Abstract
Background
Poor adherence hinders glaucoma treatment. Studies have identified demographic and clinical predictors of adherence but fewer psychological variables.
Purpose
We examined predictors from four health behavior theories and past research.
Methods
In the baseline phase of a 3-site adherence study, before any intervention, 201 participants used electronic Medication Event Monitoring System (MEMS) bottles to monitor eyedrop use for 2 months, and completed questionnaires including self-reported adherence.
Results
MEMS showed 79% adherence and self-report 94% (0.5–1.5 missed weekly doses), but correlated only rs = .31. Self-efficacy, motivation, dose frequency, and non-minority race/ethnicity predicted 35% of variance in MEMS. Cues to action, self-efficacy, and intention predicted 20% of variance in self-reported adherence.
Conclusions
Self-efficacy, motivation, intention, cues to action, dose frequency, and race/ethnicity each independently predicted adherence. Other predictors from all theories were supported in bivariate analyses, but additional study is needed. Researchers and clinicians should consider psychological predictors of adherence.
Keywords: attitude, glaucoma, medication adherence, motivation, theory
Glaucoma is a chronic, irreversible, optic neuropathy that can lead to permanent vision loss; it is the second leading cause of blindness worldwide and affects up to 60 million people (1). Eye drop medications are prescribed to preserve visual function and quality of life by reducing intraocular pressure (IOP), and regular eye drop use reduces the rate of glaucoma progression (2). However, nonadherence is an ongoing barrier to glaucoma treatment (3,4), with more than half of patients remaining nonadherent even after significant vision loss (5). Adherence has been estimated at 71% after 2 months based on electronic monitoring (6), 50% after 12 months based on population-level pharmacy data (7), and 64% after 22 months based on pharmacy data in the large-scale glaucoma adherence and persistency study (GAPS: 4).
GAPS also identified predictors of nonadherence based on chart reviews and telephone interviews for a subset of patients. Patient-level predictors of nonadherence included cost of treatment, lack of a positive outcome expectancy for benefits of treatment, and forgetting to take medication due to lack of cues to action when traveling (8), as well as low perceived susceptibility to the negative consequences of glaucoma (9). Other beliefs such as a patient’s ideas about the course of disease, the overall coherence of the illness experience, and the necessity for medication in comparison to its long-term effects have also been found to predict nonadherence in glaucoma (10). Qualitative studies support the importance of barriers (cost, confusion, forgetting, regimen complexity, and side effects), low perceived susceptibility, and poor outcome expectancy as predictors of nonadherence, and suggest other risk factors including poor patient-physician communication, medication side effects, low motivation for treatment, limited ability to administer eye drops, lack of knowledge, external stressors or competing priorities, and lack of social support (11–13).
Demographic and clinical predictors of glaucoma medication nonadherence include older patient age, living alone, taking a greater number of medications, and in some studies minority race/ethnicity, being married, and having more comorbid conditions. Other demographic and clinical variables have shown no relationship to adherence including gender, educational level, disease severity, and treatment duration (9,10,14–16).
The literature on nonadherence in other chronic diseases suggests additional predictors of nonadherence (17), which are organized into psychological theories of health behavior. As applied to glaucoma, the Health Belief Model (HBM: 18) includes some of the variables listed above: perceived susceptibility, positive outcome expectancy, perceived barriers to care such as cost, and cues to action that reduce forgetting. The HBM identifies two other possible predictors: the perceived severity of glaucoma symptoms and patients’ self-efficacy for taking medication. The Theory of Planned Behavior (TPB: 19) also includes a broad “attitude toward medication” variable that may include beliefs about susceptibility, severity, and outcome expectancy. This theory adds three other possible predictors: patients’ perceived control over their disease, perceived social norm including what they believe their physician expects in the doctor-patient relationship, and intention or commitment to take medication. The TPB explicitly states that patients’ knowledge does not affect their behavior. The information-motivation-behavioral skills model (IMB: 20), on the other hand, suggests that knowledge does affect adherence, along with behavioral skills (self-efficacy and/or functional ability) and motivation for treatment. Finally, a newer motivational model of adherence (21) suggests a central role for treatment motivation, which is in turn influenced by perceived control, mood states such as anxiety and depression, level of stress, coping efforts, and social support. In this model, knowledge is again seen as peripheral to predicting adherence behavior.
Although current knowledge about glaucoma adherence and relevant theories suggest a list of possible variables that might predict nonadherence, most studies to date have examined only a few possible predictors and have not compared their relative strength. Most studies have also given less attention to psychological predictors of adherence or examined a limited field of variables that were not closely linked to theory. We and others have argued that the science of health behavior is pre-paradigmatic, with inconsistent definitions of the terms of interest (22,23). If this is so, studies testing constructs from only a single theory are likely to miss important predictors or to obscure similarities among similarly defined constructs in different theories. Additionally, most studies have defined adherence using a single electronic or pharmacy measure even though all adherence metrics have limitations (24). Therefore, despite some high-quality prior research, predictors of adherence in glaucoma are still not well understood.
In the current study we took a theory-building rather than a theory-testing approach, examining possible predictors of adherence from multiple health behavior theories and relying on statistical methods to select the most useful ones. We collected data on more than 30 possible predictors and two different adherence metrics from 201 persons with glaucoma at three geographically and demographically distinct sites in the United States. Predictor variables were drawn from multiple health behavior theories as described above; our goal was not to directly test any one theory but rather to identify a broad range of candidate variables that might be related to glaucoma adherence. Using data from the baseline phase of a randomized controlled trial (RCT) of interventions to improve glaucoma medication adherence, we conducted an observational study to test predictors’ effects on two different measures of nonadherence, with the expectation that different operational definitions of adherence might yield different results.
