Abstract
Cytokines are involved in many functions of the immune system including initiating, amplifying and resolving immune responses. Through bioinformatics analyses of a comprehensive database of gene expression (BIGE: Body Index of Gene Expression) we observed that a small secreted protein encoded by a poorly characterized gene called meteorin-like (METRNL), is highly expressed in mucosal tissues, skin and activated macrophages. Further studies indicate that Metrnl is produced by Alternatively Activated Macrophages (AAM) and M-CSF cultured bone marrow macrophages (M2-like macrophages). In the skin, METRNL is expressed by resting fibroblasts and IFNγ-treated keratinocytes. A screen of human skin-associated diseases showed significant over-expression of METRNL in psoriasis, prurigo nodularis, actinic keratosis and atopic dermatitis. METRNL is also up-regulated in synovial membranes of human rheumatoid arthritis. Taken together, these results indicate that Metrnl represents a novel cytokine, which is likely involved in both innate and acquired immune responses.
Keywords: Meteorin-like, Cytokine, M2 macrophages, Skin, Psoriasis, Rheumatoid arthritis
1. Introduction
Cytokines are signaling molecules that play key roles in many biological processes including hematopoiesis, embryonic development, and immune responses [1]. They are produced by a variety of cell types, and many exhibit pleiotropic effects under inflammatory or homeostatic conditions [2]. Cytokines are low-molecular weight secreted proteins, usually produced by activated leukocytes, that trigger signal transduction pathways upon binding to their specific receptors. Therefore, cytokines represent key molecular messengers through which cells of the immune system communicate with each other in order to develop and control immune responses. While cytokines are crucial for mounting appropriate immune responses, their deregulation is associated with inflammatory abnormalities and/or autoimmune conditions. Blockade of many cytokines and/or their receptors has led to disease alleviation in rheumatoid arthritis, psoriasis, systematic lupus erythematosus and many other indications [3].
In the present study, we asked whether there are still small secreted proteins with cytokine-like characteristics that remain to be identified. To this end, we queried a comprehensive microarray database of gene expression in the human body, a Body Index of Gene Expression (BIGE), which contains gene expression information on more than 105 human cells and tissues [4–5]. This screen led to the identification of several unknown or poorly characterized genes encoding secreted or transmembrane proteins expressed by various cells of the immune system. We have recently reported two of these molecules; the first one is a transmembrane protein that represents a novel biomarker of activated B cells (TSPAN33) [6]; the second is a secreted protein (Isthmin 1) expressed in the skin, mucosa and by NK, NKT and Th17 cells [7].
Here, we describe that a poorly characterized small secreted protein called meteorin-like (metrnl), represents a novel cytokine likely associated with innate and possibly acquired immunity. We show that METRNL is highly expressed in barrier tissues (skin, mucosa) and is strongly induced in alternatively activated macrophages (AAM) (or M2 macrophages) and BMM (also termed as M2-like macrophages) [8–9].
METRNL is also associated with human diseases. We investigated its expression in several skin or inflammatory disorders and found that METRNL is strongly over-expressed in psoriasis. Furthermore, it is also over-expressed in human rheumatoid arthritis (RA). Taken together, our results indicate that Metrnl encodes a novel cytokine that likely plays a role in inflammatory responses and may be involved in both innate and acquired immune functions.
2. Materials and Methods
2.1. BIGE database
The BIGE database has been described [4–5]. We have used it to identify novel genes associated with several tissues or cells [10–11]. To construct the BIGE database, samples from 105 different tissues and cell types of the human body were analyzed for gene expression using U133 2.0 genearrays (Affymetrix, Santa Clara, CA). The resulting data were normalized as described [4], and probesets corresponding to METRN (232269_x_at) or METRNL (225955_at) were used to determine the expression of the METRN or METRNL-like genes in the human body.
2.2. Mice
BALB/C and C57BL/6 mice were obtained from Charles River (Wilmington, MA). Mice were housed in the regular animal facility of the University of California, Irvine. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University Of California, Irvine.
2.3. Macrophages
Bone-marrow was isolated from murine femurs and cultured in DMEM (Corning) supplemented with 10% fetal bovine serum (FBS, Life technologies), 2% penicillin-streptomycin (Mediatech, Manassas, VA) and 50ng/mL M-CSF (BioLegend, San Diego, CA) or 50ng/mL GM-CSF (BioLegend). After 3 days, non-adherent cells were removed and fresh medium was added. Bone Marrow Derived Macrophages (BMDM) were used after 7 days in culture. To obtain peritoneal cavity macrophages, peritoneal exudate cells (PEC) were collected by lavage and allowed to adhere for 2 h in DMEM supplemented with 10% FBS, and 2% penicillin-streptomycin. Non-adherent cells were then removed and fresh medium was added. Peritoneal cavity macrophages were stimulated with IL-4 (50ng/mL, BioLegend) to induce AAMs or IFNγ (50ng/mL, BioLegend). For some experiments, macrophages were also incubated with IL-13 (50ng/mL, BioLegend), LPS (100ng/mL, Sigma-Aldrich, St. Louis, MO) or PGE2 (10ng/mL, Sigma-Aldrich).
2.4. Metrnl ELISA
Mouse Metrnl ELISA was purchased from R&D systems (Minneapolis, MN), and used according to the manufacturer’s protocol.
2.5. Collection of skin samples and cell culture
Skin biopsies were collected after obtaining informed consent and approval by the University of Düsseldorf Institutional Review Board from healthy individuals undergoing plastic surgery (n = 11), or from lesional skin from individuals with common skin diseases (psoriasis vulgaris n = 12, atopic dermatitis n = 12, prurigo nodularis n = 6, actinic keratosis n = 6) as part of their diagnosis. Skin biopsies were immediately snap-frozen in liquid nitrogen and stored at −80°C.
