Abstract
Introduction
Use of post-operative radiotherapy (PORT) in non-small cell lung cancer (NSCLC) remains controversial. Limited data indicate that PORT may benefit patients with involved N2 nodes. This study evaluates this hypothesis in a large retrospective cohort treated with chemotherapy and contemporary radiation techniques.
Methods
The National Cancer Data Base (NCDB) was queried for patients diagnosed 2004–2006 with resected NSCLC and pathologically involved N2 (pN2) nodes also treated with chemotherapy. Multivariable Cox proportional hazards model was used to assess factors associated with overall survival (OS). Inverse probability of treatment weighting (IPTW) using the propensity score was used to reduce selection bias. OS was compared between patients treated with vs. without PORT using the adjusted Kaplan-Meier estimator and weighted log-rank test based on IPTW.
Results
2115 patients were eligible for analysis. 918 (43.4%) received PORT, 1197 (56.6%) did not. PORT was associated with better OS (median survival time (MST) 42 months with PORT vs. 38 months without, p=.048). This effect was significant in multivariable and IPTW Cox models (HR 0.87, 95% CI 0.78–0.98, p=.026, and HR 0.89, 95% CI 0.79–1.00, p=.046, respectively). No interaction was seen between the effects of PORT and number of involved lymph nodes (p=.615).
Conclusions
PORT was associated with better survival for patients with pN2 nodes also treated with chemotherapy. No interaction was seen between benefit of PORT and number of involved nodes. These findings reinforce the benefit of PORT for N2 disease in modern practice using the largest, most recent cohort of chemotherapy-treated pN2 patients to date.
Keywords: Non-small cell lung cancer, radiotherapy, PORT, adjuvant therapy
Introduction
The use of PORT for resected NSCLC remains controversial. A large meta-analysis of PORT trials demonstrated a survival detriment associated with PORT1, though subset analysis indicated that this detriment may not apply to those with N2 disease. Despite criticism of the PORT meta-analysis regarding inclusion of older trials which used outdated radiation equipment and techniques, as well as inclusion of unpublished data, the use of PORT has declined significantly since the publication of the PORT meta-analysis2. The detriment in overall survival seen with PORT was felt to largely be due to excessive late radiation toxicity to normal tissues, particularly the heart and lungs3,4.
More recent publications, however, have bolstered the use of PORT, especially in the setting of pN2 disease. A subset analysis of the Adjuvant Navelbine International Trialist Association (ANITA) trial suggested a benefit in overall survival for patients with N2 disease treated with PORT, regardless of the use of adjuvant chemotherapy5. Additionally, an analysis using the Surveillance, Epidemiology, and End Results (SEER) database similarly indicated that PORT was associated with improved survival for patients with N2 disease6. However, SEER analyses carry significant limitations, including lack of detail regarding radiotherapy treatment and absence of chemotherapy information.
The present study sought to determine if PORT for pN2 disease improves overall survival in patients treated with chemotherapy and contemporary radiation techniques. The NCDB NSCLC database was utilized for this analysis, which contains detailed radiation therapy information as well as receipt of chemotherapy data. The present analysis was limited to patients with pN2 disease who received chemotherapy, as the overall survival benefit conferred with the addition of chemotherapy for pN2 patients has been well established following publication of multiple randomized trials 7–10, and is considered standard of care. Additionally, all patients in this analysis were treated on linear accelerators in the three dimensional conformal radiation therapy (3DCRT) era.
Methods
The National Cancer Database (NCDB) is a large, prospectively-acquired database gathered and maintained by the American College of Surgeons (ACoS), the Commission on Cancer (CoC), and the American Cancer Society (ACS). The database draws on information gathered from CoC-accredited cancer centers nationwide and currently captures 70% of all newly-diagnoses malignancies in the United States. The dataset includes detailed information on patient characteristics, disease parameters, treatment information, and outcomes. The treatment information contains data not available in other large national databases, including detailed radiotherapy information regarding treatment site, treatment source, radiation dose (Gy), and treatment technique as well as receipt of chemotherapy information. The database is subdivided into primary sites, and an institution may apply for access to the data regarding a particular site. Emory University has been granted access to the NCDB NSCLC database, which contains 1,547,531 patients diagnosed between 1998–2011.
The primary goal of this study was to determine if PORT for pathologic stage III NSCLC improves overall survival (OS) in a modern cohort of patients treated with chemotherapy and contemporary radiotherapy techniques. A secondary goal of the analysis was to determine if a benefit from PORT may depend on the number of involved N2 nodes. To address these questions, the NCDB NSCLC lung cancer database was queried for patients diagnosed between 2004–2006 with pathologic N2 (pN2) nodal disease treated with primary resection. Currently, survival outcomes are not yet available for patients diagnosed after 2006 within the NCDB, thus the inclusion years at diagnosis for this study end at 2006. Inclusion criteria were as follows: non-small cell histology, pN2, no evidence of metastatic disease, and receipt of chemotherapy, and one lifetime cancer or cases where the reported tumor was the first of multiple diagnoses. Exclusion criteria excluded patients diagnosed at the reporting facility but treated elsewhere; patients treated with cobalt-60 teletherapy, cesium-137, gamma knife, stereotactic radiosurgery, any type of radiation other than beam, patients with radiation doses less than 3500 cGy or greater than 7000 cGy, patients treated with neoadjuvant radiotherapy, intraoperative radiotherapy, or patients with unknown radiotherapy schedules, patients with positive or unknown margin status, patients that received palliative care, and patients with unknown survival information. Patient and disease parameters were examined, including facility type, sex, age, race, insurance status and type, median income in area of residence, education, rurality or urban influence of county, Charlson-Deyo comorbidity score11,12, year of diagnosis, histology, tumor grade, pathologic T stage, treatment with chemotherapy, number of regional lymph nodes (LN) examined, and number of regional LN involved. Although chemotherapy sequence (adjuvant vs. neoadjuvant) is not coded in the NCDB, chemotherapy sequence was approximated by determining for each case the number of days between diagnosis and the definitive surgical procedure versus the number of days between diagnosis and the first administration of chemotherapy. Facility type was determined by the Commission on Cancer. Insurance was categorized as none, private, or government, which included Medicare, Medicaid, and other government insurance. Education was defined as the proportion of adults that did not graduate high school in the patient’s area of residence. OS was defined as the number of months between the most definitive surgical procedure on the primary site and the last contact or date of death.
