Abstract
Objective
This trial evaluated the efficacy of acetaminophen in reducing oxidative injury, as measured by plasma F2-Isoprostanes, in adult patients with severe sepsis and detectable plasma cell-free hemoglobin.
Design
Single center, randomized, double-blind, placebo controlled phase II trial.
Setting
Medical ICU in a tertiary, academic medical center.
Patients
Critically ill patients ≥18 years old with severe sepsis and detectable plasma cell-free hemoglobin.
Interventions
Patients were randomized 1:1 to enteral acetaminophen 1 gram every 6 hours for three days (n = 18) or placebo (n = 22) with the same dosing schedule and duration.
Measurements and Main Results
F2-Isoprostanes on study day 3, the primary outcome, did not differ between acetaminophen (30 pg/mL, IQR 24–41) and placebo (36 pg/mL, IQR 25–80, p = 0.35). However, F2-Isoprostanes were significantly reduced on study day 2 in the acetaminophen group (24 pg/mL, IQR 19 – 36) compared with placebo (36 pg/mL, IQR 23–55, p = 0.047). Creatinine on study day 3, a secondary outcome, was significantly lower in the acetaminophen group (1.0 mg/dL, IQR 0.6–1.4) compared with placebo (1.3 mg/dL, IQR 0.83 – 2.0, p = 0.039). There was no statistically significant difference in hospital mortality (acetaminophen 5.6% vs. placebo 18.2%, p = 0.355) or adverse events (AST or ALT >400)(acetaminophen 9.5% vs. placebo 4.3%, p = 0.599).
Conclusions
In adults with severe sepsis and detectable plasma cell-free hemoglobin, treatment with acetaminophen within 24 hours of ICU admission may reduce oxidative injury and improve renal function. Further study is needed to confirm these findings and determine the effect of acetaminophen on patient-centered outcomes.
Keywords: sepsis, acetaminophen, cell-free hemoglobin, F2-isoprostanes, ACROSS
Introduction
Plasma levels of cell-free hemoglobin (CFH) are elevated in patients with a variety of illnesses, including critically ill patients with sepsis (1–10). Increased levels of CFH are associated with experimental and post-operative kidney injury (3, 11) and are independently associated with increased in-hospital mortality in patients with sepsis (9). CFH may mediate poor clinical outcomes via nitric oxide scavenging and vasoconstriction (1, 2, 5, 7, 8), vascular endothelial damage (11), and the ability of the iron moiety of CFH to undergo redox cycling, leading to oxidation of lipid membranes and release of F2-isoprostanes (F2-IsoPs) (3, 12).
Acetaminophen inhibits hemoprotein-mediated lipid peroxidation owing to its ability to reduce the iron protoporphyrin radical present within CFH (13, 14). In animal studies, acetaminophen prevents oxidative injury and renal failure due to rhabdomyolysis-induced release of the hemoprotein myoglobin (14). In an observational study of adults with severe sepsis, we reported that acetaminophen administration was associated with reduced plasma levels of F2-IsoPs and was independently associated with decreased in-hospital mortality; this protective association was only observed in patients with detectable CFH (9).
Based on these data suggesting that acetaminophen could reduce oxidative injury in patients with sepsis, we conducted a randomized, double-blind placebo controlled trial to test the primary hypothesis that the treatment of critically ill adults with severe sepsis and detectable plasma CFH with acetaminophen would decrease oxidative injury as measured by plasma F2-IsoPs. We further hypothesized that acetaminophen would improve renal function as measured by serum creatinine.
Methods
Study Patients
Patients were enrolled from April 1, 2013 through December 21, 2013 in the Vanderbilt University Medical Center Medical ICU. The trial was approved by the Vanderbilt Institutional Review Board and was registered on www.clinicaltrials.gov (NCT01739361). Written informed consent was obtained from all patients or their surrogates. A data and safety monitor reviewed all adverse events and had access to all data as they were collected.
Patients with a diagnosis of severe sepsis as defined by consensus definitions (15, 16) were enrolled within 24 hours of ICU admission. Exclusion criteria included known use of acetaminophen in the previous 48 hours, history of intolerance or allergy to acetaminophen, AST or ALT > 400 U/L in the 24 hours prior to enrollment, history of chronic liver disease, patients not committed to full support, pregnancy or women of childbearing potential without a documented negative pregnancy test, patients unable to swallow and without enteral access, and patients whose treating team declined enrollment due to intended use or avoidance of acetaminophen.
As acetaminophen selectively inhibits hemoprotein-mediated lipid peroxidation, a finding supported in our observational study (9), an initial blood sample was collected after enrollment and the plasma was tested for the presence of CFH using point-of-care (POC) testing with a HemoCue® Plasma Low/Hb analyzer. Only patients with detectable CFH (≥ 10 mg/dL) were randomized. Once CFH was detected, patients immediately underwent randomization and study drug was started within 6 hours. Patients with undetectable CFH continued to be observed throughout the study period but were not randomized.
Treatment Assignments
Patients with severe sepsis and detectable CFH were randomly assigned in permuted blocks of 4 to receive either enteral acetaminophen 1g every 6 hours for three days or identical-appearing placebo with the same schedule. The duration of three days was chosen using previous observational data in patients with sepsis (9) and data to suggest safety over this duration (17). The dose of acetaminophen was chosen as the maximum Federal Drug Administration-approved dose and frequency allowed. Patients, care givers, and study personnel were blinded to the randomization scheme and group assignments during the entire study.
