Abstract
Background & Aims
Mice exposed to the hepatocellular carcinogen diethylnitrosamine at 2 weeks of age have a high risk of developing primary liver tumors later in life. Previous studies have demonstrated that diethylnitrosamine-treated mice have increased tumor burden when fed an obesigenic “Western” diet rich in lard fat and sugar. However, the role of dietary fats versus sugars in the promotion of liver cancer is poorly understood. The aim of this study was to determine how altering dietary fats versus sugars affects tumor burden in the diethylnitrosamine model.
Methods
C57BL/6N mice were treated with diethylnitrosamine at 2 weeks of age and, from 6 to 32 weeks of age, fed one of five diets that differed in fat and sugar content including normal chow, ketogenic, and Western diets.
Results
Mice fed sugar-rich diets had the greatest tumor burden irrespective of dietary fat content. In contrast, mice fed a high-fat low-sugar diet had the least tumor burden despite obesity and glucose intolerance. When evaluated as independent variables, tumor burden was positively correlated with hepatic fat accumulation, postprandial insulin, and liver IL-6, and inversely correlated with serum adiponectin. In contrast, tumor burden did not correlate with adiposity, fasting insulin, or glucose intolerance. Furthermore, mice fed high sugar diets had lower liver expression of p21 and cleaved caspase-3 compared to mice fed low sugar diets.
Conclusions
These data indicate that dietary sugar intake contributes to liver tumor burden independent of excess adiposity or insulin resistance in mice treated with diethylnitrosamine.
Keywords: Liver cancer, fructose, sugar, obesity, insulin resistance, inflammation
Introduction
Environmental risk factors for primary liver cancer include viral hepatitis, aflatoxin, alcohol, and obesity [1, 2]. A recent study of more than 900,000 adults in the United States reported the relative risk of dying from liver cancer was 4.5 times greater for men and 1.7 times higher for women with baseline body mass index (BMI) ≥ 35, compared to the reference groups with baseline BMI of 18.5 to 25 [3]. Diabetes and hyperglycemia are also highly associated with liver cancer [4–7], as is dietary intake of sugar [8]. However, the pathophysiologic mechanisms linking obesity and poor diet to liver tumor burden remain unclear and may involve a range of factors including hyperinsulinemia, insulin resistance, glucose intolerance, inflammation and hepatic steatosis.
A commonly used model of experimental primary liver cancer in mice involves a single intraperitoneal injection of the pro-carcinogen diethylnitrosamine (DEN) in neonates. DEN is metabolized in the liver by CYP450 enzymes where it is converted to an active carcinogen that causes DNA alkylation and oxidative damage, leading to development of hepatocellular adenoma (HCA) which progresses to hepatocellular carcinoma (HCC), resembling poor-prognosis HCC in humans [9–11]. In this study, we investigated the role of dietary fats and sugars on primary liver tumor burden in DEN-treated mice fed one of five diets: normal chow (NC) which is low in fat and sucrose; Western diet based on lard (WD-L) which is high in fat and sucrose; Western diet based on coconut oil (WD-C); fructose diet (FD) which is low in fat but high in sucrose and fructose; and ketogenic diet (KD) which is high in fat but low in sucrose. These diets cause dissimilar metabolic phenotypes. For example, WD-L causes obesity, peripheral insulin resistance, glucose intolerance, and hepatic steatosis compared to NC. WD-C is calorically identical to WD-L, but it induces greater hepatic lipogenesis [12], which has pathogenic and prognostic significance in primary liver cancer [13, 14]. FD was chosen because it is calorically identical to NC, but fructose induces lipogenesis and glucose intolerance in the absence of obesity [15, 16]. Finally, long-term KD promotes glucose intolerance with less hepatic steatosis compared to mice fed a WD [17]. These diets were used to determine how altering the intake of dietary sugars and fats impacts tumor burden in a mouse model of liver cancer.
Materials and Methods
Mice and DEN treatment
Male and female C57BL/6NHsd mice were purchased from Harlan Laboratories. The C57BL/6N strain was chosen over C57BL/6J because the latter has a gene mutation in nicotinamide nucleotide transhydrogenase (Nnt) that contributes to glucose intolerance and reduced insulin secretion [18]. Mice were housed and bred in a temperature-controlled room (22°C) on a 12-hour light/dark cycle in filter-top cages and with ad libitum access to food and water. Mice were time-mated to produce litters simultaneously. Offspring male C57BL/6N mice were treated with DEN (25 mg/kg) at 14 days of age via intraperitoneal (i.p.) injection as described [19]. Mice were weaned at 21 days of age and randomly allocated to cages with non-littermates of the same age. All weaned mice were fed a standard chow diet until 6 weeks of age.