Primary human cells were isolated and cultured as described [12]. For keratinocytes, keratinocyte medium (GIBCO, Invitrogen, Carlsbad, CA) was supplemented with recombinant epidermal growth factor (EGF) and bovine pituitary extract. For fibroblasts, fibroblast medium Quantum 333 (PAA, Pasching, Austria), and for endothelial cells, endothelial cell medium (EGM) MV (Lonza, Basel, Switzerland). Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats by Ficoll-Paque (GE Healthcare, Pittsburgh, PA) density-gradient centrifugation. Control samples for human spleen, kidney, brain and liver total RNA were obtained from Clontech (Mountain View, CA).
For functional analyses, recombinant cytokines (R&D Systems, BioLegend) were used to stimulate primary human keratinocytes at the following concentrations: GM-CSF (50 ng/ml), TNF-α (10 ng/ml) + IL1β (5 ng/ml), IFN-γ (50 ng/ml), IL-4 (50 ng/ml), or IL-22 (50 ng/ml) for 24 hours. Total RNA was isolated and gene expression measured by real time PCR as described [6]. qPCR results are plotted as ratios relative to the control gene 18S in universal strand cDNA [13].
2.6. Expression of METRNL in Rheumatoid Arthritis
We have previously reported the construction of a gene expression database of human rheumatoid arthritis [14]. Briefly, samples of synovial membranes of either controls (noninflammatory lesions) (N=5) or Rheumatoid Arthritis patients (N=5) were obtained and mRNA was prepared from them. cDNA was prepared and hybridized to U133 2.0 genome wide human genearrays . To measure the expression of Metrnl in Rheumatoid arthritis, a probeset corresponding to Metrnl (225955_at) was used to measure its expression in this database.
3. Results
3.1. Metrnl expression is associated with skin, mucosal tissues and cells of the immune system
We sought to identify novel genes associated with the immune system. To this end, we analyzed a comprehensive database of human gene expression (BIGE) constructed using Affymetrix U133 2.0 gene arrays [4–5] that represents more than 105 human tissues and cells. Bioinformatics analyses identified 86 novel, un-annotated or poorly characterized genes associated with immune tissues and 303 with immune cells. Eleven of the latter are predicted to encode uncharacterized secreted proteins. One of these encodes Meteorin-like (Metrnl), a secreted protein related to a Meteorin (Metrn), a known neurotrophic growth factor, which has roles in glial cell differentiation and induction of axonal extension [15]. The METRNL gene is located on human chromosome 17 (17q25.3) and mouse chromosome 11. It encodes a small secreted protein of 311 amino acids with a 45 amino acid signal peptide, predicting a mature protein of 266 amino acids (~28 kDa). The BIGE expression profiles of Meteorin (METRN) and Meteorin-like (METRNL) in the human body are shown in Figure 1A. Consistent with previous studies that have documented that Metrn is a neurotrophic growth factor [15–18], the expression of METRN is largely restricted to the Central Nervous System (CNS) (Fig 1A). In contrast, METRNL is not expressed (or expressed at very low levels) in the CNS and its expression is instead strongly associated with activated monocytes, digestive and respiratory mucosal tissues (oral cavity, esophagus, trachea, bronchi) and the skin. Table 1 shows the list of tissues with the highest expression of METRNL (full text in Supplementary Table 1). We confirmed the human microarray data by qPCR in several mouse tissues (Fig.1B). Recently, Metrnl has been reported to be expressed in adipose tissues [19]. However, data from the BIGE database indicates low expression of METRNL in human adipose tissues when compared to barrier tissues (skin, mucosa) or activated mononuclear cells (Table 1). Immunohistochemistry data on METRNL expression from the protein atlas database (www.proteinatlas.org) also supports the microarray expression data from the BIGE database.
Figure 1. Metrn and Metrnl expression in human tissues (BIGE database).
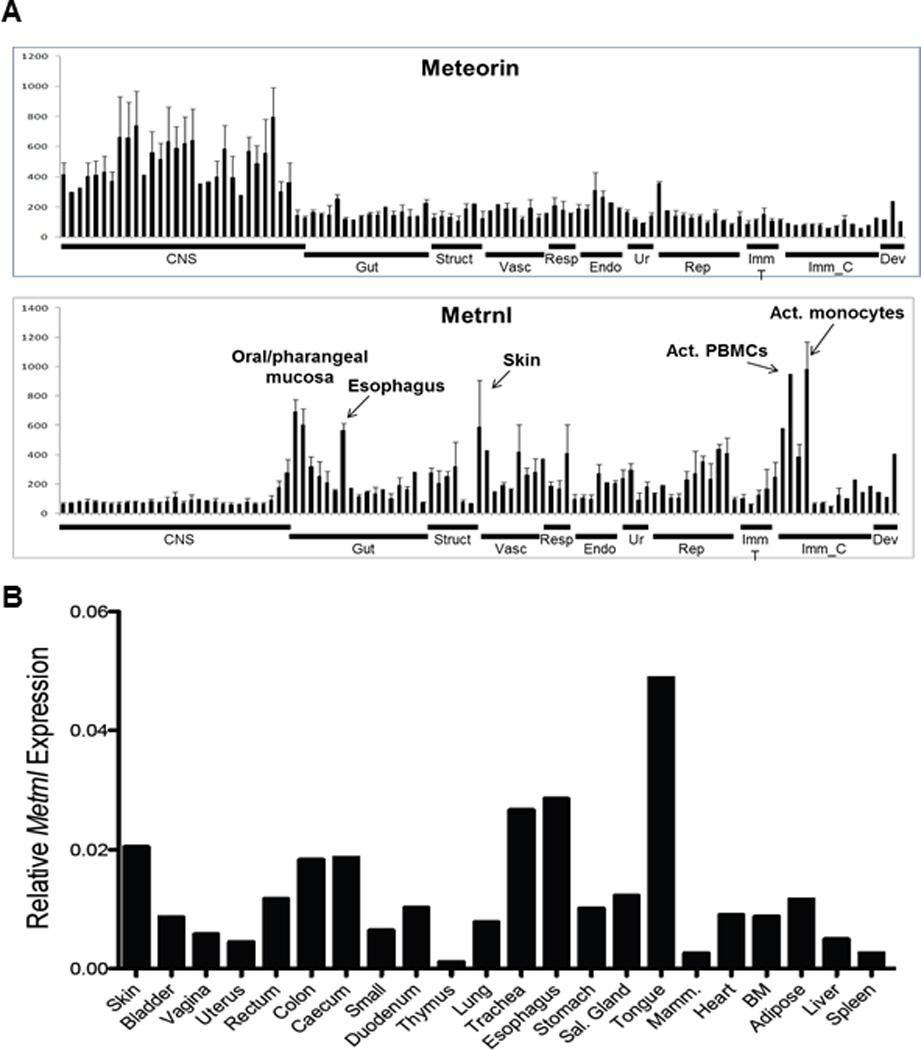
(A) BIGE expression profiles of METRN and METRNL indicating that METRN is mainly expressed in the CNS while METRNL is not expressed in the CNS but is highly expressed in activated monocytes, skin and mucosal tissues.