Measurements
Blood was collected on enrollment and in the morning of the subsequent 3 days. Blood samples were placed on ice and processed within 20 minutes of collection by centrifugation at 3000 rpm for 10 minutes at 4°C and then plasma was stored at −80°C. At the completion of the study, plasma F2-IsoPs, CFH, and acetaminophen were measured on enrollment and each study day. F2-Isofurans (F2-IsoFs), a marker of lipid peroxidation similar to F2-IsoPs but preferentially produced at high oxygen tensions (18), were also measured as a secondary endpoint. F2-IsoP and F2-IsoF measurements were made using stable isotope dilution negative ion chemical ionization gas chromatography mass spectrometry (19). Plasma from patients receiving propofol at the time of blood draw required an additional thin layer chromatography processing step to account for the lipid emulsion in propofol. CFH and acetaminophen were measured in duplicate using spectrophotometric methods (QuantiChrom™ Hemoglobin Assay Kit, Gentaur, San Jose, CA and Acetaminophen Test Kit, Fisher Scientific, Hanover Park, IL).
Statistical Analysis
The primary endpoint was plasma F2-IsoPs measured at the completion of the study (study day 3), after the last scheduled dose of study drug. Because the kidney is the most commonly reported target of oxidative injury by CFH (11, 14, 20); renal function as measured by serum creatinine was a prespecified secondary endpoint. As this was a phase II study to determine if acetaminophen has any effect on hemoprotein-mediated oxidative injury in severe sepsis, we developed a priori criteria required for a patient to be included in the primary analysis. These criteria included: the patient had to receive ≥ 4 of 12 total doses of the study drug and had to have completed at least 3 of 4 total blood draws. We also prespecified an analysis of F2-IsoPs on study day 2 as a secondary endpoint given this study day marked completion of the a priori defined minimum protocol. Given past studies of F2-IsoPs in sepsis patients (9, 21) showing a difference of 60 pg/mL between patients who did and did not receive acetaminophen and an observed standard deviation of 65 pg/mL, we calculated that we would need 40 patients in our primary analysis (20 in each arm) to have 80% power to detect this difference with an alpha of 0.05. Because we anticipated that 20% of patients with sepsis would not have detectable CFH, and that 5% would die prior to the third blood draw (9), we projected a total enrollment of 54 patients to achieve a sample size of 40 for inclusion in the primary endpoint analysis.
Data are expressed as median values with interquartile range for continuous variables and frequencies for categorical variables. Between group comparisons were conducted using the Wilcoxon’s rank-sum test for continuous variables, Fisher’s exact test for categorical variables, and Spearman’s rank correlation coefficient for correlation between two continuous variables. Kaplan-Meier survival curves were compared with the log-rank test. Linear regression was used to assess the influence of study group assignment and baseline F2-IsoP level on F2-IsoP level at completion of the study. Log transformation of variables was used in the setting of non-normally distributed residuals. IBM SPSS Statistics (version 22.0, Chicago, IL) was used for statistical analyses; a two-sided significance level of 0.05 was used for statistical inference.
Results
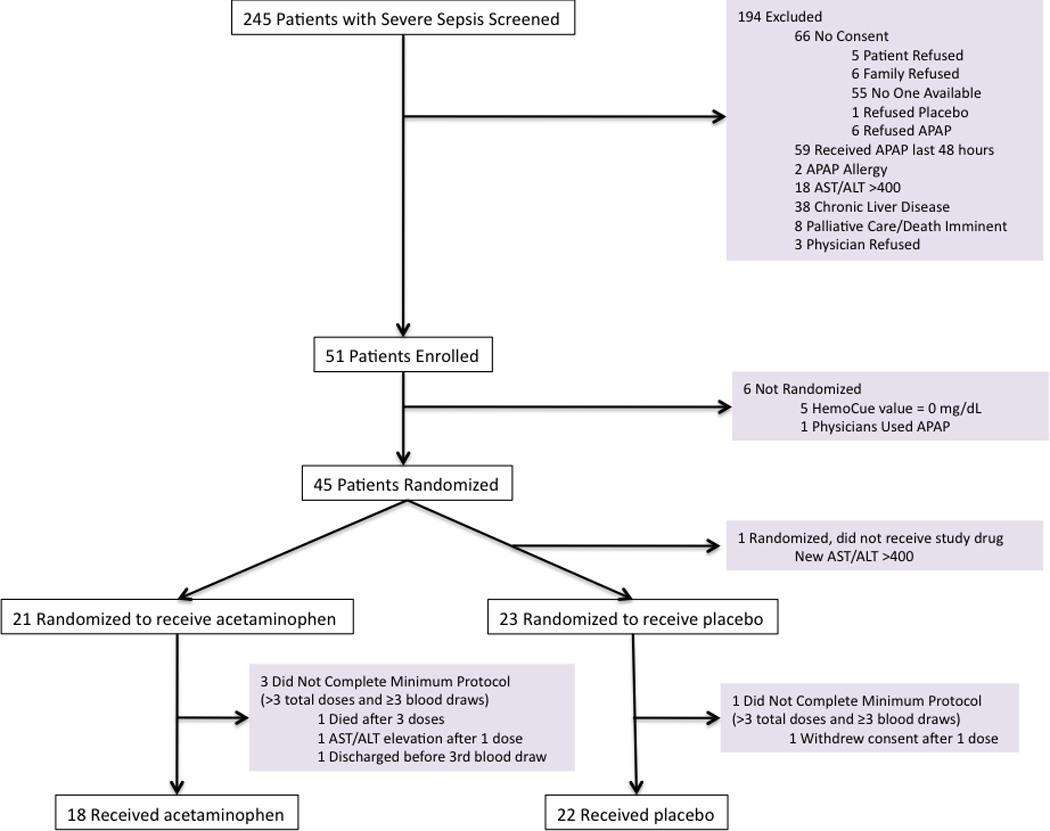
A total of 245 patients with severe sepsis were screened, of whom 51 were enrolled in the study (Figure 1). The most common exclusion criteria were receipt of acetaminophen in the 48 hours prior to screening or chronic liver disease. An additional 5 patients were not randomized due to undetectable CFH levels (9.8%) or one had acetaminophen use in the interval between screening and enrollment (1.9%). Of the remaining 45 patients randomized, 40 patients (18 acetaminophen, 22 placebo) completed the a priori defined treatment protocol of at least 4 doses of study drug and 3 blood draws, which defined our primary study population. Of these 40 patients, 9 did not have a 4th blood draw on study day 3 due to refusal of blood draw (n = 7) or discharge to another facility (n = 2).
