Abstract
In this report, the gene regulatory mechanism by which decline in arylsulfatase B (ARSB; N-acetylgalactosamine-4-sulfatase) reduces CHST11 (chondroitin-4-sulfotransferase; C4ST) mRNA expression in human colonic epithelial cells and in colonic epithelium of ARSB-deficient mice is presented. ARSB controls the degradation of chondroitin 4-sulfate (C4S) by removing the 4-sulfate group at the non-reducing end of the C4S chain, but has not previously been shown to affect C4S biosynthesis. The decline in CHST11 expression following ARSB reduction is attributable to effects of ARSB on bone morphogenetic protein (BMP)4, since BMP4 expression and secretion declined when ARSB was silenced. Inhibition of BMP4 by neutralizing antibody also reduced CHST11 expression. When C4S was more sulfated due to decline in ARSB, more BMP4 was sequestered by C4S in the cell membrane, and CHST11 expression declined. Exogenous recombinant BMP4, acting through a phospho-Smad3 binding site in the CHST11 promoter, increased the mRNA expression of CHST11. In contrast to the decline in BMP4 that followed decline in ARSB, Wnt9A mRNA expression was previously shown to increase when ARSB was silenced and C4S was more highly sulfated. Galectin-3 bound less to the more highly sulfated C4S, leading to increased nuclear translocation and enhanced galectin-3 interaction with Sp1 in the Wnt9A promoter. Silencing Wnt9A increased the expression of CHST11 in the colonic epithelial cells, and chromatin immunoprecipitation assay demonstrated enhancing effects of Wnt9A siRNA and exogenous BMP4 on the CHST11 promoter through the pSmad3 binding site. These findings suggest that cellular processes mediated by differential effects of Wnt9A and BMP4 can result from opposing effects on CHST11 expression.
Keywords: Arylsulfatase B, sulfatase, sulfotransferase, chondroitin, Wnt, BMP
1.0 Introduction
1.1 Decline in Arylsulfatase B increases chondroitin 4-sulfate
Arylsulfatase B (ARSB; N-acetylgalactosamine 4-sulfatase) is the cellular enzyme that removes 4-sulfate groups from the non-reducing end of chondroitin 4-sulfate and dermatan sulfate, and thereby regulates their degradation. Decline in ARSB leads to increases in total sulfated glycosaminoglycans (GAG) and chondroitin 4-sulfate (C4S) [1-3]. However, whether the increases in total sulfated GAGs and C4S are attributable only to inhibition of degradation or also due to an increase in production of sulfated GAGs, particularly C4S, is unknown. In this report, we address this issue, and consider how decline in ARSB affects chondroitin-4-sulfotransferase (C4ST1,2; CHST11) expression.
Decline in ARSB leads to reduced expression of BMP4 and increased expression of Wnt9A
Decline in ARSB activity increased Wnt9A expression through transcriptional effects mediated by Sp1 and galectin-3, since galectin-3 bound less to the more highly sulfated chondroitin 4-sulfate (C4S) present when ARSB activity was reduced [2]. Exposure to the common food additive carrageenan, which reduces ARSB activity, reduced expression of bone morphogenetic protein (BMP)-4 in colonic epithelium and increased Wnt9A expression and Wnt/β-catenin signling [1-4]. The experiments presented in this report examine how ARSB-mediated changes in BMP4 and Wnt9A are implicated in the regulation of CHST11 expression.
1.2 Interaction of BMP4 with glycosaminoglycans
BMP4 was also observed to interact with several glycosaminoglycans. Recombinant human BMP4 had the tightest interaction with oversulfated hyaluronan, compared to other, less sulfated glycosaminoglycans, including unmodified hyaluronan, chondroitin sulfate, and heparan sulfate [5]. Oversulfated chondroitin sulfate-E was also shown by fluorescence correlation spectroscopy with fluoresceinamine-labeled GAGs to have a high affinity for BMP-4. Osteoblast differentiation and bone mineralization were enhanced in the presence of the oversulfated CSE [6]. In other investigation, the Sulfs were reported to activate BMP4 through release from the cell surface of the HSPG-binding antagonist of BMP identified as noggin [7]. GAG sulfation was recently observed to regulate osteogenesis by supporting osteoblast functions and calcium deposition and by inhibition of osteoclasts in an in vitro model of artificial extracellular matrix with murine mesenchymal stem cells [8].