Method
Participants
Participants were 201 patients recruited from the specialty glaucoma clinics of eye institutes in Denver CO, Portland OR, and Nashville TN. Two clinics were university-affiliated and the other was independent. Data were collected during an RCT of counseling methods to improve glaucoma adherence (ClinicalTrials.gov ID# NCT01409421). The current analysis focused on data collected during the study’s baseline phase, a 2-month timeframe during which adherence was simply monitored and no interventions were provided.
Based on inclusion criteria, all participants were English-speaking adults (minimum age of 18, no upper limit) with open-angle glaucoma or ocular hypertension, were prescribed a single eye drop (either monotherapy or a single combination medication), and had a recent visual field test to measure glaucoma disease severity. Exclusion criteria were any plan for surgery in the next 6 months or any serious medical or psychological condition that in the judgment of the treating ophthalmologist would have precluded study participation. Only three patients were eliminated from the study based on the exclusion criteria, all due to cognitive impairment.
Participants had a mean age of 65.0 years (SD = 11.2 years), with a range from 20 to 93. Participants were 129 women (64.2%) and 72 men, consistent with the demographics of older Americans, and came from diverse racial and ethnic backgrounds: 17.9% were African-American, 4% Latino/Latina (with 1 participant who was both African-American and Latina), 3% Asian/Pacific Islander, 1% Native American, 69.2% White non-Hispanic, and 5% other or unspecified. All participants were fluent in English per the inclusion criteria, but three reported speaking a different language at home (1 Spanish, 1 Chinese, and 1 Hindi and Gujarati). Participants’ educational level was high: 92.7% had at least a high school diploma, 48.4% at least an undergraduate degree, 21.8% at least a master’s degree, and 3.6% a doctoral degree. This finding may be related to the location of two study sites at academic medical centers. Most participants were not working due to retirement (45.8%), unemployment (9.0%), or disability (2.5%). Most were currently married (60.1%), widowed (12.6%), or divorced (9.6%). Participants had M = 3.45 conditions besides glaucoma (SD = 2.27); the most common were heart disease (64%), chronic pain (38%) and other eye conditions (30%).
Procedure
All study procedures were conducted in accordance with the Helsinki Declaration of 1975 (revised 2000) and approved by the Colorado Multiple Institutional Review Board. All participants gave written informed consent prior to enrollment. Adherence was monitored continuously for 2 months using MEMS (Medication Event Monitoring System) bottles. MEMS are plastic bottles with a large lid that records openings via a plunger linked to a microchip. MEMS are a standard adherence monitoring method in other chronic disease states, and have been used to track eye drop adherence in prior studies (25–29). Participants were asked to store their glaucoma eye dropper in the bottle, to open it only when taking drops, and to close the bottle again afterwards. MEMS are a reactive measure because patients increase their adherence when they know they are being monitored (26). We attempted to reduce this bias by measuring MEMS-based adherence over a 2-month period, by the end of which reactivity was expected to decrease (26), and by asking participants to take medication as they normally would. Participants were informed that the study was about medication adherence and that the MEMS caps would track bottle openings, but they were not told the specific study aims.
Participants’ adherence was also assessed using a self-report scale (see below) at the time of enrollment and 2 months later. At the 2-month follow-up visit each participant was randomized to one of three conditions (usual care, reminder calls, or motivational interviewing) and the intervention phase of the RCT began; intervention-phase data were not considered in the current analysis in order to focus on naturally occurring patterns of adherence.
Measures
Participants completed a battery of self-report instruments at intake and follow-up. Questionnaire psychometrics and citations are presented in Table 1. After missing data imputation on individual items, the items on each self-report questionnaire or subscale were averaged to produce a single score for each participant.
Table 1.
Constructs, Measures, Citations, and Instrument Psychometrics
| Measure (Source) | Constructs and Relevant Theory | Reliability | Support for Validity |
|---|---|---|---|
| Items from medical records review | Age in years; gender; minority race or ethnicity (yes/no); marital status (married/not); level of education; number of comorbid medical conditions; number of comorbid psychiatric conditions (no theory basis, but prior glaucoma research supports some of these variables as predictors of adherence) | Predictive validity: chart review predicts clinical outcomes in general practice settings (35); Concurrent validity: chart reviews match patient reports in general practice settings (36) | |
| Adherence Attitude Inventory |
|
|
Construct validity: stable factors in factor analysis and across studies; convergent and discriminant validity in study with patients taking multiple medication types (32) |
| Glaucoma Treatment Compliance Assessment Tool |
|
|
Created for glaucoma; Construct validity: stable factors in factor analysis in prior studies with glaucoma, strong links to constructs in the well-validated Health Belief Model, consensus by glaucoma experts (37) |
| Composite scale from Visual Functioning Questionnaire 25 (VFQ-25) | Functional ability (22 items: IMB) | α = .90 | Created for glaucoma; Construct validity based on consensus by glaucoma experts (38); similar to the well-validated Short Form 36 (SF-36) survey (42) |
| Scale adapted for this study from VFQ-25 item #4 | Perceived severity of eye symptoms (5 items: HBM) | α = .82 | Developed for the current study as an adaptation of an existing scale (38); Construct validity based on consensus by glaucoma and symptom research experts |
| Barriers scale (created for this study): items on drug use, side effects, treatment burden, uncertainty, distress or confusion | Barriers – overall (7 items: HBM) | α = .65 | Developed for the current study based on adherence barriers noted in prior research with multiple disease states (21); Construct validity based on consensus by glaucoma and adherence experts, and by factor analysis in the current sample showing common variance except for an alcohol item |
| Diary of Ambulatory Behavioral States (DABS) control scale | Perceived control (2 items: TPB, MM) | α = .76 | Construct validity: stable factors across studies on health promotion topics (21,40); predictive validity in prior research (21) |
| Herzog motivation scale | Motivation for treatment (6 items: IMB, MM) | α = .87 | Predictive validity: predicts readiness, actual behavior in health promotion (41) |
| Subscale from SF-36 | Mood – overall (9 items: MM) | α = .88 | Construct validity: expert consensus; discriminant validity across several disease states (42) |
| State scale (20 items) from State-Trait Anxiety Inventory | Mood – anxiety (20 items: MM) | α = .90 | Construct validity: responsive to experimental manipulation and to daily variations in anxiety (43) |
| DABS mood scale | Mood – depression (4 items: MM) | α = .82 | Construct validity: stable factors across studies on health promotion topics (21,40); predictive in prior research (21) |
| Stress item: 7-point scale | Stress (1 item: MM) | retest, r = .48 (21) | Construct validity: used to measure patients’ everyday stress in an HIV adherence study (21) |
| Coping item: 7-point scale | Coping (1 item: MM) | retest, r = .39 (21) | Construct validity: used to measure patients’ everyday coping in an HIV adherence study (21) |
| Composite of Multidimensional Scale of Perceived Social Support (MSPSS), plus 2 DABS items | Social support (14 items: MM) | α = .93 | Construct validity (MSPSS): stable factor structure in healthy adults (44); divergent validity: not correlated with social desirability (44); validated for use with older adults (45) |
Note. Internal consistency estimates are Cronbach’s alphas calculated from the current sample, except for the single-item stress and coping measures in which they are retest reliability statistics based on prior research as cited.