(B) Q-PCR was used to confirm the microarray data (BIGE database) pattern of Metrnl expression.
Table 1.
| Tissue or Cell Type: | Mean intensity: |
|---|---|
| Monocytes, activated | 981.0 |
| PBMC, activated | 946.6 |
| Oral mucosa | 690.0 |
| Pharyngeal mucosa | 604.0 |
| Skin | 588.7 |
| PBMC | 579.4 |
| Esophagus | 564.4 |
| Nipple (cross section) | 435.8 |
| Pericardium | 428.8 |
| Aorta | 418.9 |
Metrnl expression data from the human BIGE database.
3.2. Metrnl is produced by Alternatively Activated Macrophages
Given the strong expression of METRNL in activated human blood monocytes (Table 1) we investigated further the conditions under which Metrnl is expressed in cells of the monocyte-macrophage cell lineage. Macrophages have been subdivided based on their activation state into classically activated (M1) or alternatively activated macrophages (AAM/M2) (27). Bacterial products and type I cytokines such as IFNγ induce M1 macrophages, while the type II cytokine IL-4 induces M2 macrophages (15, 27).
To determine whether Metrnl is associated with a specific macrophage activation pattern, we stimulated murine peritoneal macrophages with IFNγ or IL-4. As expected, IFNγ induced a robust increase in expression of M1 markers such as CXCL10 while IL-4 induced M2 markers including Arginase I (14, 27) (Fig. 2A). Metrnl expression was significantly up-regulated in IL-4-cultured macrophages, while conversely, IFNγ inhibited its expression (Fig. 2A). Consistent with the mRNA expression results, Metrnl protein levels measured by ELISA rose in response to IL-4 and IL-13 while the M1 polarizing agents IFNγ and LPS inhibited its production (Fig. 2B). These results indicate that Metrnl is produced and secreted by AAM.
Figure 2. Metrnl is expressed by alternatively activated macrophages (AAM/M2).
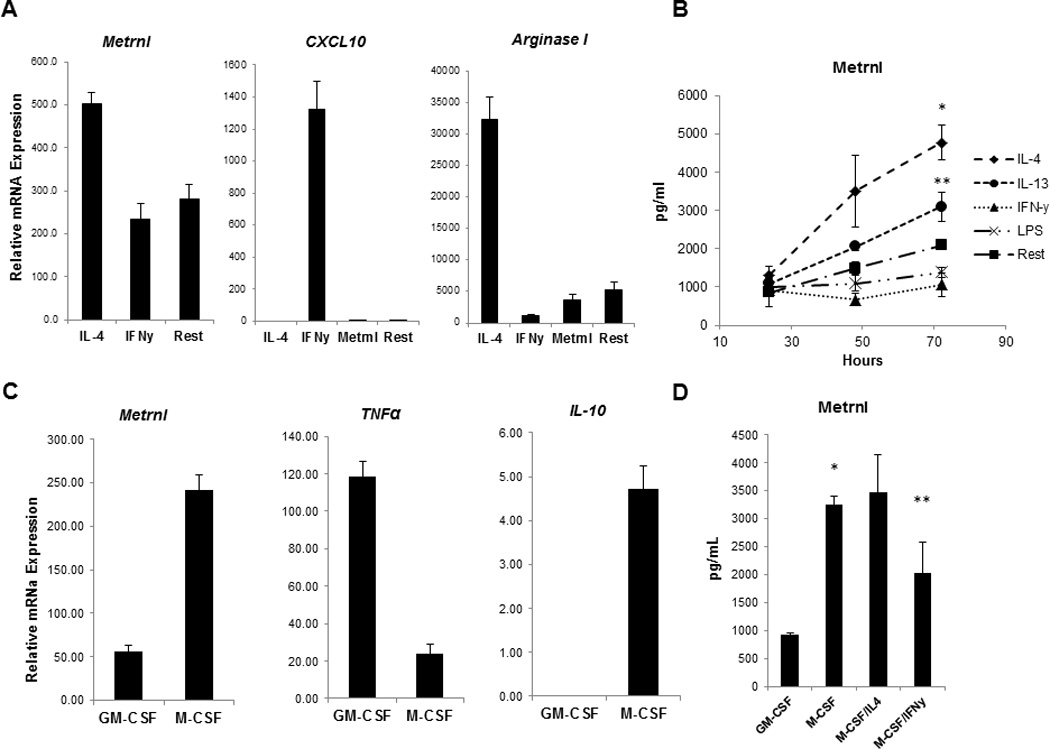
(A) Peritoneal exudate macrophages from C57BL mice were cultured under either M1 (IFNγ) or M2 (IL-4) polarizing conditions. 8 hours post stimulation Metrnl expression was measured by Q-PCR. Metrnl expression was elevated in response to IL-4 and it was suppressed by IFNγ. M1 marker CXCL10 and M2 markers Arginase I were measured in the polarized macrophages to confirm successful polarization.
