Abstract
Background
Pitch plasticity has been observed in Hybrid cochlear implant (CI) users. Does pitch plasticity also occur in bimodal CI users with traditional long-electrode CIs, and is pitch adaptation pattern associated with electrode discrimination or speech recognition performance?
Objective
Characterize pitch adaptation patterns in long-electrode CI users, correlate these patterns with electrode discrimination and speech perception outcomes, and analyze which subject factors are associated with the different patterns.
Methods
Electric-to-acoustic pitch matches were obtained in 19 subjects over time from CI activation to at least 12 months after activation, and in a separate group of 18 subjects in a single visit after at least 24 months of CI experience. Audiometric thresholds, electrode discrimination performance, and speech perception scores were also measured.
Results
Subjects measured over time had pitch adaptation patterns that fit one of the following categories: 1) “Pitch-adapting”, i.e. the mismatch between perceived electrode pitch and the corresponding frequency-to-electrode allocations decreased; 2) “Pitch-dropping”, i.e. the pitches of multiple electrodes dropped and converged to a similar low pitch; 3) “Pitch-unchanging”, i.e. electrode pitches did not change. Subjects measured after CI experience had a parallel set of adaptation patterns: 1) “Matched-pitch”, i.e. the electrode pitch was matched to the frequency allocation; 2) “Low-pitch”, i.e. the pitches of multiple electrodes were all around the lowest frequency allocation; 3) “Nonmatched-pitch”, i.e. the pitch patterns were compressed relative to the frequency allocations and did not fit either the matched-pitch or low-pitch categories. Unlike Hybrid CI users which were mostly in the pitch-adapting/matched-pitch category, the majority of bimodal CI users were in the latter two categories, pitch-dropping/low-pitch or pitch-unchanging/nonmatched-pitch. Subjects with pitch-adapting or matched-pitch patterns tended to have better low-frequency thresholds than subjects in the latter categories. Changes in electrode discrimination over time were not associated with changes in pitch differences between electrodes. Reductions in speech perception scores over time showed a weak but nonsignificant association with dropping-pitch patterns.
Conclusions
Bimodal CI users with more residual hearing may have somewhat greater similarity to Hybrid CI users and be more likely to adapt pitch perception to reduce mismatch with the frequencies allocated to the electrodes and the acoustic hearing. In contrast, bimodal CI users with less residual hearing exhibit either no adaptation, or surprisingly, a third pattern in which the pitches of the basal electrodes drop to match the frequency range allocated to the most apical electrode. The lack of association of electrode discrimination changes with pitch changes suggests that electrode discrimination does not depend on perceived pitch differences between electrodes, but rather on some other characteristics such as timbre. In contrast, speech perception may depend more on pitch perception and the ability to distinguish pitch between electrodes, especially since during multi-electrode stimulation, cues such as timbre may be less useful for discrimination.
Introduction
As CI technology and surgical techniques have improved, implantation criteria have expanded in recent years to include people with more residual hearing. This has led to the increased use of “bimodal” stimulation, the combined use of a cochlear implant (CI) with a hearing aid (HA) in the contralateral ear. As with binaural HAs or bilateral CIs, the addition of a HA to the ear opposite the CI can improve speech perception in quiet, as well as improve sound localization (review: Ching et al., 2007). More importantly, performance with bimodal stimulation can be significantly better for understanding speech in background babble compared to electric stimulation alone (Kong et al., 2005; Dorman et al., 2008), as well as for musical melody recognition (Kong et al., 2005, Dorman et al., 2008; El Fata et al., 2009). This benefit is likely to be due to added information provided by the better frequency resolution of the preserved residual hearing (Henry et al., 2005). However, there remains significant variability in the bimodal benefit, with several cases of little benefit or even interference for speech recognition compared to either ear alone in adults (Ching et al., 2007) and children (Litovsky et al., 2006).
One factor that may explain the variable outcomes in CI users is plasticity of pitch perception with CI experience (Reiss et al., 2007). Pitch plasticity is variable across CI users and may explain variability in speech perception outcomes. One possibility is that pitch perception changes may in some cases decrease pitch differences between electrodes, and thus reduce electrode discriminability and the number of usable channels for transmission of speech information. Several previous studies have found electrode discrimination to be correlated with speech perception (e.g. Collins et al., 1997; Throckmorton and Collins, 1999; Henry et al., 2000), but other studies have found no correlation (Zwolan et al., 1997).
Previous findings suggest that pitch plasticity occurs in response to frequency mismatches between electric and acoustic hearing introduced by cochlear implant programming. This mismatch arises because CI processors are programmed to analyze the range of sound frequencies needed for speech perception, and divide and allocate these frequencies to the electrodes in the CI independent of the cochlear place frequencies actually stimulated electrically. For a typical CI, the default range of frequencies analyzed can be as wide as 100-8000 Hz. Due to anatomical and design limitations, the electrode array is typically implanted to depths ranging from 8-21 mm (Lee et al., 2010), corresponding to cochlear place frequencies of no lower than 500–1500 Hz (Greenwood, 1990). This leads to a severe tonotopic mismatch between the sound frequencies analyzed versus those actually stimulated electrically in the cochlea.
Previous studies have shown that Hybrid CI users who wear HAs in both ears or a second CI in the contralateral ear can adapt pitch perception over months of experience to reduce any perceived pitch mismatch between acoustic and electric inputs due to this tonotopic mismatch (Reiss et al., 2007; 2011; 2013). Previous studies in bimodal CI users indirectly suggest that pitch perception may change in this group, as well. Several studies have shown that electrode pitches are often mismatched to and lower than the frequency-to-electrode allocations and lower than the predicted pitch based on cochlear location (e.g. Blamey et al., 1996; Boex et al., 2006; Dorman et al., 2007; McDermott et al., 2009). In contrast, pitch match experiments undertaken in bimodal CI users after little or no experience with the CI have found electrode pitches to be closer to predictions (Eddington et al., 1978; McDermott et al., 2009; Carlyon et al., 2010).
Does pitch plasticity also occur in bimodal CI users with traditional long-electrode CIs? How does pitch plasticity affect electrode discrimination and speech perception? In order to study these questions, two groups of bimodal CI users were recruited. One group was tested at multiple times from initial CI activation up to 12 months of CI use in order to directly characterize pitch adaptation patterns over time, and how electrode discrimination and speech perception are linked to these pitch changes. A second group was tested just once after at least 2 years of CI experience; 2 years is the time point by which pitch changes are generally stabilized in the absence of CI frequency-to-allocation changes. Both groups were analyzed for which subject factors might be associated with each pitch adaptation pattern.
Materials and Methods
Subjects
Experiments were conducted according to the guidelines for the protection of human subjects as set forth by the Institutional Review Board of Oregon Health & Science University, and the methods employed were approved by that board. Thirty-seven adult cochlear implant subjects with residual hearing in the contralateral, non-implanted ear participated in this study. All subjects were required to be between 18-85 years of age, and have unaided audiometric thresholds of 90 dB or better up to 1000 Hz in the non-implanted ear. Subjects were not required to be regular hearing aid users.
Two groups of subjects were studied: (1) A Post-Activation Over Time (PAOT) group of recently implanted CI users who were tested periodically (at ~0, 1, 3, 6, and 12 months after activation) in the year following implantation, and (2) an Experienced CI (ECI) group with at least 24 months of experience with the CI at the time of testing, and who were tested at one point in time. Subjects in the PAOT group were recruited by tracking implant candidates and expected surgery dates in an IRB-approved clinic database, distributing flyers to patients in their pre-operative packets, and mailing letters with detailed information about the study. Research visits were coordinated with the clinic to help patients to schedule research on the same days as clinical visits. Subjects in the ECI group were recruited similarly from the clinic database and research visits were coordinated with annual visits when applicable.
The PAOT group was made up of 19 CI users (13 male and 6 female; mean age =63.1 ± 12.2 years; mean duration of severe-profound deafness = 23.4 ± 13.7 years) and the ECI group was made up of 18 CI users (mean age = 67.2 ± 11.4 years; mean duration of severe-profound deafness = 29.7 ± 14.4 years). All except one PAOT subject were tested after implantation of a new CI; one subject, CI09, was tested after implant failure and re-implantation. No significant differences were seen between the PAOT and ECI group in age, duration of severe-profound deafness, or average low-frequency hearing loss (t-test, p>0.2).