HBM = Health Belief Model; TPB = Theory of Planned Behavior; IMB = Information-Motivation-Behavioral Skills Model; MM = Motivational Model of health behavior; VFQ-25 = Visual Functioning Questionnaire 25; DABS = Diary of Ambulatory Behavioral States; SF-36 = Short Form 36 Health Survey; MSPSS = Multidimensional Scales of Perceived Social Support.
Primary Outcome: Medication Adherence Based on MEMS
MEMS caps track bottle openings rather than actual use, but are considered a high-quality proxy measure of adherence (24). Reliability of the measure is supported by a low rate of technical errors (26) and validity is supported by correlations between MEMS and adherence-linked physiological outcomes such as viral load in HIV (30). Although MEMS are widely used to measure adherence, they may create a reactive measurement effect: In one glaucoma study the use of MEMS was associated with higher than normal adherence (27); in another a 2-week run-in period was used to rule out reactive measurement effects (28). In our own prior research we observed a return to baseline adherence 6–8 weeks after patients started using MEMS (26). MEMS data were analyzed as the overall percentage of doses taken correctly by each participant. Participants were considered adherent to a specific dose if they took once-daily medication within 22 to 26 hours after the previous dose, based on a common 2-hour window for correct timing (26,31). Because clinicians’ instructions to patients about twice- and three-times-daily medications varied, the small percentage of patients taking medication more than once per day were considered adherent if the correct number of bottle openings occurred within each 24-hour period. We tested week-by-week MEMS data for any change over time using multilevel modeling with weeks nested within participant and a person-level random effect, but found no significant trend; all MEMS data for each participant were therefore averaged into a single 0–100 count measure reflecting the percentage of days with correct adherence across the entire baseline period.
Secondary Outcome: Self-Reported Adherence
The Adherence Attitude Inventory (AAI: 32) is a 31-item measure of cognitive and behavioral items related to medication adherence. The final three items ask whether the respondent took a targeted medication (in this case, eye drops for glaucoma) correctly today, yesterday, and the day before yesterday. Studies in other chronic diseases have found that 3-day retrospective recall is a valid measure based on detection of nonadherence and correlations with known adherence predictors (33); 3-day recall also correlates significantly with physiological measures and electronic monitoring (34). In this study, the self-report recall measure had limited variability, M = 92.7, Mdn = 100, IQR = 0, which would have reduced this variable’s usefulness as an outcome measure. We were able to improve variability by averaging each participant’s recall scores from the beginning and end of the baseline phase (6 days altogether), new M = 94.2, Mdn = 100, with IQR = 13 showing increased variability. Similar to MEMS, this final self-report adherence measure reflects a percentage of days with correct adherence. Internal consistency across all 6 days was low, α = .36, but this likely reflects a true lack of consistency in participants’ adherence.
Predictor Variables from Clinical Records
Clinical and demographic information was abstracted from chart records (35,36), including participants’ age, gender, race and ethnicity (combined for analysis into a single variable reflecting minority versus non-minority), employment status (yes/no), marital status (yes/no), educational level (an ordinal-level scale by type of degree), insurance coverage (yes/no: a proxy for the cost of care), type of glaucoma (open-angle versus ocular hypertension), type of medication (one of 5 drug classes), number of doses per day, number of other medications prescribed, years since the participant started treatment, number of comorbid conditions, most recent intra-ocular pressure, most recent visual field, number of providers seen for all health conditions, and number of calls to the clinic in the past 3 months.
AAI Attitude Scales
In addition to the self-report adherence measure described above, the AAI (32) has subscales on cognitive confusion (e.g., I lose track of time and I have to take my medication late or not at all), doctor-patient relationship (my medical provider understands how difficult it is to follow my medication regimen), general self-efficacy for adherence (I fear that I am not capable of taking my medication as I should [reverse-scored]), and intention to continue in treatment (I am determined to do whatever it takes to take my medication on schedule).