1.3 Interaction of chondroitin sulfate with Wnt
Wnts have previously been reported to interact with sulfated GAGs, particularly with the 6-SO4 group of chondroitin 4,6-disulfate (chondroitin sulfate E; CSE) and heparin/heparan sulfate [9-14]. Wnts were noted to bind to the cell surface through the naturally occurring sulfated GAGs, and treatment of Wnt-responsive cells with GAG lyase reduced the Wnt activity by 50% in S2 bone stromal cells [9]. Squid CSE was shown to bind strongly to wnt-3a, as did bovine lung heparin [10]. Exogenous CSE was able to inhibit the increase in β-catenin induced by wnt-3a, further suggesting that the wnt-3a effect was modulated by CSE. CHST11 expression was markedly less in L cells that stably expressed Wnt-3a, and sustained Wnt signaling negatively regulated CHST11 expression, indicating that Wnt diffusion was regulated through CHST11 [11]. When bovine articular chondrocytes and human articular chondrocytes in culture were treated with Wnt3a, the chondrocyte development was affected, and decline in either GAG sulfation or chondroitin sulfate (CS) content diminished the response to Wnt signal from conditioned media obtained from a cell line stably transfected with Wnt3a [12].
1.4 Interaction of Wnt with heparin/heparan sulfate
Interactions of Wnts with heparin and heparan sulfate have also been reported. The extracellular Sulf-2 enzymes, which secrete 6-O endosulfatases, released Wnt ligands from heparan sulfate proteoglycans (HSPG) [13]. The model by which QSulf1, a cell surface endosulfatase, promoted Wnt signaling was also by weakening the association of Wnt ligands with the 6-OSO4 group of HSPG [14].
1.5 Interactions of ARSB withBMP4 and Wnt
In this report, we present mechanisms that integrate extracellular signals with intracellular transcriptional events, as required for developmental processes. Extracellular and intracellular signals may be integrated through modulation of ARSB activity by oxygen and the associated changes in chondroitin 4-sulfation [15]. Subsequent variation in binding to more or less sulfated C4S can then regulate other cell processes, as shown by effects on galectin-3 leading to increased transcription of versican, HIF-1α, and Wnt9A in human epithelial cells and in the ARSB-deficient mouse [2,15,16]. The studies in this report address the impact of ARSB on BMP4/Wnt mediated CHST11 expression in intestinal epithelium and provide a new perspective on the interaction between degradation and synthesis of CS.
2.0 Materials and Methods
2.1 Cell lines and animal model
The NCM460 cell line is a nontransfected, human colonic epithelial cell line, originally from the normal colonic mucosa of a 68-yr-old Hispanic male [17]. NCM460 cells were obtained and cultured in M3:10A medium (INCELL, San Antonio, TX) at 37°C in a humidified 5% CO2 environment in 6, 12, or 24 multiwell plates. Some cell preparations were exposed to λ-carrageenan (1 μg/ml; Sigma-Aldrich Co., St. Louis, MO) for 24h. HT-29 cells (ATCC #HTB-38) are a human colonic adenocarcinoma cell line, propagated in DMEM media with 10% FBS. NCM460 and HT-29 cells were treated similarly in all experiments. Both cell lines had been multiply passaged and frozen and re-cultured from frozen stock. Cells at ∼80% confluence were harvested by scraping, and spent medium was collected from control and treated wells and stored at -80°C pending further analysis.