All subjects used a standard CI electrode array from either Cochlear or MED-EL, and used the advanced combination encoder (ACE) strategy with rates ranging from 500 to 1200 pps. For the PAOT subjects, the first five columns of Table I show subject age at time of implantation, gender, implanted ear, and duration of testing after implant activation. For ECI subjects, the first five columns of Table II show subject age at time of testing, gender, implanted ear, and duration of CI use at time of testing. For both groups, the last seven columns of each table show internal CI device and pulse rate, etiology of hearing loss, duration of high-frequency severe-profound hearing loss, average contralateral low-frequency hearing loss, contralateral hearing aid model if used for each subject (“none” indicates subject did not wear a HA), specific electrodes tested, and electrodes that could not be pitch matched within the residual hearing range. Note that the majority of CI users in the study used a contralateral HA with the CI on a regular basis, but a few (CI14, CI7, CI47 in the PAOT group and CI30, CI50 in the ECI group) did not. Since all testing in this study was conducted without hearing aids, i.e. via the cochlear implant or via a headphone to the non-implanted ear, hearing aid fittings were not verified for all subjects.
Table I. Post-Activation Over Time (PAOT) group.
Demographic information for Post-Activation Over Time (PAOT) subjects tested at multiple intervals for up to 12 months after CI activation. Subject characteristics described include age in years, gender, implant ear, duration of testing in study, internal CI device and pulse rate, etiology of hearing loss (HL), duration of high-frequency (HF) severe-profound (S/P) hearing loss, average low-frequency threshold shift (averaged over 250, 500, and 1000 Hz), and contralateral hearing aid (HA) model used (“none” indicates subject did not wear a HA). The specific electrodes tested and the electrodes that could not be pitch matched (out of range) are also given in the last two columns.
| Subj | Age at implant (yrs) |
Gender | CI ear |
Dur. CI testing (mos) |
Internal Device/ pulse rate (pps) |
Etiology of HL |
Dur. HF S/P HL (yrs) |
Avg contra LF HL (dBHL) |
Contra HA model |
Elec. tested |
Elec. out of range |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CI04 | 51 | M | R | 21 | CI512/ 900 |
childhood, unknown |
30+ | 58 | Oticon Chili |
22,19, 16 |
N/A |
| CI06 | 73 | M | R | 15 | CI512/ 900 |
childhood, unknown |
30+ | 87 | Sonus | 22,18, 13 |
N/A |
| CI09 | 77 | M | L | 11 | CI512/ 900 |
noise | 35 | 72 | Resound Azure |
22,18, 12 |
N/A |
| CI13 | 43 | F | L | 8 | CI512/ 900 |
German measles |
30 + |
83 | Phonak Naida |
22,16, 12 |
N/A |
| CI14 | 67 | M | L | 14 | CI512/ 900 |
noise | 30+ | 72 | none | 22,18, 12 |
N/A |
| CI15 | 80 | F | R | 12 | CI512/ 900 |
pnuemonia, sudden SNHL |
10 | 57 | Phonak Naida |
22,18, 12 |
N/A |
| CI17 | 67 | M | R | 12 | CI512/ 900 |
noise, progressive |
15-20 | 58 | none | 22,18, 12 |
N/A |
| CI24 | 52 | M | R | 12 | CI512/ 900 |
noise, progressive |
9 | 80 | Phonak Naida |
22,18, 12 |
N/A |
| CI29 | 75 | M | L | 12 | CI512/ 900 |
noise | 20 | 68 | Siemens Intuis |
22,20, 16 |
12 |
| CI31 | 62 | F | L | 12 | CI512/ 1800 |
Meniere’s | 10-15 | 67 | Phonak Exelia |
22,18, 12 |
N/A |
| CI33 | 60 | F | R | 12 | CI512/ 900 |
multiple sclerosis |
25 | 73 | Phonak Naida |
22,18, 12 |
N/A |
| CI34 | 63 | M | R | 12.5 | CI512/ 900 |
progressive, sudden SNHL |
0-5 | 65 | Siemens RITE |
22,18, 12 |
N/A |
| CI37 | 71 | M | L | 13 | CI512/ 500 |
noise | 20+ | 52 | Phonak Extra |
22,18, 12 |
12* |
| CI38 | 78 | M | L | 12 | CI512/ 900 |
noise | 20+ | 67 | Phonak Naida |
22,18, 12 |
N/A |
| CI42 | 66 | M | R | 19.5 | Freedom/ 900 |
childhood, unknown |
50+ | 93 | Phonak F1 |
22,21, 19,18, 13,12 |
N/A |
| CI44 | 65 | F | R | 12 | Freedom/ 900 |
Progressive, unknown |
30+ | 75 | Audigy AGXO |
22,21, 18,12 |
N/A |
| CI47 | 33 | M | L | 12 | Freedom/ 900 |
chemo, progressive |
5 | 53 | none | 22,18, 12 |
all** |
| CI53 | 56 | F | R | 12 | Freedom/ 900 |
family, progressive |
20 | 40 | Phonak Naida |
22,18, 12 |
N/A |
| CI55 | 60 | M | R | 12 | Freedom/ 900 |
virus, sudden HL |
9 | 67 | Widex Inteo |
22,21, 20,19, 18,12 |
18,12 |
denotes out of range on the low frequency side at 13 months for this subject.
denotes out of range on the low-frequency side for all electrodes tested on CI activation day.
Table II. Experienced Cochlear Implant (ECI) group.
Demographic information for Experienced Cochlear Implant (ECI) subjects tested at a single visit after at least 24 months of CI experience. Subject characteristics described include age in years, gender, implant ear, duration of CI use, internal CI device and pulse rate, etiology of hearing loss (HL), duration of high-frequency (HF) severe-profound (S/P) hearing loss, average low-frequency threshold shift (averaged over 250, 500, and 1000 Hz), and contralateral hearing aid (HA) model used (“none” indicates subject did not wear a HA). The specific electrodes tested and the electrodes that could not be pitch matched (out of range) are also given in the last two columns.
| Subj | Age at implant (yrs) |
Gender | CI ear |
Dur. CI use (yrs) |
Internal Device/ pulse rate (pps) |
Etiology of HL |
Dur. HF S/P HL (yrs) |
Avg contra LF HL (dBHL) |
Contra HA model |
Elec. tested |
Elec. out of range |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CI18 | 61 | F | L | 3 | Freedom/ 900 |
unknown | 35 | 50 | Phonak Savia |
22,18, 12 |
N/A |
| CI19 | 60 | F | R | 5 | Freedom/ 900 |
scarlet fever, noise, Menieres |
50 | 70 | AVR Impact |
22-17 | 18,17 |
| CI20 | 75 | M | L | 5 | Freedom/ 900 |
noise | 35 | 65 | none | 22,18, 12 |
N/A |
| CI21 | 66 | F | R | 2 | CI512/ 500 |
genetic, measles |
57 | 82 | Resound Canta |
22,18, 12 |
N/A |
| CI25 | 53 | F | R | 2 | CI512/ 900 |
genetic, progressive |
10-15 | 75 | Siemens Pure CIC |
22,21, 19,18, 17 |
N/A |
| CI28 | 70 | M | R | 8 | N24/ 900 |
ototoxicity | 45 | 90 | Phonak Naida |
22-17 | 18,17 |
| CI30 | 64 | M | R | 3 | CI512/ 900 |
noise, family hx |
30 | 58 | none | 22-18 | 17 |
| CI32 | 82 | M | R | 7 | Freedom/ 500 |
noise | 20 | 48 | Phonak Naida |
22-17 | N/A |
| CI35 | 83 | F | R | 2 | Sonata/ 1657 |
Family hx | 12-15 | 50 | Phonak Exelia |
1-5 | 4 |
| CI40 | 73 | M | R | 6 | Freedom/ 1200 |
noise | 15-20 | 55 | Oticon Agil |
22,20, 18 |
N/A |
| CI49 | 75 | F | L | 2 | CI512/ 900 |
progressive, unknown |
10 | 70 | Widex Mind |
22-19 | N/A |
| CI50 | 48 | F | R | 2.3 | N24/ 1200 |
Progressive | 20 | 78 | none | 22,20, 18 |
N/A |
| CI54 | 78 | M | L | 4 | Freedom/ 900 |
Noise | 40 | 83 | Unitron BTE |
22.18. 12 |
N/A |
| CI56 | 61 | M | R | 12 | N24/ 1200 |
Usher Type 3 |
25 | 78 | Magnatone Monet |
22,18, 12 |
12 |
| CI63 | 69 | F | L | 2 | CI512/ 900 |
noise | 20 | 57 | Phonak Naida |
22,21, 18,17, 13,12 |
N/A |
| CI70 | 78 | M | L | 2 | CI512/ 900 |
noise, progressive |
30+ | 42 | Phonak Naida |
22,18, 12 |
N/A |
| CI71 | 71 | F | R | 5 | Freedom/ 900 | family hx, progressive | 50 | 73 | Phonak Supero | 22,18,12 | N/A |
| CI73 | 42 | F | L | 2.5 | CI512/ 900 |
progressive | 19 | 80 | Phonak Naida |
22,18,12 | N/A |
General procedures
For PAOT subjects, electric-to-acoustic pitch matching and electrode discrimination tests were conducted longitudinally at various time points during implant use. Pitch matches were typically conducted at 0, 1, 3, 6, and 12 months post-activation, but some subjects were tested up to as late as 26 months after activation. When time allowed at the implant activation day, electrode discrimination was also tested on the implant activation day, and again after at least 1 year of CI use. Speech perception scores over time were collected retrospectively for this subject group from clinical records.
