Abstract
Pharmacological mitigation of injuries caused by high-dose ionizing radiation is an unsolved medical problem. A specific nonlipid agonists of the type 2 GPCR for lysophosphatidic acid (LPA2) 2-[4-(1,3-Dioxo-1H,3H-benzoisoquinolin-2-yl)butylsulfamoyl]benzoic acid (DBIBB) when administered with a postirradiation delay up to 72 hours reduced mortality of C57BL/6 mice but not in LPA2 KO mice. DBIBB mitigated the gastrointestinal radiation syndrome, increased intestinal crypt survival and enterocyte proliferation, and reduced apoptosis. DBIBB enhanced DNA repair by augmenting the resolution of γ–H2AX foci, increased clonogenic survival of irradiated IEC-6 cells, attenuated the radiation-induced death of human CD34+ hematopoietic progenitors and enhanced the survival of the granulocyte/macrophage lineage. DBIBB also increased the survival of mice suffering of the hematopoietic acute radiation syndrome after total body irradiation. DBIBB represents the first drug candidate capable of mitigating acute radiation syndrome caused by high-dose γ-radiation to the hematopoietic and gastrointestinal system.
INTRODUCTION
Humankind faces the challenge of mitigating injuries caused by exposure to high levels of ionizing radiation as happened during the Tokai-Mura, Fukushima, and Chernobyl Nuclear Plant disasters or explosion of a nuclear device (Dainiak, et al., 2011; DiCarlo, et al., 2011; Igaki, et al., 2008). In these types of disasters, agents that can attenuate radio-sensitive organ injury can only be administered post hoc several hours, even days, after the time of radiation exposure. Drugs that attenuate radiation injury under such conditions are qualified as radiomitigators and will constitute a class of medical countermeasures against radiation – a yet nonexistent group of approved drugs. Lysophosphatidic acid (LPA) is a growth factor-like lipid mediator that has been shown to rescue cells from radiation-induced apoptosis and cellular injury in vitro and in vivo (Deng, et al., 2002). Stabilized mimics of LPA have shown radiomitigative efficacy in animal models of hematopoietic (HEM) and gastrointestinal (GI) acute radiation syndrome (ARS) (Deng, et al., 2007). In vitro receptor add-back studies and experiments conducted with cells derived from knockout (KO) mice for the Lpar2 gene indicate that this receptor subtype is necessary and sufficient to protect cells and mice from radiation-induced cell death and injury to the gut (Deng, et al., 2007; Lin, et al., 2007). Thus, improved non-lipid, drug-like analogs specific to the LPA2 GPCR could provide drug candidates suitable for the treatment of ARS. In silico drug discovery has identified 2-((3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propyl)thio)-benzoic acid (GRI977143) nonlipid compound that activates LPA2 selectively although not specifically (Kiss, et al., 2012). GRI977143, however, lacks the desired drug-like potency; it also has compromised specificity because it is an antagonist of LPA3. We undertook a medicinal chemistry approach guided by computational modeling using a pharmacophore developed from our homology model of the LPA2 ligand-binding pocket (Fujiwara, et al., 2005; Sardar, et al., 2002). This approach has led to the identification of LPA2-specific sulfamoyl benzoic acid (SBA) analogs (Patil, et al., 2014). Here we show that on of these novel LPA2-specific analogs, DBIBB is a highly effective mitigator of the acute radiation syndrome (ARS) elicited by exposure to high-dose (15.69 Gy) γ-irradiation.
RESULTS
Pharmacological properties of DBIBB
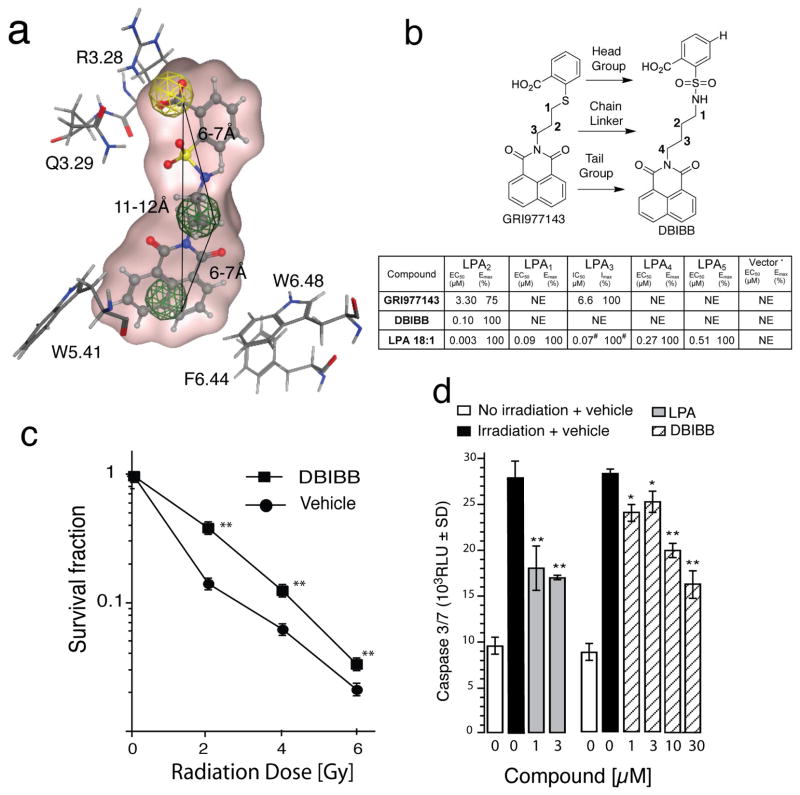
We initiated studies with a pharmacophore using GRI977143 docked into the LPA2 binding pocket as a template (Fig. 1a) to achieve LPA2 subtype agonist specificity by eliminating LPA3 antagonist activity and improving its potency. DBIBB had virtually identical overlay with to GRI977143 with the exception of the linker region between the head and tail groups, where sulfur was replaced by a sulfamoyl moiety (Fig. 1b). The carboxy group of the phenyl head group was predicted to interact with R3.28, which has been experimentally proven to be a conserved essential interaction in all EDG-family LPA and S1P receptors (Parrill, et al., 2000; Wang, et al., 2001) (Fig. 1a). The Q.3.29 residue that was previously shown to determine the specificity for LPA versus S1P in the EDG GPCR family (Wang, et al., 2001) was predicted to interact with the hydroxyl moiety of the carboxy group. When both of these residues were replaced with alanine, the ligand binding and the activation of the EDG family receptors were abolished (Wang, et al., 2001). This suggests that the position of the carboxy moiety on the phenyl head group might play an important role in binding to LPA2.
Fig. 1. Characterization of DBIBB as an LPA2 GPCR Agonist.
Panel a. DBIBB docked into the LPA2 structure-based pharmacophore (Patil, et al., 2014) incorporates three features: an anionic (head group, yellow), a hydrophobic (linker), and a hydrophobic or aromatic (tail group, green). Three-dimensional spatial arrangements and the distances between the centroids are represented in solid black lines. This model also shows the nearest vicinity of DBIBB docked into the ligand pocket with key interacting residues, shown as stick models. R3.28 is a conserved residue in all EDG family LPA and S1P receptors that interacts with the LPA phosphate. Lack of this interaction abolishes binding to either ligand. Q3.29 determines the specificity for LPA versus S1P. The W5.41, F6.44, and W5.41 residues make π-π interactions with the tail group important for activation and potency.
Panel b. Designation of the key structural motifs in GRI977143 that guided the design of DBIBB. The table shows the EC50/IC50, Emax/Imax values of GRI977143, DBIBB, and LPA 18:1 at LPA GPCR. *Vector controls were DKO MEF (LPA2), RH7777 (LPA1/3), CHO (LPA4), and B103 (LPA5) cells or engineered to express one human LPA receptor ortholog (in parenthesis). NE = no effect up to 10 μM of the ligand, the maximal concentration tested in the present experiments. Imax = % inhibition of the ~Emax50 LPA 18:1 response for a given receptor subtype using 10 μM of the antagonist. # Represents EC50 values for LPA 18:1. EC50 and IC50 concentration are given in μM for dose-response curves covering the 1 nM–10 μM range. For determination of IC50 values, dose-response curves were generated using an ~Emax50 concentration of LPA 18:1 for any given receptor subtype, and the ligand was co-applied in concentrations from 0.03 to 10 μM.