Eight-week old male C57BL/6J (The Jackson Laboratories, Bar Harbor, Maine) were housed in the Institutional Animal Care and Use Committee-approved Veterinary Medicine Unit at the Jesse Brown Veterans Affairs Medical Center (Chicago, IL). Animals were maintained with routine light-dark cycles and a standard mouse chow diet (65% carbohydrate, 20% protein and 15% fat) and drank water ad libitum. Heterozygous arylsulfatase B-deficient mice were obtained (Strain 005598; Jackson Laboratories, Bar Harbor, Maine) and bred as previously detailed [16,18]. All procedures were approved by the Animal Care Committees of the Jesse Brown VAMC and by the University of Illinois at Chicago. Weight and water consumption were measured routinely until the animals were euthanized by carbon dioxide inhalation and cervical dislocation. Colonic tissue was harvested promptly and frozen at -80°C. Subsequently, tissue was thawed and the mucosa was scraped from the underlying muscle and processed for RNA and protein extraction, as previously [19].
2.2 CHST11, CHST12, and CHST15 mRNA expression
Primers for Chst11 (mouse: chondroitin 4-sulfotransferase 1; NM_021439 NC_000076.6) were determined using Primer 3 [20]. The primers were: (F)5′-GCTGGAAGTGATGAGGATGAA-3′ and (R)5′- CAGGATGGCAGTGTTGGATAG-3′. For human CHST11 (chondroitin 4-sulfotransferase 1; NM018143), primers were: (F)5′-GTTGGCAGAAGAAGCAGAGG-3′ and (R)5′-GACATAGAGGTGGGCAAGGA-3′. Similarly, mRNA expression of CHST12 (human chondroitin 4-sulfotransferase 2; NM001243794) and of CHST15 (human chondroitin 4-sulfate 6-O sulfotransferase; NM_015892) was determined using the following primers: CHST12 (F)5′-CTTGTTCACTCCACTGCCTCT-3′ and (R)5′- TTTACTCCAGCATTCCCTTCC-3′. Expression of CHST15 was detected using the primers: (F)5′- ACTGAAGGGAACGAAAACTGG -3′ and (R)5′-CCGTAATGGAAAGGTGATGAG -3′. Real-time semi-quantitative polymerase chain reaction was performed with the M×3000P® (Stratagene, La Jolla, CA). Total RNA was extracted and purified from the cultured NCM460 and HT-29 cells and the mouse intestinal tissue by RNeasy minikit (Qiagen, Valencia, CA). Equal amounts of purified RNAs from the control and treated cells were reverse transcribed and amplified using Brilliant® SYBR Green QRT-PCR Master Mix (Stratagene) with β-actin as an internal control. Cycle threshold (Ct) was determined during the exponential phase of amplification, and compared using the formulae: Fold Change = 2Δ3 with Δ3 = Δ1- Δ2; Δ1 =Ctcontrol target gene - Ctcontrol actin and Δ2 =Cttreated target gene - Cttreated actin as previously [2].
2.3 Sulfotransferase activity
Sulfotransferase activity was determined using the Universal Sulfotransferase Activity Kit (R&D, Minneapolis, MN), which assesses the sulfotransferase activity of all the sulfotransferases in the cells or tissue tested. 3′-phosphoadenosine-5′-phosphosulfate (PAPS) is the sulfate donor for the sulfotransferase reaction. PAPS (10 μl, 1 mM), acceptor substrate (10 μl), and coupling phosphatase (3.5 μl, 100 ng/μl) were combined with 25 μl/well of the cell suspension in which overall sulfotransferase activity was to be determined in the wells of a microtiter plate. For negative controls, assay buffer was substituted for the cell suspension. The mixture was incubated for 20 minutes at 37°C, then 30 μl of malachite green was added and the mixture gently tapped. De-ionized water (100 ul) was added to each well, and color was developed. Optical density of each well was read and compared among the different wells. The amount of product formation was calculated using a phosphate standard curve, following subtraction of the reading of the negative control. The 3-inorganic phosphate released by the coupling phosphatase and detected by malachite green phosphate detection reagent, was proportional to the 3′-phosphoadenosine-5′-phosphate generated, thereby indicating the extent of the sulfotransferase reaction which utilized the sulfate group of PAPS.