For ECI subjects, electric-to-acoustic pitch matching and electrode discrimination tests were conducted in 1-2 visits at one point in time after at least 2 years of CI use.
Pitch Perception
Electric-to-acoustic pitch matches were conducted using a computer to control both electric and acoustic stimulus presentations. Electric stimuli were delivered to the CI using cochlear implant research interfaces and software. For Cochlear devices, stimulation was provided and controlled by a Freedom processor, programming pod, and NIC2 research software. For MED-EL devices, stimulation was provided and controlled by a National Instruments PCI-6533 digital input/output (IO) card, Research Interface Box 2 (RIB2), and MED-EL research software. Stimulation of each electrode consisted of a pulse train of 25 μsec biphasic pulses presented at 1200 pps with a total duration of 500 ms. The pulse rate of 1200 pps per electrode was selected to reduce the effects of any temporal cues on pitch. The electrode ground was set to monopolar stimulation with both the ball and plate electrodes active. The level of the electric stimulation for each electrode was set to a “medium loud and comfortable” current level corresponding to 6 or “most comfortable” on a visual loudness scale from 0 (no sound) to 10 (too loud).
Acoustic pure tones were delivered using an ESI Juli sound card, TDT PA5 digital attenuator and HB7 headphone buffer, and Sennheiser HD-25 headphones. Acoustic tones were presented to the contralateral ear and set to “medium loud and comfortable” levels again using the same loudness scale as for electric levels. Loudness was balanced across all tone frequencies. Tone frequencies that could not be presented loud enough to be considered a “medium loud and comfortable” level, due to the range of residual hearing in the contralateral ear, were excluded. The lowest acoustic frequency that was presented was 125 Hz, and the highest acoustic frequency, depending on the residual hearing, ranged from 1000 to 4000 Hz, sampled in 1/4 octave steps. Then, each CI electrode was loudness balanced with the acoustic tones to reduce loudness effects on electric-to-acoustic pitch comparisons. Typically, minimal adjustments were needed during loudness balancing after the initial setting of acoustic and electric levels to 6 on the visual loudness scale.
Generally, a two-interval forced-choice constant-stimulus procedure was used to obtain pitch matches. One interval contained the reference stimulus, an electric pulse train delivered to a particular electrode in the implanted ear, and the other interval contained a comparison acoustic tone delivered to the contralateral, non-implanted ear. The electric and acoustic stimuli were each 500 ms in duration and separated by a 500 ms inter-stimulus interval, with interval order randomized. The reference electrode was held constant and the comparison tone frequency varied in 1/4 octave steps within the residual hearing range, and presented in pseudorandom sequence across trials to reduce context effects (Reiss et al., 2007; 2012). At least 6 trials were presented for each comparison frequency. In each trial, the subject was asked to indicate which interval had the higher pitch.
The exact same pseudorandom sequence was used in each run and session. Due to time constraints, pitch matches were conducted for a subset of electrodes within the residual hearing frequency range of the non-implanted ear. The electrodes tested for each subject are listed in Tables I and II.
The averaged pitch matched responses were used to construct psychometric functions for each cochlear implant electrode that was tested. Pitch matches were computed as the 50% point interpolated from a 3-point smoothed psychometric function (for examples, see Figure 2 of Reiss et al., 2012). The range of pitch-matched frequencies was similarly computed as those falling between the 25% and 75% points on the psychometric function, and comparison with subjective reports indicate a correspondence of this range with diffuseness of pitch, e.g. narrow ranges indicate tonal percepts whereas broad ranges indicate noise-like or buzzy percepts.
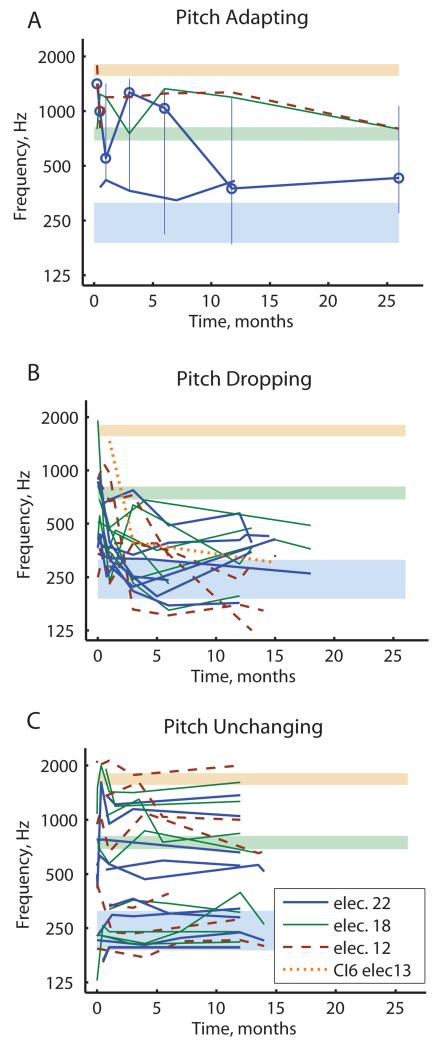
Figure 2.
Pooled population results for the PAOT subject groups tracked over time, with plots separated by pitch adaptation category. Different colors and line styles indicate different electrodes; only data for electrodes 22, 18 and 12 are shown except where indicated (see legend). Shaded regions indicate frequency-to-electrode allocations for the corresponding colored electrodes. A) Pitch-adapting subjects generally showed pitch changes in the direction of aligning with the frequency allocations (CI4 for electrode 22 and CI15 for electrodes 22 and 18), but not so much for electrode 12 for CI15. Vertical lines indicate the pitch match range for electrode 22 of CI15 (highlighted by circles) eventually overlaps with the frequency-to-electrode allocation for that electrode. B) Pitch-dropping subjects showed pitch drops for all electrodes independent of frequency allocations (CI6, CI9, CI13,CI24, CI29, CI31, CI37, CI42); note pitch drops for electrodes 18, 12 and 13 toward the electrode 22 allocation (thin green, dashed brown, and dotted orange lines, respectively). C) Pitch-unchanging subjects showed generally stable pitch for all electrodes without convergence toward frequency allocations (CI14, CI17, CI33, CI34, CI38, CI44, CI47, CI53, CI55); note that the apical electrode 22 (thick blue lines) remained high in pitch for several of these subjects.