Panel c. DBIBB enhances the clonogenic survival of IEC-6 cells. IEC-6 cells were irradiated with increasing doses of γ-irradiation from a 137Cs-source at a dose rate of 4.4 Gy/min and plated. Cultures were treated ~30 min postirradiation with 10 μM DBIBB or vehicle and surviving colonies (± SD) were counted on postirradiation day eight. (**p < 0.001 over control, n=3)
Panel d. DBIBB and LPA inhibit radiation-induced caspase 3/7 activation in irradiated IEC-6 cells. IEC-6 cells were irradiated with 10 Gy at a dose rate of 4.4 Gy/min from a 137Cs source. Test compounds were added to serum-free medium 1 h after irradiation at the concentrations listed. Activity of caspases 3/7 (± SD) was measured 24 h after irradiation. (*p < 0.05, **p < 0.001 over irradiated vehicle control, n=4)
The ligand properties of GRI977143, DBIBB, and LPA 18:1 were characterized at LPA1, LPA3, LPA4, LPA5, and S1P1 receptors (Table insert in Fig. 1b). Because GRI977143 is both a selective agonist of the LPA2 receptor and a weak antagonist of LPA3 (Kiss, et al., 2012), we also examined whether DBIBB had antagonist or allosteric modulatory action on LPA1/3/4/5 GPCR. The pharmacological characterization of DBIBB at LPA2 showed that had nanomolar potency (IC50 ~100 nM, a 33-fold increase over GRI977143); it was a specific agonist without activating or inhibiting LPA1/3/4/5 receptors. Importantly, DBIBB lacked LPA3 antagonism. Altogether, these experiments indicated that DBIBB is a specific agonist of the LPA2 receptor.
Radioprotective efficacy of DBIBB in vitro
Exposure to high levels of radiation can trigger apoptotic cell death. We have shown earlier that LPA treatment within 1–2 h after exposure of cultured cells to γ-irradiation can rescue apoptotically-committed cells (Deng, et al., 2007; Kiss, et al., 2012). The rat intestinal crypt epithelium-like cell line IEC-6 is a nontransformed cell type with expression of LPA2 that undergoes radiation- and genotoxic stress-induced apoptosis that is attenuated by LPA (Deng, et al., 2002; Deng, et al., 2004; Deng, et al., 2007). Clonogenic survival assay was performed to assess whether DBIBB treatment increases IEC-6 cell survival when administered ~30 min after exposure to γ-irradiation. DBIBB applied postirradiation significantly (p < 0.01) increased clonogenic survival of IEC-6 cells in the 2 – 6 Gy dose range (Fig. 1c). The increased clonogenicity of IEC-6 cells could be due to the reduction of radiation-induced apoptosis and re-entry into the cell cycle. To test this hypothesis, we measured activation of the main executioner caspases 3/7 in IEC-6 cells exposed to γ-irradiation. DBIBB dose-dependently reduced caspase 3/7 activation when applied 1 h postirradiation to IEC-6 cells (Fig. 1d). IEC-6 cells express multiple subtypes of LPA receptors (Deng, et al., 2007). To determine to role of the LPA2 receptor in the radiomitigative actions of DBIBB we used MEF from LPA1 x LPA2 double knockout (DKO) mice reconstituted with only the human LPA2 ortholog. We have previously shown that LPA can protect these cells against radiation- or genotoxin-induced apoptosis (Deng, et al., 2007; E, et al., 2009; Kiss, et al., 2012; Kiss, et al., 2013). LPA2 MEF and vector-transduced DKO MEF were exposed to 15 Gy of γ-irradiation at a dose rate of ~4.4 Gy/min and treated with 1 and 3 μM of either LPA or DBIBB one hour after irradiation. Apoptosis was monitored by measuring caspase 3/7, 8, and 9 activities or DNA fragmentation. LPA and DBIBB reduced the activity of initiator caspases 8, 9, and the executioner caspases 3/7 (Fig. 2a–c) in LPA2 MEF but had no significant attenuating effect in vector control MEF. In LPA2 MEF but not in vector control MEF, DBIBB dose-dependently reduced DNA fragmentation 4 h after irradiation (Fig. 2d). LPA has been shown to rescue apoptotically-committed cells after Adriamycin-induced DNA damage (Lin, et al., 2007). DBIBB also reduced caspase 3/7 activity and DNA fragmentation in LPA2 MEF treated with Adriamycin (Fig. 2e & Supplemental Fig. 1). Poly[ADP-ribose] synthase 1 (PARP-1) is an important enzyme that regulates DNA repair, transcription, and cell survival (Ko and Ren, 2012). ADP-ribosylation enhances kinase activity of ATM and DNA-PK, inhibits kinase activity of ATR, and enables nuclear retention and transcriptional activity of p53 and NFκB, in turn promoting DNA repair and cell survival (Aguilar-Quesada, et al., 2007; Kedar, et al., 2008; Ko and Ren, 2012). PARP-1 is inactivated by cleavage by caspase 3 from a 116 KDa active form into an 89 KDa inactive enzyme. Because LPA and DBIBB inhibited caspase 3 activity, we measured PARP-1 cleavage 4h after irradiation in LPA2-MEF and Vector-MEF (Fig. 2f). DBIBB and LPA inhibited PARP-1 cleavage in LPA2-MEF but were ineffective in Vector-MEF cells.
Fig. 2. DBIBB inhibits radiation- and genotoxic stress-induced apoptosis in vitro.
Panel a. The effect of DBIBB on radiation-induced activation of initiator caspase 8. DBIBB and LPA inhibited apoptosis in LPA2 MEF but were inactive in vector MEF. Increasing concentrations of the compounds or vehicle (3 μM BSA for LPA and 0.1% v/v DMSO for other compounds) were added to the cells 1 h after irradiation with 15 Gy at 4.4 Gy/min from a 137Cs source. Caspase 8 activation was measured 4 h postirradiation. Open bars indicate cells from vehicle-treated non-irradiated controls. Bars and data points represent the mean ± SD of at least three independent experiments (*p < 0.05, **p < 0.01, ***p < 0.001 based on Student’s t-test in this and subsequent panels).
Panel b. Effects of DBIBB and LPA on radiation-induced activation of initiator caspase 9. Experimental conditions and statistical methods were the same as in panel A.
Panel c. DBIBB dose-dependently inhibits activation of executional caspases 3/7 in LPA2 MEF. Cells were treated with indicated concentrations of the DBIBB 1 h after radiation exposure and caspase activity (mean ± SD, n=3) was measured 4 h later.
Panel d. Inhibition of radiation-induced DNA fragmentation by LPA and DBIBB in DKO MEF reconstituted with LPA2. DBIBB and LPA (symbols as in panel A) selectively inhibited DNA fragmentation in LPA2 MEF but showed no significant mitigative action in vector MEF cells. The ligands or vehicle was added to the cells 1 h after irradiation, and DNA fragmentation (mean ± SD, n=3) was measured 4 h after irradiation.
Panel e. LPA2 MEF or vector transduced MEF cells were pretreated with LPA or DBIBB, and apoptosis was induced using 1.7 μM Adriamycin 1 h later. Caspase 3/7 activity (± SD, n=3) was measured 5 h after induction of apoptosis. **p < 0.01 and ***p < 0.001 using Student’s t-test relative to vehicle.
Panel f. DBIBB and LPA reduce PARP-1 cleavage induced by γ–irradiation. Subconfluent LPA2- or Vector-MEF cells were serum-starved 1 h before the irradiation and were irradiated with 15 Gy. The cells were treated postirradiation with 10 μM DBIBB, 3 μM LPA, or vehicle (3 μM cc BSA + 0.1% DMSO). Four hours after the irradiation the samples were collected and 30 μg of cell lysates were separated on a 10 % SDS-PAGE and processed for western blotting. Note that LPA and DBIBB decreased the cleavage of PARP-1 in LPA2-MEF but failed to do so in Vector-MEF (representative of 3 experiments).