Figure 1. Patient Screening, Enrollment, and Follow-up.
Baseline Characteristics
At baseline, patients had similar severity of illness, number of organ failures, need for mechanical ventilation, and vasoactive medications (Table 1). Patients in the placebo group were older and had higher baseline levels of F2-IsoPs, IsoFs, and creatinine compared to the acetaminophen group. Baseline levels of CFH were similar between groups. The lung (46%) and urinary tract (30%) were the most common sources of severe sepsis.
Table 1.
Baseline Characteristics
| Characteristic | Acetaminophen n = 18 |
Placebo n = 22 |
|
|---|---|---|---|
| Age (years) | 50 (41 – 64) | 58 (47 – 63) | |
| Men (n, %) | 9 (50%) | 12 (54.5%) | |
| White Race (n, %) | 17 (94.4%) | 19 (86.4%) | |
| APACHE II score | 21 (15.7 – 29) | 22 (16.5 – 24.2) | |
| SOFA score | 5.5 (4 – 8.5) | 6 (5 – 8) | |
| Severe Sepsis Organ Failures | |||
| Cardiovascular | 10 (55.6%) | 16 (72.7%) | |
| Renal | 14 (77.8%) | 17 (77.3%) | |
| Respiratory | 8 (44.4%) | 9 (40.9%) | |
| Hematologic | 3 (16.7%) | 3 (13.6%) | |
| Metabolic | 9 (50%) | 11 (50%) | |
| Source of Severe Sepsis | |||
| Respiratory | 10 (55.6%) | 9 (40.9%) | |
| Urinary | 6 (33.3%) | 6 (27.3%) | |
| Skin | 1 (5.6%) | 1 (4.5%) | |
| Abdominal | 3 (16.7%) | 4 (18.2%) | |
| Central Nervous System | 0 | 1 (4.5%) | |
| Vascular Catheter/Bloodstream | 3 (16.6%) | 4 (18.1%) | |
| Vasoactive Medications (n, %) | 8 (44.4%) | 10 (45.5%) | |
| Mechanical Ventilation | |||
| Invasive (n, %) | 8 (44.4%) | 6 (27.3%) | |
| Non-invasive (n, %) | 1 (5.6%) | 1 (4.5%) | |
| ARDS (n, %) | 5 (27.8%) | 7 (31.8%) | |
| Cell-free Hemoglobin (mg/dl) | 10 (10 – 30) | 20 (10 – 32) | |
| Maximum Temperature (°C) | 37.7 (37 – 38.5) | 37.9 (37.2 – 38.4) | |
| Lactic Acid (mEq/L) (n = 33) | 1.75 (1.22 – 3.25) | 1.8 (1 – 3.75) | |
| AST (U/L) (n = 35) | 38 (24.5 – 59.5) | 41 (24 – 67) | |
| ALT (U/L) (n = 35) | 31.5 (18 – 53.7) | 34 (18.5 – 45.5) | |
| Creatinine (mg/dL) | 1.63 (0.8 – 2.75) | 2.06 (1.02 – 3.59) | |
| F2-Isoprostanes (pg/mL) | 33 (24 – 43) | 40 (29 – 61) | |
| F2-Isofurans (pg/mL) | 45 (34 – 82) | 56 (31 – 94) | |
Data given as median (25th percentile – 75th percentile) or number (percentage) of patients
APACHE = acute physiology and chronic health evaluation; SOFA = sequential organ failure assessment; ARDS = acute respiratory distress syndrome; AST = aspartate aminotransferase; ALT = alanine aminotransferase
Efficacy of Acetaminophen on Oxidative Injury
In the 31 of 40 (77%) patients who had plasma samples available from study day 3, there was no significant difference in F2-IsoPs levels between the acetaminophen (30 pg/mL, IQR 24 – 41) and placebo groups (36 pg/mL, IQR 25 – 80, p = 0.35) in a univariate analysis or when adjusted for baseline F2-IsoP levels (p = 0.21). However, on study day 2 when 39 of 40 patients had plasma samples available, there was a significant reduction in F2-IsoPs in the acetaminophen group (24 pg/mL, IQR 19 – 36) compared with placebo (36 pg/mL, IQR 23 – 55, p = 0.047) (Figure 2). Because plasma processing with an additional thin layer chromatography step for patients who were receiving propofol at the time of blood draw could introduce systematic differences in measured F2-IsoP levels, we performed a sensitivity analysis after removing the patients who were receiving propofol at the time of blood draw (n = 5 on both study days 2 and 3, 3 patients in placebo group, 2 patients in acetaminophen group). There remained a statistically significant decrease in F2-IsoPs in the acetaminophen group on day 2 (23 pg/mL, IQR 18 – 36) compared with placebo (42 pg/mL, IQR 23 – 73, p = 0.016), and the difference in F2-IsoPs on study day 3 approached significance (30 pg/mL, IQR 23 – 39) compared with placebo (49 pg/mL, 26 – 86, p = 0.058). There was no difference in F2-IsoF levels on study day 2 or 3 between the acetaminophen and placebo groups; however when patients receiving propofol were removed from the analysis, there was a significant reduction in F2-IsoF levels on study day 3 in the acetaminophen group (27 pg/mL, IQR 20 – 49) compared with placebo (57 pg/mL, IQR 42 – 83, p = 0.029) (Figure 2).