For a pitch match result to be considered valid, the psychometric function had to reach 100%, i.e., at least one acoustic tone had to be judged as higher in pitch than the electrode-evoked pitch 100% of the time. In some cases, the electric stimulation produced a pitch sensation too high-pitched for the subject to consistently rank any acoustic tones as always higher in pitch, due to the upper limit of the low-frequency hearing range available for comparison in the non-implanted ear. If this occurred, the psychometric function never reached 100%, and the pitch matches were recorded as “out of range”; similarly, in a few cases, the psychometric function never started from 0 on the low frequency side. These out-of-range electrodes from the high- and low-frequency sides are both noted in Tables I and II.
Electrode discrimination
Six PAOT (CI14, CI42, CI44, CI47, CI53, and CI55) and eight ECI subjects (CI19, CI21, CI25, CI28, CI30, CI32, CI35, and CI49) were also tested on electrode discrimination with one or more electrodes. The C levels and T levels were verified for the individual electrodes tested. A 4-interval, forced-choice procedure was used to measure discrimination for each adjacent electrode pair. The stimuli were four 500 ms electric pulse trains of 500 ms duration with a 500 ms inter-stimulus interval. The reference electrode was selected to be the apical electrode of the pair. The probe electrode was stimulated in a randomly selected interval while the reference electrode was stimulated in the three remaining intervals. Stimulus intensity was randomly roved within the top 40% of the electrical dynamic range, or the range between C and T levels, for each electrode and interval to reduce loudness cues for discrimination (Henry et al., 2000).
The subject was asked to respond by indicating on a touch screen which interval had the probe electrode. For each run, 30 trials were presented and discrimination score was calculated as percent correct identification of the probe interval.
Speech perception tests
Speech perception scores collected in the clinic were analyzed retrospectively for PAOT subjects to determine whether there was a relationship of speech perception changes to pitch perception changes over time. Tests administered clinically included the Hearing In Noise Test (HINT; Nilsson, Soli, and Sullivan, 1994) and the AZBio sentence in noise test (Auditory Potential, LLC; Spahr et al., 2012). Subjects were either tested with 2 lists of 10 recorded HINT sentences in quiet or 1 list of 20 recorded AZBio sentences in quiet or in a background of four talkers at a +10 dB signal-to-noise ratio (SNR). All stimuli were presented in the sound field at 70 dB SPL, with both speech and noise presented from a front-facing loudspeaker at a distance of 1 m.
Results
Pitch changes over time after implant activation
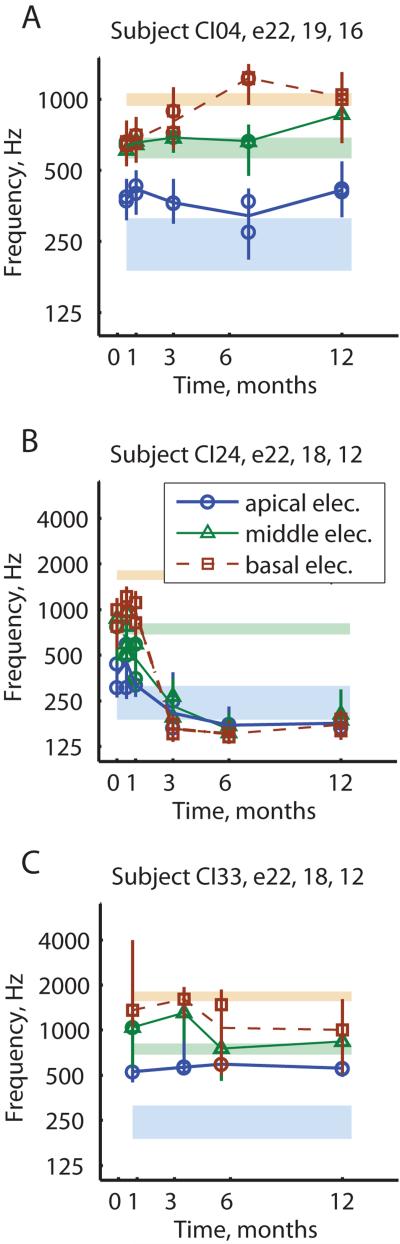
Figure 1 shows three representative examples of how electric-to-acoustic pitch matches changed over time in the PAOT group. Figure 1A shows an example of a subject, CI04, whose electrode pitch percepts for electrodes 22, 19, and 16 (thick blue lines with circles, thin green lines with triangles, and dashed brown lines with squares, respectively) grew farther apart over time to more closely match their frequency-to-electrode allocations (shaded regions with colors corresponding to respective electrodes). In this case, the pitch changes were in the direction of reducing the mismatch between electric pitch sensations and acoustic frequencies allocated to those electrodes and heard simultaneously in the other ear; we call this pattern “pitch adapting”. Another subject, CI15, had two electrodes that were pitch-adapting, but the third electrode was mal-adapting and dropped in pitch slightly (Fig. 2A). The pitch match range (vertical lines) is also shown for electrode 22 for that subject (circles) to indicate that the pitch match range broadened over time to overlap and align with the frequency-to-electrode allocation.
Figure 1.
Examples of three different subjects with different pitch adaptation trends over time. Different colors/symbols/line styles indicate pitch match results for different electrodes: blue circles for the most apical electrode 22, green triangles for the middle electrode, and brown squares for the most basal electrode tested. Vertical lines indicate the pitch match range for each measurement. Light shaded regions indicate the frequency-to-electrode allocations for the corresponding colored respective electrodes listed at the top of each subplot. A) Subject with pitch-adapting pattern. B) Subject with pitch-dropping pattern. C) Subject with pitch-unchanging pattern.
Figure 1B shows an example of another subject, CI24, whose electrode pitch percepts for electrodes 22, 18, and 12 (blue circles, green triangles, and brown squares, respectively) all dropped over time to approach the same low pitch around the lower boundary of the frequency-to-electrode allocation for electrode 22 (bottom blue shaded region). These pitch changes exacerbated the pitch mismatch between electrodes and acoustic hearing; we call this pattern “pitch dropping”. Some subjects showed pitch drops for all electrodes. Some other subjects only showed pitch drops for the most basal electrode, with the apical electrode adapting and the middle electrode unchanged in pitch. In fact, two subjects had pitch drops in the basal electrode such that there was a pitch reversal between the middle and basal electrodes (CI29, CI37). Another subject showed an increase in the apical electrode pitch to align with the frequency-to-electrode allocation even as the middle and basal electrodes dropped in pitch (CI13).
Figure 1C shows an example of a third subject, CI33, who showed minimal changes in pitch over time for electrodes 22, 18, and 12 which remained clustered around the frequency-to-electrode allocation for electrode 18; we call this pattern “pitch unchanging”. These three examples represent the three main types of pitch adaptation patterns seen in bimodal CI users.
Pitch adaptation patterns were classified quantitatively based on individual electrodes and for the group of electrodes for each subject. Each electrode’s pitch adaptation pattern was classified based on 1) the magnitude of pitch change and 2) the change in overlap in pitch match range with frequency-to-electrode allocation. If the magnitude of the pitch change was less than 0.3 octaves, the electrode was classified as “unchanging”. If the magnitude of the pitch change was greater than or equal to 0.3 octaves, then a) increased overlap in pitch match and frequency-to-electrode ranges was classified as “adapting; b) decreased overlap was classified as “mal-adapting” or more specifically for negative pitch changes as “dropping” for all but one electrode out of those tested (CI34, electrode 22).
The pitch adaptation category for each subject was based on a more global assessment of all three electrodes, which led to three main subject categories: 1) If at least two electrodes were adapting or already aligned in pitch, the subject was classified as “pitch-adapting”; 2) If both the middle and basal electrodes were dropping in pitch in a direction away from the frequency-to-electrode allocation, or if one electrode (typically the basal electrode) was dropping in pitch away from the allocation by more than 1 octave, then the subject was classified as “pitch-dropping”; 3) The remaining subjects were classified as “pitch-unchanging”, and typically had at least two electrodes unchanging and unaligned in pitch.