The DNA damage caused by ionizing radiation must be effectively repaired to allow cell cycle progression, avoid oncogenic or lethal mutations, and aberrant chromosomes that could lead to mitotic catastrophe (Kirsch, et al., 2010). The phosphorylated histone 2AX on residue S139 (γ–H2AX) is a marker of DNA double-strand breaks that is a marker to assess the effectiveness of DNA repair by non-homologous end-joining (Lukas, et al., 2011). The effect of DBIBB on the resolution of γ–H2AX-positive cells was compared using flow cytometry (Fig. 3 a & b). LPA2 and vector–MEF were pretreated with 10 μM DBIBB for 15 min and γ–H2AXhigh cells were measured using flow cytometry from 0.5 to 6 h after 15 Gy irradiation. In LPA2 MEF, DBIBB accelerated and significantly augmented the resolution of γ–H2AX foci as measured by γ–H2AXhigh expression compared to vehicle treatment. In contrast, in vector MEF DBIBB failed to elicit a significant decrease in γ–H2AX. Taken together, DBIBB accelerated DNA repair by non-homologous end-joining indicated by the resolution γ–H2AXhigh nuclei.
Fig. 3. DBIBB accelerates the resolution of γH2AXhigh cells following irradiation and activates the ERK1/2 prosurvival kinases.
Panel a. Time course of γH2AXhigh resolution in LPA2 MEF and Vector control MEF cells treated with DBIBB or vehicle. Cells were pretreated for 15 minutes with 10 μM DBIBB or vehicle (0.1 % DMSO) and irradiated with 15 Gy at 4.4 Gy/min. At times indicated the cells were stained with anti-phospho-H2AX eFluor660 and 104 events were recorded per sample using a LSR II flow cytometer. In the 30 min panel we indicate the position of the gate (black line) used in subsequent panels to identify γH2AXhigh cell subpouplations with the highes intensity of γH2AX staining. Blue lines are vehicle treated, red lines are DBIBB treated samples. Note the separation of DBIBB treated γH2AXhigh cells from vehicle treated cells and the lack of sapartaion between DBIBB and vehicle treated Vector control MEF (orange and green).
Panel b. Attenuation of γ–H2AXhigh (mean ± SD, n=3) expression by DBIBB in LPA2 and vector transduced MEF using flow cytometry. This panel shows the quantification of the experiment shown in panel a. Open bars represent vehicle treated, filled bars DBIBB treated samples. Representative of n=3 experiments. Note that DBIBB accelerated the resolution of γ–H2AX expressing cells by significantly reducing γH2AXhigh cells in LPA2 MEF (red) but not in vector MEF (blue) at every time point tested. * Denotes p < 0.05 between DBIBB versus vehicle treated LPA2 MEF, # denotes p < 0.05 between DBIBB treated LPA2 versus vector transduced MEF.
Panel c. DBIBB and LPA activates ERK1/2 phosphorylation and concomitantly reduce γ–H2AX levels in LPA2-MEF but not in Vector-MEF cells. Cells were irradiated and 4 h later processed for western blotting as in panel b. Note the robust ERK1/2 phosphorylation and the decreased γH2AX levels in the LPA2-MEF cells only (representative of 3 experiments).
LPA-mediated activation of the extracellular response-regulated kinases 1/2 (ERK1/2) is a key signaling step in DNA repair as well as an important step in DNA repair, cell survival, and proliferation. DBIBB and LPA increased the phosphorylated active forms of ERK1/2 while concomitantly reduced the level of γ–H2AX in LPA2-MEF. In contrast, both ligands were ineffective in Vector-MEF cells (Fig. 3c).
Efficacy of DBIBB mitigating the acute GI-ARS and HEM-ARS
DBIBB was tested using a murine GI-ARS model of partial-body irradiation (PBI) with shielding of the bone marrow contained in the tibiae, fibulae and paws that effectively spares ~5% of the bone marrow. ((Boggs, 1984; Booth, et al., 2012); PBI-BM5, lemental Fig. 2). A 15.69 Gy radiation dose delivered at 1.47 Gy/min from a 137Cs in our facility lead to a ~LD70/30 mortality in 10–12-week-old mice without supportive care other than gel food diet starting on postirradiation day 4. Acute toxicology studies with daily administrations of up to 10 mg/kg of DBIBB for 10 days showed no visually observable adverse effects and pathological findings at necropsy, indicating the lack of toxicity mice (data not shown). In this model mice developed GI-ARS with peak mortality starting 8 d postirradiation and subsiding by postirradiation day 12. Crypt counts in the small intestine culled on postirradiation days 1, 5, 10, and 20 from PBI-BM5 mice showed a precipitous decrease, reaching nadir in the day 5 sample and gradually recovering to counts near those seen in sham irradiated mice (Supplemental Fig. 3a). The number of apoptotic cell nuclei stained with TUNEL reaction show an increase around the nadir of crypt counts and gradually diminish as crypt numbers rebound (Supplemental Fig. 3b). These histopathological features are consistent with the manifestation of GI-ARS. Thus, cohorts of C57BL/6 mice have been subjected to this model with or without subcutaneous treatment with 1 or 10 mg/kg of DBIBB starting 26 ± 2 h and repeated at 48 ± 2 h and 72 h ± 2 h postirradiation. The endpoint of the study was mortality at postirradiation day 30, by which time the delayed and life-threatening effects resulting from GI- and HEM-ARS have manifested. In the vehicle-treated group, only one mouse out of 14 per group survived to day 30, indicating that the 15.69 Gy dose resulted in 93% lethality (LD90/30, Fig. 4a). Although only two mice in the group treated with 1 mg/kg DBIBB survived to day 30, the mean survival time in this dose group increased significantly to 15 days from 8 days in the vehicle group (p < 0.002, in Fisher’s exact test), effectively shifting morbidity and mortality characteristic of the GI-ARS to the typical period of the HEM-ARS. The group that received treatments with 10 mg/kg DBIBB showed a significant increase in survival, with 10 of 14 mice remaining alive on day 30 (p < 0.001). Subsequent to the peak of the GI-ARS mortality on postirradiation day 12, only 14% (2 mice) of vehicle control mice were alive, whereas survival in the 1 mg/kg and 10 mg/kg treatment groups were 57% (8 of 14 mice) and 93% (13 of 14 mice), respectively. These results collectively indicate that DBIBB administration starting at 26 ± 2 h postirradiation at 10 mg/kg significantly mitigated both 12-day and 30-day mortality.
Fig. 4. DBIBB mitigates the GI-ARS.
Panel a. Kaplan-Meier survival plots for groups of 15 C57BL/6 mice exposed to 15.69 Gy of 137Cs γ–irradiation at a dose rate of ~1.5 Gy/min using a PBI-BM5 model. DBIBB dissolved in 1% ethanol/2% propanediol in phosphate-buffered saline (vehicle) was administered starting at +26 ± 2 h postirradiation for 3 days as a single daily subcutaneous injection. DBIBB dose-dependently increased survival during the GI-ARS phase (days 8–12) and also the HEM-ARS phase (days 15–20), particularly in the group treated with a 10 mg/kg daily dose.
Panel b. Mean crypt count per circumference in jejunum sections in groups (mean ± SD, n=4) of C57BL/6 mice taken on postirradiation day 4.5. Crypt counts were scored on H&E-stained paraffin-embedded sections by a board-certified histopathologist blind to the treatments. Crypt counts showed a dose-dependent increase that reached significance in the 3 and 10 mg/kg dose groups (Student’s t-test). The irradiation and treatment regimen was identical to that in panel a.
Panel c. The number of TUNEL-positive apoptotic and Ki67-positive proliferating cells was counted using adjacent slides used for crypt counting in panel b. The count of TUNEL-positive nuclei (yellow bars, mean ± SD, n=4) showed a DBiBB dose-dependent decrease that reached significance in the 10 mg/kg treatment group. Conversely, the number of Ki67 marker-positive nuclei (grey bars, mean ± SD, n=4) showed an increasing trend that also reached significance in the 10 mg/kg dose group. The immune-positive nuclei were counted using the Nuclear Quantification algorithm (version 9) of the Aperio Slide Digitizer instrument.