Statistical Analysis
Statistical analysis was conducted using SAS Version 9.313. Descriptive statistics for each variable were reported. The univariate association of each covariate with receipt of postoperative RT was assessed using the chi-square test for categorical covariates and ANOVA for numerical covariates. The univariate association of each covariate with OS was assessed using Cox proportional hazards models and log-rank tests. A multivariable Cox model was fit including postoperative RT and the covariates. A backward variable selection method was used to select the covariates applying an alpha =.20 removal criteria. Additionally, a model was fit to test for an interaction between postoperative RT and number of positive LN.
To reduce the treatment selection bias, a propensity score weighting method was also implemented14. Propensity scores were calculated in order to model the main effect of treatment. A logistic regression model was used to calculate propensity scores including the covariates that were marginally associated with survival in univariate or multivariable analysis (p-value<0.20): sex, insurance, income, education, urban/rural, Charlson-Deyo comorbidity score, histology, grade, pathologic T stage, regional LN positive, regional LN examined, and age. A second set of propensity scores was created in order to model an interaction between treatment and number of LN positive (<3 vs. ≥3). The four possible combinations of treatment and number of LN positive were used as the outcome in the propensity score model so that covariates would be balanced across all four groups. A nominal logistic regression model was used to calculate propensity scores instead of a binary logistic regression. The number of regional LN examined was not included in the second propensity score model because it was too strongly related to number of LN positive.
Inverse probability of treatment weights (IPTW) were calculated from the propensity scores and represented the inverse probability of a participant receiving the observed treatment based on their characteristics. IPTW estimates were further stabilized by multiplying them by the marginal probability of receiving the treatment observed. For all analysis, the weights were normalized to add up to the original sample size. The effectiveness of the weighting was evaluated by calculating the standardized differences of the covariates between patients treated with and without PORT, weighting by the IPTW in the total sample and within each positive LN group15. The treatment effects were recalculated using the IPTW with a Cox model including receipt of PORT; and a Cox model including receipt of PORT, number of LN positive, and their interaction. Adjusted Kaplan-Meier survival curves using IPTW and the weighted log-rank test were generated comparing treatment groups16.
Results
In the NSCLC NCDB, 2115 patients diagnosed between 2004–2006 were eligible for analysis. Complete patient characteristics are presented in Table 1. Of the eligible patients, 918 (43.4%) received PORT and 1197 (56.6%) did not. As for the chemotherapy sequencing, 1730 (81.79%) received adjuvant chemotherapy, 192 (9.1%) received neoadjuvant chemotherapy, and the sequence was unknown for 193 (9.1%). Patients were more likely to receive PORT if treated at a Comprehensive Community Cancer Center (CCCC) or other community center (vs. academic center, p<0.001), had private insurance (p<0.001), lived in an area with median income between $30,000–$45,999 (vs. <$30,000 or > $46,000, p=0.038), had Charlson-Deyo score of 0 (vs. 1 or 2+, p=0.001), had ≤3 regional lymph nodes examined (p<0.001), or were younger in age (p<0.001).
Table 1.
Patient Descriptive Statistics According to Receipt of Post-Operative Radiotherapy (PORT)
| PORT | ||||
|---|---|---|---|---|
| Characteristic | Total (n=2115) | No (N=1197) | Yes (N=918) | P value* |
| Facility Type | <0.001† | |||
| Community Cancer Program/Other | 170 (8.0%) | 81 (6.77%) | 89 (9.69%) | |
| Comprehensive Community Cancer Program | 1098 (51.9%) | 594 (49.62%) | 504 (54.9%) | |
| Academic/Research Program (Includes NCI) | 847 (40%) | 522 (43.61%) | 325 (35.4%) | |
| Sex | 0.340 | |||
| Male | 991 (46.9%) | 550 (45.95%) | 441 (48.04%) | |