Figure 2. Plasma F2-Isoprostanes and F2-Isofurans Measured at Baseline and Each Study Day.
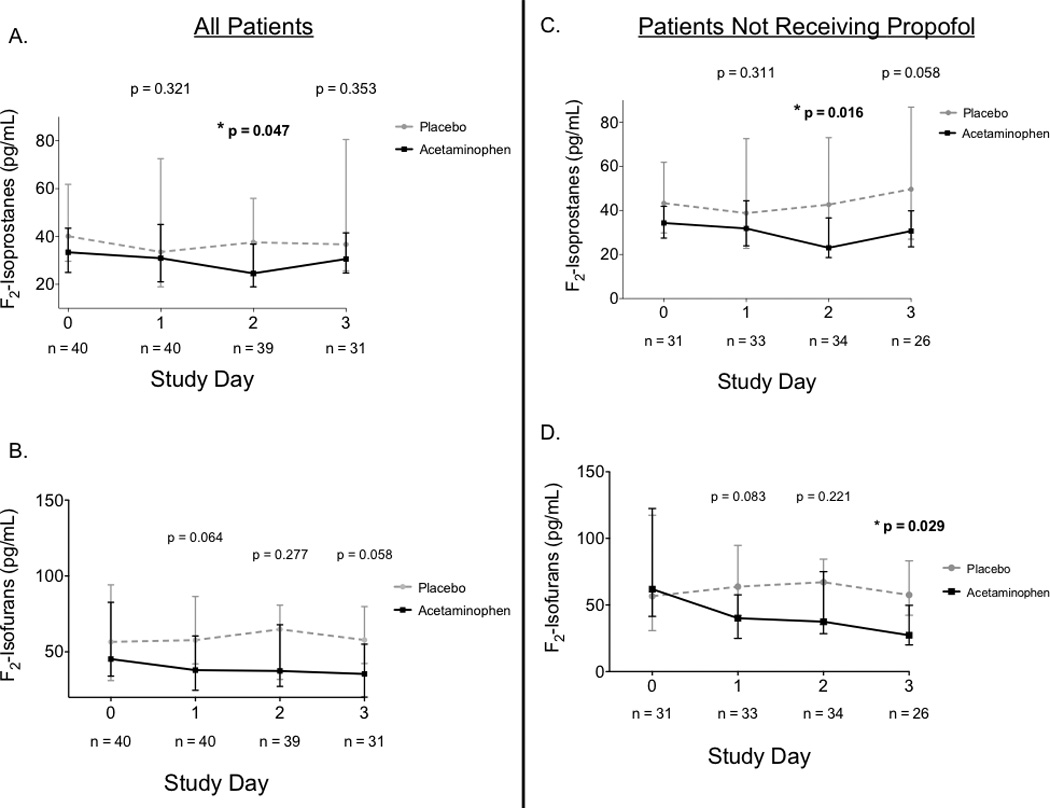
F2-Isoprostane and F2-Isofuran levels were compared between groups on each study day (panels A and B) and in patients who were not receiving propofol at the time of blood draw (panels C and D) to account for differences in sample processing with this group of patients. P-values represent between-group comparisons at each time point. Study day 3 was the primary endpoint of the study and study day 2 was analyzed as a secondary endpoint. Squares and circles represent medians and bars represent interquartile ranges.
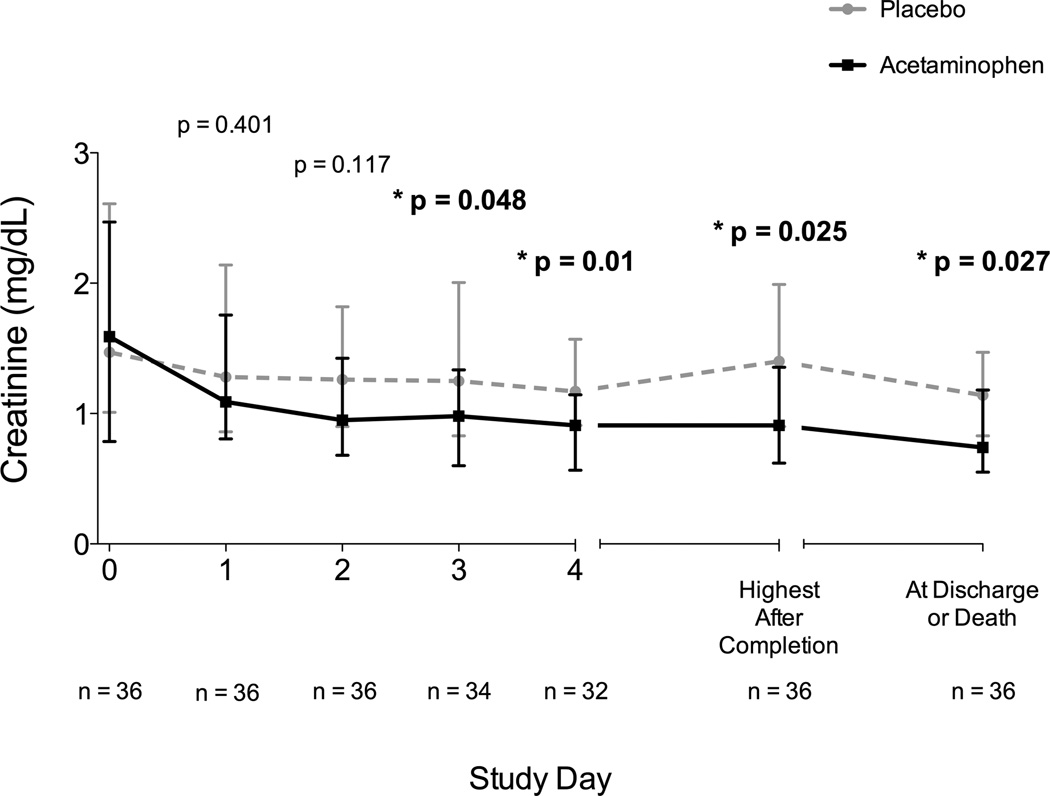
Serum creatinine was significantly lower in the acetaminophen group on study day 3 (1.04 mg/dL, IQR 0.61– 1.44) compared with placebo (1.36 mg/dL, IQR 0.83 – 2.02, p = 0.039). This difference persisted after completion of the study drug at day 4 (0.93 mg/dL, IQR 0.55 – 1.2 vs 1.3 mg/dL, IQR 0.97 – 3.45, p = 0.004), for the highest creatinine measured during the remainder of hospitalization, and for creatinine measured on the day of discharge or death. After removing the 4 patients from the analysis whose creatinine levels were manipulated by renal replacement therapy at any point during the study (3 in placebo group, 1 in acetaminophen group), this significant reduction of creatinine in the acetaminophen group during and after the study period persisted (Figure 3). In a linear regression model controlling for baseline creatinine, acetaminophen significantly reduced creatinine at study day 3 (Beta −0.48, 95% CI −0.86 to −0.1, p = 0.014). In agreement with past animal studies (11), we found that increased baseline CFH correlated strongly with subsequent creatinine after completion of the study (rs = 