DBIBB mitigates radiation-induced apoptosis, crypt loss and augments cell proliferation
We repeated the PBI model by culling four mice on postirradiation day 4.5 and determining the number of crypts, TUNEL, and Ki67 proliferation marker-positive nuclei in sections of the jejuna. In this experiment, C57BL/6 mice were again exposed to our PBI-BM5 model and treated with 1, 3, or 10 mg/kg of DBIBB or vehicle at 26 ± 2 h, 48 ± 2 h, and 72 ± 2 h postirradiation. Surviving crypts were counted on H&E-stained sections taken at 3.5 and 7.5 cm distal to the pylorus. Jejuna from mice treated with DBIBB showed a dose-dependent increase in the number of surviving crypts compared to the vehicle control (Fig. 4b). Ki67-positive proliferating nuclei also showed a dose-dependent increase that reached significance compared to the vehicle group in the 10 mg/kg treatment group (Fig. 4c). TUNEL-positive apoptotic nuclei counts showed a dose-dependent decrease in the treatment groups (Fig. 4c). These results taken together indicate that the radiomitigative mechanism of action of DBIBB is mediated in part via reduction of apoptosis and increased cell proliferation leading to increased crypt survival.
DBIBB mitigates mortality during HEM-ARS period
Mice treated with DBIBB after PBI exposure to 15.69 Gy showed little mortality after day 15 during the manifestation of HEM-ARS. This trend was most pronounced in the 10 mg/kg dose group. We hypothesized that this lack of mortality during the HEM-ARS period could be due not only to recovery of the hematopoietic system from the shielded bone marrow but also to protective and regenerative effects of DBIBB on hematopoiesis. To test this hypothesis, we exposed C57BL/6 mice to 8.5 Gy total body irradiation (TBI) at 0.82 Gy/min (~LD60/30). Mice were injected subcutaneously with 1 or 10 mg/kg DBIBB starting 26 ± 2 h and repeated at 48 ± 2 h and 72 h ± 2 h postirradiation (Fig. 5a). Thirty-day survival, in the 1 mg/kg treatment group although 20% higher than in the vehicle control group but it did not reach significance using Fisher’s exact test. In contrast, the 10 mg/kg cohort showed a 50% survival benefit over the control (p=0.007). In this treatment group mortality began on day 16 and no animal was lost after day 18 that is consistent with the typical manifestation of the HEM-ARS.
Fig. 5. DBIBB mitigates radiation injury to the hematopoietic system.
Panel a. DBIBB applied decreases mortality in a TBI model of HEM-ARS. Wild type C57BL/6 mice were exposed to an LD70/30 dose of 8.5 Gy using 0.82 Gy/min. The mice were treated with single daily subcutaneous injections starting at +24 h postirradiation and continuing for 3 more days (n= 15 mice/group). DBIBB caused a significant increase in 30-day survival (p = 0.007 calculated using log-rank test relative to vehicle). The low-dose 1 mg/kg group showed a significant increase in mean survival time (p < 0.01) but not in 30-day survival over the control group.
Panel b. Effect of delayed postirradiation administration of DBIBB in the TBI model. Administration of DBIBB was tested in a paradigm when the start of treatment was delayed from +26 ± 2 h to +72 ± 2 h after irradiation. Mice in the 26-h delayed administration group received three additional daily doses of 10 mg/kg at 26 ± 2 h, 48 ± 2 h, 72 ± 2 h, and 96 ± 2 h postirradiation, whereas mice in the 72 h delayed administration group were injected at 72 ± 2 h and 96 ± 2 h postirradiation with DBIBB but received vehicle at 26 ± 2 h and 48 ± 2 h postirradiation. Survival in the three groups is shown in the Kaplan-Meier plots. Irradiation parameters were identical as in panel a.
Panel c. The radiomitigative action of DBIBB requires the LPA2 receptor. WT and LPA2 KO mice (n=15, age 16–18 weeks) on the C57BL/6 background were exposed to identical irradiation and treatment conditions as shown in panel a. Treatment with DBIBB at 10 mg/kg failed to reduce 30-day mortality significantly (p = 0.3, Fisher’s exact test) and also failed to increase mean survival time in LPA2 KO mice, whereas the same treatment in WT mice significantly (p<0.01) reduced mortality over the vehicle control.
Panel d. Effect of DBIBB on the survival and hematopoietic differentiation of irradiated human CD34+ progenitor cells. Purified CD34+ hematopoietic progenitor cells in serum-free Iscove’s MDM were irradiated with 4 Gy 137Cs γ–irradiation at a dose rate of ~0.85 Gy/min. After irradiation, cells were treated with a 1–10 μM concentration of DBIBB for 4 h. Cells were plated at 4.000 cells/plate/ml in triplicate in MethoCult medium 4 h after irradiation. Colonies of the different lineages were identified by their characteristic morphology and counted on postirradiation day 14. Note that DBIBB increased the total colony number and colonies of the granulocyte/macrophage lineage. Data are the mean ± SD of three independent experiments with cells from three different donors (*p > 0.05 and **p > 0.01 relative to vehicle).
We also tested whether DBIBB could mitigate mortality when the start of treatment was delayed from +26 ± 2 h to +72 ± 2 h after irradiation. Mice in the 26-h delayed administration group received three additional daily doses of 10 mg/kg DBIBB at 26 ± 2 h, 48 ± 2 h, 72 ± 2 h, and 96 ± 2 h postirradiation, whereas mice in the 72 h delayed administration group were injected once more at 96 ± 2 h postirradiation with DBIBB but received vehicle at 26 ± 2 h and 48 ± 2 h postirradiation keeping the number of handling and injections identical in all groups. On day 30, only 3 out of 15 vehicle control mice were alive (Fig. 5b). In the group of mice treated with DBIBB starting with a +24 h postirradiation delay, 13 out of 15 survived (87%), whereas in the +72 h delayed administration group, 14 out of 15 mice (93%) remained alive on day 30. No deaths occurred in either treatment group prior to day 12 or after day 20. In the vehicle group mice began dying on day 8 and continued to die until postirradiation day 25. These results suggest that DBIBB shows efficacy in mitigating HEM-ARS even if treatment is delayed by 72 ± 2 h after TBI.
We next examined whether DBIBB can rescue LPA2 KO mice from HEM-ARS. WT or LPA2 KO mice were exposed to 8.5 Gy TBI and treated with three daily subcutaneous 10 mg/kg doses of DBIBB or vehicle starting at +26 ± 2 h postirradiation. Treatment of LPA2 KO mice with DBIBB caused no significant mitigation of mortality (p = 0.3, Fig. 5c), whereas the same treatment caused a significant reduction in mortality in WT mice (p < 0.007). We evaluated whether DBIBB treatment increases the abundance of cKit-Lin-Sca-1 positive hematopoietic progenitors in the shielded bone marrow from the tibia isolated on postirradiation day 4 from mice exposed to the PBI-BM model. These experiments showed an increase in the number of Kit-Lin-Sca-1 positive cells in the tibial bone marrow of DBIBB treated mice but even though the experiments were repeated three times the increase did not reach significance due to the low number of these cells (n=6 mice per group – Supplemental Fig. 4).
To extend the radioprotective effect of DBIBB on hematopoiesis between species, we tested human hematopoietic progenitor cells, which predominantly express LPA2 among multiple LPA receptor subtypes (Supplemental Fig. 5). Purified CD34+ progenitor cells derived from human cord blood were irradiated with 4 Gy prior to 2-h treatment with increasing concentration of DBIBB or vehicle. After this treatment the cells were transferred to MethoCult™ medium for the determination of survival and clonogenic capacity (Fig. 5d). DBIBB significantly increased the total number of colonies and specifically enhanced the survival of the granulocyte/macrophage lineages. This in vitro result corroborates our findings concerning the radiomitigation in DBIBB treated TBI mice and extends it to hematopoietic progenitors of the human species.