| Female | 1124 (53.1%) | 647 (54.05%) | 477 (51.96%) | |
| Age | <0.001† | |||
| Median | 64 | 65 | 62 | |
| Range | 27–89 | 27–89 | 30–84 | |
| Race | 0.402 | |||
| White | 1831 (87 5%) | 1026 (86 95%) | 805 (88 17%) | |
| Other | 262 (12 5%) | 154 (13 05%) | 108 (11 83%) | |
| Insurance | <0 001† | |||
| Not Insured | 41 (2 0%) | 23 (1 95%) | 18 (1 99%) | |
| Private Insurance | 963 (46 3%) | 492 (41 77%) | 471 (52 1%) | |
| Govt Insurance | 1078 (51 8%) | 663 (56 28%) | 415 (45 91%) | |
| Income | 0 038† | |||
| < $30 000 | 252 (12 5%) | 151 (13 36%) | 101 (11 43%) | |
| $30 000 $34 999 | 387 (19 2%) | 195 (17 26%) | 192 (21 72%) | |
| $35 000 $45 999 | 539 (26 8%) | 297 (26 28%) | 242 (27 38%) | |
| $46 000 + | 836 (41 5%) | 487 (43 1%) | 349 (39 48%) | |
| Urban/Rural | 0 292 | |||
| Metro Area | 1655 (82 9%) | 937 (83 66%) | 718 (81 87%) | |
| Urban/Rural | 342 (17 1%) | 183 (16 34%) | 159 (18 13%) | |
| Charlson Deyo Score | 0 001† | |||
| 0 | 1292 (61 1%) | 692 (57 81%) | 600 (65 36%) | |
| 1 | 638 (30 2%) | 396 (33 08%) | 242 (26 36%) | |
| 2+ | 185 (8 7%) | 109 (9 11%) | 76 (8 28%) | |
| Year of Diagnosis | 0 079 | |||
| 2004 | 673 (31 8%) | 357 (29 82%) | 316 (34 42%) | |
| 2005 | 694 (32 8%) | 405 (33 83%) | 289 (31 48%) | |
| 2006 | 748 (35 4%) | 435 (36 34%) | 313 (34 1%) | |
| Histology | 0 761 | |||
| Large cell carcinomas | 131 (6 2%) | 76 (6 35%) | 55 (5 99%) | |
| Squamous cell carcinomas | 502 (23 7%) | 288 (24 06%) | 214 (23 31%) | |
| Adenocarcinomas | 1407 (66 5%) | 787 (65 75%) | 620 (67 54%) | |
| Adenosquamous carcinomas | 75 (3 5%) | 46 (3 84%) | 29 (3 16%) | |
| Grade | 0 085 | |||
| 1 | 107 (5 1%) | 66 (5 51%) | 41 (4 47%) | |
| 2 | 877 (41 5%) | 520 (43 44%) | 357 (38 89%) | |
| 3 | 936 (44 3%) | 509 (42 52%) | 427 (46 51%) | |
| 4 | 81 (3 8%) | 46 (3 84%) | 35 (3 81%) | |
| Unknown | 114 (5 4%) | 56 (4 68%) | 58 (6 32%) | |
| AJCC Pathologic T stage | 0 092 | |||
| T0/1 | 712 (33 8%) | 386 (32 36%) | 326 (35 67%) | |
| T2 | 1147 (54 4%) | 671 (56 24%) | 476 (52 08%) | |
| T3 | 114 (5 4%) | 69 (5 78%) | 45 (4 92%) | |
| T4 | 134 (6 4%) | 67 (5 62%) | 67 (7 33%) | |
| Regional Nodes Positive | 0 314 | |||
| 1 2 | 987 (49 5%) | 568 (50 49%) | 419 (48 22%) | |
| > 3 | 1007 (50 5%) | 557 (49 51%) | 450 (51 78%) | |
| Median | 3 | 2 | 3 | |
| Range | 1 34 | 1 34 | 1 20 | |
| Regional Nodes Examined | <0 001† | |||
| 1 3 | 221 (11 7%) | 93 (8 74%) | 128 (15 53%) | |
| 4 6 | 344 (18 2%) | 194 (18 23%) | 150 (18 2%) | |
| 7 9 | 351 (18 6%) | 217 (20 39%) | 134 (16 26%) | |
| >9 | 972 (51 5%) | 560 (52 63%) | 412 (50%) | |
| Median | 10 | 10 | 9 5 | |
| Range | 1 68 | 1 68 | 1 55 | |
Abbreviations: NCI: National Cancer Institute; AJCC: American Joint Committee on Cancer
ANOVA for numerical covariates and chi-square test for categorical covariates.
Significant
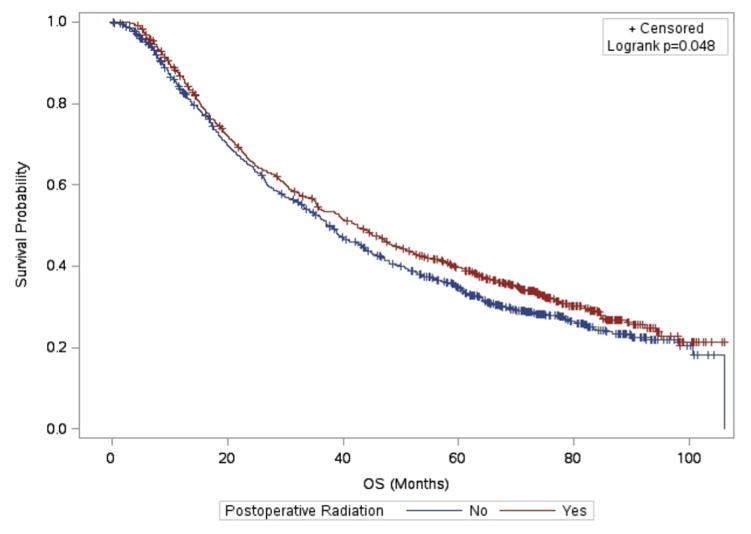
Median OS for patients treated with PORT was significantly longer than for those not treated with PORT on propensity-weighted log-rank analysis (42 months vs. 38 months, p=0.048, see Figure 1). The 5 year OS rate was 39.8% for those who received PORT vs. 34.7% for those who did not receive PORT. The complete results of the univariate and multivariable analysis of overall survival may be found in Table 2 and Table 3, respectively. On univariate analysis of survival, use of PORT was associated with a strong trend toward better survival (HR 0.91, 95% CI 0.82–1.01, p=0.071). On multivariable analysis, PORT was significantly associated with better OS (HR 0.87, 95% CI 0.77–0.98, p=0.021). Other factors associated with better survival on multivariable analysis were younger age (p<0.001), female sex (p<0.001), living in a higher income area (p=0.028), living in an urban/rural vs. metro county (p=0.034), adenocarcinoma histology compared to adenosquamous carcinoma(p=0.003), lower T stage (p<0.001), 1–2 involved lymph nodes (LN) vs. ≥3 (p<0.001), and higher number of examined LN (p<0.001). A summary of the IPTW analyses of overall survival may be found in Table 4. On IPTW Cox analysis, PORT was significantly associated with better OS (HR 0.89, 95% CI 0.79–1.00), p=0.046). No interaction was seen between the effect of PORT and the number of positive LN (p=0.615).