Population results for electrodes 22, 18, and 12 from the PAOT group are shown in Figure 2, divided into subgroups based on subject pitch adaptation pattern category. Figures 2A-C show pooled data from subjects classified as “pitch-adapting”, “pitch-dropping”, and “pitch-unchanging”, respectively. Multiple subjects are shown in each subplot. Each electrode is indicated by a different color and line style: thick blue lines for electrode 22, thin green lines for electrode 18, and dashed brown lines for electrode 12. Note that the pitch-adapting subject shown in Fig. 1A is only represented by electrode 22 in Fig. 2A, because data for electrodes 18 and 12 were not collected for this subject. Data from electrode 13 is shown for one subject in the pitch-dropping group as orange in Fig. 2B. As can be seen, the majority of bimodal CI users had either “pitch-dropping” (Fig. 2B; n=8) or “pitch-unchanging” (Fig. 2C; n=9) adaptation patterns; unlike Hybrid CI users, very few had “pitch-adapting” patterns (Fig. 2A; n=2).
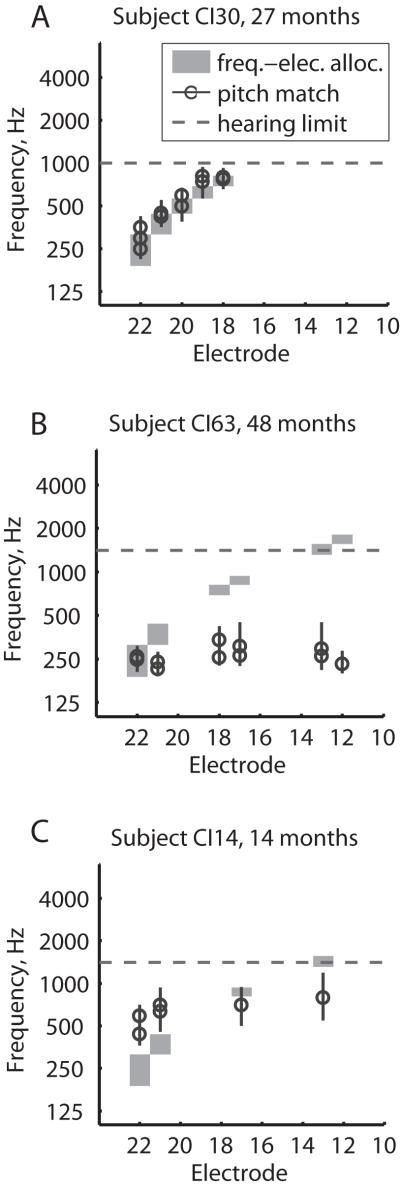
Pitch adaptation patterns in experienced CI users
Figure 3 shows examples of electric-to-acoustic pitch matches at a single time point for three subjects in the ECI group. Note that these data are plotted versus electrode instead of versus time as in Figures 1-2. Figure 3A shows an example of a subject, CI30, whose pitch percepts (black circles) at 27 months post-activation generally matched the frequency allocations for those electrodes (gray shaded regions). This “matched-pitch” pattern is consistent with and parallels the “pitch-adapting” PAOT group.
Figure 3.
Examples of three ECI subjects with different pitch patterns across electrodes. All subjects here had at least 2 years of experience with the CI. Circles indicate pitch matches, and shaded regions indicate frequency-to-electrode allocations for the corresponding electrodes. Vertical lines indicate the pitch match range for each measurement. Horizontal dashed lines indicate the upper frequency limit of loudness-balanced residual hearing. Symbols indicate the pitch match results plotted versus electrode number. A) Subject with a matched-pitch pattern, i.e. pitch matches that were aligned with the frequency-to-electrode allocations for each electrode. B) Subject with a low-pitch pattern, i.e. pitch matches that were all low and below the corresponding frequency-to-electrode allocations. C) Subject with a nonmatched-pitch pattern that was compressed relative to the frequency-to-electrode allocations.
Figure 3B shows an example of another subject, CI63, whose pitch percepts were low for multiple electrodes at 48 months post-activation, and generally aligned with the lowest frequency-to-electrode allocation for electrode 22. This “low-pitch” pattern parallels the “pitch-dropping” PAOT groups.
Finally, Figure 3C shows an example of a subject, CI14, whose pitch percepts were compressed relative to the frequency-to-electrode allocation; this “nonmatched-pitch” pattern parallels the “pitch-unchanging” PAOT group, and may reflect the original tonotopic mapping for those electrodes.
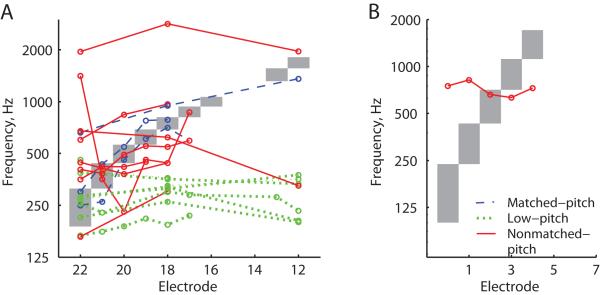
Population results for all subjects from the ECI group are shown in Figure 4, with different colors and line styles indicating pattern classification. As can be seen in Fig. 4A, blue dashed lines indicate “matched-pitch” or pitch that aligned with the frequency allocations, green dotted lines indicate “low-pitch” or pitch that was mostly aligned with the frequency allocation for electrode 22, and red solid lines indicate “nonmatched-pitch” that was neither low nor aligned, and also tended to be compressed but was not always so. Figure 4A shows the subjects with standard frequency-to-electrode allocations from Cochlear (Frequency Table 22: 188-7938 Hz) Figure 4B shows one other subject with a MED-EL device who had different frequency-to-electrode allocations plotted as gray areas. As with the PAOT group data in Fig.2, the majority of subjects had either “low-pitch” (n=7) or “nonmatched-pitch” (n=9) patterns, whereas only three subjects had “matched-pitch” (n=3).
Figure 4.
Population summary plot of pitch versus electrode patterns for all ECI subjects. Different colors and line styles indicate the pitch adaptation category, with blue dashed lines indicating matched-pitch subjects (CI19, CI30, CI70), green dotted lines indicating low-pitch subjects (CI18, CI20, CI21, CI25, CI63, CI71), and red solid lines indicating nonmatched-pitch subjects (CI28, CI32, CI40, CI49, CI50, CI54, CI56, CI73). Grey shaded regions indicate the frequency-to-electrode allocations. A) All subjects with standard frequency-to-electrode allocations from Cochlear. Note that one subject in the matched-pitch category had a pitch match with a pitch center higher than the frequency-to-electrode allocation for electrode 22, but also had a wide pitch match range for this electrode that still overlapped with the frequency allocation (not shown). B) One subject with different frequency-to-electrode allocations from MED-EL (CI35) in the nonmatched-pitch group.
Correlations of pitch adaptation pattern with residual hearing and other factors
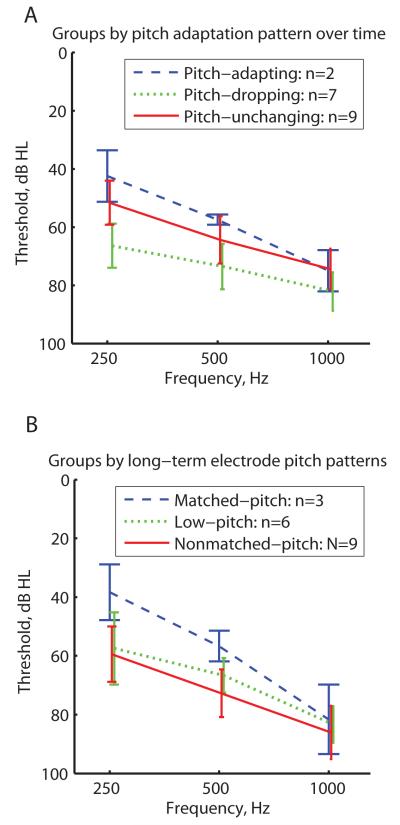
Figure 5 shows the averaged audiograms for each PAOT and ECI group. Unaided audiometric thresholds were obtained at the ~12 month time point for the PAOT group or at the time of testing for the ECI group. PAOT groups are shown in Fig. 5A and ECI groups are shown in Fig. 5B, with each group coded by color and symbol. One interesting observation is that both the pitch-adapting PAOT and matched-pitch ECI groups (blue dashed lines in Figs. 5A and 5B, respectively) had overall better thresholds than the other groups (green dotted and solid red lines), though none of these differences were statistically significant at any frequency (2-sided rank-sum test, p>0.05). When the PAOT and ECI groups are combined, however, the difference between pitch-adapting/matched pitch and the pitch-dropping/low-pitch groups becomes barely significant at 250 Hz (P=0.043, 2-sided rank-sum test) and 500 Hz (P=0.046).
