], we wondered whether apoB promoter in liver could also be more methylated than in WT mice. We analyzed six CpG sites in mice apoB promoter; from those three of them were significantly hypermethylated in the KO compared to WT mice (Figure 4F). After restoring hepatic SAMe levels, apoB mRNA levels re-established as binding to HUR and methylation levels in the three CpG sites did (Figure 4F).
Factors that regulate cytosolic lipid storage such as PLIN2 also influence VLDL secretion [29]. We have found that the HFD induces hepatic TG storage and increases VLDL-apoE secretion in GNMT-KO mice, whereas deletion of PLIN2 in GNMT-KO mice results in the reduction of hepatic TG levels [19]. We wanted to know whether absence of PLIN2 in GNMT-KO mice could disturb VLDL assembly, VLDL-apoE secretion and VLDL clearance. PLIN2 deletion in GNMT-KO mice leads to the increased VLDL-TG secretion, decreased VLDL-PE secretion and increased size of VLDL already observed in GNMT-KO mice when compared to their controls (Figure S5A). Surprisingly, the absence of PLIN2 in GNMT-KO mice led to an increase in VLDL-PC secretion not found in GNMT-KO mice (Figure S5A) supporting a described role of PLIN2 binding PC [30, 31]. In PLIN2-KO mice VLDL-TG secretion was also increased while no other changes were found (Figure S6A and S6B). Turnover of TG stores for secretion in VLDL and serum TG levels were decreased in the double KO mice if compared to their controls (Figure S5B and S5C). VLDL-apoE and -apoB secretion changed in the same way as in GNMT-KO mice leading also to the secretion of apoE-enriched VLDL particles (Figure S5D). Thus, the storage of TG in lipid droplets in GNMT-KO mice doesn′t have any effect over VLDL-apoE secretion. The increased apoE availability is directly linked with the excess SAMe levels.
DISCUSSION
VLDL assembly depends on the hepatic availability of lipids, certain proteins and multiple enzymatic reactions [32]. Specific features of VLDL, among other factors, define the rate of VLDL clearance from the blood stream [33]. VLDL particles export lipids from liver to tissues and when lipid supply is excessive, metabolic homeostasis in peripheral tissues may disrupt [34]. Excess SAMe levels in GNMT-KO mice induce PC synthesis, through the PEMT pathway [19]. The PEMT pathway is required to ensure VLDL secretion [20], and the conversion of PE into PC seems to be involved in formation of lipid droplets [35], in which mobilization of lipids represents a regulated step in VLDL secretion [15, 16]. The increased PEMT flux in GNMT-KO mice is responsible for the rise in VLDL-TG secretion, which is linked with increased mobilization of hepatic TG stores, and higher MTP and DGAT activity. Inhibition of DGAT1 reduces liver fibrosis in mice with NASH [36] while that of DGAT2 results in decreased hepatosteatosis but higher fibrosis and liver damage [37]. In 8 month-old GNMT-KO mice, liver fibrosis is more prominent [17] and DGAT activity is increased to a higher degree than in 3 month-old KO mice, while DGAT2 mRNA is decreased 40% as compared to their WTs. No changes have been found in DGAT2 or DGAT1 mRNA in 3 month-old KO mice (data not shown). All these suggest a role of DGAT1 and DGAT2 in the progression of NAFLD in GNMT-KO mice.
The increased PEMT flux also causes a reduction in hepatic PE [19], reproduced in the decreased VLDL-PE secretion. Surprisingly, even though PC synthesis was increased in GNMT-KO mice no changes were found in VLDL-PC secretion; however, deletion of PLIN2 in GNMT-KO mice, in which PLIN2 expression is increased [19], results in the rise of VLDL-PC secretion suggesting a role of PLIN2 in the retention and catabolism of PC. PLIN2 binds lipids such as PC with high affinity, which supports this hypothesis [31]. As expected, these changes were not observed in PLIN2-KO mice in which PC synthesis was not increased.