Diets
From 6 weeks of age, mice were fed one of five experimental diets. Food was provided ad libitum in order to prevent alterations in food-seeking behavior and disruptions in normal set-points for food intake (n=6–12 mice per diet group). Only 6 of 8 mice in the KD group completed the study, as 2 mice were euthanized before study completion due to wounds incurred from fighting. The euthanized mice did not show evidence of tumor burden. Sample size sufficient to detect a 20% change in tumor number was estimated a priori using a power analysis based on group means and standard deviations previously reported [35]. Dietary intervention was delayed until 4 weeks after treatment with the carcinogen in order to distinguish tumor initiation by the carcinogen from promotion of lesion growth by the diets. Weight of food consumed per cage was measured bi-weekly from diet initiation until study completion. kCals consumed were calculated based on kCal content per gram of food for each diet. kCals consumed per cage were then divided by the number of mice in the cage to estimate kCals consumed per mouse. Diets were prepared in-house according to methods previously established in our laboratory [20], with modifications to macronutrient (i.e. fat and carbohydrate) content. All diets contained (w/w): 4% Mineral Mix AIN-76 (Harlan Teklad), 1% Vitamin Mix AIN-76 (Harlan Teklad), 0.4% choline bitartrate, 0.3% methionine, and 2% gelatine.
NC contained (w/w): 6% wheat bran, 67% uncooked corn starch, 17% casein, and 3% safflower oil. FD contained (w/w): 6% wheat bran, 21% uncooked corn starch, 31% sucrose, 15% fructose, 17% casein, and 3% safflower oil. WD-L and WD-C contained (w/w): 18% uncooked corn starch, 5% wheat bran, 21% sucrose, either 23% lard or coconut oil, 18% casein, and 3% safflower oil. KD contained (w/w): 71% lard, 16% casein, and 6% safflower oil. All animal studies were performed according to policies established by the UVA Institutional Animal Care and Use Committee and criteria outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86–23 revised 1985).
Insulin sensitivity and glucose tolerance testing
Insulin and glucose measurements were obtained from mice at 16 weeks of study diet. Blood samples for insulin and glucose measurements were collected in the random-fed state at 19:00 hours, and in the basal state after a 12-hour overnight fast. Glucose was measured in whole blood using an Accu-Chek glucometer (Roche Diagnostics) and insulin was measured in serum using an ELISA colorimetric assay kit (Crystal Chem) according to the manufacturer’s protocol. Glucose tolerance testing was performed as we have described [21] in mice at 16 weeks of study diet following a 12-hour overnight fast. Insulin sensitivity was estimated using the HOmeostatic Model Assessment of Insulin Resistance (HOMA-IR) method [22], calculated using the formula: .
Tissue collection and tumor analysis
Mice were euthanized at 32 weeks of age (26 weeks of experimental diet) in the random-fed state between 09:00–11:00 hours. Tumor multiplicity, which represents the number of surface-hemorrhaging tumors per liver, was counted. The diameter of each visible tumor (≥0.5 mm in diameter) was used to calculate tumor volume using the formula: . Individual tumor volumes were summed to calculate total liver tumor burden per animal. Tumor burden values were used throughout the study to test for correlations with diet-related parameters as a representation of both tumor size and number. Mice without visible tumors were excluded from tumor burden, multiplicity, and all other analyses (n=1–2 per group). The large lobe of the liver was kept for histology, and the remaining liver was divided into non-tumor-involved and tumor-involved tissue, snap-frozen in liquid nitrogen, and stored at −80°C until further biochemical analyses. The weights of both gonadal and subcutaneous fat pads were summed and represented as combined adipose weight per animal.
Liver histology
The large lobe of the liver was fixed in 10% neutral-buffered formalin and paraffin-embedded for microtome sectioning (5μm thick) and hematoxylin & eosin (H&E) staining. Slides were digitally scanned using an Aperio ScanScope (SC System) to produce high resolution images (resolution: 0.25μm per pixel). Histological analysis was performed in a blinded manner. Hyperplastic foci (HPF) were identified by the presence of focal basophilia. HCA was characterized by the presence of basophilic cells and cells containing glycogen and fat, resembling human HCA. HCC was distinguished from pre-neoplastic lesions if three or more of the standard criteria were met: undifferentiated trabecular structure; enlarged, mild-moderately polymorphic hyperchromatic nuclei with enlarged nucleoli; presence of basophilia; increased abundance of mitotic figures; and invasive growth (27–28). HCC which developed within HCA was classified as HCC foci, and HCC which arose from hepatic tissue was classified as bona fide HCC.
Liver and serum cytokines
Liver cytokines were measured in non-tumor-involved liver tissue by qPCR as we have described [23]. Cyclophilin A was used as a housekeeping gene. Primer sequences are listed in Supplemental Table 1. Serum cytokines were measured by ELISA colorimetric assay kits in serum collected at the time of harvest (IL-6 and TNFα, Cayman Chemical; leptin and adiponectin, Boster Biosciences).
Liver fat content
The liver lipid extraction method was adapted from Folch [24] as we have described [23]. Colorimetric assay kits were used to measure triglyceride (Pointe Scientific) and cholesterol (Infinity, Thermo Scientific) content of the lipid extracts according to the manufacturers’ protocols.
Western blotting
Liver tissue was homogenized in RIPA buffer (10mM TRIS pH 8.0, 0.5mM EGTA, 1% Triton X-100, 0.2% SDS, 100mM NaCl) with protease inhibitors cocktail (Roche) and phosphatase inhibitors (2mM sodium orthovanadate, 1mM sodium pyrophosphate, 10mM sodium fluoride, 250nM microcystin). Antibodies used for immunoblotting were: phospho-p70S6K (T389) (Cell Signaling 9206S), phospho-AMPK (T172) (Cell Signaling 2535P), AMPK (Cell Signaling 2793S), p53 (Santa Cruz 6243), acetyl-p53 (K379) (Cell Signaling 2570S), SIRT1 (Millipore 07-131), p21 (Abcam 7960), cleaved CASP3 (Cell Signaling 9664), Cyclin D1 (Santa Cruz 753), and 14-3-3 (Santa Cruz 1657).