Figure 1.
Adjusted Kaplan-Meier Survival Estimates and Weighted Log Rank Test
Table 2.
Univariate Analysis of Overall Survival
| Covariate | Level | N | OS (Months)
|
||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | HR P-value | Log-rank P-value | |||
| Postoperative Radiation | Yes | 918 | 0.91 (0.82–1.01) | 0.073 | 0.071 |
| No | 1197 | - | - | ||
|
| |||||
| RT Regional Dose | >60 Gy | 67 | 1.04 (0.78–1.39) | 0.793 | 0.226 |
| 50–60 Gy | 436 | 0.88 (0.77–1.01) | 0.066 | ||
| <50 Gy | 415 | 0.92 (0.80–1.05) | 0.207 | ||
| No post-op radiation | 1197 | - | - | ||
|
| |||||
| Facility Type | Academic/Research Program (Includes NCI) | 847 | 0.84 (0.69–1.02) | 0.083 | 0.219 |
| Comprehensive Community Cancer Program | 1098 | 0.86 (0.71–1.04) | 0.122 | ||
| Community Cancer Program/Other | 170 | - | - | ||
|
| |||||
| Sex | Male | 991 | 1.29 (1.16–1.43) | <.001 | <.001† |
| Female | 1124 | - | - | ||
|
| |||||
| Patient Age | 2115 | 1.01 (1.01–1.02) | <.001 | - | |
|
| |||||
| Race | Other | 262 | 0.93 (0.79–1.09) | 0.376 | 0.375 |
| White | 1831 | - | - | ||
|
| |||||
| Insurance | Not Insured | 41 | 1.14 (0.81–1.62) | 0.450 | <.001† |
| Private Insurance | 963 | 0.79 (0.71–0.88) | <.001 | ||
| Govt. Insurance | 1078 | - | - | ||
|
| |||||
| Income | < $30,000 | 252 | 1.25 (1.06–1.48) | 0.010 | 0.017† |
| $30,000 – $34,999 | 387 | 1.19 (1.02–1.37) | 0.023 | ||
| $35,000 – $45,999 | 539 | 1.16 (1.01–1.32) | 0.031 | ||
| $46,000 + | 836 | - | - | ||
|
| |||||
| Urban/Rural | Urban/Rural | 342 | 1.01 (0.88–1.17) | 0.875 | 0.876 |
| Metro Area | 1655 | - | - | ||
|
| |||||
| Charlson-Deyo Score | 2+ | 185 | 1.22 (1.02–1.47) | 0.034 | 0.014† |
| 1 | 638 | 1.15 (1.03–1.29) | 0.016 | ||
| 0 | 1292 | - | - | ||
|
| |||||
| Year of Diagnosis | 2004 | 673 | 0.94 (0.83–1.07) | 0.356 | 0.332 |
| 2005 | 694 | 1.04 (0.91–1.18) | 0.584 | ||
| 2006 | 748 | - | - | ||
|
| |||||
| Histology | Squamous cell carcinomas | 502 | 1.11 (0.98–1.25) | 0.111 | 0.002† |
| Large cell carcinomas | 131 | 1.22 (0.98–1.51) | 0.074 | ||
| Adenosquamous carcinomas | 75 | 1.56 (1.20–2.04) | <.001 | ||
| Adenocarcinomas | 1407 | - | - | ||
|
| |||||
| Grade | Unknown | 114 | 1.07 (0.77–1.50) | 0.689 | 0.038† |
| 4 | 81 | 1.64 (1.16–2.33) | 0.005 | ||
| 3 | 936 | 1.20 (0.93–1.55) | 0.155 | ||
| 2 | 877 | 1.14 (0.88–1.48) | 0.309 | ||
| 1 | 107 | - | - | ||
|
| |||||
| AJCC Pathologic T | 4 | 134 | 1.64 (1.32–2.03) | <.001 | <.001† |
| 3 | 114 | 1.49 (1.17–1.88) | <.001 | ||
| 2 | 1147 | 1.25 (1.11–1.40) | <.001 | ||
| 0/1 | 712 | - | - | ||
|
| |||||
| Regional Nodes Positive | >=3 | 1007 | 1.30 (1.17–1.45) | <.001 | <.001† |
| 1–2 | 987 | - | - | ||
|
| |||||
| Regional Nodes Examined | >9 | 972 | 0.79 (0.67–0.94) | 0.009 | 0.073 |
| 7–9 | 351 | 0.82 (0.67–1.01) | 0.056 | ||
| 4–6 | 344 | 0.82 (0.67–1.00) | 0.047 | ||
| 1–3 | 221 | - | - | ||
Abbreviations: OS: Overall Survival; HR: Hazard Ratio; RT: Radiotherapy; NCI: National Cancer Institute; AJCC: American Joint Committee on Cancer
Significant
Table 3.