High levels of SAMe in GNMT-KO mice leads to the secretion of enlarged, PE-poor and TG and apoE enriched VLDL particles. These features facilitate VLDL clearance from blood. We have also observed that in a group of patients with NAFLD serum TG levels are decreased and VLDL size increased when hepatic GNMT protein levels are lower. Moylan et al (2014) [25] have recently found that patients with severe NAFLD exhibited significant down-regulation of MAT1A and GNMT genes. We do not know whether MAT protein expression, involved in SAMe synthesis, may be decreased in this group of NAFLD patients but the enlarged VLDL size, decreased hepatic PE levels and increased PC/PE ratio suggest that the balance between GNMT and MAT activity results in increased hepatic SAMe levels which enhance the PEMT flux. Unfortunately, SAMe content could not be measured in liver biopsies from patients with NAFLD used in the present study. All this supports the hypothesis that administration of SAMe to improve liver disease and lipoprotein metabolism will have beneficial effects only in that group of patients in which hepatic SAMe content is decreased. When SAMe content is increased, probably a methionine deficient diet could be the chosen treatment according to the results obtained previously [19] and in this work. In the absence of apoE, VLDL catabolism from the blood is heavily impaired [38] resulting in increased levels of TG and decreased uptake of TG-rich lipoproteins as have been observed in apoE deficient mice [39]. Since over-expression of apoE markedly reduces apoB-containing lipoproteins in plasma [13], we propose that the high hepatic SAMe levels leads to VLDL-apoE enrichment. This will explain the increased VLDL clearance in GNMT-KO mice, a feature more marked in animals fed the HFD in which clearance is also higher and the heart and liver TG storage is increased, which could be taken as a consequence of excessive lipid supply to these tissues. The fact that no changes in serum fatty acids or ketone bodies together with the lack of increase in CD36 protein levels or in genes related with lipogenesis in GNMT-KO animals fed the HFD support this proposal. CD36 is upregulated in liver of NASH patients [40] and CD36 mRNA increases in animals fed a HFD after partial hepatectomy [41]. However, in the obese fa/fa Zucker rats, when CD36 mRNA levels are increased, the protein content in hepatocytes does not change [42]. mTOR selectively regulates the expression of CD36 at a translational level [43,44]. We have found that phosphorylation of S6 and 4E-BP1, which are mTOR targets, (data not shown) are decreased in GNMT-KO mice fed the HFD while CD36 mRNA is increased (data not shown) suggesting that downregulation of mTOR pathway in this model could suppress, at least in part, CD36 translation.
ApoE secretion in VLDL was increased as a consequence of higher liver apoE content, which is also modulated by hepatic SAMe levels. In HepG2 apoE is partially degraded through proteasome [28] and partially through lysosomal enzymes [27]; however our results show that in hepatocytes from GNMT-KO mice and their WT other mechanisms are involved in regulating apoE availability. Interestingly, apoE can escape degradation and can be re-secreted when following LDLr-mediated internalization by liver cells [45]. This is an attractive hypothesis since VLDL clearance is increased in GNMT-KO mice; therefore, apoE could be recycle by the cell several times until final degradation occurs, as has been suggested before [45].
Factors that regulate cytosolic lipid storage such as PLIN2 or Cideb also influence VLDL secretion [29, 46]. We observed that the HFD induces hepatic TG storage and increases VLDL-apoE secretion in GNMT-KO mice whereas absence of PLIN2 in GNMT-KO mice results in the reduction of hepatic TG levels, so we thought that cytosolic lipid storage could influence apoE secretion in VLDL. However, while deletion of PLIN2 in GNMT-KO mice resulted in decreased turnover of TG for secretion in VLDL, apoE secretion was still increased as VLDL clearance from blood, discarding this hypothesis. Excess SAMe levels in GNMT-KO mice liver are associated with decreased apoB secretion. We found that liver content in apoB100 was decreased as a consequence of decreased apoB mRNA. We also found that 3CpG islands were hypermethylated in apoB promoter and that apoB mRNA binding with mRNA stabilizing HUR protein was decreased in GNMT-KO mice, suggesting that apoB mRNA synthesis and stability were decreased in GNMT-KO mice.
In conclusion, all these findings show that in NAFLD increased SAMe disrupts VLDL features and enhances VLDL clearance from the blood stream which may lead to improvement in serum lipid profile in NAFLD patients. However, the increased lipoprotein lipid supply to tissues could also impair intracellular lipid homeostasis and be a cause of lipid storage in muscle, heart or adipose tissue being involved in the extrahepatic complications of NAFLD.
Supplementary Material
Acknowledgments
This work was supported by Gobierno Vasco IT-336-10 (to P.A.), Etortek bioGUNE 2011 (to P.A and M.L.M-C), Saiotek (to P.A), Gobierno vasco IT-661-13 (to A.G-O). Instituto de Salud Carlos III ( PI10/00067 and PI13/01299 to C.G-M). Educacion Gobierno Vasco 2012 (to M.L.M.-C), FIS PI11/01588 (to M.L.M.-C), Asociacion Espanola contra el Cancer (to M.L.M-C), Sanidad Gobierno Vasco 2012 (to M.V.R.), and NIH AT-1576 (to S.C.L., M.L.M.-C., and J.M.M.). CIBERehd is funded by Instituto de Salud Carlos III. MM-U. was a recipient of a predoctoral fellowship from the Basque Government.