Statistical analyses
Group results are presented as mean ± standard error of the mean (SEM) and compared by one-way ANOVA followed by Fisher’s PLSD post-hoc test. Tumor burden (mm3) and multiplicity values were log2-transformed to stabilize group variance which is appropriate when standard error is proportional to changes in the mean. One-way ANOVA and Fisher’s PLSD post-hoc tests were performed on the log2-transformed data. Scatter plots were analyzed by linear regression to determine line of best-fit, followed by Pearson’s correlation analysis to measure the correlation coefficient between two variables. Statistical significance was accepted at p<0.05. Statistical analyses were performed using GraphPad Prism v6.00.
Results
Dietary effects on tumor burden
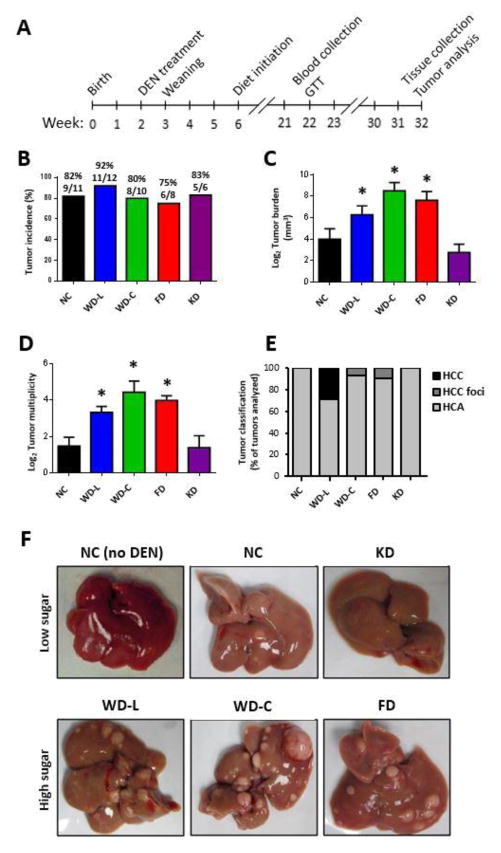
Fig. 1A illustrates the study design. In brief, C57BL/6N mice were injected with DEN (25 mg/kg i.p.) at 14 days of age. The mice were weaned at 3 weeks of age and randomized to cages with non-littermates to avoid litter bias. From 6 weeks of age, mice were fed one of five diets with varying sugar and fat content. Metabolic analyses were performed at 22 weeks of age (16 weeks of study diet) and mice were euthanized at 32 weeks of age (26 weeks of study diet). We observed that tumor incidence was similar between diet groups (Fig. 1B); however, tumor burden and multiplicity was significantly greater in all mice fed high-sugar diets (WD-L, WD-C and FD) (Fig. 1C–D) compared to mice fed NC. Mice fed KD, which was high in fat but low in sugar, had low tumor burden that was comparable to mice fed NC diet. Histological analysis identified the majority of tumors as HCA, but instances of HCC and HCC foci were observed in livers of mice fed the high-sugar diets (Fig. 1E and Supplemental Fig. 1). The mouse with the median tumor burden for each diet group is shown as a representative image in Fig. 1F.
Fig. 1. Dietary nutrient content has a strong influence on liver tumor burden and multiplicity in mice treated with DEN.
(A) Diagram of study design. Tumor incidence (B), average tumor burden (C), and multiplicity (D) of DEN-treated mice at 32 weeks of age. Data presented as mean±SEM. * indicates a significant difference compared to NC group, p<0.05 (n=5–11). (E) Histological classification of tumors observed (n=4–6). HCC=hepatocellular carcinoma, HCA=hepatocellular adenoma. (F) Representative images of livers of DEN-treated mice and a liver from an age-matched mouse fed NC (NC no DEN) shown for comparison.