DISCUSSION
Earlier studies have shown that the pan-LPA receptor agonist octadecenyl thiophosphate decreased the loss of intestinal stem cells and promoted crypt survival in WT but not in LPA2 KO mice (Deng, et al., 2007). The weak LPA2-selective agonist GRI977143 decreased mortality in a HEM-ARS model when administered at 24 h postirradiation. LPA2 receptors are widely expressed and are detectable in intestinal (Munoz, et al., 2012), hematopoietic (Evseenko, et al., 2013; Ortlepp, et al., 2013), and embryonic stem cells (Costa, et al., 2013) which play an important role in postirradiation tissue regeneration. Starting with a pharmacophore based on GRI977143, we developed DBIBB which is a specific agonists of the LPA2 receptor subtype without detectable modulatory effects of the other LPA GPCR and the S1P1 receptor. DBIBB was characterized using multiple in vitro and in vivo assays to evaluate its effects on cell and organ injury caused by γ-radiation and Adriamycin. DBIBB exerted dose-dependent inhibition of caspases 3/7/8, and 9, reduces DNA fragmentation, enhanced DNA repair via LPA2 and increased clonogenic survival of IEC-6 cells. Taken together, these results establish that specific activation of LPA2 alone is sufficient to protect cells from radiation- and chemical genotoxin-induced cell death.
Preserving the genetic integrity of the DNA after ionizing radiation exposure is a pivotal step that can decide whether the cell will be able to clear the checkpoints in cell cycle progression and prevent mitotic catastrophe due to chromosomal aberrations. A radiation mitigator ideally should augment the repair of radiation-induced DNA damage. Thus, the present findings showing that the resolution of phosphorylated form of the histone H2AX was accelerated in LPA2 MEF but not in vector MEF after exposure to DBIBB is of importance. Kirsch et al. proposed that due to incomplete DNA repair some intestinal epithelial cells may undergo aberrant mitosis and die by mitotic catastrophe and not by apoptotic death (Kirsch, et al., 2010). These authors showed that p53 and its downstream target the cyclin-dependent kinase inhibitor p21 can attenuate this type of cell loss that manifests within the first 4 hours after radiation injury. LPA2 has been shown to activate p53 (Jiang, et al., 2011; Kortlever, et al., 2008; Lee, et al., 2013) and p21 (Kanehira, et al., 2012; Wu, et al., 2011). Thus, DBIBB via LPA2 might activate p53 and downstream p21 to exert its radiomitigating effect. However, in our studies DBIBB was administered >24 h postirradiation. To what extent is the late phase of radiation-induced cell loss leading to GI-ARS involves mitotic catastrophe-mediated versus apoptotic cell death will have to be addressed in future studies. Kirsch et al. showed that inhibition of the intrinsic apoptotic pathway by knocking out Bak-1 and Bax in hematopoietic and endothelial cells prevented the manifestation of the HEM-ARS-related in mice. Our results demonstrating that DBIBB inhibited the activation of the intrinsic apoptotic pathway are consistent with such a mechanism and might provide an explanation for the increased survival of mice in the HEM-ARS model. Whether DBIBB-mediated attenuation of the intrinsic apoptosis pathway or mitotic catastrophe play in the postirradiation survival of radiation-resistant Bmi1 and/or radiation-sensitive Lgr5 marker positive intestinal stem cells will have to be examined in future experiments.
The application of LPA2 MEF together with Vector-transduced DKO-MEF control cells in every step of the in vitro characterization of DBIBB provided important genetic evidence that the radiation mitigating actions of this compound are mediated by the LPA2 receptor. DBIBB protected rat intestinal crypt-derived IEC-6 cell line, and human CD34+ hematopoietic progenitor cells that endogenously expresses LPA2 among other LPA receptor subtypes establishing its cross-species efficacy as a radiomitigator. Our efforts to demonstrate that DBIBB enhances the population of Lim-cKit-Sca-1 positive hematopoietic progenitor cells in the shielded bone marrow on postirradiation day four showed an increment but further extensive experiments will be necessary to characterize the effects of this compound on hematopoiesis that are beyond the scope of the present study. DBIBB in murine ARS models showed a significant radiomitigative effect on the GI system indicated by decreased apoptosis, increased crypt survival, increased cell proliferation, and regeneration. These effects, on the GI tract combined with the increased recovery of the hematopoietic system, which might also indirectly regulate GI-recovery via soluble factors, could provide an explanation for the increased survival of mice following exposure to high does of γ-radiation. DBIBB was effective in mitigating HEM-ARS in the TBI model when administration began up to 72 h postirradiation. There are few reports in the literature on radioprotective and radiomitigative compounds (Anzai, et al., 2013; Basile, et al., 2012; Berbee, et al., 2011; Burdelya, et al., 2008; Gaberman, et al., 2013; Gao, et al., 2012; Geiger, et al., 2012; Haydont, et al., 2007; Kim, et al., 2013; Saha, et al., 2011; Sridharan, et al., 2013; Wang, et al., 2013), however, most of these interventions lose efficacy when administered 24 h postinjury or they are efficacious only in treating single-organ injury. The effective protection we detected with delayed administration of DBIBB indicates that the initial tissue damage sustained during the first 72 postirradiation hours can be effectively treated. The signal transduction mechanisms responsible for this delayed-onset radiomitigative effect linked to selective activation of LPA2 in radiosensitive stem/progenitor cells in the tissues remain unclear but are likely to involve multiple downstream secondary targets (E, et al., 2009; Lin, et al., 2007; Lin, et al., 2010).
The results of our study collectively support the potential medicinal utility of specific LPA2 receptor agonists in mitigating GI and HEM injury elicited by high doses of ionizing radiation. The delayed efficacy of DBIBB at 72 h after radiation injury lends previously unavailable experimental evidence in support of the concept of radiation mitigation and underlines the feasibility treatment protocols beginning days after exposure. The present results provide evidence that a single pharmacological agent might be capable of attenuating both HEM and GI injury caused by high doses of ionizing radiation.
SIGNIFICANCE
Medical treatment of complex injuries caused by high-dose ionizing radiation is an unsolved medical problem. Radiation mitigators — a new class of radiation countermeasures effective when administered several hours, even days, after radiation exposure are needed. Activation of the LPA2 GPCR for the growth factor-like lipid mediator lysophosphatidic acid (LPA) was shown to attenuate cell injury caused by ionizing radiation; however, no LPA2-specific agonist has been identified before. We identified 2-[4-(1,3-Dioxo-1H,3H-benzo[de]isoquinolin-2-yl)butylsulfamoyl] benzoic acid (DBIBB) as a novel LPA2 specific agonist. Using MEF from LPA1/2 double knockout mice reconstituted with only the LPA2 GPCR, DBIBB was found to rescue these cells from radiation- or genotoxin-induced death and enhanced DNA repair by reducing γ–H2AX. In wild type mice, but not in LPA2 KO mice, DBIBB reduced mortality caused by total-body γ-irradiation and was effective even when treatment started +72 h postexposure. In a partial-body irradiation model with shielding of ~5% of the bone marrow, DBIBB dose-dependently decreased mortality, increased the number of surviving intestinal crypts and reduced the number of apoptotic nuclei. DBIBB also reduced radiation-induced death of human CD34+ hematopoietic progenitor cells. DBIBB represents the first radiomitigator small molecule drug candidate capable of reducing mortality caused by the hematopoietic and gastrointestinal acute radiation syndromes.