Glaucoma Treatment Compliance Assessment Tool (GTCAT)
Participants’ knowledge and attitudes were also measured using the GTCAT (37), a measure based on the HBM. GTCAT subscales measure knowledge (e.g., all of my vision could be lost due to glaucoma), perceived susceptibility (I think I will go blind in 5 years if I DO NOT use my eye drops), outcome expectancy (there are things I can do to control my glaucoma), specific self-efficacy to use eye drops (I can place the eye drops into my eye correctly without any assistance, a variable that was distinct from global self-efficacy as measured by the AAI, r = .14), cues to take medication (sometimes the drops aren’t with me when it is time to take them), and barriers due to side effects (sometimes the drops are painful or uncomfortable to take).
Functioning and Symptoms
Daily functioning was measured using the Visual Functioning Questionnaire (VFQ-25: 38) total score (e.g., do you accomplish less than you would like because of your vision?). To measure perceived severity of glaucoma symptoms, the authors expanded a VFQ-25 item about eye pain into a new 5-item scale on eye symptoms including stinging, aching, or itching. Symptom items were recommended by the authors with clinical expertise in glaucoma treatment and selected by the first author based on common symptoms reported in the glaucoma adherence literature. Because this symptoms measure (expanded from one VFQ-25 item to five) was new in this study, we examined Cronbach’s alpha and found that these items grouped together into a single scale with good reliability, α = .82.
Barriers to Care
Specific barriers were identified based on our team’s prior adherence research (17,21,39) and evaluated with items on alcohol use, drug use, side effects, treatment burden, uncertainty about the need for treatment, and distress or confusion. Because the Barriers to Care instrument was new in this study, we calculated Cronbach’s alpha for all items together and then examined the contribution of each individual item to the measure’s internal consistency reliability. One item – about alcohol use – failed to load with the other items and substantially reduced the Cronbach’s alpha. We therefore dropped that item, and a final Barriers to Care scale score was calculated as an average of 7 of the original 8 items with a final α= .65.
Perceived Control
Participants’ level of perceived control over everyday situations was measured using a 3-item subscale from the Diary of Ambulatory Behavioral States (DABS: 21,40; e.g., feelings today – Are you able to handle difficulties?)
Motivation
Motivation for treatment was measured using a 7-item scale that outperforms the widely used stage-of-change measure psychometrically (41: e.g., I am planning to take my medication as prescribed).
Mental Health Variables
Mood was evaluated using the 9-item SF-36 mental health subscale (42: e.g., have you felt calm and peaceful?). Anxiety was also measured using the state version of the State-Trait Anxiety Inventory (43: e.g., I feel anxious), and depressed mood was measured using a 4-item scale from the DABS (21,40: e.g., How are you feeling today? Sad?).
Stress and Coping
Stress and coping were each measured using a single 7-point item developed based on previous research using daily survey items to predict health behavior (21: e.g., in the last 7 days, how well do you feel you have been able to cope with stress?).
Social Support
Perceived social support was measured using the Multidimensional Scales of Perceived Social Support (MSPSS: 44,45; e.g., my friends really try to help me). We also tested two DABS social support items from our prior research on HIV adherence (21), but these correlated so strongly with the MSPSS that all items from the original instrument and our two additional items were averaged into a single scale with α= .93.
Data Analysis
Primary analyses were performed in SPSS version 20 (IBM Corporation, Armonk NY). Baseline data were at least 90% complete for all variables; missing values were screened and appeared to be either missing at random or missing completely at random. To minimize bias missing data were therefore handled using the NORM program (46) for multiple imputation of missing values prior to further analysis, which was then conducted on a complete dataset. All variables were initially screened for normality and missing data; because both dependent variables showed a strong negative skew, nonparametric Spearman correlations were used to screen predictors in bivariate analyses. Significant univariate predictors (p < .05) were then included in a single combined model to identify the best predictors of each adherence variable. Poisson regression with robust standard errors was used for the combined analysis, which addressed non-normality in the adherence outcomes by considering them as count data. Predictors that emerged as significant in each multiple regression model were examined for multicollinearity, and results were tested in sensitivity analyses by leaving out one variable at a time when there was at least moderate shared variance with another predictor and/or the criterion variable (r > .30). In all cases, conclusions remained unchanged.
Results
Sample Representativeness
A total of 260 eligible patients were approached for the study and 77.3% agreed. Participants were more often female (63.7% versus 49.3%), χ2(2) = 4.44, p = .04, African-American (19.9% versus 6.0%), χ2(2) = 4.71, p = .03, and slightly younger (M = 65.0 years versus 69.9), t(251) = 2.69, p = .008, than those who refused participation. There were no differences between participants and nonparticipants in the percentage of Latino/Latina patients or the percentage of patients with insurance (ps > .65). A higher percentage of women and younger age are consistent with the general characteristics of study volunteers (47), but the higher percentage of African-American participants and absence of any difference in the percentage of Latino/Latina patients are not typical of volunteers and show sample diversity.
Differences between Study Sites
Participants were similar across sites in terms of age, F(2, 190) = 0.89, p = .41, gender, χ2(2) = 3.52, p = .17, and marital status, χ2(8) = 9.30, p = .32. Compared to the other two sites, the Portland OR clinic’s participants were significantly more likely to be White, χ2(2) = 22.1, p < .001, and were more highly educated, F(2, 189) = 5.22, p = .006. Scores differed across sites on 10 predictor variables (all ps < .001) – confusion, self-efficacy, outcome expectancy, perceived control, number of doses per day, number of comorbidities, number of other medications, visual field results, intra-ocular pressure, and treatment duration – so study site was included as a covariate in the Poisson regression models.