(B) Metrnl protein levels were measured by ELISA in supernatants from macrophages cultured in the presence of IL-4, IL-13, IFNγ and LPS for 24, 48 and 72hrs. Production of Metrnl was increased in peritoneal cavity macrophages in response to IL-4 (*P < 0.001) and IL-13 (**P < 0.02).
(C) The Metrnl expression levels were measured Q-PCR in GM-CSF -or M-CSF– derived BMM (GM-BMM and BMM, respectively). Metrnl was elevated in BMM and paralleled expression of another BMM cytokine (IL-10).
(D) Metrnl levels were measured by ELISA in GM-BMM and BMM. Metrnl levels were elevated in supernatants from BMM when compared to GM-BMM (*P < 0.0001). Culturing BMM in the presence of IL-4 led to an increase of Metrnl production whereas IFNγ suppressed Metrnl production (**P < 0.02).
Each experiment was done in triplicate and performed three times. A representative experiment (out of three) is shown.
3.3. Metrnl is produced by M-CSF cultured bone marrow derived macrophages
Granulocyte macrophage (GM-CSF) or macrophage (M-CSF) colony stimulating factors are hematopoietic growth factors that induce various phenotypic and functional changes in macrophage cell lineage populations [8]. M-CSF is produced by many tissues and it controls tissue macrophage numbers [20]. In contrast, GM-CSF production is associated with inflammatory conditions where it plays a role in the development of inflammatory Dendritic Cells (DC) [21–22].
GM-CSF and M-CSF have different effects on macrophages. Culturing murine bone marrow cells with GM-CSF or M-CSF has been linked to the M1- or M2- activation states of macrophages, respectively [9, 23]. GM-CSF-bone marrow-derived macrophages (GM-BMM) display a “pro-inflammatory” cytokine profile and have therefore been termed “M1-like” while M-CSF-generated macrophages (BMM) are associated with “anti-inflammatory” mechanisms and have been named “M2-like” [8, 23].
To further explore Metrnl expression pattern in macrophage cell lineage, we generated GM-BMM and BMM and measured the levels of Metrnl expression by qPCR. Consistent with previous reports, GM-BMM exhibit strong expression of TNFα. Conversely, Metrnl expression was strongly induced in BMM and paralleled production of IL-10 (Fig. 2C). Consistent with the qPCR data, BMM secrete high levels of Metrnl and this production was suppressed by IFNγ (Fig. 2D). We conclude that Metrnl is a cytokine produced by macrophages under M2/BMM-polarizing conditions.
We also measured Metrnl expression in THP1 cells (a human monocyte cell line). Interestingly, Metrnl is expressed by resting THP1 cells (suggesting that THP-1 cells exhibit a basal activation state) but its expression increased significantly upon PGE2 treatment (Supplementary Fig.1). Since PGE2 inhibits the expression of pro-inflammatory cytokines (i.e. TNFα) production in THP-1 cells [24], PGE2 is considered an anti-inflammatory stimulus in these cells. These observations further suggest that Metrnl is produced by M2 macrophages and may represent an anti-inflammatory protein.
3.4. Metrnl expression is associated with several human skin diseases
Tanaka et al [25] observed up-regulation of METRNL expression in Familial Primary Localized Cutaneous Amyloidosis (FPLCA), an autosomal dominant disorder associated with chronic itching and skin lichenification. We sought to investigate the expression of METRNL in several human skin diseases. We performed qPCR of METRNL mRNA in cDNA from biopsy samples of either normal skin or lesional skin from patients with psoriasis, Atopic Dermatitis (AD), basal cell carcinoma, scleroderma, actinic keratosis, and other skin diseases (Table 2). We found significant up-regulation of METRNL expression in psoriasis, AD, prurigo nodularis and actinic keratosis (Table 2), but the most significant up-regulation was observed in psoriasis (Fig. 3A, Table 2). These observations strongly suggest that METRNL is involved in the pathology of these diseases.
Table 2. METRNL gene expression in different human skin diseases.
Q-PCR analysis show a statistically significant up-regulation of Metrnl in Psoriasis (p<0.002), Atopic Dermatitis (p<0.034), Prurigo Nodularis (p<0.045) and Actinic Keratosis (p<0.017).
| Diseases | Patients* | Healthy Controls * | P value |
|---|---|---|---|
| Inflammatory | |||
| Atopic Dermatitis | 685.3 (523.6–744.2) | 436.3 (230.6–630.1) | 0.034 |
| Psoriasis | 1024 (869.8–1176.0) | 436.3 (230.6–630.1) | 0.002 |
| Prurigo Nodularis | 646.9 (515.4–744.2) | 436.3 (230.6–630.1) | 0.045 |
| Lupus Erythematous | 414.8 (342.4–564) | 436.3 (230.6–630.1) | 0.755 |
| Lichen Planus | 499.7 (453.4–586.7) | 436.3 (230.6–630.1) | 0.427 |
| Neoplastic | |||
| Actinic Keratosis | 739.7 (608.7–1167.8) | 436.3 (230.6–630.1) | 0.017 |
| Basal Cell Carcinoma | 411.5 (189.5–624.5) | 436.3 (230.6–630.1) | 0.784 |
| Squamous Cell Carcinoma | 328.5 (268.4–613.9) | 436.3 (230.6–630.1) | 0.949 |
Median; Interquartile range in parentheses. Healthy controls N=10; Patients, a minimum N=6 was used.
Figure 3. In skin, METRNL is expressed in IFNγ-treated keratinocytes and is associated with several skin disorders.