Physiologic effects
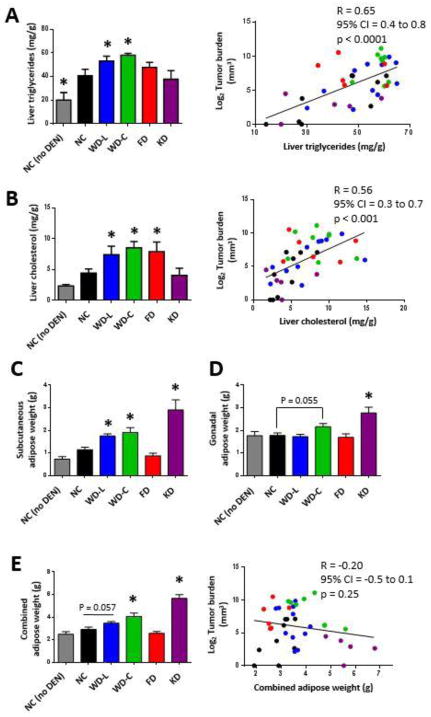
DEN treatment significantly increased liver triglyceride stores in all dietary groups compared to control mice that did not receive DEN (Fig. 2A). When comparing only DEN-injected mice, those fed WD-L and WD-C had significantly increased liver triglycerides compared with mice fed NC (Fig. 2A). Liver cholesterol levels were significantly elevated in mice fed high sugar diets (WD-L, WD-C, and FD) compared with those fed NC (Fig. 2B). When evaluated as independent parameters, both liver triglyceride (Fig. 2A) and cholesterol (Fig. 2B) significantly correlated with tumor burden. There were no differences in overall body weights between groups, but compared to mice fed NC, mice fed WD-C and KD had greater adiposity, whereas mice fed FD had a trend for lower adiposity (Figs. 2C–E). Overall, there was no correlation between tumor burden and adiposity or body weight (Fig. 2E and Supplemental Fig. 2B). This was particularly emphasized by the low tumor burden and high fat mass in mice fed KD and the high tumor burden and low fat mass of mice fed FD. Food consumption monitoring showed a trend for increased kCal intake in mice fed diets rich in fat (WD-L, WD-C, KD) (Supplemental Fig. 2A). Mice fed WD-C and FD had larger liver weight as a percentage of body weight compared to mice fed NC, while mice fed KD had smaller liver weight as a percentage of body weight (Supplemental Figs. 2B–D).
Fig. 2. Liver tumor burden is highly correlated with liver fat content, but does not correlate with adiposity.
Triglycerides (A) and cholesterol (B) were measured in non-tumor-involved liver lipid extracts from mice at 32 weeks of age. Data on left are averaged per group and data sets on right represent the correlation between lipid and tumor burden for each individual mouse. Weights of subcutaneous adipose (C), gonadal adipose (D), and combined subcutaneous and gonadal adipose (E) of mice at 32 weeks of age. Data in bar graphs presented as mean±SEM. * indicates a significant difference compared to NC, p<0.05 (n=5–11).
Metabolic effects
At 16 weeks of diet, serum insulin and glucose levels were measured in random-fed (Figs. 3A and 3B) and overnight-fasted mice (Figs. 3C and 3D). Mice fed WD-L and WD-C had significantly elevated serum insulin levels in the postprandial fed state compared to mice fed NC. Furthermore, serum insulin levels in the postprandial state positively correlated with tumor burden when compared as independent parameters irrespective of diet (Fig. 3A). Postprandial fed blood glucose levels were similar between all diet groups (Fig. 3B). Fasting hyperglycemia was observed in mice fed WD-L and WD-C (Fig. 3C), but fasting hyperinsulinemia was only observed in mice fed WD-C (Fig. 3D). HOMA-IR calculations indicated that mice fed WD-L and WD-C were insulin resistant under basal fasting conditions (Fig. 3E). Furthermore, mice fed WD-L and KD diet displayed marked glucose intolerance compared to mice fed NC (Fig. 3F). In sum, of the metabolic parameters evaluated, post-prandial insulin was the major factor associated with increased tumor burden. In contrast, tumor burden was not independently associated with fasting glucose, fasting insulin, glucose tolerance, or HOMA-IR (Supplemental Figs. 3A through 3D).
Fig. 3. Liver tumor burden is associated with post-prandial insulin, but not other parameters of whole-body glucose metabolism.
Fed serum insulin (A) and glucose (B) levels, and fasted serum insulin (C) and glucose (D) levels after 16 weeks of study diet. (E) HOMA-IR derived from basal blood glucose and insulin levels. (F) Blood glucose levels in mice over time and integrated area under the curve (AUC) for glucose tolerance. Data in bar graphs and line graphs presented as mean±SEM. * indicates a significant difference compared to NC, p<0.05 (n=5–11).