We thank technical support from Jose Antonio Lopez Gomez and Unidad de formacion e investigacion UFI11/20, University of Basque Country UPV/EHU
Abbreviations
- apo
apolipoprotein
- CD
control diet
- DGAT
diacylglycerol acyltransferase
- DG
diglyceride
- DZA
3-deazaadenosine
- FA
fatty acid
- FC
Free cholesterol
- GNMT-Glycine N-methyltransferase
KB, ketone bodies
- KO
knockout
- MAT
methionine adenosyltransferase
- MDD
methionine deficient diet
- MTP
microsomal triglyceride transfer protein
- NAFLD
non-alcoholic fatty-liver disease
- NASH
non-alcoholic steatohepatitis
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PEMT
phosphatidylethanolamine N-methyltransferase
- PLIN
perilipin
- qRT-PCR
real-time polymerase chain reaction
- SAH
S-adenosylhomocysteine
- SAMe
S-adenosylmethionine
- TG
triglyceride
- TGL
triglyceride lipase
- VLDL
very-low-density lipoprotein
- WT
wild type
Footnotes
None of the authors have conflict of interest to declare
Authors contribution: MMU, MVR, AGO, CGM, MMC, JM and PA study concept and design; MMU, DM, LFA; OF, DFA, VGD, IMG, IA, XB, ZL, CW, SL, RF and CGM acquisition of data; MMU, MVR, MMC, JM and PA analysis and interpretation of data; MMU and PA drafting of the manuscript; MMU, MVR, MMC, JM and PA critical revision of the manuscript for important intellectual content; PA study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scott R, Donoghoe M, Watts GF, O'Brien R, Pardy C, Taskinen MR, et al. Impact of metabolic syndrome and its components on cardiovascular disease event rates in 4900 patients with type 2 diabetes assigned to placebo in the FIELD randomised trial. Cardiovasc Diabetol. 2011;10:102–2840-10-102. doi: 10.1186/1475-2840-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ooi EM, Ng TW, Watts GF, Chan DC, Barrett PH. Effect of fenofibrate and atorvastatin on VLDL apoE metabolism in men with the metabolic syndrome. J Lipid Res. 2012;53:2443–2449. doi: 10.1194/jlr.P029223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2013 doi: 10.1002/hep.26717. [DOI] [PubMed] [Google Scholar]
- 4.Fabbrini E, deHaseth D, Deivanayagam S, Mohammed BS, Vitola BE, Klein S. Alterations in fatty acid kinetics in obese adolescents with increased intrahepatic triglyceride content. Obesity (Silver Spring) 2009;17:25–29. doi: 10.1038/oby.2008.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujita K, Nozaki Y, Wada K, Yoneda M, Fujimoto Y, Fujitake M, et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772–780. doi: 10.1002/hep.23094. [DOI] [PubMed] [Google Scholar]
- 6.Cano A, Buque X, Martinez-Una M, Aurrekoetxea I, Menor A, Garcia-Rodriguez JL, et al. Methionine adenosyltransferase 1A gene deletion disrupts hepatic very low-density lipoprotein assembly in mice. Hepatology. 2011;54:1975–1986. doi: 10.1002/hep.24607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jo H, Choe SS, Shin KC, Jang H, Lee JH, Seong JK, et al. Endoplasmic reticulum stress induces hepatic steatosis via increased expression of the hepatic very low-density lipoprotein receptor. Hepatology. 2013;57:1366–1377. doi: 10.1002/hep.26126. [DOI] [PubMed] [Google Scholar]
- 8.Borchardt RA, Davis RA. Intrahepatic assembly of very low density lipoproteins. Rate of transport out of the endoplasmic reticulum determines rate of secretion. J Biol Chem. 1987;262:16394–16402. [PubMed] [Google Scholar]
- 9.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 10.Jiang ZG, Robson SC, Yao Z. Lipoprotein metabolism in nonalcoholic fatty liver disease. J Biomed Res. 2013;27:1–13. doi: 10.7555/JBR.27.20120077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mensenkamp AR, Havekes LM, Romijn JA, Kuipers F. Hepatic steatosis and very low density lipoprotein secretion: the involvement of apolipoprotein E. J Hepatol. 2001;35:816–822. doi: 10.1016/s0168-8278(01)00249-5. [DOI] [PubMed] [Google Scholar]
- 12.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 13.Shimano H, Yamada N, Katsuki M, Shimada M, Gotoda T, Harada K, et al. Overexpression of apolipoprotein E in transgenic mice: marked reduction in plasma lipoproteins except high density lipoprotein and resistance against diet-induced hypercholesterolemia. Proc Natl Acad Sci U S A. 1992;89:1750–1754. doi: 10.1073/pnas.89.5.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbons GF, Khurana R, Odwell A, Seelaender MC. Lipid balance in HepG2 cells: active synthesis and impaired mobilization. J Lipid Res. 1994;35:1801–1808. [PubMed] [Google Scholar]