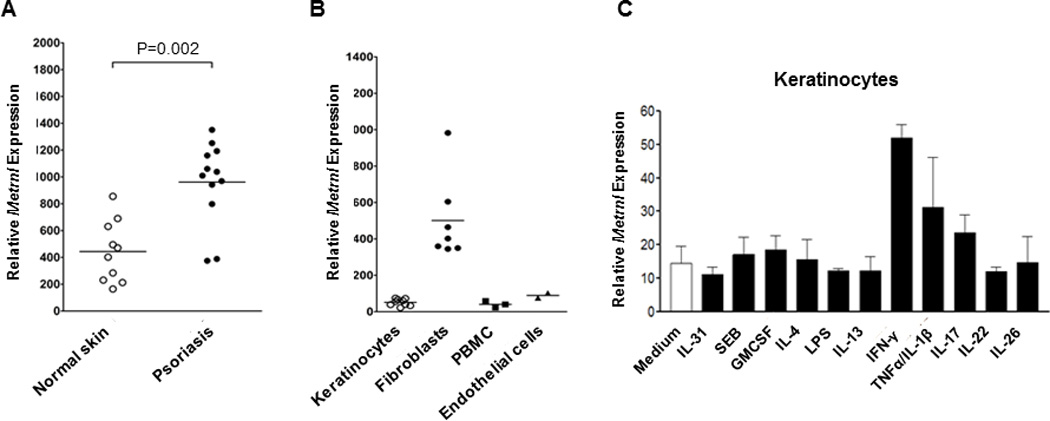
(A) Analysis of METRNL expression in various human diseases indicates strong over-expression in psoriasis (p<0.002)
(B) Comparison of METRNL expression in human keratinocytes, fibroblasts, PBMC and endothelial cells indicate that the highest levels are expressed by fibrobasts.
(C) Human keratinocytes were cultured with a range of activating agents prior to measuring METRNL expression levels. METRNL expression was highest in IFNγ-induced keratinocytes.
3.5. In skin, Metrnl is expressed by resting fibroblasts and by IFNγ-stimulated keratinocytes
As shown in Fig 1A, skin is one of the highest sites of METRNL expression. The strong expression of METRNL in various skin conditions (Fig 3) and by AAMs (Fig 2) suggested the involvement of the latter cells in the pathology of these diseases. To analyze this further, we sought to determine whether other skin cells produce METRNL. To this end, we tested METRNL expression by human keratinocytes, fibroblasts, PBMCs and endothelial cells. Under resting conditions, METRNL is expressed by fibroblasts but not by keratinocytes, PBMCs or endothelial cells (Fig.3B). However, METRNL expression is strongly induced in keratinocytes by IFNγ (Fig 3C). The latter observation suggests that the over-expression of METRNL in psoriasis may be due to the Th1 nature of this disease [26–27].
3.6. METRNL is over-expressed in human Rheumatoid Arthritis
We have previously constructed a database of gene expression in human rheumatoid arthritis using samples of synovial membranes of patients affected by this disease [14]. We queried the expression of METRNL in this database, and, as shown in Figure 4, it is significantly up-regulated in rheumatoid arthritis. This result further supports a role for METRNL in chronic inflammatory diseases.
Figure 4. METRNL is over-expressed in Rheumatoid Arthritis (RA).
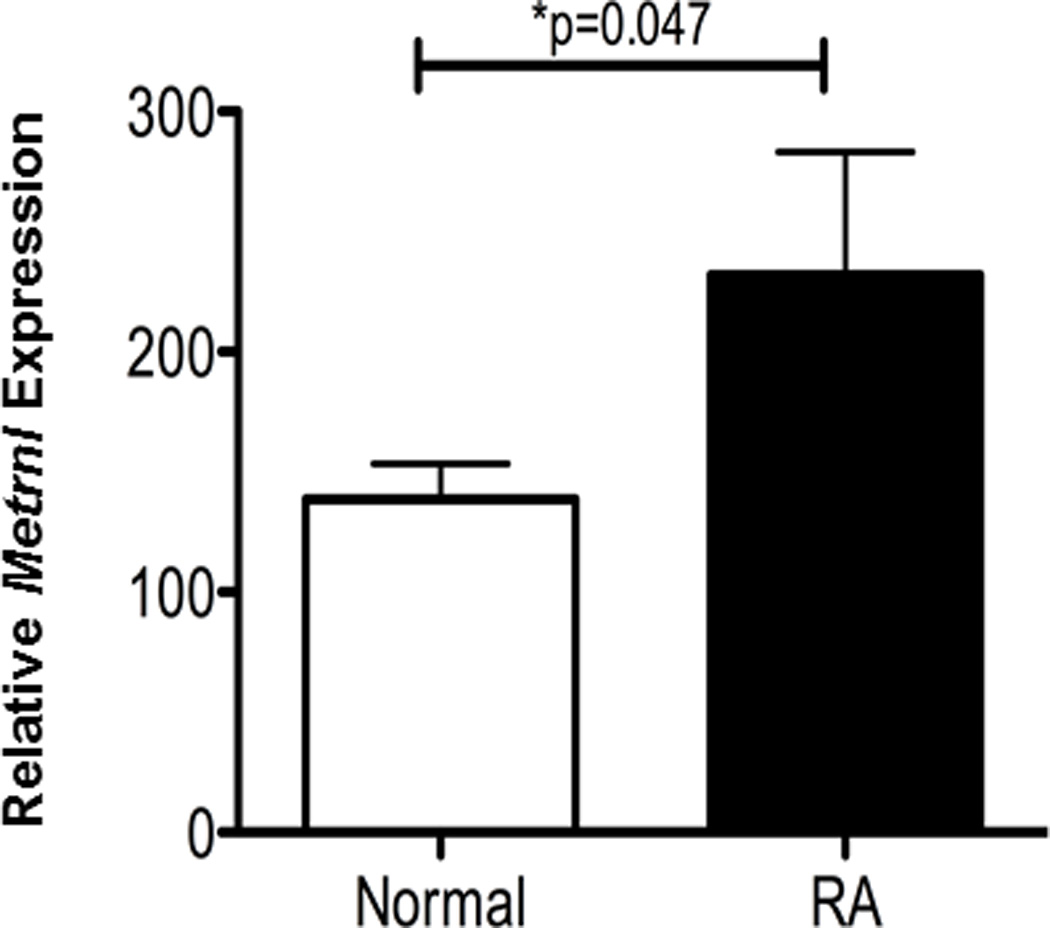
Microarray data from Affymetrix U133 2.0 Plus genearray analyses of synovial membranes of patients with RA or normal controls indicate that METRNL is over-expressed in RA lesions (*P<0.047). N=5 for both patients and controls (from ref 18).