Adherence to Glaucoma Treatment
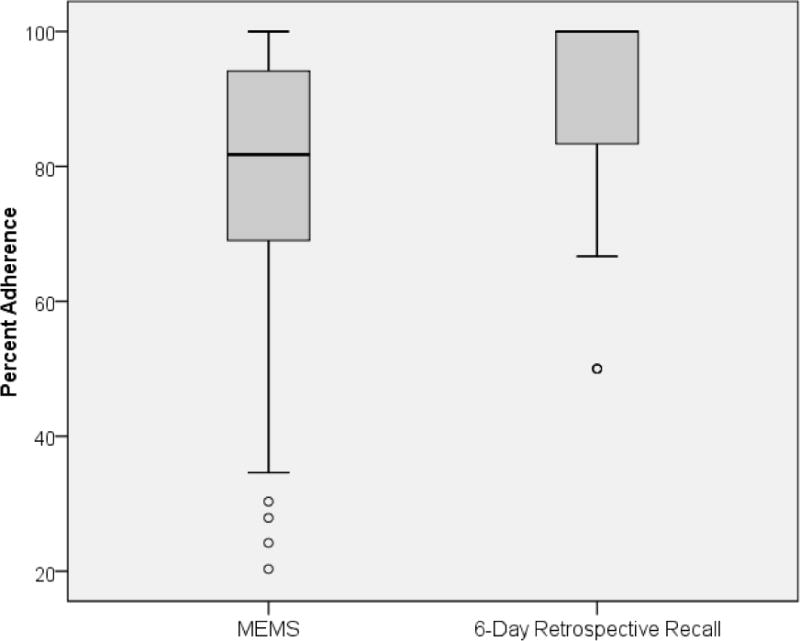
Boxplots in Figure 1 show median adherence (dark line) for each measure, which was 100% for the self-report measure and 81% for MEMS. Mean adherence levels were slightly lower, M = 94.1 (SD = 11.3) for self-report and M = 78.5 (SD = 17.8) for MEMS. The self-report measure had less variability (IQR = 13.0) than MEMS (IQR = 25.0). On the self-report scale at least three-quarters of participants achieved the likely therapeutic adherence level of 80% over 2 months, and more than half of participants did based on MEMS. The bivariate correlation between the two adherence measures was only rs = .31, so predictors of each adherence measure were examined separately.
Figure 1.
Median and Variability for Two Measures of Adherence
Predictors of Adherence based on MEMS
In univariate analyses, MEMS-based adherence was individually predicted by variables from all four psychological theories (Table 2). Race/ethnicity and educational level predicted adherence, but other demographic variables did not. Clinical predictors of adherence based on MEMS included higher number of doses per day and better visual field test results. The strongest predictors based on multiple Poisson regression modeling were self-efficacy for adherence, motivation for treatment, greater number of doses per day, and non-minority race or ethnicity. Other psychological predictors of adherence including fewer cues to action, absence of barriers, absence of confusion, intention to adhere, higher functional ability, absence of negative mood, and absence of anxiety were significant predictors of adherence in univariate analyses but nonsignificant in the multiple Poisson regression model. The total model accounted for 35% of the variability in adherence based on MEMS.
Table 2.
Predictors of Adherence based on MEMS
| Predictor | Univariate Spearman rho | Multiple Poisson Regression Model |
|---|---|---|
| Higher Educational Level (D) | +.17 | |
| Minority Race/Ethnicity (D) | −.21 | β = −.28 (SE = .13), p = .025 |
| More Doses Per Day (C) | +.15† | β = .55 (SE = .14), p < .001 |
| Better Visual Field Test Results (C) | +.18 | |
| Fewer Cues to Action (HBM) | −.27 | |
| Higher Barriers – Overall (HBM) | −.15 | |
| Higher Barriers – Confusion (HBM) | −.24 | |
| Greater Self-Efficacy for Adherence (HBM, IMB) | +.30 | β = .20 (SE = .09), p = .025 |
| Greater Intention to Adhere (TPB) | +.27 | |
| Higher Functional Ability (IMB) | +.20 | |
| Greater Motivation for Treatment (IMB, MM) | +.29 | β = .32 (SE = .14), p = .019 |
| More Negative Overall Mood (MM) | −.16 | |
| More Anxiety (MM) | −.14 |
Effect in the opposite direction from what was predicted based on prior research.
Note. Regression model results are after controlling for nonsignificant between-site differences. In the multiple regression model, 35% of the total variance in adherence was accounted for (pseudo R-square computed from log likelihood values of the final model compared to the null model). All significant univariate predictors were included in the multiple regression analysis, but only the significant multiple regression results are presented. D = demographic predictor, C = clinical predictor, HBM = variable from the Health Belief Model, TPB = variable from the Theory of Planned Behavior, IMB = variable from the Information-Motivation-Behavioral Skills model, MM = variable from the Motivational Model.
Predictors of Adherence based on Self-Report
In univariate analyses, self-reported adherence was predicted by similar variables from all four psychological theories (Table 3). No demographic or clinical variables predicted this self-reported adherence measure. The strongest predictors of self-reported adherence based on multiple Poisson regression modeling were cues to action, self-efficacy for adherence, and intention to adhere. Other psychological predictors of adherence including absence of confusion, higher functional ability, motivation for treatment, absence of negative mood, absence of anxiety, and presence of social support were significant in univariate analyses but non-significant in the multiple Poisson regression model. The total model accounted for 20% of the variability in adherence based on self-report.
Table 3.