- 15.Wiggins D, Gibbons GF. The lipolysis/esterification cycle of hepatic triacylglycerol. Its role in the secretion of very-low-density lipoprotein and its response to hormones and sulphonylureas. Biochem J. 1992;284(Pt 2):457–462. doi: 10.1042/bj2840457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilham D, Ho S, Rasouli M, Martres P, Vance DE, Lehner R. Inhibitors of hepatic microsomal triacylglycerol hydrolase decrease very low density lipoprotein secretion. FASEB J. 2003;17:1685–1687. doi: 10.1096/fj.02-0728fje. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Chantar ML, Vazquez-Chantada M, Ariz U, Martinez N, Varela M, Luka Z, et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology. 2008;47:1191–1199. doi: 10.1002/hep.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noga AA, Zhao Y, Vance DE. An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J Biol Chem. 2002;277:42358–42365. doi: 10.1074/jbc.M204542200. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Una M, Varela-Rey M, Cano A, Fernandez-Ares L, Beraza N, Aurrekoetxea I, et al. Excess S-adenosylmethionine reroutes phosphatidylethanolamine towards phosphatidylcholine and triglyceride synthesis. Hepatology. 2013;58:1296–1305. doi: 10.1002/hep.26399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimaki-Mogami T, Suzuki K, Takahashi A. The role of phosphatidylethanolamine methylation in the secretion of very low density lipoproteins by cultured rat hepatocytes: rapid inhibition of phosphatidylethanolamine methylation by bezafibrate increases the density of apolipoprotein B48-containing lipoproteins. Biochim Biophys Acta. 1996;1304:21–31. doi: 10.1016/s0005-2760(96)00100-2. [DOI] [PubMed] [Google Scholar]
- 21.Bharadwaj KG, Hiyama Y, Hu Y, Huggins LA, Ramakrishnan R, Abumrad NA, et al. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J Biol Chem. 2010;285:37976–37986. doi: 10.1074/jbc.M110.174458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo T, Al-Haideri M, Treskova E, Worgall TS, Kako Y, Goldberg IJ, et al. Lipoprotein lipase-mediated selective uptake from low density lipoprotein requires cell surface proteoglycans and is independent of scavenger receptor class B type 1. J Biol Chem. 2000;275:30355–30362. doi: 10.1074/jbc.M910327199. [DOI] [PubMed] [Google Scholar]
- 23.BLIGH EG, DYER WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz JI, Ochoa B. Quantification in the subnanomolar range of phospholipids and neutral lipids by monodimensional thin-layer chromatography and image analysis. J Lipid Res. 1997;38:1482–1489. [PubMed] [Google Scholar]
- 25.Moylan CA, Pang H, Dellinger A, Suzuki A, Garrett ME, Guy CD, et al. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology. 2014;59:471–482. doi: 10.1002/hep.26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy SK, Yang H, Moylan CA, Pang H, Dellinger A, Abdelmalek MF, et al. Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:1076–1087. doi: 10.1053/j.gastro.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye SQ, Reardon CA, Getz GS. Inhibition of apolipoprotein E degradation in a post-Golgi compartment by a cysteine protease inhibitor. J Biol Chem. 1993;268:8497–8502. [PubMed] [Google Scholar]
- 28.Wenner C, Lorkowski S, Engel T, Cullen P. Apolipoprotein E in macrophages and hepatocytes is eegraded via the proteasomal pathway. Biochem Biophys Res Commun. 2001;282:608–614. doi: 10.1006/bbrc.2001.4611. [DOI] [PubMed] [Google Scholar]
- 29.Magnusson B, Asp L, Bostrom P, Ruiz M, Stillemark-Billton P, Linden D, et al. Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler Thromb Vasc Biol. 2006;26:1566–1571. doi: 10.1161/01.ATV.0000223345.11820.da. [DOI] [PubMed] [Google Scholar]