2.4 BMP-4 inhibition by neutralizing antibody
BMP4 blocking antibody (Clone 66119, MAB757, R&D) was used to inhibit the effects of BMP4 in the NCM460 cells. The effectiveness of the blocking antibody in limiting detectable BMP4 was confirmed by BMP4 ELISA (RayBiotech, Inc., Norcross, GA), using recommended procedures. ELISA showed progressive decline in measurable BMP4 with increasing antibody concentration, ranging from 25 ng/ml to 100 ng/ml × 24 h. Antibody concentration of 100 ng/ml × 24 h was used in subsequent experiments.
2.5 ARSB activity and measurements of total sulfated glycosaminoglycans and chondroitin-4-sulfate
ARSB activity measurements and determinations of sulfated glycosaminoglycans, and chondroitin-4-sulfate in the NCM460 and HT-29 cells were performed as previously reported [2,3]. A fluorimetric assay was performed with the substrate 4-methylumbilliferyl sulfate (4-MUS) using 20 μl of cell homogenate and 80 μl of assay buffer (0.05M Na acetate buffer, pH 5.6) were combined with 100 μl of substrate (5mM 4-MUS in assay buffer) in wells of a microplate. The microplate was incubated for 30 minutes at 37°C, and the reaction was stopped by 150 μl of stop buffer (Glycine-Carbonate buffer, pH 10.7), and fluorescence was measured at 360 nm (excitation) and 465 nm (emission) in an microplate reader (FLUOstar, BMG LABTECH, Inc., Cary, NC).
The Blyscan™ assay kit (Biocolor Ltd, Newtownabbey, N. Ireland) was used for detection of the sulfated glycosaminoglycans (GAGs), based on the reaction of 1,9-dimethylmethylene blue with the sulfated oligosaccharides in the GAG chains. C4S content was determined following immunoprecipitation with C4S antibody (LY111, 0.5 μg/ml, Seikagaku, Tokyo, Japan). C6S antibody (0.5 μg/ml; LSBio, Seattle, WA) was used to detect C6S, and total CS-56 antibody (1:100; SCBT) to detect total CS, using previously reported methods [15].
2.6 Chromatin immunoprecipitation assay with detection of CHST11 promoter oligonucleotide by phospho-Smad3 antibody and by phospho-Smad1,5,9(8) antibody
BMP4 transcriptional effects were previously reported to be mediated by phospho-Smad3, which is increased and undergoes nuclear translocation following BMP4 exposure [21-25]. NCM460 cells tested included: untreated control, control-silenced by siRNA, ARSB-silenced by siRNA, treated with exogenous human recombinant BMP4 (20 ng/ml × 24 h; R&D, Minneapolis, MN), or exposed to the common food additive carrageenan (1 μg/ml × 24 h) which reduces ARSB activity. The nuclear chromatin from the various cell preparations was prepared and processed, as previously described [2,16]. Primers that encompassed the phospho-Smad3 binding site in the CHST11 promoter were selected, and the targeted promoter oligonucleotide (5′-GTCT-3′) was amplified by QRT-PCR. Binding of phospho-Smad3 antibody (Cell Signaling Technology, Danvers, MA) and of phospho-Smad 1,5,9 antibody (Cell Signaling) to the CHST11 promoter oligonucleotide was compared among the different nuclear chromatin preparations, noting that Smad8 is also named as Smad9. Agarose gel electrophoresis was performed to illustrate the variation in phospho-Smad3 binding to the different nuclear preparations and to confirm the lack of binding of the phospho-Smad1,5,9 antibody to the CHST11 promoter oligonucleotide.
2.7 Wnt9A and Arylsulfatase B silencing by siRNA
Silencing of Wnt9A and of ARSB was performed using commercial siRNAs (Qiagen). SiRNA sequences for ARSB (NM_000046) silencing were: sense: 5′-GGGUAUGGUCUCUAGGCA-3′ and antisense: 5′-UUGCCUAGAGACCAU ACCC-3′. DNA target sequence for Wnt9A siRNA was: 5′ - CCGTGTGGACTTCCACAACAA - 3′. Silencing was performed using 75 ng of siRNA (0.3 μL; Qiagen) in 100 μL of serum-free medium with 6 μL of HighPerfect Transfection reagent (Qiagen), added dropwise to ∼25,000 cells in 1100 cc of serum-free media in wells of a 12-well plate, and then maintained for 24 h under the standard cell culture conditions [19].