Predictors of Self-Reported Medication Adherence (6-Day Recall)
| Predictor | Univariate Spearman rho | Multiple Poisson Regression Model |
|---|---|---|
| Fewer Cues to Action (HBM) | −.35 | β = −.32 (SE = .139), p = .011 |
| Higher Barriers – Confusion (HBM) | −.28 | |
| Greater Self-Efficacy for Adherence (HBM, IMB) | +.33 | β = .38 (SE = .16), p = .018 |
| Greater Intention to Adhere (TPB) | +.32 | β = .28 (SE = .11), p = .009 |
| Higher Functional Ability (IMB) | +.21 | |
| Greater Motivation for Treatment (IMB, MM) | +.22 | |
| More Negative Overall Mood (MM) | −.21 | |
| More Anxiety (MM) | −.17 | |
| Greater Social Support (MM) | +.18 |
Note. Regression model results are after controlling for nonsignificant between-site differences. In the multiple regression model, 20% of the total variance in adherence was accounted for (pseudo R-square computed from log likelihood values of the final model compared to the null model). All significant univariate predictors were included in the multiple regression analysis, but only the significant multiple regression results are presented. D = demographic predictor, C = clinical predictor, HBM = variable from the Health Belief Model, TPB = variable from the Theory of Planned Behavior, IMB = variable from the Information-Motivation-Behavioral Skills model, MM = variable from the Motivational Model.
Discussion
In a large, relatively diverse sample, participants with glaucoma or ocular hypertension had overall adherence in the 79%–94% range, depending on how adherence was measured. This level of adherence is higher than in some prior studies (6–8) but consistent with other studies that used MEMS (26–29). Because our data were collected during the first 2 months of MEMS monitoring when reactivity is greatest, they likely reflect an artificially high adherence level that would be expected to decrease over time. However, variables that predict nonadherence even during this time of relatively high adherence are likely to be useful later in treatment as well.
Predictors of Adherence in Glaucoma
Prior research including the large-scale GAPS study has suggested both medication-related and psychological predictors of nonadherence in glaucoma, and many of these were replicated in the current study. Lower adherence was predicted by some demographic and clinical variables, including number of doses per day, severity of glaucoma based on visual field, minority race/ethnicity, and educational level. However, contrary to some prior research, adherence was unrelated to age or marital status. Furthermore, other than the number of doses of medication per day, no medication characteristic such as drug class or side effects predicted adherence.
Psychological variables were stronger predictors of adherence, including constructs drawn from the HBM – cues to action, barriers including confusion, and self-efficacy – from the TPB – intention and perceived social support – from the IMB – motivation, self-efficacy, and functional ability – and from the motivational model – mood, motivation, and social support. Similar to the findings in GAPS (8), forgetting to take medication and barriers such as the cost of treatment each contributed to nonadherence, but contrary to the GAPS findings outcome expectancies and perceived susceptibility to negative consequences of glaucoma were not strong predictors. Despite these findings, most of the variability in adherence remained unexplained.
In multiple Poisson regression models, the most consistent predictor was general self-efficacy for adherence, which was included in both of the final models. This was followed by motivation for treatment, intention to take medication, higher number of doses per day, non-minority race/ethnicity, and cues to action, each of which was included in only one of the final models. Each of these predictors made independent contributions to the models, suggesting that they influence adherence in different ways. Other significant univariate predictors may contribute variance based on their relationship with one of these stronger predictors.
Limitations
One notable finding in this study was the substantial disagreement between two well-validated adherence measures. Although this is not necessarily surprising for a construct with no gold-standard metric and is consistent with prior studies in other health conditions (30,31), it creates a dilemma for adherence researchers. Discrepancies between adherence measures may be explained by several factors. First, the self-report measure had only seven choices (adherent on 0 through 6 out of 6 days measured), which contributed to its more limited variability. Second, the MEMS measure was based on data collected daily over 8 weeks, permitting a much higher level of variability that might have contributed to a slightly lower estimate – i.e., if the MEMS data had to be aggregated into just seven categories, they also might show half of individuals at close to 100% adherence. Third, method variance (a tendency for all data collected in the same way to correlate with each other) might explain some correlations between self-reported predictor variables and self-reported adherence, while the MEMS measure did not share method variance with self-report predictors. Overall, our results suggest that adherence is a complex construct and that the exact level of nonadherence detected is dependent on how the data are collected (24).
A second limitation of the current study is that we took an empirical approach to theory building using candidate predictor variables from multiple theories of health behavior. This strategy may be useful in developing new, integrative theories of adherence to guide future research. However, a significant limitation of this study and of multiple regression in general is that findings are data-dependent and may not replicate. The moderately large sample size in the current study reduces concern about sample dependence. We also note that prior studies testing more limited subsets of variables have identified similar predictors of glaucoma nonadherence, which also reduces concern about cross-validation; the current study might be considered as a more comprehensive replication of several previous investigations. Nevertheless, further research would be helpful to confirm the specific predictors and parameter estimates identified in this study. Another concern is that most of the instruments used to measure health behavior constructs in this study were not originally developed for glaucoma and their validity for use in this population is unproven. Although we did not note any obvious validity problems based on patterns in the data or participant report, our results may not generalize to other operational definitions of the same constructs. Given these limitations, we believe the current results are most useful for hypothesis generation and selection of variables in future studies, rather than proving or disproving a specific theory about the determinants of adherence.
Finally, it is important to note that theories like the TPB and the motivational model do more than identify lists of predictors; they also specify how those variables relate to one another and the paths by which a key variable like intention or motivation mediates the effects of other variables on behavior. Although key mediator variables from both of these theories were identified as strong direct predictors of adherence, other variables that are believed to have more distal relationships to behavior were not as strongly supported. This pattern of results is entirely consistent with what a theory positing mediated effects would predict. Additional study using more powerful theory-testing methods such as structural equation modeling (SEM) is needed to determine whether any of the theories used to identify candidate variables for this study would be supported or not supported overall. Furthermore, even though we used established scales to link our findings to prior research, it might be possible to create even more effective predictors of adherence by using SEM to combine individual scale items across constructs.