- 30.Senthivinayagam S, McIntosh AL, Moon KC, Atshaves BP. Plin2 inhibits cellular glucose uptake through interactions with SNAP23, a SNARE complex protein. PLoS One. 2013;8:e73696. doi: 10.1371/journal.pone.0073696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIntosh AL, Storey SM, Atshaves BP. Intracellular lipid droplets contain dynamic pools of sphingomyelin: ADRP binds phospholipids with high affinity. Lipids. 2010;45:465–477. doi: 10.1007/s11745-010-3424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundaram M, Yao Z. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr Metab (Lond) 2010;7 doi: 10.1186/1743-7075-7-35. 35-7075-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rensen PC, Herijgers N, Netscher MH, Meskers SC, van Eck M, van Berkel TJ. Particle size determines the specificity of apolipoprotein E-containing triglyceride-rich emulsions for the LDL receptor versus hepatic remnant receptor in vivo. J Lipid Res. 1997;38:1070–1084. [PubMed] [Google Scholar]
- 34.Perman JC, Bostrom P, Lindbom M, Lidberg U, StAhlman M, Hagg D, et al. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J Clin Invest. 2011;121:2625–2640. doi: 10.1172/JCI43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horl G, Wagner A, Cole LK, Malli R, Reicher H, Kotzbeck P, et al. Sequential synthesis and methylation of phosphatidylethanolamine promote lipid droplet biosynthesis and stability in tissue culture and in vivo. J Biol Chem. 2011;286:17338–17350. doi: 10.1074/jbc.M111.234534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, et al. Diacylglycerol acyltranferase 1 anti-sense oligonucleotides reduce hepatic fibrosis in mice with nonalcoholic steatohepatitis. Hepatology. 2008;47(2):625–35. doi: 10.1002/hep.21988. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45(6):1366–74. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 38.Cole LK, Dolinsky VW, Dyck JR, Vance DE. Impaired phosphatidylcholine biosynthesis reduces atherosclerosis and prevents lipotoxic cardiac dysfunction in ApoE−/− Mice. Circ Res. 2011;108:686–694. doi: 10.1161/CIRCRESAHA.110.238691. [DOI] [PubMed] [Google Scholar]
- 39.Karavia EA, Papachristou DJ, Kotsikogianni I, Giopanou I, Kypreos KE. Deficiency in apolipoprotein E has a protective effect on diet-induced nonalcoholic fatty liver disease in mice. FEBS J. 2011;278:3119–3129. doi: 10.1111/j.1742-4658.2011.08238.x. [DOI] [PubMed] [Google Scholar]
- 40.Bechmann LP, Gieseler RK, Sowa JP, Kahraman A, Erhard J, Wedemeyer I, et al. Apoptosis is associated with CD36/fatty acid translocase upregulation in nonalcoholic steatohepatitis. Liver Int. 2010;30(6):850–9. doi: 10.1111/j.1478-3231.2010.02248.x. [DOI] [PubMed] [Google Scholar]
- 41.Sydor S, Gu Y, Schlattjan M, Bechmann LP, Rauen U, Best J, et al. Steatosis does not impair liver regeneration after partial hepatectomy. Lab Invest. 2013;93(1):20–30. doi: 10.1038/labinvest.2012.142. [DOI] [PubMed] [Google Scholar]
- 42.Buqué X, Cano A, Miquilena-Colina ME, García-Monzón C, Ochoa B, Aspichueta P. High insulin levels are required for FAT/CD36 plasma membrane translocation and enhanced fatty acid uptake in obese Zucker rat hepatocytes. Am J Physiol Endocrinol Metab. 2012;303(4):E504–14. doi: 10.1152/ajpendo.00653.2011. [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Yan Y, Hu L, Zhao L, Yang P, Moorhead JF, et al. Rapamycin-mediated CD36 translational suppression contributes to alleviation of hepatic steatosis. Biochem Biophys Res Commun. 2014;447(1):57–63. doi: 10.1016/j.bbrc.2014.03.103. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, Hu L, Zhao L, Yang P, Moorhead JF, Varghese Z, et al. Inflammatory Stress Increases Hepatic CD36 Translational Efficiency via Activation of the mTOR Signalling Pathway. PLoS One. 2014;9(7):e103071. doi: 10.1371/journal.pone.0103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rensen PC, Jong MC, van Vark LC, van der Boom H, Hendriks WL, van Berkel TJ, et al. Apolipoprotein E is resistant to intracellular degradation in vitro and in vivo. Evidence for retroendocytosis. J Biol Chem. 2000;275:8564–8571. doi: 10.1074/jbc.275.12.8564. [DOI] [PubMed] [Google Scholar]
- 46.Ye J, Li JZ, Liu Y, Li X, Yang T, Ma X, et al. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 2009;9:177–190. doi: 10.1016/j.cmet.2008.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.