0.427, p = 0.009). Acetaminophen attenuated this response to baseline CFH. When controlling for baseline CFH levels, patients in the acetaminophen group had significantly lower creatinine on study day 3 and 4 compared to placebo (Beta −0.5, 95% CI −0.95 to −0.06, p = 0.027 and Beta −1.17, 95% CI −2.1 to −0.15, p = 0.026, respectively).
Figure 3. Creatinine Measured at Enrollment, Each Study Day, and After Study Completion in Patients Never Requiring Renal Replacement Therapy.
In patients never receiving any type of renal replacement therapy during or after the study (n = 36), creatinine levels were similar at baseline (study day 0) and were significantly reduced in the acetaminophen group on study day 3, the day following study completion (day 4), through the remainder of hospitalization and at discharge or death. P-values represent between-group comparisons at each time point. Squares and circles represent medians and bars represent interquartile ranges.
Effect of Acetaminophen on Other Clinical Endpoints
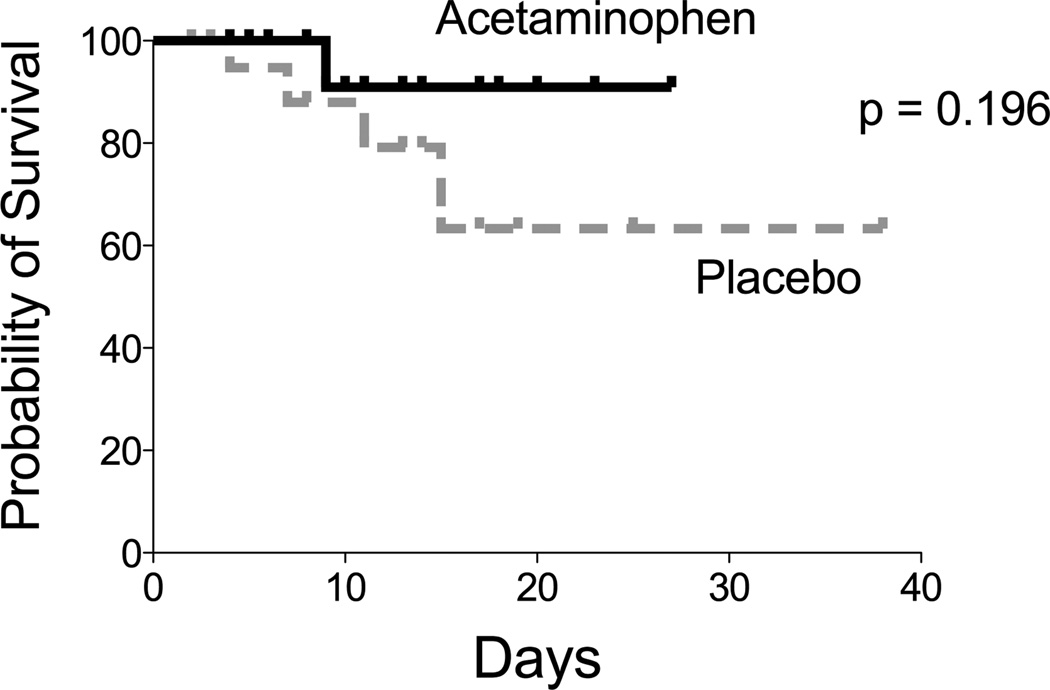
Acetaminophen modestly but significantly decreased the maximum temperature observed on study day 3 (36.9 °C, IQR 36.9 – 37.1) compared with placebo (37.1 °C, IQR 36.9 – 37.4, p = 0.049). There were no significant differences between groups during the study period in heart rate or mean arterial blood pressure. By study day 3, there were more patients alive and free of organ failures in the acetaminophen group versus placebo (55% vs 30%, p = 0.18) but this difference was not significant. Differences in ventilator-free days (acetaminophen 28 days, IQR 23–28 vs placebo 27 days, IQR 20–28, p = 0.527) and in-hospital mortality (acetaminophen 5.6% vs placebo 18.2%, p = 0.355) were also not significantly different between groups (Figure 4).