Multivariable Analysis of Overall Survival
| Covariate | Level | OS (Months)
|
||
|---|---|---|---|---|
| Hazard Ratio | HR P-value | Type3 P-value | ||
| Postoperative Radiation | Yes | 0.87 (0.77–0.98) | 0.021 | 0.021† |
| No | - | - | ||
|
| ||||
| Sex | Male | 1.23 (1.09–1.38) | <.001 | <.001† |
| Female | - | - | ||
| Patient Age | 1.01 (1.01–1.02) | <.001 | <.001† | |
|
| ||||
| Insurance | Not Insured | 1.29 (0.87–1.90) | 0.201 | 0.087 |
| Private Insurance | 0.90 (0.78–1.03) | 0.133 | ||
| Govt. Insurance | - | - | ||
|
| ||||
| Income | < $30,000 | 1.26 (1.03–1.53) | 0.022 | 0.028† |
| $30,000 – $34,999 | 1.25 (1.06–1.48) | 0.009 | ||
| $35,000 – $45,999 | 1.14 (0.99–1.32) | 0.075 | ||
| $46,000 + | - | - | ||
|
| ||||
| Urban/Rural | Urban/Rural | 0.83 (0.70–0.99) | 0.034 | 0.034† |
| Metro Area | - | - | ||
|
| ||||
| Histology | Squamous cell carcinomas | 1.00 (0.87–1.16) | 0.966 | 0.013† |
| Large cell carcinomas | 1.21 (0.95–1.54) | 0.130 | ||
| Adenosquamous carcinomas | 1.55 (1.16–2.07) | 0.003 | ||
| Adenocarcinomas | - | - | ||
|
| ||||
| AJCC Pathologic T stage | 4 | 1.58 (1.25–2.01) | <.001 | <.001† |
| 3 | 1.33 (1.01–1.76) | 0.042 | ||
| 2 | 1.25 (1.10–1.42) | <.001 | ||
| 0/1 | - | - | ||
|
| ||||
| Regional Nodes Positive | >=3 | 1.48 (1.30–1.68) | <.001 | <.001† |
| 1–2 | - | - | ||
|
| ||||
| Regional Nodes Examined | >9 | 0.59 (0.48–0.72) | <.001 | <.001† |
| 7–9 | 0.67 (0.54–0.84) | <.001 | ||
| 4–6 | 0.68 (0.54–0.84) | <.001 | ||
| 1–3 | - | - | ||
Abbreviations: OS: Overall Survival; HR: Hazard Ratio; AJCC: American Joint Committee on Cancer
Significant
Number of observations in the original data set = 2115.
Number of observations used = 1730.
Backward selection with an alpha level of removal of .20 was used. The following variables were removed from the model: Charlson/Deyo score, facility type, grade, year of diagnosis, and race.
Table 4.
Analysis of Overall Survival Using Inverse Probability of Treatment Weighting with the Propensity Score
| Covariate | OS (Months)
|
||
|---|---|---|---|
| Hazard Ratio | HR P-value | Type3 P-value | |
| Main Effects Model a | |||
| Postoperative Radiation: Yes vs. No | 0.89 (0.79–1.00) | 0.046 | 0.046 |
|
| |||
| Interaction Model b | |||
| Interaction: Postoperative Radiation with Regional Lymph Nodes Positive | - | - | 0.615 |
Abbreviations: OS: Overall Survival; HR: Hazard Ratio
Model included postoperative radiation. Number of observations used was 1730.
Model included postoperative radiation, regional lymph nodes positive, and their interaction. Number of observations used was 1831.
Discussion
The role of PORT in resected stage III lung cancer has remained controversial since publication of the PORT meta-analysis in 1998. Though an OS detriment was observed in patients receiving PORT, this study has been criticized due to the use of antiquated radiotherapy equipment and techniques. Such outdated factors include use of cobalt-60 equipment, which leads to inhomogeneous dose distribution, along with unsophisticated 2-D field design. Taken together, these factors likely increased the volume of normal tissue exposed to high dose radiation. Such technical factors likely substantially worsened the treatment mortality associated with PORT by simultaneously limiting the effectiveness of the therapy and increasing the likelihood of severe radiation pneumonitis3. The obsolete nature of the PORT meta-analysis data limits its applicability to modern practice, yet limited data have emerged to determine the potential role for PORT in the setting of involved N2 nodes5,6,17–28. Data justifying the use of PORT in addition to adjuvant chemotherapy are even more limited, with the largest study being the ANITA secondary analysis (224 pN2 patients, 118 received chemotherapy)5.
The present study suggests that in this retrospective cohort, PORT was associated with an OS benefit in patients with pN2 disease who received chemotherapy. The addition of PORT was associated with significantly prolonged median survival, from 38 to 42 months. This effect did not appear to be dependent on the number of involved lymph nodes. Although a larger series using SEER data indicated that the benefit of PORT may be limited to those with over 50% of resected lymph nodes involved with disease, the lack of chemotherapy data in that study limits its relevance to modern practice26.
The use of the NCDB affords significant advantages over previous studies designed to address the efficacy of PORT for pN2 NSCLC. With a large patient population and comprehensive data including detailed radiotherapy information such as radiation dose, treatment site, treatment source and treatment technique coupled with receipt of chemotherapy data, the NCDB allows for an analysis of PORT in patients treated with more modern, up-to-date therapies. By limiting the analysis to those treated with contemporary radiotherapy and chemotherapy, this retrospective cohort provides data that suggest that PORT may be beneficial for pN2 patients treated in current practice.
Continuous advancements in radiotherapy technology over the last few decades and the resultant improvements in the therapeutic ratio of radiation likely contribute to the emerging benefit of PORT in the era of adjuvant chemotherapy. The advent of three dimensional (3D) treatment planning, 3D conformal radiotherapy, and Intensity-Modulated Radiotherapy (IMRT) have allowed for delivery of more conformal radiotherapy for lung cancer with less toxicity to surrounding normal lung and thoracic structures29–36. Implementation into radiotherapy practice of on-board imaging (OBI), which involves the acquisition of images by the treatment machine to improve setup accuracy, and respiratory motion control during radiotherapy delivery have allowed for reduced setup error, which has in turn reduced field margins and the volume of normal tissue irradiated37–43. In the setting of unresectable disease, rates of pneumonitis have continued to decline in the last few decades. The rate of grade 3 pneumonitis in the Radiation Therapy Oncology Group (RTOG) trial 9410, which completed enrollment in 1998, was 11%44. A more recent study, RTOG 0617, which completed enrollment in 2011, revealed a grade 3 pneumonitis rate of 5%45 A previous report showed that when meta-analysis of PORT data was limited to trials which used linear accelerators, PORT was associated with better local control and overall survival17. It should be noted, however, that the trials analyzed by Billiet et al. were underpowered, limiting the conclusions able to be drawn46. The association between PORT and better OS seen in this study is not surprising, as the analysis was limited to the most recent complete data available in the NCDB and patients treated with cobalt were excluded.