Figure 5.
Average unaided audiometric thresholds for PAOT group (A) and the ECI group (B). Audiometric thresholds were obtained at the ~12-month time point for the PAOT subjects or at the time of testing for the ECI subjects. Different colors and line styles indicate different pitch adaptation categories (see legend). A non-significant trend of better thresholds was seen for the pitch-adapting PAOT subgroup (blue dashed lines in A) and matched-pitch ECI subgroup (blue dashed lines in B) compared to the other subgroups.
Due to variation across electrodes within subjects in the PAOT subjects, especially in the pitch-dropping group, this analysis was repeated to assess individual electrode differences in pitch adaptation pattern versus audiometric threshold at the center of each electrode’s frequency allocation. A significant difference was found between pitch-adapting electrodes versus pitch-dropping electrodes (mean thresholds of 67.2 dB versus 84.2 dB; P=0.003), and between pitch-unchanging versus pitch-dropping electrodes (mean threshold of 68.2 dB; P=0.016), with pitch-dropping electrodes associated with higher frequency-to-electrode allocation thresholds. These electrodes also tended to be the more basal electrodes, consistent with this association and the typical pattern of sloping high-frequency hearing loss.
No significant differences in chronological age or duration of severe-profound deafness were seen between the different adaptation groups (2-sided rank-sum test, p>0.05; not shown).
Electrode discrimination
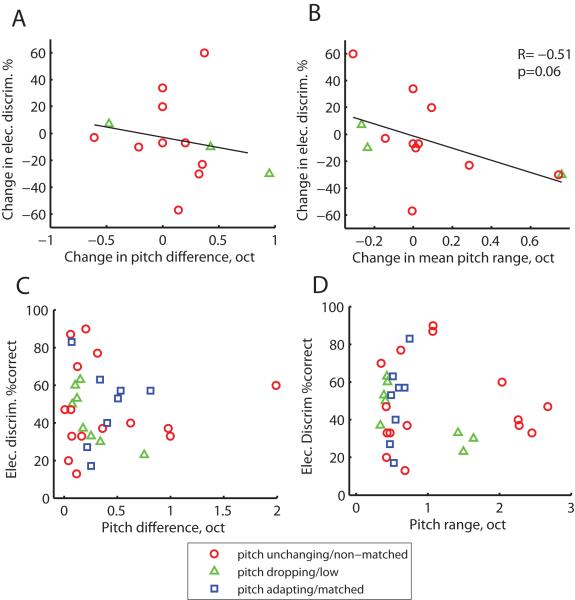
Figure 6 shows the population summary of electrode discrimination plotted as a function of electrode pitch differences and ranges. PAOT subjects are shown in Fig. 6A-B and ECI subjects are shown in Fig. 6C-D. Note that electrode discrimination data for PAOT subjects were only obtained from subjects in the pitch-unchanging (red circles) and pitch-dropping (green triangles) groups. No association was observed between changes in interelectrode pitch difference and changes in electrode discrimination performance over time in the PAOT group (Fig. 6A), between changes in interelectrode pitch difference and changes in electrode discrimination in the ECI group (Fig. 6C), or between changes in mean electrode geometric pitch range and changes in electrode discrimination in the ECI group (Fig. 6D). No significant differences in electrode discrimination was seen between any of the groups (rank-sum test).
Figure 6.
Population summary of electrode discrimination scores plotted as a function of electrode pitch differences and ranges, in units of octaves, for PAOT subjects (A-B) and ECI subjects (C-D). A. No association was observed between changes in interelectrode pitch difference and electrode discrimination performance over time in the PAOT group. Note that electrode discrimination data were only obtained from subjects in the pitch-unchanging (red circles) and pitch-dropping (green triangles) groups. B. A weak correlation (p=0.06, 2-tailed Pearson correlation test) was observed between changes in mean geometric pitch range (averaged for electrode pair) and electrode discrimination scores over time in the PAOT group. C. No association was observed between electrode pair pitch difference and electrode discrimination for ECI subjects. D. No association was observed between mean electrode pitch range and electrode discrimination for ECI subjects.
However, an interesting pattern was observed between changes in mean geometric pitch range and changes in electrode discrimination scores over time. That is, greater pitch range or increase of diffuseness seemed to be associated with poorer electrode discrimination and narrower pitch range or decrease of diffuseness seemed to be associated with improved electrode discrimination. This negative correlation, however, was not quite significant (R= −0.51, p=0.06, two-tailed Pearson correlation test).
Speech perception over time
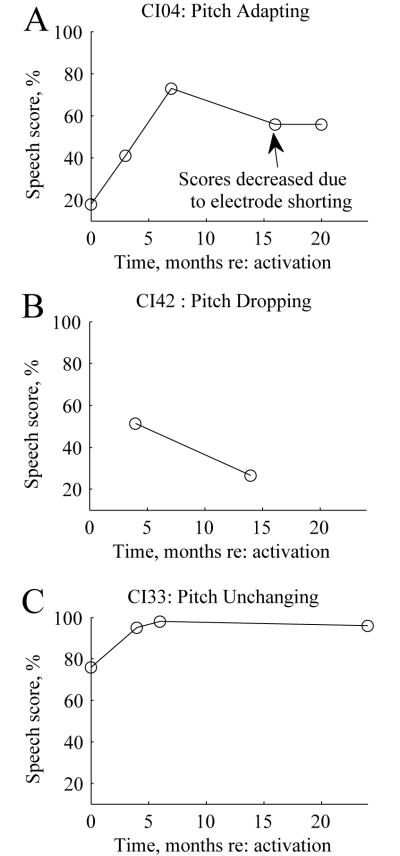
Examples of speech perception scores over time are shown in Figure 7 for the subjects with pitch changes over time shown in Fig. 1. All scores shown in these examples were obtained using AZBio sentence lists for the CI-only in quiet. Note that the subjects with the pitch-adapting and the pitch-unchanging patterns exhibited monotonically increasing speech scores over time, while the subject with the pitch-dropping pattern exhibited a drop in speech perception over time. This suggests a potential relationship of pitch drops to speech perception drops over time.
Figure 7.
Examples of speech perception scores over time for PAOT subjects with different representative pitch adaptation patterns. A. AZBio sentence scores over time for a pitch-adapting subject (shown in Fig. 1A). Note that the drop in speech scores at 16 months was explained by shorting of several electrodes. B. HINT sentence scores for a dropping-pitch subject (similar to the subject shown in Fig. 1B). C. AZBio sentence scores for an unchanging-pitch subject (shown in Fig. 1C).
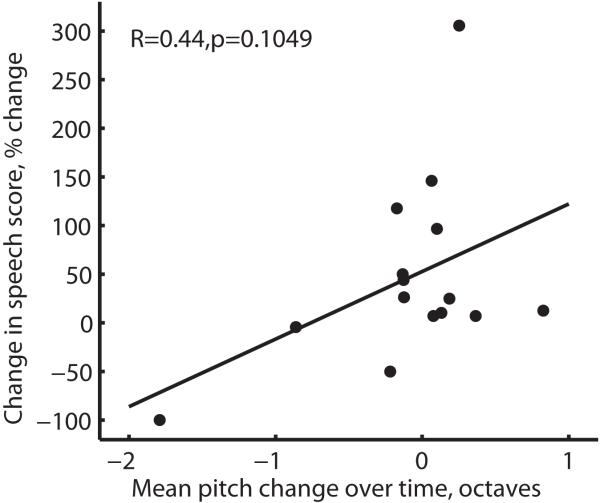
To quantify this potential relationship, changes in speech scores over time versus mean pitch changes over time were plotted for the population of subjects in Figure 8. Pitch changes between initial activation and the latest measurement were averaged over 3 electrodes tested in each subject. Speech scores were obtained using either AZBio or HINT sentences in quiet at approximately the same time points as the pitch measurements, with the same sentence test used longitudinally within each subject, and are plotted as a percent change relative to the original score in Figure 8. As can be seen, a modest but nonsignificant relationship was observed (R=0.44, p=0.105, two-tailed Pearson correlation test) with the linear fit shown.