EXPERIMENTAL PROCEDURES
Induction of apoptosis
In all experiments, apoptosis was induced either by direct γ-irradiation or by the radiomimetic chemotherapeutic Adriamycin. In radiation-induced apoptosis experiments using caspase activation or DNA fragmentation assays as endpoints, MEF cells were plated the day before the irradiation in 48-well plates at a density of 2 × 104 cells/well. One hour before irradiation, the full-growth medium was changed to serum-free medium, and the cell cultures were exposed to a dose of 15 Gy 137Cs-γ-irradiation (4.4 Gy/min). One hour postirradiation MEF cells were treated either with vehicle (BSA or DMSO), LPA (1–3 μM), DBIBB (1, 3 or 10 μM). Caspase activation and DNA fragmentation were measured 4 h after the irradiation. For the Adriamycin-induced (1.7 μM) apoptosis experiments, MEF cells were plated in 48-well plates (2 × 104 cells/well) and cultured overnight in a complete growth medium. The next morning, the growth medium was replaced by serum-starved medium supplemented with 0.1% (w/v) BSA. Cells were pretreated for 1 h with LPA (1–3 μM), DBIBB (1, 3 or 10 μM), or vehicle (BSA for LPA or 0.1% DMSO for DBIBB). Caspase activation and/or DNA fragmentation were measured to assess apoptosis 5 h after Adriamycin exposure. In the irradiation-induced apoptosis experiments of IEC-6 cells, the cells were plated in 48-well plates in a complete medium (3.5 × 104 cells/well). On the next day the complete medium was replaced with serum-starvation medium for 18–24 h before the irradiation. To induce apoptosis, IEC-6 cells were exposed to 10 or 15 Gy γ-irradiation as noted for a given experiment at a rate of 4.4 Gy/min. One hour after irradiation, the medium was replaced with a fresh serum-starvation medium and IEC-6 cells were treated either with vehicle (BSA or 0.1 DMSO), LPA (1–3 μM), or DBIBB (1, 3, 10, and 30 μM). Six hours after irradiation, the medium was changed and the cells were treated with fresh compounds at the concentrations mentioned above. Caspase activation was measured 24 h after irradiation. In a separate plate, sister cultures were plated for non-irradiated vehicle treatment (BSA or DMSO) at the same density as used for irradiation.
Caspase activation assay
To measure caspase activation, the cells were lysed in 50 μl Caspase-Glow ® reagent. Caspase – Glo 3/7, Caspase – Glo 8, and Caspase – Glo 9 were purchased from Promega (Madison, WI) and used as described previously (Kiss, et al., 2013). The caspase activity was calculated as mean ± SD for triplicate wells for every experimental group.
DNA fragmentation ELISA
DNA fragmentation was quantified by using the Cell Death Detection ELISAPLUS kit (Roche Diagnostics; Penzberg, Germany) and normalized to protein concentration. Protein concentration was measured using the BCA Protein Assay Kit (Thermo Fisher Scientific; Waltham, MA). DNA fragmentation was determined and expressed as absorbance at 405 nm/min/mg protein as described previously (Kiss, et al., 2013).
Quantification γ–H2AXhigh levels
LPA2-DKO-MEF and Vector-DKO-MEF cells were plated in at 1.5 × 105 cells/well density in 1.5 ml DMEM supplemented with 2mM L-Glutamine and 10% FBS. Next day, the cells were serum starved for 2.5 hours, pretreated for 15 minutes with either 10 μM DBIBB, 10 μM LPA, or vehicle (0.1 % DMSO) and irradiated with 15 Gy at 4.4 Gy/min. After irradiation the medium was replaced with fresh serum-free medium containing either vehicle or DBIBB. At times indicated (15 min – 6 h) the cells were trypsinized, centrifuged and stained with anti-phospho-H2AX (S139) eFluor660 (Ebioscience, San Diego, CA) using the Foxp3/Transcription Factor Staining Buffer Set (Biolegend Inc., San Diego CA) following the manufacturer’s protocol. Ten thousand events per sample were measured with flow cytometry using a LSR II instrument (Becton Dickinson Inc.) and analyzed with the FACSDiva software (Becton Dickinson Inc.). Gating was set in all samples to reveal intensively stained γH2AXhigh population of cells that allowed to follow the time course of resolution i.e. the progression of ds-DNA repair.
For western blotting, cell lysates were harvested 4 h after irradiation in RIPA buffer (PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS). Protein concentrations were determined using the Pierce™ BCA method (Thermo Scientific). For detection of phosphorylated phosphorylated ERK1/2 (Cell Signaling), 20 μg of protein samples was separated on a 12% SDS-PAGE gel and transferred onto a nitrocellulose membrane. For detection of phosphorylated H2AX (Cell Signaling) and β-actin (Sigma Aldrich), 15 μg of protein samples was separated on a 16% SDS-PAGE gels. Membrane was blocked with 5% milk in TBS, 0.05% Tween-20 (TBST) for 1.5 h followed by overnight incubation with primary antibodies at 4°C in 5% BSA TSBT. Membrane was washed with TBST and incubated with secondary antibodies for 1 h, and finally washed in TBST. For monitoring PARP-1 cleavage MEF cells were plated day before the experiment. Next day when the cells were serum starved 1 h before the irradiation and irradiated with 15 Gy at 4.3 G/min. The cells were treated 10 min after the irradiation with 10 μM DBIBB or 3 μM LPA, or vehicle (3 μM cc BSA + 0.1% DMSO). Four hours after the irradiation the samples were collected for protein extraction and 30 μg of the cell lysates was run on a 10 % SDS-PAGE. The rabbit anti- PARP-1 antibody was from Cell Signaling and used at 1:1000 dilution. Pierce™ SuperSignal West Pico ECL kit (Thermo Scientific) was used to visualize the membrane.
Mouse PBI and TBI irradiation models
Experimental procedures were reviewed and approved by the IACUC of the University of Tennessee Health Science Center. Eight-to-ten-week old C57BL/6 female mice (Jackson Laboratories, Bar Harbor, ME) or LPA2 KO mice on the C57BL/6 background (kind gift of Dr. Jerold Chun, Scripps Institute, La Jolla, CA) bred in our colony, were acclimated for at least one week in the vivarium. All irradiations were performed using a Cs137 γ-source (J.L. Shepherd & Assoc. Mark I, Model 25, San Fernando, CA). Mice were housed in Allentown Biocontainment cages (Allentown Inc., Allentown, NJ) in the Regional Biocontainment Laboratory of the University of Tennessee Health Science Center Memphis. The mice received no supportive care but were provided gel-food packs (Diet Gel 76A, Clear H2O, Inc., γ-irradiation sterilized) on postirradiation day 4 and were feed sterilized chow (Harlan Teklad LM-485 mouse/rat diet, γ-irradiation sterilized) throughout the study, ad libitum with autoclaved tap water. Animals were group housed with a maximum of 4 animals per cage using Autoclaved bedding, enrichment blocks, and nestlets.
Radiation field mapping and calibration by ion chamber dosimetry was done by manufacturer at installation. Referencing dosimetry was conducted in May 2012, indicating an average dose rate of 1.47 Gy/min. In addition, routine validation and quality control measurements of exposure rates and exposure rate mapping in the chamber at positions of interest was conducted by a Certified Health Physicist using a calibrated RadCal 0.6 cc therapy grade ion chamber/electrometer system. High-dose thermoluminescent dosimeters were used in most irradiations to validate the actual dose delivered to the mice (MD Anderson Cancer Center Radiation Dosimetry Services). The isodose field was validated using Gafchromic (radiochromic) film for high-dose dosimetry (10–50 Gy, Ashland Inc., Covington, KY).
The PBI model with bone marrow sparring we used is very similar to that used by Booth and colleagues of the MCART Consortium (Booth, et al., 2012). In our PBI model, mice were anesthetized with ketamine (87 mg/kg) and xylazine (13 mg/kg, IP) and placed in a plexiglass restrainer such that the legs below the knee were shielded. The shielding protected both tibiae, fibulae and the paws from radiation that contain ~ 5 % of the bone marrow (Boggs, 1984). The effectiveness of shielding was verified in randomly selected mice by histological examination (see below Supplemental Fig.2). Mice placed into the isodose field of the irradiator were irradiated in groups of eight.
During the model development phase, mice were exposed to radiation doses from 6 to 18 Gy and a dose-response curve was generated using logit-probit analysis for day-10 and day-30 mortality. In this model a 15.69 Gy delivered at a dose rate of 1.47 Gy/min yielded an empirical ~LD85/10 mortality with some seasonal variation with the peak occurring between postirradiation days 8–10. Histological examination of the intestinal radiation damage confirmed the development of the GI-ARS in all animals.