Figure 4. In-hospital Survival.
All 40 patients who completed the a priori trial protocol were followed through death or hospital discharge and were included in the analysis of in-hospital survival.
Protocol Adherence and Adverse Events
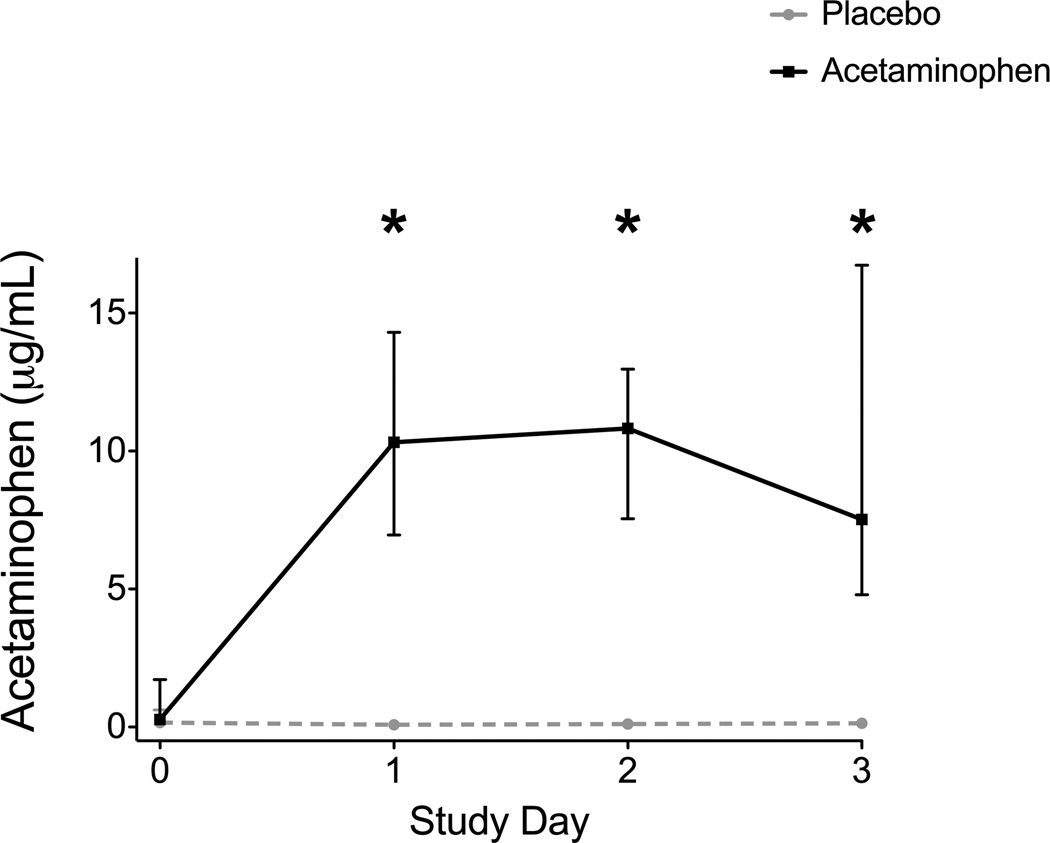
Acetaminophen was detectable and significantly increased in the plasma of the acetaminophen group on every study day after enrollment compared to the placebo group (Figure 5). Among the 40 patients in the primary analysis, 7 (38%) acetaminophen patients and 10 (45%) placebo patients missed any dose of study drug (p = 0.75) with a median number of doses missed of 2 (IQR 1–5). Missed doses were due to nothing by mouth status (42%), inability to swallow with a lack of enteral access (35%), and patient refusal (23%). No off-protocol acetaminophen was received by any patient during the 3 day study period.
Figure 5. Plasma Acetaminophen Levels on Enrollment and Each Study Day.
Acetaminophen levels were negligible at baseline and increased significantly every study day in those receiving acetaminophen compared to placebo. * = p < 0.001 for between group comparisons at each time point. Squares and circles represent medians and bars represent interquartile ranges.
Although the primary analysis included 40 patients who were able to complete the a priori defined protocol, adverse event data was collected on all patients exposed to any amount of study drug. Among these 44 patients, there was no occurrence of rash or other hypersensitivity. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were recorded if measured for clinical purposes; however measurement of AST and ALT was not a mandated part of the study since the dose used in this study is approved by the FDA and has a good safety profile (17). Of the patients who were exposed to any amount of study drug and who had AST or ALT measured as part of their routine ICU care, there were 2 (9.5%) patients in the acetaminophen group and 1 (4.3%) in the placebo group (p = 0.599) who had a new AST or ALT elevation above 400 U/L after enrollment. Among the 40 patients in the primary analysis who received ≥ 4 doses of study drug, the median highest measured AST at the completion of the study in the acetaminophen group (75 U/L, IQR 24–75) or during the remainder of the patient’s hospitalization (29 U/L, IQR 25 – 184) was not statistically different from placebo (study completion, 49 U/L, IQR 42 – 410, p = 0.699) (remainder of hospitalization, 49 U/L, IQR 21 – 101, p = 0.934).