Figure 8.
Population summary of changes in speech scores over time versus mean pitch changes over time in PAOT subjects. Pitch changes between initial activation and the latest measurement were averaged over 3 electrodes tested in each subject (electrodes 22; 19, 18 or 16; 13 or 12). Speech scores were obtained using either AZBio or HINT sentences in quiet at approximately the same time points as the pitch measurements. A modest but nonsignifcant relationship was seen between relative speech score changes and overall electrode pitch perception changes (p=0.105, Pearson two-tailed correlation test).
The speech scores for one pitch-dropping subject, CI24, were omitted from this analysis since that subject exhibited fluctuating speech scores along with temporary dizziness and increased electrode impedances after implantation. This subject’s AZBio sentence scores dropped from 96% to 82% between 1-3 months, dropped further to 62% at 5 months (around the time the pitch drops stabilized for all electrodes as shown in Fig. 1B), and recovered to 87% at 7 months and 96% at 12 months. Over this time frame, impedance did not change between 1-3 months, but increased from 8.5 to 13 kOhms and 8.9 to 10.3 kOhms for electrodes 22 and 18 respectively between 3-5 months around the same time as the onset of vestibular symptoms. These symptoms were attributed to benign paroxysmal positional vertigo (BPPV). At 7 months, the dizziness spontaneously recovered and impedances came back down to 7.7-7.8 kOhms.
Threshold and comfortable stimulation levels tended to increase over time for all subjects, and showed no consistent relationship to pitch or speech perception changes.
Discussion
Bimodal CI users with traditional long-electrode CIs exhibited pitch plasticity, but different trends were observed than in Hybrid CI users. Subjects in the PAOT group tracked over time after CI activation showed one of three different pitch adaptation patterns: pitch-adapting, pitch-dropping, or pitch-unchanging. Very few bimodal CI users adapted pitch in directions that reduced the acoustic-electric mismatch (pitch-adapting); the majority exhibited drops in pitch for the more basal electrodes (pitch-dropping) or showed no pitch changes over time (pitch-unchanging). This contrasts with previous studies in Hybrid CI users, most of whom adapted pitch to reduce the acoustic-electric mismatch (Reiss et al., 2007; Reiss et al., 2013).
The audiometric differences between groups with different adaptation patterns in this study suggests a possibility that individuals with more residual hearing, including those implanted with the Hybrid CI, are more likely to be pitch-adapting, whereas those with less hearing are more likely to be pitch-dropping or exhibit no pitch changes over time. However, because of the small number of bimodal CI users who exhibited the pitch-adapting pattern compared to other patterns, these differences were only barely significant at 250 and 500 Hz when the PAOT and ECI group were combined.
It is not clear what mechanism is driving the pitch-dropping adaptation pattern, but abnormal spread of excitation with hearing aid amplification may be one factor; broad excitation may cause amplitude modulation of each electrode to be temporally correlated with lower acoustic frequencies than those allocated to the electrode. Another possibility raised by the association of impedance changes with drops in both speech and pitch perception in one subject (CI24) is that pitch drops may be caused by the growth of fibrotic tissue or bone growth. Local growth of tissue around the electrode could conceivably lead to local impedance increases, and in turn force the electrical current path to shift toward neurons apical to the insertion; such effects may be more likely in long-electrode CI subjects due to the higher likelihood of implant trauma and tissue response with a deeper insertion.
Alternatively, the dizziness of this subject may have indicated endolymphatic hydrops, suggested to be associated with increased impedance and fluctuations in speech perception scores (Lustig et al., 2003). However, four other subjects also exhibited dizziness, but of these half exhibited pitch-dropping patterns (CI13, CI42) and the other half had unchanging pitch (CI34, CI44); only one of these subjects also exhibited drops in speech perception (CI42). Thus, there is no consistent association of reported dizziness with changes in pitch perception or speech perception.
Of the subjects with unchanging pitch patterns, it should be noted that these include all three subjects in the PAOT group (CI14, CI17, and CI47) who did not use a hearing aid in the contralateral ear. These three subjects may not have had sufficient acoustic hearing in their daily life to perceive an acoustic-electric pitch mismatch to drive pitch plasticity. In the ECI group, one subject (CI30) also did not wear a hearing aid in the contralateral ear at the time of testing, but had perfectly aligned pitch matches; however, this subject may have started out with already-aligned pitch matches, or may have initially worn a hearing aid long enough for adaptation to occur.
The remaining subjects with unchanging pitch patterns did wear a hearing aid, so their lack of pitch adaptation is not explained by lack of acoustic amplification. Instead, these subjects may be adapting pitch perception in a different way. Recent data has shown that experienced bimodal CI users exhibit abnormally broad binaural fusion of CI electrodes and contralateral acoustic tones differing in pitch by as much as 3-4 octaves (Reiss et al., 2014). While Hybrid CI users experience acoustic-electric pitch mismatches within as well as across ears (between residual hearing in implanted ear and contralateral ear and electric hearing in the implanted ear), bimodal CI users experience mismatches across ears only (between residual hearing in the contralateral non-implanted ear and electric hearing in the implanted ear). When there is a mismatch only across ears, it may be simpler for the brain to adapt binaural integration of pitch, i.e. fusion, than to adapt tonotopic mapping. In fact, when pitch and binaural fusion was measured over time for one of the subjects in that study, that subject exhibited no changes in pitch (despite an acoustic-electric mismatch), but fusion increased to reduce the perception of the mismatch (Reiss et al., in press). Thus, the subjects who exhibited no changes in pitch in this study may have similarly adapted their binaural pitch fusion instead.
Pitch perception patterns were also measured for multiple electrodes in the ECI subjects who had at least 24 months of CI experience at the time of testing. These subjects were very similar to the PAOT group in that they also exhibited three different types of pitch versus electrode patterns: matched-pitch, low-pitch, and nonmatched-pitch. These parallel the respective pitch-adapting, pitch-dropping, and pitch-unchanging PAOT groups, though it should be noted that the low-pitch group may reflect a combination of pitch-dropping and pitch-unchanging trends, since some subjects may have had little high-frequency nerve survival and thus may have had low pitch patterns to start with.
As with the PAOT group, the majority of subjects exhibited either low-pitch or nonmatched-pitch patterns. The finding of low-pitch patterns is consistent with the previous literature; several other studies conducted in experienced CI users have also found electrode pitch to be mismatched to and lower than the frequency-to-electrode allocations (e.g. Blamey et al., 1996; Boex et al., 2006; Dorman et al., 2007; McDermott et al., 2009), while some studies conducted immediately after CI activation have found electrode pitch to be closer to predictions (Eddington et al., 1978; McDermott et al., 2009; Carlyon et al., 2010). However, this is the first study to demonstrate, in the PAOT group, that these low-pitch patterns could also be due to pitch plasticity rather than limited high-frequency nerve survival or stimulation of spiral ganglion cells instead of neurites near the hair cells.
The dropping-pitch pattern in particular may be clinically significant because of the resulting reductions in pitch differences between electrodes over time. For example, if inter-electrode pitch differences are important for electrode discrimination, electrode discrimination performance could become worse over the duration of implant use in such subjects. In this study, we did not find electrode discrimination changes to be significantly associated with changes in pitch differences between electrodes. A weak relationship was detected between electrode discrimination and electrode pitch bandwidth or “sharpness”, but this was not significant. These results suggest that electrode discrimination does not depend solely on perceived pitch differences between electrodes, but may also depend on additional attributes such as timbre; this is consistent with a previous study that reported electrode discrimination to be better modeled as a multidimensional analysis of multiple attributes rather than based on a single attribute (Collins et al., 1997). Alternatively, very small pitch differences may be sufficient for successful electrode discrimination, especially when pitch bandwidth is narrow.