Inflammation
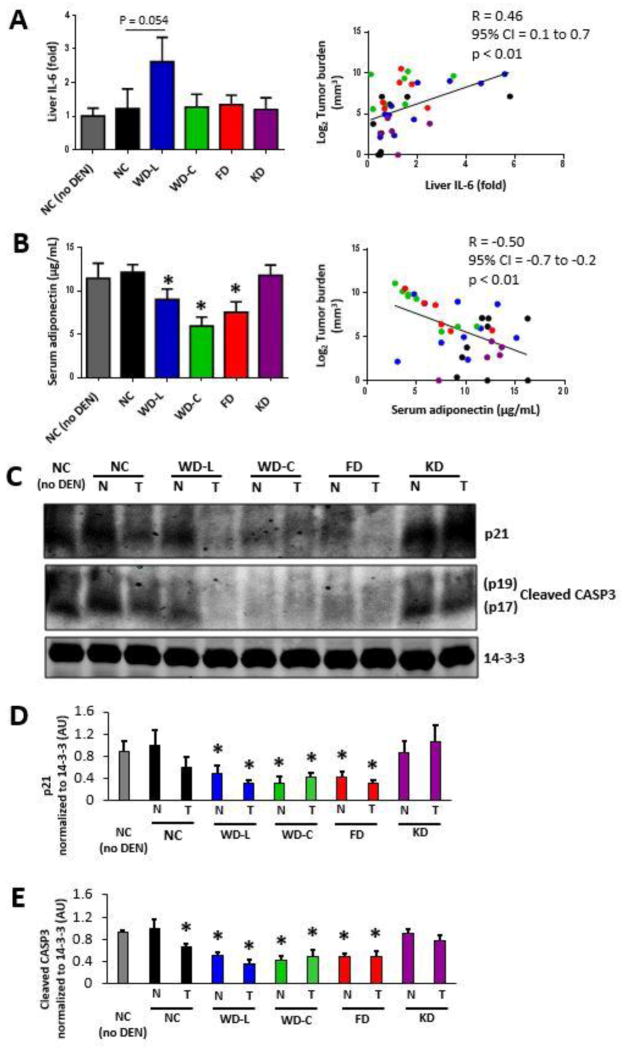
To examine whether diet-induced inflammation was associated with liver tumor burden, we measured mRNA expression of IL-6, IL-1β, and TNFα as markers of inflammation in non-tumor involved liver tissue. Although no individual diet significantly increased liver IL-6 expression, there was a significant positive association between tumor burden and liver IL-6 expression at the level of individual mice (Fig. 4A, right). In contrast, while mice fed the WD-C had significantly increased IL-1β and TNFα expression, neither IL-1β nor TNFα were independently associated with tumor burden (Supplemental Figs. 4A and 4B). Furthermore, serum inflammatory markers IL-6 or TNFα were not related to tumor burden (Supplemental Figs. 5A and 5B). Collectively, of the liver and serum inflammatory markers measured, only liver IL-6 mRNA expression significantly correlated with tumor burden across diet groups.
Fig. 4. Liver tumor burden is positively correlated with liver IL-6, but negatively associated with serum adiponectin and apoptosis.
(A) Fold-change in mRNA expression of IL-6 in non-tumor-involved liver tissue. Left; IL-6 expression level averaged by diet. Right; IL-6 expression compared to tumor burden for each mouse in the study. (B) Adiponectin concentration in serum collected from mice at 32 weeks of age averaged per diet (left) and compared to tumor burden in each mouse (right) (for A–B. n=5–11). (C) Protein expression in tumor tissue and non-tumor-involved liver tissue from mice at final harvest. 14-3-3 was used as a loading control. Protein expression was measured in at least three independent mice for each group; one representative set is shown. Quantitation of Western blot band densities for p21 (D) and cleaved CASP3 (E) (for D-E, n=3). Data in bar graphs presented as mean±SEM. * indicates a significant difference compared to NC, p<0.05.
Serum adipokines
Leptin and adiponectin are adipokines altered with obesity and both hormones are linked to insulin sensitivity and cancer. Serum analysis of each mouse identified an inverse correlation between serum adiponectin and both dietary sugar intake (WD-L, WD-C, and FD) and tumor burden (Fig. 4B). In contrast, leptin was not independently associated with tumor burden despite significantly higher concentrations in mice fed Western diets (WD-L and WD-C) (Supplemental Fig. 5C).
Liver protein expression
To examine potential mechanisms by which diet affects liver tumor burden, we performed western blot analysis of proteins in both tumor tissue and non-tumor liver tissue. We first examined expression and activation of proteins involved in genotoxic stress response, growth and survival. AMP-activated protein kinase (AMPK) is known to be phosphorylated and activated under conditions of genotoxic stress [25]. Consistent with this, we observed that AMPK was hyper-phosphorylated in liver tissue of mice treated with DEN compared to non-DEN-treated mice (Supplemental Figs. 6A and 6C). However, we did not detect any cancer-specific difference in the regulation of AMPK targets including p53 expression [26] or mTORC1 activity (as determined by p70S6K phosphorylation at T389) (Fig. 4C and Supplemental Figs. 6A, 6B, and 6D). In sum, DEN increased both AMPK and mTORC1 activity, but their activity was not related to diet or tumor burden in the DEN model of liver cancer. Sirtuin 1 (SIRT1) is an NAD+-dependent deacetylase that can also be activated under genotoxic stress. Active SIRT1 can deacetylate p53 at K379, resulting in reduced p53 activity as well as degradation [27]. SIRT1 was not differentially expressed between groups (Supplemental Figs. 6A and 6F); however, DEN treatment led to decreased acetylated p53 at K379 in all diet groups except KD (Supplemental Figs. 6A and 6E).
We next examined the expression of proteins involved in cell cycle regulation and apoptosis. Compared to mice fed low-sugar diets (NC and KD), mice fed the high-sugar diets (WD-L, WD-C, and FD) had lower p21 protein expression (Fig. 4C and 4D) and less cleaved caspase-3 (Fig. 4C and 4E). Together, these data indicate that high sugar diets are associated with anti-apoptosis (reduced caspase-3 cleavage) and increased cell cycle progression (reduced p21 expression).
Discussion
The etiology of obesity-related primary liver cancer is thought to evolve through stages of non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), fibrosis and cryptogenic cirrhosis [28]. Obesity is a risk factor for NAFLD; therefore, it is considered to be a proximal contributor to the progression of liver cancer [2, 29–31]. However, it remains unclear whether obesity per se or the chronic intake of obesigenic macronutrients is most important in promoting liver cancer tumor growth [32–34]. The purpose of this study was to investigate the interplay between dietary nutrients under controlled and well-defined conditions using the DEN model of murine liver cancer.