4. Discussion
We sought to identify novel or poorly characterized genes encoding either transmembrane or secreted proteins associated with the immune system. To this end, we analyzed a comprehensive database of human gene expression (BIGE database: [4–5]). One of the genes we identified encodes a poorly characterized small secreted protein called Meteorin-like. METRNL is related to another gene that encodes Meteorin, a known neurotrophic factor [15]. Unlike METRN, which is expressed in the CNS, METRNL is strongly expressed in skin and mucosal tissues (which collectively represent barrier tissues) as well as in M2-polarized macrophages. The Immgen database ((http://www.immgen.org/) confirms that Metrnl is produced by various macrophage populations as well as dendritic cells and some granulocytes, but not by any lymphoid populations (T, B, NK or NKT cells). Furthermore, METRNL is over-expressed in several human skin diseases including psoriasis. METRNL encodes a small secreted protein (~28KDa) produced by several cell types whose expression is regulated by other cytokines (IL4 induces its expression in macrophages, IFNγ in keratinocytes). These observations strongly suggest that METRNL encodes a cytokine involved in innate immunity or inflammatory responses. Very little is known about METRNL. A report identified it as a gene associated with adipose tissues [19], but our human expression data indicates that its expression in other non-fat tissues (ie skin, mucosa or monocytes) is significantly higher (Figure 1A) than in adipocyte-predominant tissues. Recently, Metrnl has been shown to be expressed by muscle cells following exercise and adipose tissue in response to cold exposure [28] . Metrnl was shown to improve glucose tolerance and stimulate an eosinophil-dependent increase in IL-4 expression which promoted alternative activation of adipose tissue macrophages [28]. We should note that the expression of METRNL in the human body (Fig. 1A) indicates homeostatic expression in various tissues. This suggests that it may have other non-inflammatory activities that have yet to be defined.
Given its high expression in skin, we decided to explore its expression in certain skin pathologies. We found it significantly up-regulated in Psoriasis, atopic dermatitis (AD), Prurigo nodularis and Actinic keratosis (Table 2). However, the highest up-regulation was observed in Psoriasis (Fig 3A), a chronic inflammatory disease of the skin which affects 2–3% of the Caucasian population [29]. This disorder is characterized by thickened, scaly plaques which result from hyper-proliferation of the skin’s epidermal layer due to premature maturation of keratinocytes and dermal inflammatory infiltrates [27, 30]. Psoriasis has been considered a type 1 associated autoimmune disease with an inflammatory infiltrate that includes plasmacytoid DCs, CD11c+ DCs as well as type I associated immune cells including CD8+ and CD4+ T cells, the latter corresponding to Th1 and Th17 subsets [26]. However, alternatively activated macrophages have also been described in psoriasis [31]. In addition, M-CSF has been shown to play a role in accelerated wound healing and control of dermal macrophage and Langerhans-cell development under both homeostatic and inflammatory conditions [9].
AD is a chronic skin disorder characterized by dry, scaly skin and itchy red, splotchy lesions [32–33], which often become colonized by bacteria and fungi [33]. AD is one of the most common disorders, affecting 15–30% of children and 2–10% of adults [34]. In contrast to psoriasis, however, AD has traditionally been linked to type II immune responses which are characterized by elevated IgE production, activation of mast cells, eosinophilia and induction of Th2 cells [35–36]. However, recent evidence suggests a biphasic immunological response in which early stages of inflammation are characterized by Th2 responses and chronic lesions exhibit strong Th1, Th17 and Th22 infiltrates [36–38].
Due to their skin location, keratinocytes are among the first cells to encounter invading microorganisms [39–40]. These observations strongly suggest that METRNL is a component of innate immunity during skin inflammation, and may explain why METRNL is up-regulated in psoriasis (which is widely considered a Th1-associated disease). Keratinocytes express a wide range of receptors that allow them to distinguish between different pathogens and help initiate appropriate immune responses. They do this by producing various cytokines and chemokines such as IL-1, TNF, IL-10, CCL20, CXCL9-11, CCL27 and CXCL1 [41–42]. The over-expression of METRNL in psoriasis may be due to IFNγ-induced keratinocytes. This model would also account for the Th1/Th17 association with psoriasis [26, 43].
Several studies have documented that both psoriasis and AD exhibit a strong genetic component and multiple human genome screens have identified four susceptibility loci that overlap between these two diseases: 1q21, 3q21, 17q25 and 20p (34, 41). This suggests that common genes affect the skin pathogenicity in both psoriasis and AD. Interestingly, the METRNL gene is located in one of these susceptibility loci (human chromosome 17q25), which makes it a strong candidate to be involved in the pathogenesis of these diseases.
While the strong expression of METRNL in skin suggested a role for it in skin pathologies (Fig 1A), we also explored the expression of METRNL in other chronic inflammatory diseases. METRNL is significantly up-regulated in the synovial membranes of rheumatoid arthritis patients (Fig. 4). The latter observation again supports a role for METRNL in inflammatory diseases. We conclude that METRNL encodes a novel cytokine that is very likely involved in both innate (through its expression in skin and mucosal tissues) and acquired (through its expression in activated macrophages) immunity.