The TBI model we used was similar to that described by Plett et al. (Plett, et al., 2012) in the MCART Consortion of the NIAID. In our TBI model, the 8–10 week old C57BL/6 or LPA2 KO mice (15–16 animals/group) were exposed to 8.5 Gy γ-irradiation from the Cs137 γ-source at a dose-rate of ~0.82 Gy/min in a rotating chamber. This radiation dose that yielded an LD60/30 mortality with mean survival time of 22 ± 2.6 day (SD) was selected based on a partial dose-response curve that spanned the dose-range between 8 to 10 Gy in 0.5 Gy increments. The mice were housed and maintained under identical conditions as described above for the PBI model without supportive care. Twenty-six ± two hours after the irradiation the animals were treated with either vehicle or DBIBB via subcutaneous injection. Animal survival was recorded twice daily up to 30 days, which was the endpoint of these experiments. Statistical testing of the survival data was done by Fisher’s exact test.
Supplementary Material
Highlights.
DBIBB is an LPA2 GPCR-specific agonist butylsulfamoyl benzoic acid analog
DBIBB protects cells from radiation injury and enhances DNA repair via LPA2
DBIBB decreases mortality due to GI and HEM acute radiation syndromes
DBIBB is an effective radiomitigator with postirradiation administration up to 72 h
Acknowledgments
We thank Drs. Tony Marion and Dan Rosson for their help with the flow cytometry, Ms. Ashley Ezekiel-Yacoubian for her assistance with slide digitization and morphometric analyses, Jin Emerson-Cobb for her editorial help, and Prof. Thomas MacVittie for critical reading of the manuscript. This work was funded by grants from NIAID AI080405 (GT), the American Cancer Society 122059-PF-12-107-01-COD (JIF), by Award Number I01BX007080 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (G.T.), and the Van Vleet Endowment (GT).
Footnotes
Contributions
R.P., S.P., and D.D.M. performed the synthetic experiments. E.Sz. performed apoptosis experiments. J.I.F. performed the computational chemistry studies. D.B.N., K.G.L., and S.C.L. performed the pharmacological characterization of the compounds. A.B. performed the hematopoiesis experiments. Y.F. performed in vivo experiments. F.T., E.Sz., A.B. & G.T. performed the statistical analysis of the data. L.B. performed the histopathology, K. E.-T., A. Bo., J.S. C.R.Y., J.S., and G.I.B. developed and characterized the TBI and PBI murine ARS models. G.T. designed the experiments and wrote the paper with input from all coauthors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar-Quesada R, Munoz-Gamez JA, Martin-Oliva D, Peralta A, Valenzuela MT, Matinez-Romero R, Quiles-Perez R, Menissier-de Murcia J, de Murcia G, Ruiz de Almodovar M, et al. Interaction between ATM and PARP-1 in response to DNA damage and sensitization of ATM deficient cells through PARP inhibition. BMC Mol Biol. 2007;8:29. doi: 10.1186/1471-2199-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzai K, Ueno M, Matsumoto KI, Ikota N, Takata J. Gamma-tocopherol-N,N-dimethylglycine ester as a potent post-irradiation mitigator against whole body X-irradiation-induced bone marrow death in mice. J Radiat Res. 2013 doi: 10.1093/jrr/rrt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H. In: Methods in Neurosciences. Conn PM, Sealfon SC, editors. Chapter 19. San Diego: Academic Press; 1995. pp. 366–428. [Google Scholar]
- Basile LA, Ellefson D, Gluzman-Poltorak Z, Junes-Gill K, Mar V, Mendonca S, Miller JD, Tom J, Trinh A, Gallaher TK. HemaMax, a recombinant human interleukin-12, is a potent mitigator of acute radiation injury in mice and non-human primates. PLoS One. 2012;7:e30434. doi: 10.1371/journal.pone.0030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbee M, Fu Q, Garg S, Kulkarni S, Kumar KS, Hauer-Jensen M. Pentoxifylline enhances the radioprotective properties of gamma-tocotrienol: differential effects on the hematopoietic, gastrointestinal and vascular systems. Radiat Res. 2011;175:297–306. doi: 10.1667/RR2399.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs DR. The total marrow mass of the mouse: a simplified method of measurement. Am J Hematol. 1984;16:277–286. doi: 10.1002/ajh.2830160309. [DOI] [PubMed] [Google Scholar]
- Booth C, Tudor G, Tudor J, Katz BP, MacVittie TJ. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012;103:383–399. doi: 10.1097/hp.0b013e318266ee13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Sourris K, Lim SM, Yu QC, Hirst CE, Parkington HC, Jokubaitis VJ, Dear AE, Liu HB, Micallef SJ, et al. Derivation of endothelial cells from human embryonic stem cells in fully defined medium enables identification of lysophosphatidic acid and platelet activating factor as regulators of eNOS localization. Stem Cell Res. 2013;10:103–117. doi: 10.1016/j.scr.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Dainiak N, Gent RN, Carr Z, Schneider R, Bader J, Buglova E, Chao N, Coleman CN, Ganser A, Gorin C, et al. Literature review and global consensus on management of acute radiation syndrome affecting nonhematopoietic organ systems. Disaster Med Public Health Prep. 2011;5:183–201. doi: 10.1001/dmp.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Balazs L, Wang DA, Van Middlesworth L, Tigyi G, Johnson LR. Lysophosphatidic acid protects and rescues intestinal epithelial cells from radiation- and chemotherapy-induced apoptosis. Gastroenterology. 2002;123:206–216. doi: 10.1053/gast.2002.34209. [DOI] [PubMed] [Google Scholar]
- Deng W, Poppleton H, Yasuda S, Makarova N, Shinozuka Y, Wang DA, Johnson LR, Patel TB, Tigyi G. Optimal lysophosphatidic acid-induced DNA synthesis and cell migration but not survival require intact autophosphorylation sites of the epidermal growth factor receptor. The Journal of biological chemistry. 2004;279:47871–47880. doi: 10.1074/jbc.M405443200. [DOI] [PubMed] [Google Scholar]
- Deng W, Shuyu E, Tsukahara R, Valentine WJ, Durgam G, Gududuru V, Balazs L, Manickam V, Arsura M, VanMiddlesworth L, et al. The lysophosphatidic acid type 2 receptor is required for protection against radiation-induced intestinal injury. Gastroenterology. 2007;132:1834–1851. doi: 10.1053/j.gastro.2007.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, Bader JL, Coleman CN, Weinstock DM. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5(Suppl 1):S32–44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ES, Lai YJ, Tsukahara R, Chen CS, Fujiwara Y, Yue J, Yu JH, Guo H, Kihara A, Tigyi G, et al. Lysophosphatidic acid 2 receptor-mediated supramolecular complex formation regulates its antiapoptotic effect. J Biol Chem. 2009;284:14558–14571. doi: 10.1074/jbc.M900185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evseenko D, Latour B, Richardson W, Corselli M, Sahaghian A, Cardinal S, Zhu Y, Chan R, Dunn B, Crooks GM. Lysophosphatidic acid mediates myeloid differentiation within the human bone marrow microenvironment. PLoS One. 2013;8:e63718. doi: 10.1371/journal.pone.0063718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Sardar V, Tokumura A, Baker D, Murakami-Murofushi K, Parrill A, Tigyi G. Identification of residues responsible for ligand recognition and regioisomeric selectivity of lysophosphatidic acid receptors expressed in mammalian cells. J Biol Chem. 2005;280:35038–35050. doi: 10.1074/jbc.M504351200. [DOI] [PubMed] [Google Scholar]