Of the 5 diets tested in this study, 2 diets served as known controls. The lard-based Western diet is known to increase liver tumor burden compared to a normal chow diet [35, 36], and the mechanism is widely thought to be a consequence of obesity-related metabolic disturbances. However, the other 3 test diets (coconut oil-based Western diet, lipogenic high fructose diet, and ketogenic diet) have not previously been evaluated in a mouse model of liver cancer. By comparing these diets, we observed that adiposity can be uncoupled from primary liver tumor burden. For example, mice fed a ketogenic diet rich in lard had low tumor burden despite marked adiposity. In addition, mice fed a lipogenic fructose diet were lean but had considerable tumor burden compared to mice fed NC diet. The NC and FD diets had equivalent fat and carbohydrate compositions, but the majority of carbohydrates in FD were from sugars (sucrose and fructose), whereas the carbohydrates in NC were from uncooked starch (complex glucose polysaccharides). Importantly, mice fed the fructose diet developed considerable tumor burden despite being the leanest group. In addition, FD feeding not increase other cancer-related phenotypes including liver inflammation or glucose intolerance compared to normal chow-fed controls. It is notable that the DEN-treated C57BL/6N mice fed Western diets gained increased adiposity, but did not gain significantly more body mass compared to mice fed NC. In other studies, DEN-treated C57BL/6J mice fed Western diets have a more robust increase in both body mass and adiposity; e.g. Park et al. [33]. The reason for the lack of a large fold-change in body mass in this study is related to the fact that C57BL/6N mice fed NC diet are larger than baseline C57BL/6J mice fed NC diet. For example, the NC-fed mice in this study weighed on average 41 grams, which is a higher body mass compared to adult C57BL/6J mice in similar studies [33]. This body weight phenotype difference is consistent with recent studies comparing the C57BL/6J and C57BL/6N strains, wherein C57BL/6N mice gained more body weight over time than C57BL/6J mice fed NC diet [37, 38]. Because the C57BL/6N mice in our study gained considerable body weight with chow feeding, the high calorie diets did not have pronounced obesigenic effects. This result strongly supports a role for dietary sugar intake in tumor burden that is independent of obesity and obesity-related phenotypes.
We also investigated whether altering only the type of dietary fat could affect tumor burden. Lard fat is composed of 95% long-chain fatty acids. Consumption of high-fat high-sucrose lard-based diets results in impaired whole-body glucose tolerance. In contrast, coconut oil is composed of 63% medium-chain fatty acids that are highly saturated, have less deleterious effects on whole-body glucose tolerance than lard-based diets (Fig 3F), but exacerbate liver fat accumulation [12]. We observed that both WD-L and WD-C had increased tumor burden compared to NC; however, mice fed WD-C had the greatest tumor burden and tumor multiplicity of all diets. One possibility is that the lipogenic nature of medium chain fat diets contributes to worsening disease progression. These findings may have implications for dietary consideration in Southeast Asian populations that readily consume coconut oil and also have among the greatest incidence of hepatitis and liver cancer worldwide [39, 40].
Numerous studies have linked the pro-inflammatory cytokines IL-6, IL-1β, and TNFα to liver cancer. Liver inflammation accompanies steatosis in the development of NASH [28, 41] and liver cancer in humans [29, 42, 43]. We observed that liver IL-6 expression was positively associated with tumor burden. Recently, a study by Park et al. demonstrated that IL-6 knockout mice treated with DEN and fed a lard-based Western diet had less liver tumor burden than wild type controls fed the same diet [35]. Although this study demonstrates that loss of IL-6 is protective against liver cancer, it should be noted that the IL-6 mutant mice were leaner than controls, had lower liver triglycerides, and had lower serum insulin levels [35]. Thus, one possibility is that the beneficial alterations in serum insulin or liver fat content in IL-6 knockout mice confer this protection from liver cancer.
In the present study, liver steatosis was highly associated with liver tumor development. Compared to mice fed NC, mice fed diets with the highest sugar content (WD-L, WD-C, and FD) had the greatest liver lipid content and tumor burden. Since insulin and IL-6 are potent activators of lipogenesis [44, 45], one possible scenario is that high sugar consumption coupled with elevated postprandial insulin and IL-6 expression provide the stimuli to promote tumor growth. The concept that lipogenesis is an important driver of cancer progression has been suggested previously [14, 46, 47]. Consistent with this concept is our observation that mice fed diets low in sugar (NC and KD) had the lowest postprandial insulin, IL-6 expression, liver lipid content and lowest tumor burden.
Importantly, our data is in line with a recent prospective human study that identified hyperinsulinemia as a more prominent risk factor for HCC than obesity [48].
Adiponectin inhibits the proliferation of liver cancer cells by increasing apoptosis, and low serum adiponectin is linked with poor-prognosis HCC in patients [49]. Previous studies have shown that excess sugar consumption is sufficient to decrease serum adiponectin levels in rats [50] and humans [51]. In the present study, we observed that serum adiponectin levels were decreased in mice fed diets containing high sugar. We also identified that lower serum adiponectin levels were associated with less cleaved caspase-3 and a decrease in p21 expression in liver tissue. Together, these data support a possible scenario whereby excess sugar intake leads to a reduction in serum adiponectin that consequently impairs apoptosis and/or enables cell cycle progression.