2.8 Co-immunoprecipitation of C4S and BMP4
Chondroitin 4-sulfate antibody (2 μg/mg protein; LY111 Amsbio LLC, Lake Forest, CA) was used to immunoprecipitate NCM460 cells following ARSB silencing and control silencing, as well as with untreated control cell preparations. The BMP4 protein that co-immunoprecipitated with C4S was detected by ELISA, as above (2.4).
2.9 Treatment of cell membrane by chondroitinase ABC and keratanase
Membrane preparations were obtained from NCM460 cells treated with ARSB siRNA, or control siRNA, λ-carrageenan (1 μg/ml × 24h), an inhibitor of ARSB [1], chondroitinase ABC (0.1 U/ml; Sigma) or keratanase (0.1 U/ml; Sigma) for 1 h at 37°C, or from untreated control cells. The membrane fraction was prepared following repeated centrifugations, as previously detailed [26]. Cells were harvested in PBS containing 2 mM EDTA, disrupted by sonication in ice-cold sonication buffer [20 mM Tris-HCl, pH 7.5, 0.25 M sucrose, 10 mM MgCl2, and 2 mM EDTA] with a complete protease inhibitor mixture, then centrifuged at 10,000g for 10 minutes at 4°C [26]. The pellet was discarded, and the resulting supernatants were centrifuged at 100,000g for 1 hour at 4°C. The resulting pellet (membrane fraction) was solubilized in RIPA buffer (50 nM Tris-HCl containing 0.15 M NaCl, 1% Nonidet P40, 0.5% deoxycholic acid, and 0.1% SDS, pH 7.4). The BMP4 in the membrane fraction was measured by ELISA. BMP4 was also measured in the spent media and the cell lysate of the NCM460 cells following treatment with ARSB siRNA, control siRNA, chondroitinase ABC, and combinations of these treatments.
2.10 Determination of ratio of chondroitin 4-sulfate to chondroitin 6-sulfate
NCM460 cells in which ARSB was silenced by siRNA, or treated by scrambled control siRNA, or untreated control cells were grown in 24-well plates. Sandwich ELISA was performed using C4S antibody (LY111, 0.5 μg/ml, Seikagaku, Tokyo, Japan) or C6S antibody (0.5 μg/ml; LSBio, Seattle, WA), with total CS-56 antibody (1:100; SCBT) as the coating antibody. Antibody signal intensity was compared for C4S and C6S antibodies, with signal intensity for CS-56 antibody considered as the signal for total CS.
2.11 Statistics
Statistical significance was determined using InStat (GraphPad, San Diego, CA) software. For all determinations, at least three independent biological samples were tested, with technical replicates of each measurement. Mean values were compared using one-way ANOVA tests, with Tukey-Kramer post-test for multiple comparisons, unless stated otherwise. Some comparisons were performed using unpaired t-tests, two-tailed. P-values of ≤0.05 were recognized as significant. In the figures, * represents p≤0.05, ** p≤0.01, and *** p≤0.001.