The OS benefit imparted by chemotherapy for resected NSCLC results from reduction in both local and distant failure8,47. Per the ANITA study, adjuvant chemotherapy reduced distant failure from 28% to 25% and local failure from 18% to 12%. In this study, at least 82% of patients received adjuvant chemotherapy and 9% received neoadjuvant chemotherapy, though the chemotherapy sequence would not be expected to affect survival advantage afforded by chemotherapy10. The use of PORT allows for further reductions in the rate of local failure, improving survival beyond that afforded by chemotherapy alone. A meta-analysis of concurrent versus sequential chemoradiotherapy in the definitive treatment setting for stage III NSCLC demonstrated that improved local control leads to better overall survival48. Thus, improvements in local control in the resected stage III patient population using PORT would also be likely to improve survival. Although the current analysis lacks local control data (local control is not recorded within the NCDB), it would be expected that the overall survival benefit seen in this study may be related to reductions in local failure.
The value of PORT for N2 disease is currently being evaluated in a prospective randomized trial in Europe. The Intergroupe Francophone de Cancerologie Thoracique initiated the Lung Adjuvant Radiotherapy Trial (LungART), a phase III randomized trial assessing the benefit of conformal PORT following surgery for patients with completely resected pN2 disease49. Patients may receive either neoadjuvant or adjuvant chemotherapy and must be treated using small volume 3D-conformal radiotherapy. The target volumes specified on this trial involve only the bronchial stump, the ipsilateral hilum, and the involved mediastinal lymph node stations, plus a small margin. The treated area within the chest is therefore significantly reduced relative to the volumes treated during the earlier days of PORT (see figure 2). The trial further specifies a prescription dose of 54 Gy. Both the target volumes and the prescription dose used in LungART are consistent with the current standards of practice.. The results of LungART will be informative and will definitively answer the question of the value of PORT for pN2 NSCLC.
Figure 2.

Representative “Beam’s Eye View” from a single anterior-posterior field from a modern course of postoperative radiotherapy (A) and out-of-date postoperative radiotherapy (B) for a patient with a completely-resected right upper lobe tumor found to have involved N2 nodes. The borders for the field on the right (B) were derived from the specifications of the Medical Research Council (MRC) Lung Cancer Working Party randomized trial of postoperative radiotherapy, a trial included in the PORT meta-analysis. The MRC study mandated coverage of the entire mediastinum, bilateral hila, bronchial stump, and, in the case of an upper lobe tumor, the bilateral supraclavicular fossae. These field specifications resulted in significantly higher volumes of normal heart and lung in the treatment field than what is currently acceptable. Additionally, with older radiotherapy equipment (such as Cobalt-60 units), less penetrating, lower energy beams were used, which resulted in higher superficial dose relative to the dose at the desired target depth. This created significant dose inhomogeneity with the highest dose level deposited in uninvolved lung, chest wall, and heart. Contoured normal structures seen in this figure are the lungs (purple), the heart (pink), and the esophagus (orange).
This analysis does carry some limitations beyond its retrospective design. Though the NCDB does have advantages over other large datasets, including large sample size, consistency of data drawn from across the United States, inclusion of chemotherapy data, and detailed radiotherapy information, the NCDB is limited by the potential for miscoding and incremental survival data. Also, although the most modern available cohort from the NCDB was used, some of the patients included in the analysis were treated up to 10 years ago. Additionally, follow-up time is somewhat short particularly given that late radiation toxicities can influence survival. The analysis also lacks toxicity, treatment compliance, and quality of life data, which is important information for this group of patients that can be relatively tenous after undergoing surgical resection and adjuvant chemotherapy. Such factors may have influenced long-term survival results. Additionally, staging techniques used within this group are not available, which could have also affected survival outcomes. However, contemporary staging procedures such as positron emission tomography-computed tomography (PET/CT) and magnetic resonance imaging (MRI) of the brain were considered standard of care at the time of the study period, so lack of staging information would likely not change the conclusions of this study significantly.
This study did analyze radiation dose (< 50 Gy, 50–60 Gy, and > 60 Gy) as a variable for overall survival in univariate analysis (table 1), and it did not appear to be significant. However, more specific radiation details such as dose to normal tissues including lung V20, mean lung dose, and mean heart dose were not available within this dataset. Dosimetric data would be valuable; however this very specific radiation data will likely only be available in the context of randomized trials and is beyond the capabilities of the NCDB.
Caution should be taken when interpreting studies, such as the present one, which are based on retrospective patient cohorts. In this study, for example, the better survival seen in patients treated with PORT suggests a benefit in patients with resected NSCLC. However, patients treated with PORT in this cohort tended to be younger and have lower co-morbidity, which also suggests that selection bias may have affected the results.
Despite these limitations, the present analysis provides data supporting the benefit of PORT in OS for patients with pN2 disease. This conclusion is in line with current practice guidelines, which indicate that PORT is an acceptable therapy to be given in addition to chemotherapy for patients with resected pN2 disease. The survival benefit of PORT appears to be additive in this retrospective population when given along with chemotherapy and shows no dependence upon number of involved lymph nodes. Though caution should be taken when interpreting studies based on retrospective cohorts, evidence of the value of PORT in the adjuvant treatment paradigm in patients with pN2 NSCLC continues to build. The results of the pending LungART randomized trial should provide a more definitive answer to this persistent clinical question.