Conclusion
With multiple competing theories that may overlap or omit key predictors, it has been argued that the science of health behavior is still in a pre-paradigmatic state (22,23). Within the subfield of medication adherence, previous studies have often examined predictors from only a single theory or have omitted psychological predictors and examined only medication-related variables without a clear linkage to theory. The current study, which included a large and diverse sample, replicates most of the findings from prior glaucoma adherence research and was designed to consider a wider range of variables than those examined previously. This study also adds to the literature by presenting results simultaneously for two different measures of medication adherence, allowing for comparison and contrast across different operational definitions of the criterion variable. Although our results may be sample-specific and should be regarded as preliminary findings for future theory-building, the integration of constructs from multiple theories can provide important direction for the field of adherence. Ultimately, replication or non-replication of findings across many large-scale studies is necessary for our understanding of treatment adherence to advance.
Acknowledgments
Author Note: This research was supported by a grant from Merck & Co., Inc., with additional research infrastructure support from the Colorado Clinical and Translational Science Institute, NIH grant #1UL1RR025780-01. The sponsor’s representative participated in design of the study and review of the manuscript, and Dr. Fitzgerald’s contributions are gratefully recognized with co-authorship credit. The authors wish to acknowledge Laurra Aagaard, Gordon Barker, Sandy Owings, Mary Preston, Scott Ruark, Christopher Shelvock, and Christina Sheppler for their contributions to this study.
Footnotes
Drs. Schmiege, Mansberger, Kammer, and Fitzgerald declare that they have no other conflict of interest.
Authors’ COI Disclosure and Adherence to Ethical Standards
In the past 3 years Dr. Kahook has also served as a speaker for Merck & Co; Dr. Cook has served as a consultant for Takeda Inc., Covance Market Access Inc., Academic Impressions Inc., Competency & Credentialing Institute, and Medical Simulation Corp.
All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. This study was reviewed for human subjects and confidentiality (HIPAA) compliance and approved by the Colorado Multiple Institutional Review Board.
Contributor Information
Paul F. Cook, University of Colorado College of Nursing
Sarah J. Schmiege, University of Colorado College of Nursing
Steven Mansberger, Devers Eye Institute.
Jeffrey Kammer, Vanderbilt Eye Institute.
Timothy Fitzgerald, Merck & Co., Inc.
Malik Y. Kahook, University of Colorado School of Medicine
References
- 1.Friedman DS, Wolfs RC, Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi GC, Pasinetti GM, Scudeller L, Radaelli R, Bianchi PE. Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol. 2010;21:410–414. doi: 10.5301/EJO.2010.6112. [DOI] [PubMed] [Google Scholar]
- 3.Gordon ME, Kass MA. Validity of standard compliance measures in glaucoma compared with an electronic eyedrop monitor. In: Cramer JA, Spilker B, editors. Patient Compliance in Medical Practice and Clinical Trials. New York: Raven Press; 1991. pp. 163–173. [Google Scholar]
- 4.Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: Methodology and findings of the glaucoma adherence and persistency study (GAPS) Invest Ophthalmol Vis Sci. 2007;48:5052–5057. doi: 10.1167/iovs.07-0290. [DOI] [PubMed] [Google Scholar]
- 5.Meichenbaum DC, Turk D. Facilitating Treatment Adherence: A Practitioner’s Guidebook. New York: Plenum Press; 1987. [Google Scholar]
- 6.Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically: The Travatan Dosing Aid Study. Ophthalmol. 2009;116:191–199. doi: 10.1016/j.ophtha.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Schmier JK, Covert DW, Robin AL. First-year treatment patterns among new initiators of topical prostaglandin analogs. Curr Med Res Opin. 2009;25:851–858. doi: 10.1185/03007990902791132. [DOI] [PubMed] [Google Scholar]
- 8.Friedman DS, Hahn SF, Gelb L, et al. Doctor-patient communication, health-related beliefs, and adherence in glaucoma: Results from the glaucoma adherence and persistency study. Ophthalmol. 2008;115:1320–1327. doi: 10.1016/j.ophtha.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Friedman DS, Okeke CO, Jampel HD, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmol. 2009;116:1097–1105. doi: 10.1016/j.ophtha.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Rees G, Leong O, Crowston JG, Lamoureux EL. Intentional and unintentional nonadherence to ocular hypotensive treatment in patients with glaucoma. Ophthalmol. 2010;117:903–908. doi: 10.1016/j.ophtha.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Taylor SA, Galbraith SM, Mills RP. Causes of non-compliance with drug regimens in glaucoma patients: A qualitative study. J Ocul Pharmacol Ther. 2002;18:401–409. doi: 10.1089/10807680260362687. [DOI] [PubMed] [Google Scholar]
- 12.Lacey J, Cate H, Broadway DC. Barriers to adherence with glaucoma medications: A qualitative research study. Eye. 2009;23:924–932. doi: 10.1038/eye.2008.103. [DOI] [PubMed] [Google Scholar]
- 13.Tsai J, McClure CA, Ramos S, Schlundt DG. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12:393–398. doi: 10.1097/00061198-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Khandekar R, Shama ME, Mohammed AJ. Noncompliance with medical treatment among glaucoma patients in Oman–a cross-sectional descriptive study. Ophthal Epidemiol. 2005;12:303–309. doi: 10.1080/09286580500224602. [DOI] [PubMed] [Google Scholar]