3.0 Results
3.1 Decline in ARSB inhibits CHST11 (chondroitin-4-sulfotransferase 1) expression and sulfotransferase activity in human intestinal epithelial cells and in mouse colonic epithelium
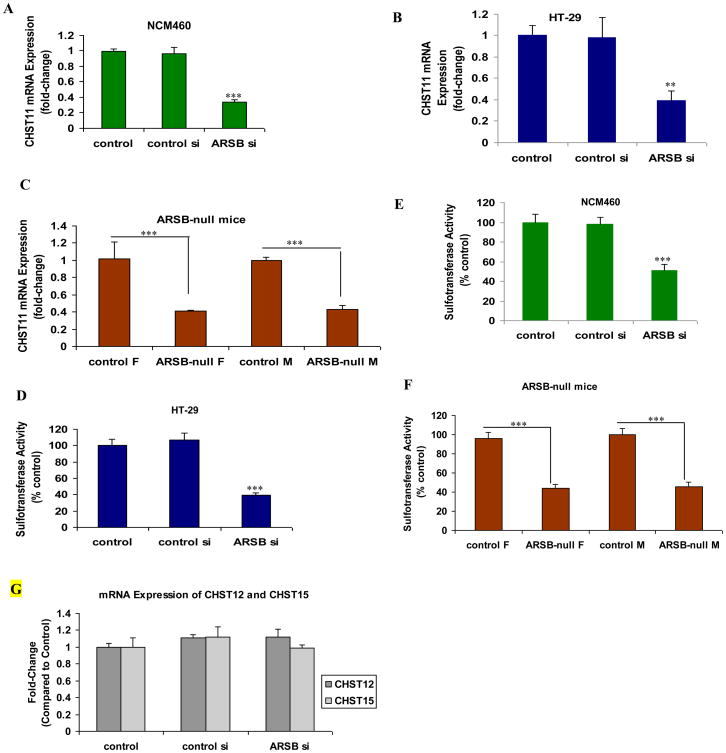
ARSB was previously shown to be effectively silenced by siRNA in the human colonic NCM460 and HT-29 cells [2]. The mRNA expression of CHST11 was determined by QRT-PCR following ARSB silencing and was significantly reduced in both of the colonic epithelial cell lines following ARSB silencing (p<0.001) (Fig. 1A, 1B). Significant reduction in expression of CHST11 was also evident in the colonic epithelium of the ARSB-null mice (Fig. 1C). Consistent with the decline in expression, the overall sulfotransferase activity was also significantly reduced in the cells and in the ARSB-null mice (p<0.001) (Fig. 1D, 1E, 1F). Expression of CHST12 (chondroitin 4-sulfotransferase 2) and CHST15 [carbohydrate (N-acetylgalactosamine 4-sulfate.6-O) sulfotransferase 15] was unaffected by ARSB silencing in the colonic epithelium of the ARSB null-mice (Fig. 1G).
Figure 1. Expression of CHST11 and sulfotransferase activity are reduced when ARSB is silenced in human colonic epithelial cells and in ARSB-null mice.
A, B. In NCM460 and HT-29 cells, marked decline in mRNA expression of CHST11 followed ARSB silencing (p<0.001, p<0.01; one-way ANOVA with Tukey-Kramer post-test; n=6).
C. In the ARSB-null mice, the Chst11 expression in the mouse colonic epithelium was significantly reduced, compared to wild-type C57BL/6J mice (p=0.001, unpaired t-test, two-tailed; n=6).
D, E. Sulfotransferase activity in the NCM460 and HT-29 cells was significantly reduced following ARSB silencing (one-way ANOVA with Tukey-Kramer post-test; n=6 for NCM460; n=3 for HT-29).
F. Sulfotransferase activity was significantly reduced in the ARSB-null mice, compared to the wild-type C57BL/6J mice (unpaired t-test, two-tailed; n=6).