In summary, this study demonstrates the powerful influence of nutrition on primary liver cancer growth and progression. The matrix of diets used in this study provides strong evidence that dietary sugar consumption is more significant for tumor growth than over-nutrition (e.g. excess dietary fat), adiposity, and/or insulin resistance. These data reduce the complexity of the metabolic milieu associated with liver tumor growth and narrow attention on roles for adiponectin, post-prandial hyperinsulinemia, and liver lipogenesis. Future nutritional studies in mice are necessary to determine whether established liver tumor growth can be stalled or reversed if sugar is removed from the diet. If so, these data would provide pre-clinical evidence to support testing dietary intervention in patients diagnosed with primary liver cancer.
Supplementary Material
Acknowledgments
Financial Support
This project received financial support from the University of Virginia Research and Development Funds, NIH R21 AA022154 and R01 DK101803 (to K.L.H.), and R01DK096076 (to N.L.). F.L.B is a Hope Funds for Cancer Research Fellow, fellowship 14-06-04.
List of Abbreviations
- BMI
Body mass index
- DEN
Diethylnitrosamine
- HCA
Hepatocellular adenoma
- HCC
Hepatocellular carcinoma
- NC
Normal chow
- WD-L
Western diet-lard
- WD-C
Western diet-coconut oil
- FD
Fructose diet
- KD
Ketogenic diet
- HOMA-IR
Homeostatic model assessment of insulin resistance
- HPF
Hyperplastic foci
- mTOR
Mammalian target of rapamycin
- AMPK
AMP-activated protein kinase
- SIRT1
Sirtuin 1
- CASP3
Caspase-3
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
Footnotes
Conflict of Interest
All authors declare no conflict of interest.
Author Contributions
M.E.H., S.H.C., and K.L.H. designed the study and performed experiments. J.D.Y.C., F.L.B., D.S.B., N.L., and C. Li performed experiments. C. Lackner scored histology. M.E.H., F.L.B., and K.L.H. wrote and edited the manuscript with input from other authors. We thank Dr. Nigel Turner (University of New South Wales) for advice with diets and Dr. Lindsay E. Wu (University of New South Wales) for discussions concerning the DEN model. P21 antibody was kindly provided by Dr. Zhen Yan (University of Virginia).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 6.Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360–1365. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 7.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 8.Rossi M, Lipworth L, Maso LD, Talamini R, Montella M, Polesel J, et al. Dietary glycemic load and hepatocellular carcinoma with or without chronic hepatitis infection. Ann Oncol. 2009;20:1736–1740. doi: 10.1093/annonc/mdp058. [DOI] [PubMed] [Google Scholar]
- 9.Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther. 1996;71:57–81. doi: 10.1016/0163-7258(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 10.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 11.Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90:367–386. doi: 10.1111/j.1365-2613.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner N, Hariharan K, TidAng J, Frangioudakis G, Beale SM, Wright LE, et al. Enhancement of muscle mitochondrial oxidative capacity and alterations in insulin action are lipid species dependent: potent tissue-specific effects of medium-chain fatty acids. Diabetes. 2009;58:2547–2554. doi: 10.2337/db09-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evert M, Calvisi DF, Evert K, De Murtas V, Gasparetti G, Mattu S, et al. AKT/mTOR activation induces a module of metabolic changes contributing to growth in insulin-induced hepatocarcinogenesis. Hepatology. 2012 doi: 10.1002/hep.25600. [DOI] [PubMed] [Google Scholar]
- 14.Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S, et al. Increased Lipogenesis, Induced by AKT-mTORC1-RPS6 Signaling, Promotes Development of Human Hepatocellular Carcinoma. Gastroenterology. 2011;140:1071–1083. e1075. doi: 10.1053/j.gastro.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009;9:252–264. doi: 10.1016/j.cmet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messier C, Whately K, Liang J, Du L, Puissant D. The effects of a high-fat, high-fructose, and combination diet on learning, weight, and glucose regulation in C57BL/6 mice. Behav Brain Res. 2007;178:139–145. doi: 10.1016/j.bbr.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Garbow JR, Doherty JM, Schugar RC, Travers S, Weber ML, Wentz AE, et al. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol. 2011;300:G956–967. doi: 10.1152/ajpgi.00539.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes. 2006;55:2153–2156. doi: 10.2337/db06-0358. [DOI] [PubMed] [Google Scholar]
- 19.Vesselinovitch SD, Mihailovich N. Kinetics of diethylnitrosamine hepatocarcinogenesis in the infant mouse. Cancer Res. 1983;43:4253–4259. [PubMed] [Google Scholar]