Acknowledgments
Research reported in this publication was supported in part by the Biostatistics & Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The data used in the study are derived from a de-identified NCDB file. The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Footnotes
Conflicts of Interest: none
References
- 1.Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT Meta-analysis Trialists Group. Lancet. 1998 Jul 25;352(9124):257–263. [PubMed] [Google Scholar]
- 2.Bekelman JE, Rosenzweig KE, Bach PB, Schrag D. Trends in the use of postoperative radiotherapy for resected non-small-cell lung cancer. International journal of radiation oncology, biology, physics. 2006 Oct 1;66(2):492–499. doi: 10.1016/j.ijrobp.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 3.Munro AJ. What now for postoperative radiotherapy for lung cancer? Lancet. 1998 Jul 25;352(9124):250–251. doi: 10.1016/S0140-6736(98)22030-7. [DOI] [PubMed] [Google Scholar]
- 4.Phlips P, Rocmans P, Vanderhoeft P, Van Houtte P. Postoperative radiotherapy after pneumonectomy: impact of modern treatment facilities. International journal of radiation oncology, biology, physics. 1993 Oct 20;27(3):525–529. doi: 10.1016/0360-3016(93)90375-6. [DOI] [PubMed] [Google Scholar]
- 5.Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. International journal of radiation oncology, biology, physics. 2008 Nov 1;72(3):695–701. doi: 10.1016/j.ijrobp.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 6.Lally BE, Zelterman D, Colasanto JM, Haffty BG, Detterbeck FC, Wilson LD. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006 Jul 1;24(19):2998–3006. doi: 10.1200/JCO.2005.04.6110. [DOI] [PubMed] [Google Scholar]
- 7.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. The New England journal of medicine. 2004 Jan 22;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 8.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. The lancet oncology. 2006 Sep;7(9):719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 9.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. The New England journal of medicine. 2005 Jun 23;352(25):2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 10.Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010 Jul 1;28(19):3138–3145. doi: 10.1200/JCO.2009.27.6204. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992 Jun;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 13.Faries DE, Leon AC, Haro JM, Obenchain RL. Analysis of Observational Health Care Data Using SAS. Cary, N.C: SAS Institute; 2010. [Google Scholar]
- 14.Imbens GW. The role of the propensity score in estimating dose-response functions. Biometrika. 2000 Sep 1;87(3):706–710. [Google Scholar]
- 15.Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Medical decision making: an international journal of the Society for Medical Decision Making. 2009 Nov-Dec;29(6):661–677. doi: 10.1177/0272989X09341755. [DOI] [PubMed] [Google Scholar]
- 16.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Statistics in medicine. 2005 Oct 30;24(20):3089–3110. doi: 10.1002/sim.2174. [DOI] [PubMed] [Google Scholar]
- 17.Billiet C, Decaluwe H, Peeters S, et al. Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: A meta-analysis. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2013 Oct 4; doi: 10.1016/j.radonc.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Burdett S, Rydzewska L, Tierney JF, Fisher DJ Group PM-aT. A closer look at the effects of postoperative radiotherapy by stage and nodal status: updated results of an individual participant data meta-analysis in non-small-cell lung cancer. Lung cancer. 2013 Jun;80(3):350–352. doi: 10.1016/j.lungcan.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Butof R, Kirchner K, Appold S, et al. Potential clinical predictors of outcome after postoperative radiotherapy of non-small cell lung cancer. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft … [et al] 2014 Mar;190(3):263–269. doi: 10.1007/s00066-013-0501-4. [DOI] [PubMed] [Google Scholar]
- 20.Hald SM, Bremnes RM, Al-Shibli K, et al. CD4/CD8 co-expression shows independent prognostic impact in resected non-small cell lung cancer patients treated with adjuvant radiotherapy. Lung cancer. 2013 May;80(2):209–215. doi: 10.1016/j.lungcan.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani C, Levra NG, Filippi AR, et al. Postoperative radiotherapy for patients with completely resected pathologic n2 non-small-cell lung cancer: a retrospective analysis. Clinical lung cancer. 2013 Mar;14(2):194–199. doi: 10.1016/j.cllc.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Matsuguma H, Nakahara R, Ishikawa Y, et al. Postoperative radiotherapy for patients with completely resected pathological stage IIIA-N2 non-small cell lung cancer: focusing on an effect of the number of mediastinal lymph node stations involved. Interactive cardiovascular and thoracic surgery. 2008 Aug;7(4):573–577. doi: 10.1510/icvts.2007.174342. [DOI] [PubMed] [Google Scholar]
- 23.Moretti L, Yu DS, Chen H, et al. Prognostic factors for resected non-small cell lung cancer with pN2 status: implications for use of postoperative radiotherapy. The oncologist. 2009 Nov;14(11):1106–1115. doi: 10.1634/theoncologist.2009-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawyer TE, Bonner JA, Gould PM, et al. The impact of surgical adjuvant thoracic radiation therapy for patients with nonsmall cell lung carcinoma with ipsilateral mediastinal lymph node involvement. Cancer. 1997 Oct 15;80(8):1399–1408. doi: 10.1002/(sici)1097-0142(19971015)80:8<1399::aid-cncr6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.Shen WY, Ji J, Zuo YS, et al. Comparison of efficacy for postoperative chemotherapy and concurrent radiochemotherapy in patients with IIIA-pN2 non-small cell lung cancer: An early closed randomized controlled trial. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2013 Oct 31; doi: 10.1016/j.radonc.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Urban D, Bar J, Solomon B, Ball D. Lymph node ratio may predict the benefit of postoperative radiotherapy in non-small-cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2013 Jul;8(7):940–946. doi: 10.1097/JTO.0b013e318292c53e. [DOI] [PubMed] [Google Scholar]