Discussion
This randomized, double-blind, placebo-controlled trial of acetaminophen for the reduction of oxidative injury in patients with severe sepsis and detectable CFH demonstrated that acetaminophen may reduce oxidative injury as measured by plasma levels of F2-IsoPs and IsoFs and was effective in decreasing creatinine compared with placebo. Although levels of F2-IsoPs were not different at day 3, this analysis included fewer patients (n = 31) than the analysis at day 2 (n = 39) which showed a significant reduction of F2-IsoPs with acetaminophen.
CFH can be detected in the plasma in a number of disease states, such as sickle cell-anemia (1), malaria (20), patients undergoing hemodialysis (2), cardiac bypass (3, 4), and red blood cell transfusion (5). In all of these patient populations, CFH has been associated with poor clinical outcomes thought to be mediated by the propensity of CFH to scavenge nitric oxide (1, 2, 5, 7, 8) and damage the vascular endothelium (11), as well as the detrimental ability of the iron moiety of CFH to oxidize lipid membranes and release F2-IsoPs and IsoFs (3, 12). CFH-related iron deposition and production of the highly vasoconstrictive F2-IsoPs and IsoFs is thought to be the primary mechanism of renal injury in CFH and other hemoprotein-mediated disease states (11, 12, 20) such as rhabdomyolysis.
Acetaminophen, at therapeutic concentrations, is a potent inhibitor of hemoprotein-mediated lipid peroxidation owing to its ability to reduce the ferryl radical present in CFH (13). This reductive capacity of acetaminophen inhibits the ability of CFH to cause oxidative damage and induce organ injury. In an animal model of rhabdomyolysis leading to acute kidney injury induced by the hemoprotein myoglobin, animals treated with acetaminophen had reduced F2-IsoPs and creatinine levels and improved creatinine clearance (14).
Sepsis is characterized in part by oxidant injury (21), kidney injury (22), and high mortality (23, 24). Animal models of sepsis support CFH as a mediator of morbidity and mortality; in these models end-organ damage and risk of mortality could be attenuated by rescue therapies directed at CFH (25). Using observational data, we (9) and others (10) have recently described CFH as a potential injurious mediator in humans with sepsis, the presence of which is associated with oxidative injury and increased mortality. We also reported that acetaminophen use in patients with sepsis was associated with lower plasma levels of F2-IsoPs and was independently associated with decreased in-hospital mortality in patients with detectable CFH (9).
The current randomized trial suggests that acetaminophen may reduce oxidative and renal injury in patients with sepsis and detectable CFH. Although there was no significant difference in the primary outcome of F2-IsoP on study day 3, the analysis of the secondary outcome of patients on study day 2 showed a significant reduction in F2-IsoP in the acetaminophen group. There are a number of possible explanations for the discrepant findings at day 2 and day 3. First, the study day 3 analysis was underpowered to detect a difference with 8 patients missing from the analysis compared to study day 2 and the original study power analysis may have overestimated the anticipated effect size. Second, the additional thin layer chromatography step required for patients receiving propofol may have introduced a systematic bias into the F2-IsoP measurements. However, removing patients receiving propofol from the analysis did not change the study results significantly. Although there was no substantial change in the F2-IsoP analysis after removing patients receiving propofol, we cannot predict how the inclusion or exclusion of these patients in future, larger trials will affect F2-IsoP analysis. Third, acetaminophen might have an early effect on F2-IsoPs that is not durable in patients with multiple potential sources of oxidative injury. Finally, it is possible that acetaminophen may lack efficacy in reducing F2-IsoPs and any difference only reflects a baseline imbalance. Potentially of greater clinical relevance was the robust and consistent reduction in creatinine seen in the acetaminophen group which is in agreement with past animal studies (14) and was consistent across a number of different analyses. As even small elevations of creatinine are common in sepsis and associated with substantially increased morbidity and mortality (22, 26), new therapies for this complication are needed.
The current trial has some limitations. First, the small sample size, single center, and surrogate endpoints limit the generalizability of the results. Second, there were baseline imbalances in age, creatinine, and F2-IsoP levels which may influence the results. The safety profile of acetaminophen at this dose and schedule appears to be acceptable in regards to laboratory values and clinical outcomes in critically ill adults with severe sepsis; however AST and ALT monitoring was not mandated in the protocol which may reduce the sensitivity of detecting adverse events. Finally, this trial was conducted only in medical ICU patients with severe sepsis, limiting the generalizability of the data to other patient populations.
This trial may have a number of implications for future research in critically ill patients with sepsis. Enteral acetaminophen is a common, inexpensive, and easily administered drug with an acceptable safety profile during short-term use in healthy subjects (17) and in the current study of critically ill patients with severe sepsis. Although the reduction in biomarkers of oxidative injury in the study was not consistent across all analyses, the biologic plausibility of benefit with acetaminophen in patients with sepsis and detectable CFH, ease and safety of administration, consistent although non-significant improvement across all clinical outcomes, and the finding of improved renal function should warrant a larger trial in this patient population.
Conclusions
Although there was no significant reduction in plasma levels of F2-IsoPs in the acetaminophen group on study day 3, acetaminophen significantly improved F2-IsoPs at day 2 compared to placebo and improved renal function during and after the completion of the study in 40 patients with severe sepsis. Further study with larger sample sizes and heterogeneous patient populations is warranted to determine whether acetaminophen improves important clinical outcomes such as incidence of acute kidney injury and mortality in patients with severe sepsis.