- Gaberman E, Pinzur L, Levdansky L, Tsirlin M, Netzer N, Aberman Z, Gorodetsky R. Mitigation of Lethal Radiation Syndrome in Mice by Intramuscular Injection of 3D Cultured Adherent Human Placental Stromal Cells. PLoS One. 2013;8:e66549. doi: 10.1371/journal.pone.0066549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Fish BL, Szabo A, Doctrow SR, Kma L, Molthen RC, Moulder JE, Jacobs ER, Medhora M. Short-term treatment with a SOD/catalase mimetic, EUK-207, mitigates pneumonitis and fibrosis after single-dose total-body or whole-thoracic irradiation. Radiat Res. 2012;178:468–480. doi: 10.1667/RR2953.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger H, Pawar SA, Kerschen EJ, Nattamai KJ, Hernandez I, Liang HP, Fernandez JA, Cancelas JA, Ryan MA, Kustikova O, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med. 2012;18:1123–1129. doi: 10.1038/nm.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydont V, Gilliot O, Rivera S, Bourgier C, Francois A, Aigueperse J, Bourhis J, Vozenin-Brotons MC. Successful mitigation of delayed intestinal radiation injury using pravastatin is not associated with acute injury improvement or tumor protection. Int J Radiat Oncol Biol Phys. 2007;68:1471–1482. doi: 10.1016/j.ijrobp.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Igaki H, Nakagawa K, Uozaki H, Akahane M, Hosoi Y, Fukayama M, Miyagawa K, Akashi M, Ohtomo K, Maekawa K. Pathological changes in the gastrointestinal tract of a heavily radiation-exposed worker at the Tokai-mura criticality accident. J Radiat Res. 2008;49:55–62. doi: 10.1269/jrr.07058. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Xie X, Li Z, Wang Z, Zhang Y, Ling ZQ, Pan Y, Chen Y. Functional cooperation of RKTG with p53 in tumorigenesis and epithelial-mesenchymal transition. Cancer Res. 2011;71:2959–2968. doi: 10.1158/0008-5472.CAN-10-4077. [DOI] [PubMed] [Google Scholar]
- Kanehira M, Kikuchi T, Ohkouchi S, Shibahara T, Tode N, Santoso A, Daito H, Ohta H, Tamada T, Nukiwa T. Targeting lysophosphatidic acid signaling retards culture-associated senescence of human marrow stromal cells. PLoS One. 2012;7:e32185. doi: 10.1371/journal.pone.0032185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedar PS, Stefanick DF, Horton JK, Wilson SH. Interaction between PARP-1 and ATR in mouse fibroblasts is blocked by PARP inhibition. DNA Repair (Amst) 2008;7:1787–1798. doi: 10.1016/j.dnarep.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SB, Ly P, Kaisani A, Zhang L, Wright WE, Shay JW. Mitigation of Radiation-Induced Damage by Targeting EGFR in Noncancerous Human Epithelial Cells. Radiat Res. 2013;180:259–267. doi: 10.1667/RR3371.1. [DOI] [PubMed] [Google Scholar]
- Kirsch DG, Santiago PM, di Tomaso E, Sullivan JM, Hou WS, Dayton T, Jeffords LB, Sodha P, Mercer KL, Cohen R, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327:593–596. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss GN, Fells JI, Gupte R, Lee SC, Liu J, Nusser N, Lim KG, Ray RM, Lin FT, Parrill AL, et al. Virtual screening for LPA2-specific agonists identifies a nonlipid compound with antiapoptotic actions. Mol Pharmacol. 2012;82:1162–1173. doi: 10.1124/mol.112.079699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss GN, Lee SC, Fells JI, Liu J, Valentine WJ, Fujiwara Y, Thompson KE, Yates CR, Sumegi B, Tigyi G. Mitigation of radiation injury by selective stimulation of the LPA(2) receptor. Biochimica et biophysica acta. 2013;1831:117–125. doi: 10.1016/j.bbalip.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HL, Ren EC. Functional aspects of PARP1 in DNA repair and transcription. Biomolecules. 2012;2:524–548. doi: 10.3390/biom2040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortlever RM, Brummelkamp TR, van Meeteren LA, Moolenaar WH, Bernards R. Suppression of the p53-dependent replicative senescence response by lysophosphatidic acid signaling. Mol Cancer Res. 2008;6:1452–1460. doi: 10.1158/1541-7786.MCR-08-0066. [DOI] [PubMed] [Google Scholar]
- Lee SJ, No YR, Dang DT, Dang LH, Yang VW, Shim H, Yun CC. Regulation of hypoxia-inducible factor 1alpha (HIF-1alpha) by lysophosphatidic acid is dependent on interplay between p53 and Kruppel-like factor 5. The Journal of biological chemistry. 2013;288:25244–25253. doi: 10.1074/jbc.M113.489708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FT, Lai YJ, Makarova N, Tigyi G, Lin WC. The lysophosphatidic acid 2 receptor mediates down-regulation of Siva-1 to promote cell survival. J Biol Chem. 2007;282:37759–37769. doi: 10.1074/jbc.M705025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ME, Herr DR, Chun J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010;91:130–138. doi: 10.1016/j.prostaglandins.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J, Lukas C, Bartek J. More than just a focus: The chromatin response to DNA damage and its role in genome integrity maintenance. Nat Cell Biol. 2011;13:1161–1169. doi: 10.1038/ncb2344. [DOI] [PubMed] [Google Scholar]
- Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. Embo J. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortlepp C, Steudel C, Heiderich C, Koch S, Jacobi A, Ryser M, Brenner S, Bornhauser M, Brors B, Hofmann WK, et al. Autotaxin is expressed in FLT3-ITD positive acute myeloid leukemia and hematopoietic stem cells and promotes cell migration and proliferation. Exp Hematol. 2013;41:444–461. e444. doi: 10.1016/j.exphem.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Parrill AL, Wang D, Bautista DL, Van Brocklyn JR, Lorincz Z, Fischer DJ, Baker DL, Liliom K, Spiegel S, Tigyi G. Identification of Edg1 receptor residues that recognize sphingosine 1-phosphate. J Biol Chem. 2000;275:39379–39384. doi: 10.1074/jbc.M007680200. [DOI] [PubMed] [Google Scholar]
- Patil R, Fells JI, Szabó E, Lim KG, Norman DD, Balogh A, Patil SA, Strobos J, Miller DD, Tigyi GJ. Design and Synthesis of Sulfamoyl Benzoic Acid Analogs with Subnanomolar Agonist Activity Specific to the LPA2 Receptor. Med Chem Report. 2014 doi: 10.1021/jm5007116. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil R, Szabó E, Fells JI, Balogh A, Lim KG, Norman DB, Patil S, Strobos J, Miller DD, Tigyi GJ. Design and synthesis of sulfamoyl benzoic acid analogs with subnanomolar agonist activity specific to the LPA2 receptor. J Med Chem. 2014 doi: 10.1021/jm5007116. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett PA, Sampson CH, Chua HL, Joshi M, Booth C, Gough A, Johnson CS, Katz BP, Farese AM, Parker J, et al. Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012;103:343–355. doi: 10.1097/HP.0b013e3182667309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 2011;6:e24072. doi: 10.1371/journal.pone.0024072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardar VM, Bautista DL, Fischer DJ, Yokoyama K, Nusser N, Virag T, Wang DA, Baker DL, Tigyi G, Parrill AL. Molecular basis for lysophosphatidic acid receptor antagonist selectivity. Biochim Biophys Acta. 2002;1582:309–317. doi: 10.1016/s1388-1981(02)00185-3. [DOI] [PubMed] [Google Scholar]
- Sridharan V, Tripathi P, Sharma S, Corry PM, Moros EG, Singh A, Compadre CM, Hauer-Jensen M, Boerma M. Effects of late administration of pentoxifylline and tocotrienols in an image-guided rat model of localized heart irradiation. PLoS One. 2013;8:e68762. doi: 10.1371/journal.pone.0068762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DA, Lorincz Z, Bautista DL, Liliom K, Tigyi G, Parrill AL. A single amino acid determines lysophospholipid specificity of the S1P1 (EDG1) and LPA1 (EDG2) phospholipid growth factor receptors. J Biol Chem. 2001;276:49213–49220. doi: 10.1074/jbc.M107301200. [DOI] [PubMed] [Google Scholar]
- Wang J, Kulkarni A, Chintala M, Fink LM, Hauer-Jensen M. Inhibition of protease-activated receptor 1 ameliorates intestinal radiation mucositis in a preclinical rat model. Int J Radiat Oncol Biol Phys. 2013;85:208–214. doi: 10.1016/j.ijrobp.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Khandoga AL, Goyal P, Fells JI, Perygin DH, Siess W, Parrill AL, Tigyi G, Fujiwara Y. Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J Biol Chem. 2009;284:17304–17319. doi: 10.1074/jbc.M109.003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Mukherjee A, Lebman DA, Fang X. Lysophosphatidic acid-induced p21Waf1 expression mediates the cytostatic response of breast and ovarian cancer cells to TGFbeta. Mol Cancer Res. 2011;9:1562–1570. doi: 10.1158/1541-7786.MCR-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.