- 20.Hoehn KL, Turner N, Swarbrick MM, Wilks D, Preston E, Phua Y, et al. Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab. 2010;11:70–76. doi: 10.1016/j.cmet.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taddeo EP, Laker RC, Breen DS, Akhtar YN, Kenwood BM, Liao JA, et al. Opening of the mitochondrial permeability transition pore links mitochondrial dysfunction to insulin resistance in skeletal muscle. Mol Metab. 2014;3:124–134. doi: 10.1016/j.molmet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berglund ED, Li CY, Poffenberger G, Ayala JE, Fueger PT, Willis SE, et al. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes. 2008;57:1790–1799. doi: 10.2337/db07-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow JD, Lawrence RT, Healy ME, Dominy JE, Liao JA, Breen DS, et al. Genetic inhibition of hepatic acetyl-CoA carboxylase activity increases liver fat and alters global protein acetylation. Mol Metab. 2014;3:419–431. doi: 10.1016/j.molmet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 25.Cao C, Lu S, Kivlin R, Wallin B, Card E, Bagdasarian A, et al. AMP-activated protein kinase contributes to UV- and H2O2-induced apoptosis in human skin keratinocytes. The Journal of biological chemistry. 2008;283:28897–28908. doi: 10.1074/jbc.M804144200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.He G, Zhang YW, Lee JH, Zeng SX, Wang YV, Luo Z, et al. AMP-activated protein kinase induces p53 by phosphorylating MDMX and inhibiting its activity. Molecular and cellular biology. 2014;34:148–157. doi: 10.1128/MCB.00670-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 28.Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, et al. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636–1642. doi: 10.1053/j.gastro.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Regimbeau JM, Colombat M, Mognol P, Durand F, Abdalla E, Degott C, et al. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transpl. 2004;10:S69–73. doi: 10.1002/lt.20033. [DOI] [PubMed] [Google Scholar]
- 31.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 32.Juakiem W, Torres DM, Harrison SA. Nutrition in cirrhosis and chronic liver disease. Clin Liver Dis. 2014;18:179–190. doi: 10.1016/j.cld.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Montella M, Crispo A, Giudice A. HCC, diet and metabolic factors: Diet and HCC. Hepat Mon. 2011;11:159–162. [PMC free article] [PubMed] [Google Scholar]
- 34.Mandair DS, Rossi RE, Pericleous M, Whyand T, Caplin M. The impact of diet and nutrition in the prevention and progression of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2014 doi: 10.1586/17474124.2014.894879. [DOI] [PubMed] [Google Scholar]
- 35.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill-Baskin AE, Markiewski MM, Buchner DA, Shao H, DeSantis D, Hsiao G, et al. Diet-induced hepatocellular carcinoma in genetically predisposed mice. Hum Mol Genet. 2009;18:2975–2988. doi: 10.1093/hmg/ddp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahle M, Horsch M, Fridrich B, Seelig A, Schultheiss J, Leonhardt J, et al. Phenotypic comparison of common mouse strains developing high-fat diet-induced hepatosteatosis. Mol Metab. 2013;2:435–446. doi: 10.1016/j.molmet.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholson A, Reifsnyder PC, Malcolm RD, Lucas CA, MacGregor GR, Zhang W, et al. Diet-induced obesity in two C57BL/6 substrains with intact or mutant nicotinamide nucleotide transhydrogenase (Nnt) gene. Obesity (Silver Spring) 2010;18:1902–1905. doi: 10.1038/oby.2009.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42 (Suppl 3):S206–214. doi: 10.1016/S1590-8658(10)60507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Misra A, Singhal N, Khurana L. Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: role of dietary fats and oils. J Am Coll Nutr. 2010;29:289S–301S. doi: 10.1080/07315724.2010.10719844. [DOI] [PubMed] [Google Scholar]
- 41.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 42.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 43.Zen Y, Katayanagi K, Tsuneyama K, Harada K, Araki I, Nakanuma Y. Hepatocellular carcinoma arising in non-alcoholic steatohepatitis. Pathol Int. 2001;51:127–131. doi: 10.1046/j.1440-1827.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 44.Brass EP, Vetter WH. Stimulation of Lipogenesis by Interleukin-6 and Misoprostol-Free Acid in Isolated Rat Hepatocytes. Am J Ther. 1995;2:706–710. doi: 10.1097/00045391-199509000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952–964. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 46.Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117–8126. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- 47.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 48.Aleksandrova K, Boeing H, Nothlings U, Jenab M, Fedirko V, Kaaks R, et al. Inflammatory and metabolic biomarkers and risk of liver and bilary tract cancer. Hepatology. 2014 doi: 10.1002/hep.27016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saxena NK, Fu PP, Nagalingam A, Wang J, Handy J, Cohen C, et al. Adiponectin modulates C-jun N-terminal kinase and mammalian target of rapamycin and inhibits hepatocellular carcinoma. Gastroenterology. 2010;139:1762–1773. 1773 e1761–1765. doi: 10.1053/j.gastro.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ran J, Hirano T, Fukui T, Saito K, Kageyama H, Okada K, et al. Angiotensin II infusion decreases plasma adiponectin level via its type 1 receptor in rats: an implication for hypertension-related insulin resistance. Metabolism: clinical and experimental. 2006;55:478–488. doi: 10.1016/j.metabol.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Rezvani R, Cianflone K, McGahan JP, Berglund L, Bremer AA, Keim NL, et al. Effects of sugar-sweetened beverages on plasma acylation stimulating protein, leptin and adiponectin: relationships with metabolic outcomes. Obesity. 2013;21:2471–2480. doi: 10.1002/oby.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.