The World Health Organization-International Union of Immunological Societies (WHO-IUIS) Nomenclature Subcommittee on Interleukin Designation has defined the following criteria for interleukin designation [44]: i) the molecule must have been purified, molecularly cloned and expressed; ii) its nucleotide and inferred amino acid sequence should be distinct from any currently known interleukin and from any other already described molecule; iii) the molecule must be a natural product of cells of the immune system; and iv) the molecule should mediate a potentially important function in immune responses.
METRNL meets all of these criteria. It has been cloned and expressed, and it exhibits a unique nucleotide and amino sequence. Here, we demonstrate that the main cellular sources of METRNL are M2 macrophages and barrier tissues (skin and mucosa). Insofar as immune function, Rao et. al. [28] have shown that Metrnl promotes the development of alternatively activated macrophages through an eosinophil-dependent increase in IL-4. The fact that AAMs produce Metrnl (Fig 2), and that its expression in vivo favors the alternative activation of macrophages [28], strongly suggests that it represents an ‘amplification loop’ that promotes the widespread alternative activation of macrophages, possibly leading to anti-inflammatory effects. Taken together, these results strongly suggest that METRNL may be involved in functions that have been associated with M2-polarized macrophages including wound healing, tissue remodeling or Th2 responses [45] and may participate in certain inflammatory responses where M2 macrophages or other Metrnl-producing cells are involved.
We conclude that METRNL encodes a novel cytokine, and suggest that this gene and its product should be renamed Interleukin 39 (IL-39), a name that better describes the nature of its product and follows from the last interleukin described (IL-38) [46].
Supplementary Material
Abbreviations
- AAM
alternatively activated macrophages
- BIGE
body index of gene expression
- FPLCA
Familial Primary Localized Cutaneous Amyloidosis
- AD
atopic dermatitis
- METRN
Meteorin
- METRNL
Meteorin-like
- RA
rheumatoid arthritis
Footnotes
This work was supported by a NIAID grant R21AI096278-01 and NIH T32 AI060573
References
- 1.Oppenheim JJ. Cytokines: past, present, and future. Int J Hematol. 2001;74:3–8. doi: 10.1007/BF02982543. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello CA. Historical insights into cytokines. Eur J Immunol. 2007;37(Suppl 1):S34–S45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Shea JJ, Kanno Y, Chan AC. In Search of Magic Bullets: The Golden Age of Immunotherapeutics. Cell. 2014;157:227–240. doi: 10.1016/j.cell.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J, Hever A, Willhite D, Zlotnik A, Hevezi P. Effects of RNA degradation on gene expression analysis of human postmortem tissues. FASEB J. 2005;19:1356–1358. doi: 10.1096/fj.04-3552fje. [DOI] [PubMed] [Google Scholar]
- 5.Roth RB, Hevezi P, Lee J, Willhite D, Lechner SM, Foster AC, Zlotnik A. Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics. 2006;7:67–80. doi: 10.1007/s10048-006-0032-6. [DOI] [PubMed] [Google Scholar]
- 6.Luu VP, Hevezi P, Vences-Catalan F, Maravillas-Montero JL, White CA, Casali P, Llorente L, Jakez-Ocampo J, Lima G, Vilches-Cisneros N, Flores-Gutierrez JP, Santos-Argumedo L, Zlotnik A. TSPAN33 is a novel marker of activated and malignant B cells. Clin Immunol. 2013;149:388–399. doi: 10.1016/j.clim.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valle-Rios R, Maravillas-Montero JL, Burkhardt AM, Martinez C, Buhren BA, Homey B, Gerber PA, Robinson O, Hevezi P, Zlotnik A. ISM1 is a novel secreted protein expressed in skin, mucosal tissues and NK, NKT and Th17 cells. Journal of Interferon and Cytokine Research. 2014;34:795–801. doi: 10.1089/jir.2013.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacey DC, Achuthan A, Fleetwood AJ, Dinh H, Roiniotis J, Scholz GM, Chang MW, Beckman SK, Cook AD, Hamilton JA. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J Immunol. 2012;188:5752–5765. doi: 10.4049/jimmunol.1103426. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 10.Hevezi P, Moyer BD, Lu M, Gao N, White E, Echeverri F, Kalabat D, Soto H, Laita B, Li C, Yeh SA, Zoller M, Zlotnik A. Genome-wide analysis of gene expression in primate taste buds reveals links to diverse processes. PLoS One. 2009;4:e6395. doi: 10.1371/journal.pone.0006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber PA, Hevezi P, Buhren BA, Martinez C, Schrumpf H, Gasis M, Grether-Beck S, Krutmann J, Homey B, Zlotnik A. Systematic identification and characterization of novel human skin-associated genes encoding membrane and secreted proteins. PLoS One. 2013;8:e63949. doi: 10.1371/journal.pone.0063949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, Muller A, McClanahan TK, Dieu-Nosjean MC, Orozco R, Ruzicka T, Lehmann P, Oldham E, Zlotnik A. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC) J Immunol. 2000;164:3465–3470. doi: 10.4049/jimmunol.164.7.3465. [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Soto H, Hevezi P, Roth RB, Pahuja A, Alleva D, Acosta HM, Martinez C, Ortega A, Lopez A, Araiza-Casillas R, Zlotnik A. Gene array analysis comparison between rat collagen-induced arthritis and human rheumatoid arthritis. Scand J Immunol. 2008;68:43–57. doi: 10.1111/j.1365-3083.2008.02117.x. [DOI] [PubMed] [Google Scholar]