- 15.Djafari F, Lesk MR, Harasymowycz PJ, Desjardins D, Lachaine J. Determinants of adherence to glaucoma medical therapy in a long-term patient population. J Glaucoma. 2009;18:238–243. doi: 10.1097/IJG.0b013e3181815421. [DOI] [PubMed] [Google Scholar]
- 16.Muir KW, Santiago-Turla C, Stinnett SS, et al. Health literacy and adherence to glaucoma therapy. Am J Ophthalmol. 2006;142:223–226.e2. doi: 10.1016/j.ajo.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Cook PF. Adherence to medications. In: O’Donohue WT, Levensky ER, editors. Promoting Treatment Adherence: A Practical Handbook for Health Care Providers. Thousand Oaks, CA: Sage; 2006. pp. 183–202. [Google Scholar]
- 18.Mansberger SL. Are you compliant with addressing glaucoma adherence? Am J Ophthalmol. 2010;149:1. doi: 10.1016/j.ajo.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ajzen I, Fishbein M. Questions raised by a reasoned action approach: Comment on Ogden (2003) Health Psychol. 2004;23:431–434. doi: 10.1037/0278-6133.23.4.431. [DOI] [PubMed] [Google Scholar]
- 20.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25:462–473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 21.Cook PF, McElwain CJ, Bradley-Springer L. Feasibility of a PDA diary method to study daily experiences in persons living with HIV. Res Nurs Health. 2010;33:221–234. doi: 10.1002/nur.20381. [DOI] [PubMed] [Google Scholar]
- 22.Larsen KR, Voronovich ZA, Cook PF, Pedro L. Addicted to constructs: Science in reverse? Addictions. 2013;108:1532–1533. doi: 10.1111/add.12227. [DOI] [PubMed] [Google Scholar]
- 23.Cane J, OConnor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci. 2012;7:37. doi: 10.1186/1748-5908-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chesney MA. The elusive gold standard: future perspectives for HIV adherence assessment and intervention. J Acquir Immune Defic Syndr. 2006;43:S149–S155. doi: 10.1097/01.qai.0000243112.91293.26. [DOI] [PubMed] [Google Scholar]
- 25.Cook PF, Bremer RW, Ayala AJ, Kahook MY. Feasibility of a motivational interviewing delivered by a glaucoma educator to improve medication adherence. Clin Ophthalmol. 2010;4:1091–1101. doi: 10.2147/OPTH.S12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook PF, Schmiege SJ, McClean M, Aagaard L, Kahook MY. Practical and analytic issues in the electronic assessment of adherence. West J Nurs Res. 2011;34:598–620. doi: 10.1177/0193945911427153. [DOI] [PubMed] [Google Scholar]
- 27.Robin AL, Novak GD, Covert DW, Crockett RS, Marcic TS. Adherence in glaucoma: Objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144:533–540. doi: 10.1016/j.ajo.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Richardson C, Brunton L, Olleveant N, et al. A study to assess the feasibility of undertaking a randomized controlled trial of adherence with eye drops in glaucoma patients. Patient Prefer Adher. 2013;7:1025–1039. doi: 10.2147/PPA.S47785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmol. 2011;118:2398–2402. doi: 10.1016/j.ophtha.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Golin CE, Miller JG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 31.Choo PF, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Lewis SJ, Abell N. Development and evaluation of the Adherence Attitude Inventory. Res Soc Work Pract. 2002;12:107–123. [Google Scholar]
- 33.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 34.Mathews WC, Mar-Tang M, Ballard C, et al. Prevalence, predictors, and outcomes of early adherence after starting or changing antiretroviral therapy. AIDS Patient Care STDs. 2002;16:157–172. doi: 10.1089/10872910252930867. [DOI] [PubMed] [Google Scholar]
- 35.Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. J Am Med Assoc. 2000;283:1715–1722. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
- 36.Pakhomov SV, Jacobsen SJ, Chute CG, Roger VL. Agreement between patient-reported symptoms and their documentation in the medical record. Am J Manag Care. 2008;14:530–539. [PMC free article] [PubMed] [Google Scholar]
- 37.Barker GT, Cook PF, Kahook M, Kammer J, Mansberger SL. Psychometric properties of the Glaucoma Treatment Compliance Assessment Tool. May 5–9: Association for Research in Vision and Ophthalmology Conference; Seattle WA. 2013. Session #346, Presentation # 3522. [Google Scholar]
- 38.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire (VFQ-25) Arch Ophthalmol. 2001;119:1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 39.Cook PF. Patients’ and health care practitioners’ attributions about adherence problems as predictors of medication adherence. Res Nurs Health. 2008;31:261–273. doi: 10.1002/nur.20256. [DOI] [PubMed] [Google Scholar]
- 40.Kamarck TW. The Diary of Ambulatory Behavioral States: A new approach to the assessment of psychosocial influences on ambulatory cardiovascular activity. In: Krantz DS, Baum A, editors. Technology and Methods in Behavioral Medicine. Mahwah, NJ: Erlbaum; 1998. pp. 163–185. [Google Scholar]
- 41.Herzog TA, Blagg CO. Are most precontemplators contemplating smoking cessation? Assessing the validity of the stages of change. Health Psychol. 2007;26:222–231. doi: 10.1037/0278-6133.26.2.222. [DOI] [PubMed] [Google Scholar]
- 42.McHorney CA, Ware JE, Lu JFR, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 44.Dahlem NW, Zimet GD, Walker RR. The multidimensional scale of perceived social support: A confirmation study. J Clin Psychol. 1991;47:756–761. doi: 10.1002/1097-4679(199111)47:6<756::aid-jclp2270470605>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 45.Stanley MA, Beck JG, Zebb BJ. Psychometric properties of the MSPSS in older adults. Aging Ment Health. 1998;2:186–193. [Google Scholar]
- 46.Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001;6:330–351. [PubMed] [Google Scholar]
- 47.Kazdin AE. Research Design in Clinical Psychology. 2. Boston: Allyn and Bacon; 1992. [Google Scholar]