Acknowledgements
Sources of Funding
Supported in part by the Vanderbilt CTSA grant UL1 TR000445 from NCRR/NIH, NIH T32 HL087738, UL1 RR024975-01, HL103836, HL090785, HL117676, GM15431, and Courtney’s Race for the ARDS Cure and the Courtney Charneco Family
Dr. Janz received support for article research from the National Institutes of Health (NIH). Dr. Bastarache received support for article research from the NIH and the American Heart Association. Her institution received grant support. Dr. Rice consulted for Avisa, LLC and GlaxoSmithKline, LLC (Member of DSMB) and received support for article research from the NIH. His institution received grant support from the NIH NHLBI. Dr. Bernard received support for article research from the NIH. His institution received grant support from the NIH. Dr. Oates received support for article research from the NIH and served as a board member for Cumberland Pharmaceuticals. His institution received grant support from the NIH and has a patent (Use patent for acetaminophen in the treatment of diseases induced by hemeprotein catalyzed lipid peroxidation). Dr. Ware received support for article research from the NIH. Her institution received grant support from the NIH.
We would like to thank all of the patients who participated in this study, the intensive care unit personnel who assisted with the study, and Arthur P Wheeler, MD who served as the data and safety monitor.
Abbreviations
- CFH
cell-free hemoglobin
- F2-IsoP
F2-Isoprostanes
- F2-IsoF
F2-Isofurans
- PaO2
partial pressure of arterial oxygen
- FiO2
fraction of inspired oxygen
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- APACHE
Acute Physiology and Chronic Health Evaluation
- ICU
intensive care unit
- SOFA
sequential organ failure assessment
Footnotes
Authors’ Contributions
DRJ, JAB, TWR, GRB, JAO, LJR, and LBW were involved in the study design. DRJ, MAW, GS, and NW collected the data. DRJ and LBW performed the statistical analysis. DRJ drafted the manuscript and all authors participated in the revision of this manuscript. All authors read and approved the final manuscript.
These data were presented in abstract form at the American Thoracic Society International Conference, May 18, 2014 (Publication # A6568)
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
References
- 1.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 2.Meyer C, Heiss C, Drexhage C, Kehmeier ES, Balzer J, Mühlfeld A, Merx MW, Lauer T, Kühl H, Floege J, Kelm M, Rassaf T. Hemodialysis-induced release of hemoglobin limits nitric oxide bioavailability and impairs vascular function. J Am Coll Cardiol. 2010;55:454–459. doi: 10.1016/j.jacc.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 3.Billings FT, Ball SK, Roberts LJ, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. 2011;50:1480–1487. doi: 10.1016/j.freeradbiomed.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermeulen Windsant IC, Hanssen SJ, Buurman WA, Jacobs MJ. Cardiovascular surgery and organ damage: time to reconsider the role of hemolysis. J Thorac Cardiovasc Surg. 2011;142:1–11. doi: 10.1016/j.jtcvs.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Curr Opin Hematol. 2009;16:515–523. doi: 10.1097/MOH.0b013e32833157f4. [DOI] [PubMed] [Google Scholar]
- 6.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299:2304–2312. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minneci PC, Deans KJ, Zhi H, Yuen PST, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donadee C, Raat NJH, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janz DR, Bastarache JA, Peterson JF, Sills G, Wickersham N, May AK, Roberts LJ, Ware LB. Association Between Cell-Free Hemoglobin, Acetaminophen, and Mortality in Patients With Sepsis: An Observational Study. Crit Care Med. 2013 doi: 10.1097/CCM.0b013e3182741a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamzik M, Hamburger T, Petrat F, Peters J, de Groot H, Hartmann M. Free hemoglobin concentration in severe sepsis: methods of measurement and prediction of outcome. Crit Care. 2012;16:R125. doi: 10.1186/cc11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baek JH, D'Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeder BJ, Svistunenko DA, Cooper CE, Wilson MT. The radical and redox chemistry of myoglobin and hemoglobin: from in vitro studies to human pathology. Antioxid Redox Signal. 2004;6:954–966. doi: 10.1089/ars.2004.6.954. [DOI] [PubMed] [Google Scholar]
- 13.Ouellet M, Percival MD. Mechanism of acetaminophen inhibition of cyclooxygenase isoforms. Arch Biochem Biophys. 2001;387:273–280. doi: 10.1006/abbi.2000.2232. [DOI] [PubMed] [Google Scholar]
- 14.Boutaud O, Moore KP, Reeder BJ, Harry D, Howie AJ, Wang S, Carney CK, Masterson TS, Amin T, Wright DW, Wilson MT, Oates JA, Roberts LJ. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci USA. 2010;107:2699–2704. doi: 10.1073/pnas.0910174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101:1481–1483. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 16.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 17.Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, Harris SC. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296:87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 18.Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ. Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc Natl Acad Sci USA. 2002;99:16713–16718. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milne GL, Yin H, Brooks JD, Sanchez S, Jackson Roberts L, Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Meth Enzymol. 2007;433:113–126. doi: 10.1016/S0076-6879(07)33006-1. [DOI] [PubMed] [Google Scholar]
- 20.Barsoum RS. Malarial acute renal failure. J Am Soc Nephrol. 2000;11:2147–2154. doi: 10.1681/ASN.V11112147. [DOI] [PubMed] [Google Scholar]
- 21.Ware LB, Fessel JP, May AK, Roberts LJ. Plasma biomarkers of oxidant stress and development of organ failure in severe sepsis. Shock. 2011;36:12–17. doi: 10.1097/SHK.0b013e318217025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014 doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 23.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 25.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassú AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepúlveda N, Smith A, Soares MP. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010;2:51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 26.Linder A, Fjell C, Levin A, Walley KR, Russell JA, Boyd JH. Small acute increases in serum creatinine are associated with decreased long-term survival in the critically ill. Am J Respir Crit Care Med. 2014;189:1075–1081. doi: 10.1164/rccm.201311-2097OC. [DOI] [PubMed] [Google Scholar]