- 15.Nishino J, Yamashita K, Hashiguchi H, Fujii H, Shimazaki T, Hamada H. Meteorin: a secreted protein that regulates glial cell differentiation and promotes axonal extension. EMBO J. 2004;23:1998–2008. doi: 10.1038/sj.emboj.7600202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HS, Han J, Lee SH, Park JA, Kim KW. Meteorin promotes the formation of GFAP-positive glia via activation of the Jak-STAT3 pathway. J Cell Sci. 2010;123:1959–1968. doi: 10.1242/jcs.063784. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Andrade N, Torp M, Wattananit S, Arvidsson A, Kokaia Z, Jorgensen JR, Lindvall O. Meteorin is a chemokinetic factor in neuroblast migration and promotes stroke-induced striatal neurogenesis. J Cereb Blood Flow Metab. 2012;32:387–398. doi: 10.1038/jcbfm.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorgensen JR, Thompson L, Fjord-Larsen L, Krabbe C, Torp M, Kalkkinen N, Hansen C, Wahlberg L. Characterization of Meteorin--an evolutionary conserved neurotrophic factor. J Mol Neurosci. 2009;39:104–116. doi: 10.1007/s12031-009-9189-4. [DOI] [PubMed] [Google Scholar]
- 19.Li ZY, Zheng SL, Wang P, Xu TY, Guan YF, Zhang YJ, Miao CY. Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression. CNS Neurosci Ther. 2014;20:344–354. doi: 10.1111/cns.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Jr., Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmid MA, Kingston D, Boddupalli S, Manz MG. Instructive cytokine signals in dendritic cell lineage commitment. Immunol Rev. 2010;234:32–44. doi: 10.1111/j.0105-2896.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- 22.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O'Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 23.Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;178:5245–5252. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- 24.Choi SS, Gatanaga M, Granger GA, Gatanaga T. Prostaglandin-E2 regulation of tumor necrosis factor receptor release in human monocytic THP-1 cells. Cell Immunol. 1996;170:178–184. doi: 10.1006/cimm.1996.0150. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka A, Lai-Cheong JE, van den Akker PC, Nagy N, Millington G, Diercks GF, van Voorst Vader PC, Clements SE, Almaani N, Techanukul T, Hide M, South AP, McGrath JA. The molecular skin pathology of familial primary localized cutaneous amyloidosis. Exp Dermatol. 2010;19:416–423. doi: 10.1111/j.1600-0625.2010.01083.x. [DOI] [PubMed] [Google Scholar]
- 26.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 27.Palfreeman AC, McNamee KE, McCann FE. New developments in the management of psoriasis and psoriatic arthritis: a focus on apremilast. Drug Des Devel Ther. 2013;7:201–210. doi: 10.2147/DDDT.S32713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM. Meteorin-like Is a Hormone that Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699–711. doi: 10.1038/nri1689. [DOI] [PubMed] [Google Scholar]
- 30.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Djemadji-Oudjiel N, Goerdt S, Kodelja V, Schmuth M, Orfanos CE. Immunohistochemical identification of type II alternatively activated dendritic macrophages (RM 3/1+3, MS-1+/-, 25F9-) in psoriatic dermis. Arch Dermatol Res. 1996;288:757–764. doi: 10.1007/BF02505293. [DOI] [PubMed] [Google Scholar]
- 32.Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Physician. 2012;86:35–42. [PubMed] [Google Scholar]
- 33.Schmitt J, Langan S, Deckert S, Svensson A, von Kobyletzki L, Thomas K, Spuls P. Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. J Allergy Clin Immunol. 2013;132:1337–1347. doi: 10.1016/j.jaci.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Saito H. Much atopy about the skin: genome-wide molecular analysis of atopic eczema. Int Arch Allergy Immunol. 2005;137:319–325. doi: 10.1159/000086464. [DOI] [PubMed] [Google Scholar]
- 35.Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. J Clin Cell Immunol. 2011;2 doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Cesare A, Di Meglio P, Nestle FO. A role for Th17 cells in the immunopathogenesis of atopic dermatitis? J Invest Dermatol. 2008;128:2569–2571. doi: 10.1038/jid.2008.283. [DOI] [PubMed] [Google Scholar]
- 37.Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974–982. doi: 10.1111/all.12184. [DOI] [PubMed] [Google Scholar]
- 38.Eyerich K, Pennino D, Scarponi C, Foerster S, Nasorri F, Behrendt H, Ring J, Traidl-Hoffmann C, Albanesi C, Cavani A. IL-17 in atopic eczema: linking allergen-specific adaptive and microbial-triggered innate immune response. J Allergy Clin Immunol. 2009;123:59–66. doi: 10.1016/j.jaci.2008.10.031. e54. [DOI] [PubMed] [Google Scholar]
- 39.Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat Immunol. 2013;14:978–985. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- 40.Singh A, Morris RJ. Innate immunity and the regulation and mobilization of keratinocyte stem cells: are the old players playing a new game? Exp Dermatol. 2012;21:660–664. doi: 10.1111/j.1600-0625.2012.01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14:289–301. doi: 10.1038/nri3646. [DOI] [PubMed] [Google Scholar]
- 43.Novelli L, Chimenti MS, Chiricozzi A, Perricone R. The new era for the treatment of psoriasis and psoriatic arthritis: perspectives and validated strategies. Autoimmun Rev. 2014;13:64–69. doi: 10.1016/j.autrev.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Nomenclature for secreted regulatory proteins of the immune system (interleukins). WHO-IUIS Nomenclature Subcommittee on Interleukin Designation. Eur Cytokine Netw. 1991;2:309–310. [PubMed] [Google Scholar]
- 45.Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol. 2013;120:163–184. doi: 10.1016/B978-0-12-417028-5.00006-5. [DOI] [PubMed] [Google Scholar]
- 46.Clavel G, Thiolat A, Boissier MC. Interleukin newcomers creating new numbers in rheumatology: IL-34 to IL-38. Joint Bone Spine. 2013;80:449–453. doi: 10.1016/j.jbspin.2013.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