- 27.Wisnivesky JP, Halm EA, Bonomi M, Smith C, Mhango G, Bagiella E. Postoperative radiotherapy for elderly patients with stage III lung cancer. Cancer. 2012 Sep 15;118(18):4478–4485. doi: 10.1002/cncr.26585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou B, Xu Y, Li T, et al. A multicenter retrospective analysis of survival outcome following postoperative chemoradiotherapy in non-small-cell lung cancer patients with N2 nodal disease. International journal of radiation oncology, biology, physics. 2010 Jun 1;77(2):321–328. doi: 10.1016/j.ijrobp.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong JG, Burman C, Leibel S, et al. Three-dimensional conformal radiation therapy may improve the therapeutic ratio of high dose radiation therapy for lung cancer. International journal of radiation oncology, biology, physics. 1993 Jul 15;26(4):685–689. doi: 10.1016/0360-3016(93)90289-8. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Liao Z, Wei X, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT) International journal of radiation oncology, biology, physics. 2006 Dec 1;66(5):1399–1407. doi: 10.1016/j.ijrobp.2006.07.1337. [DOI] [PubMed] [Google Scholar]
- 31.Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. International journal of radiation oncology, biology, physics. 2007 May 1;68(1):94–102. doi: 10.1016/j.ijrobp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 32.Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. International journal of radiation oncology, biology, physics. 2005 Feb 1;61(2):318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 33.Singh AK, Lockett MA, Bradley JD. Predictors of radiation-induced esophageal toxicity in patients with non-small-cell lung cancer treated with three-dimensional conformal radiotherapy. International journal of radiation oncology, biology, physics. 2003 Feb 1;55(2):337–341. doi: 10.1016/s0360-3016(02)03937-8. [DOI] [PubMed] [Google Scholar]
- 34.Grills IS, Yan D, Martinez AA, Vicini FA, Wong JW, Kestin LL. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. International journal of radiation oncology, biology, physics. 2003 Nov 1;57(3):875–890. doi: 10.1016/s0360-3016(03)00743-0. [DOI] [PubMed] [Google Scholar]
- 35.Fang LC, Komaki R, Allen P, Guerrero T, Mohan R, Cox JD. Comparison of outcomes for patients with medically inoperable Stage I non-small-cell lung cancer treated with two-dimensional vs. three-dimensional radiotherapy. International journal of radiation oncology, biology, physics. 2006 Sep 1;66(1):108–116. doi: 10.1016/j.ijrobp.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Graham MV, Purdy JA, Emami B, Matthews JW, Harms WB. Preliminary results of a prospective trial using three dimensional radiotherapy for lung cancer. International journal of radiation oncology, biology, physics. 1995 Dec 1;33(5):993–1000. doi: 10.1016/0360-3016(95)02016-0. [DOI] [PubMed] [Google Scholar]
- 37.Bissonnette JP, Purdie TG, Higgins JA, Li W, Bezjak A. Cone-beam computed tomographic image guidance for lung cancer radiation therapy. International journal of radiation oncology, biology, physics. 2009 Mar 1;73(3):927–934. doi: 10.1016/j.ijrobp.2008.08.059. [DOI] [PubMed] [Google Scholar]
- 38.Onishi H, Kuriyama K, Komiyama T, et al. A new irradiation system for lung cancer combining linear accelerator, computed tomography, patient self-breath-holding, and patient-directed beam-control without respiratory monitoring devices. International journal of radiation oncology, biology, physics. 2003 May 1;56(1):14–20. doi: 10.1016/s0360-3016(02)04414-0. [DOI] [PubMed] [Google Scholar]
- 39.Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Medical physics. 2006 Oct;33(10):3874–3900. doi: 10.1118/1.2349696. [DOI] [PubMed] [Google Scholar]
- 40.Ramsey CR, Langen KM, Kupelian PA, et al. A technique for adaptive image-guided helical tomotherapy for lung cancer. International journal of radiation oncology, biology, physics. 2006 Mar 15;64(4):1237–1244. doi: 10.1016/j.ijrobp.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Michalski JM, Graham MV, Bosch WR, et al. Prospective clinical evaluation of an electronic portal imaging device. International journal of radiation oncology, biology, physics. 1996 Mar 1;34(4):943–951. doi: 10.1016/0360-3016(95)02189-2. [DOI] [PubMed] [Google Scholar]
- 42.Van de Steene J, Van den Heuvel F, Bel A, et al. Electronic portal imaging with on-line correction of setup error in thoracic irradiation: clinical evaluation. International journal of radiation oncology, biology, physics. 1998 Mar 1;40(4):967–976. doi: 10.1016/s0360-3016(97)00925-5. [DOI] [PubMed] [Google Scholar]
- 43.Miles EF, Kelsey CR, Kirkpatrick JP, Marks LB. Estimating the magnitude and field-size dependence of radiotherapy-induced mortality and tumor control after postoperative radiotherapy for non-small-cell lung cancer: calculations from clinical trials. International journal of radiation oncology, biology, physics. 2007 Jul 15;68(4):1047–1052. doi: 10.1016/j.ijrobp.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 44.Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. Journal of the National Cancer Institute. 2011 Oct 5;103(19):1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradley JD, Paulus R, Komaki R, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non-small cell lung cancer: Results on radiation dose in RTOG 0617. Unpulished Data, Presented at American Society of Clinical Oncology Annual Meeting; May 31–June 4, 2013; 2013. [Google Scholar]
- 46.Le Pechoux C, Arriagada R, Pignon JP. Need for new powered trials to assess the role of post-operative radiotherapy for stage III non-small cell lung cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2014 Jul 15; doi: 10.1016/j.radonc.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Group NM-aC. Arriagada R, Auperin A, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010 Apr 10;375(9722):1267–1277. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010 May 1;28(13):2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 49.Le Pechoux C, Dunant A, Pignon JP, et al. Need for a new trial to evaluate adjuvant postoperative radiotherapy in non-small-cell lung cancer patients with N2 mediastinal involvement. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007 Mar 1;25(7):e10–11. doi: 10.1200/JCO.2006.09.6263. [DOI] [PubMed] [Google Scholar]