We did find a modest but nonsignificant correlation between drops in overall pitch perception for all electrodes and reductions (or lack of improvement) in speech perception scores over time. Unlike electrode discrimination, perception of speech or other complex sounds involves stimulation of multiple electrodes, and timbre cues may be less useful for discrimination of complex sounds encoded with multiple electrodes. Perception of complex sounds may thus depend more on the ability to easily distinguish pitch between electrodes. Further study is needed to clarify the interrelationship between pitch, electrode discrimination, and speech perception.
Acknowledgments
We thank the OHSU Cochlear Implant Team for assistance with subject recruitment, Aaron Parkinson of Cochlear and Bob Wolford of MED-EL for providing implant equipment and programming tools, and Colin Irwin of Cochlear and Joshua Stohl of MED-EL for cochlear implant research software support. This research was supported by grants P30DC010755 and P30DC005983 from the National Institutes of Deafness and Communication Disorders, National Institutes of Health.
Footnotes
Conflicts of Interest and Sources of Funding: The fifth author (C.E.L.) is on the Med-El audiology advisory board. The sixth author (F.M.W.) is on the Med-El surgical advisory board. The seventh author (S.O.M.) is on the advisory board of Med-EL, Advanced Bionics, and Cochlear Limited. This research was supported by grant P30DC010755 from the National Institutes of Deafness and Communication Disorders, National Institutes of Health.
References
- Blamey PJ, Dooley GJ, Parisi ES, Clark GM. Pitch comparisons of acoustically and electrically evoked auditory sensations. Hear. Res. 1996;99:139–50. doi: 10.1016/s0378-5955(96)00095-0. [DOI] [PubMed] [Google Scholar]
- Boex C, Baud L, Cosendai G, Sigrist A, Kos MI, Pelizzone M. Acoustic to electric pitch comparisons in cochlear implant subjects with residual hearing. J Assoc Res Otolaryngol. 2006;7(2):110–124. doi: 10.1007/s10162-005-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyon RP, Macherey O, Frijans JH, Axon PR, Kalkmann RK, Boyle P, Baguley DM, Briggs J, Deeks JM, Briaire JJ, Barreau X, Dauman R. Pitch comparisons electric stimulation of a cochlear implant and acoustic stimuli presented to a normal-hearing contralateral ear. J. Assoc. Res. Otolaryngol. 2010;11(4):625–40. doi: 10.1007/s10162-010-0222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching TY, van Wanrooy E, Dillong H. Binaural-bimodal fitting or bilateral implantation for managing severe to profound deafness: a review. Trends Amplif. 2007;11(3):161–92. doi: 10.1177/1084713807304357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Zwolan TA, Wakefield GH. Comparison of electrode discrimination, pitch ranking, and pitch scaling data in post-lingually deafened adult cochlear implant subjects. J. Acoust. Soc. Am. 1997;101:440–455. doi: 10.1121/1.417989. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Gifford RH, Spahr AJ, McKarns SA. The benefits of combining acoustic and electric stimulation for the recognition of speech, voice and melodies. Audiol. Neurootol. 2008;13:105–112. doi: 10.1159/000111782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman MF, Spahr T, Gifford R, Loiselle L, McKarns S, Holden T, Skinner M, Finley C. An electric frequency-to-place map for a cochlear implant patient with hearing in the nonimplanted ear. J. Assoc. Res. Otolaryngol. 2007;8(2):234–40. doi: 10.1007/s10162-007-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddington DK, Dobelle WH, Brackmann DE, Mladejovsky MG, Parkin JL. Auditory prostheses research with multiple channel intracochlear stimulation in man. Ann Otol Rhinol Laryngol. 1978;87(6 Pt 2):1–39. [PubMed] [Google Scholar]
- El Fata F, James CJ, Laborde ML, Fraysse B. How much residual hearing is ‘useful’ for music perception with cochlear implants? Audiol. Neurootol. 2009;14(Suppl 1):14–21. doi: 10.1159/000206491. [DOI] [PubMed] [Google Scholar]
- Greenwood D. A cochlear frequency-position function for several species--29 years later. J Acoust Soc Am. 1990;87(6):2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Henry BA, McKay CM, McDermott HJ, Clark GM. The relationship between speech perception and electrode discrimination in cochlear implantees. J. Acoust. Soc., Am. 2000;108(3 pt. 1):1290–80. doi: 10.1121/1.1287711. [DOI] [PubMed] [Google Scholar]
- Kong YY, Stickney GS, Zeng FG. Speech and melody recognition in binaurally combined acoustic and electric hearing. J. Acoust. Soc. Am. 2005;117:1351–61. doi: 10.1121/1.1857526. [DOI] [PubMed] [Google Scholar]
- Lee J, Nadol JB, Eddington DK. Depth of electrode insertion and postoperative performance in humans with cochlear implants: A histopathologic study. Audiol Neurotol. 2010;15:323–331. doi: 10.1159/000289571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Johnson PM, Goday SP. Benefits of bimodal cochlear implants and/or hearing aids in children. Int. J. Audiol. 2006b;45(7):78–91. doi: 10.1080/14992020600782956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig LR, Yeagle J, Niparko JK, Minor LB. Cochlear implantation in patients with bilateral Ménière’s syndrome. Otol. Neurotol. 2003;24(3):397–403. doi: 10.1097/00129492-200305000-00009. [DOI] [PubMed] [Google Scholar]
- McDermott H, Sucher C, Simpson A. Electro-Acoustic Stimulation. Audiol Neurootol. 2009;14(Suppl 1):2–7. doi: 10.1159/000206489. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Soli SD, Sullivan JA. Development of the hearing in noise test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am. 1994;95(2):1085–99. doi: 10.1121/1.408469. [DOI] [PubMed] [Google Scholar]
- Reiss LAJ, Ito RA, Eggleston JL, Wozny DR. Abnormal binaural spectral integration in cochlear implant users. J. Assoc.. Res. Otolaryngol. 2014;15(2):235–48. doi: 10.1007/s10162-013-0434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LAJ, Turner CW, Karsten SA, Gantz BJ. Plasticity in human pitch perception induced by tonotopically mismatched electro-acoustic stimulation. Neuroscience. 2014;256:43–52. doi: 10.1016/j.neuroscience.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LA, Lowder ML, Karsten SA, Turner CW, Gantz BJ. Effects of extreme tonotopic mismatches between bilateral cochlear implants on electric pitch perception: A case study. Ear Hear. 2011;32(4):536–40. doi: 10.1097/AUD.0b013e31820c81b0. PMID: 21307775, or PMC3120897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LA, Perreau AE, Turner CW. Effects of lower frequency-to-electrode allocations on speech and pitch perception with the Hybrid short-electrode cochlear implant. Audiol. Neurotol. 2012;17(6):357–72. doi: 10.1159/000341165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LAJ, Turner CW, Erenberg SR, Gantz BJ. Changes in pitch with a cochlear implant over time. J. Assoc. Res. Otolaryngol. 2007;8(2):241–57. doi: 10.1007/s10162-007-0077-8. PMID: 17347777 or PMC2538353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahr AJ, Dorman MF, Litvak LM, van Wie S, Gifford RH, Loizou PC, Loiselle LM, Oakes T, Cook S. Development and validation of the AzBio sentence lists. Ear. Hear. 2012;33(1):112–117. doi: 10.1097/AUD.0b013e31822c2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throckmorton CS, Collins LM. Investigation of the effects of temporal and spatial interactions on speech-recognition skills in cochlear implant subjects. J. Acoust. Soc., Am. 1999;105:861–873. doi: 10.1121/1.426275. [DOI] [PubMed] [Google Scholar]
- Zwolan TA, Collins LM, Wakefield GH. Electrode discrimination and speech recognition in postlingually deafened adult cochlear implant subjects. J. Acoust. Soc., Am. 1997;102:3673–3685. doi: 10.1121/1.420401. [DOI] [PubMed] [Google Scholar]