G. Expression of CHST12 and CHST15 was unaffected in the ARSB-null mice (n=6).
[ARSB=arylsulfatase B; CHST11=carbohydrate (chondroitin 4) sulfotransferase 11; CHST12=carbodhydrate (chondroitin 4) sulfotransferase 12; CHST15=carbohydrate (N-acetylgalactsosamine 4-sulfate 6-O) sulfotransferase; F=female; M=male; si-small interfering siRNA]
3.2 Decline in ARSB inhibits BMP4 expression and secretion
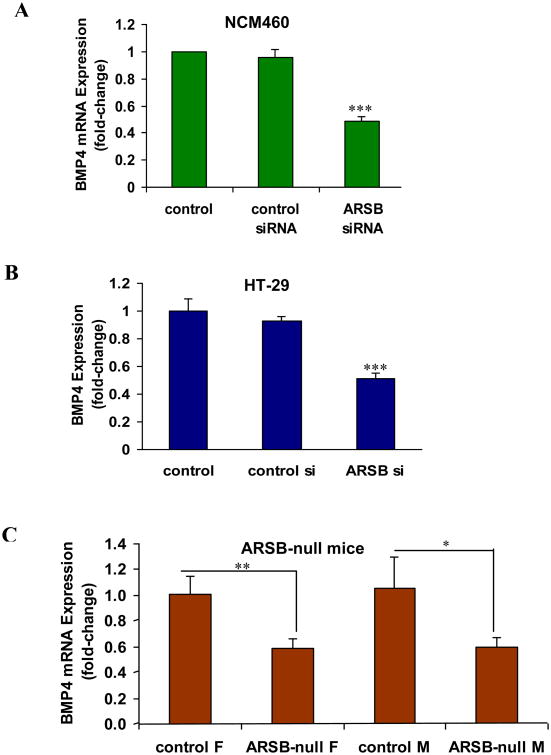
Following ARSB silencing in the NCM460 and HT-29 cells, BMP4 mRNA expression was markedly reduced (Fig. 2A, 2B). Similarly, in the ARSB-null mice, the expression of BMP4 in the colonic epithelium was significantly reduced, compared to the expression in the control mice (Fig. 2C).
Figure 2. In the colonic epithelial cell lines and in the ARSB-null mice, BMP4 expression was reduced when ARSB was silenced.
A. Silencing ARSB significantly reduced the expression of BMP4 in the NCM460 cells (p<0.001, one-way ANOVA with Tukey-Kramer post-test; n=6).
B. Similarly, in HT-29 cells, the BMP4 expression was markedly reduced when ARSB was silenced cells (p<0.001, one-way ANOVA with Tukey-Kramer post-test; n=6).
C. In the ARSB null mice, BMP4 mRNA was significantly less than in the control wild-type mice (p=0.011 for female mice, p=0.0341 for male mice; unpaired t-test, two-tailed; n=6).
[ARSB=arylsulfatase B; BMP4=bone morphogenetic protein; F=female; M=male; si-=small interfering siRNA]
3.3 BMP-4 secretion is inhibited due to increased BMP4 binding to more highly sulfated chondroitin 4-sulfate present when ARSB is reduced; Chondroitinase ABC increases secretion
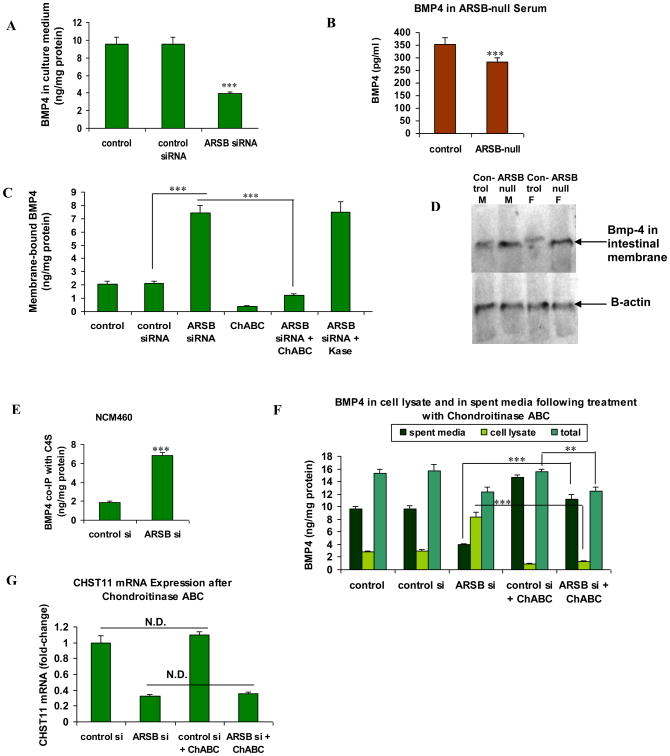
BMP4 secretion into the spent media of the NCM460 cells was significantly reduced when ARSB was silenced (Fig. 3A). Also, BMP4 in the serum of ARSB-null mice was significantly less than in the control C57BL/6J mice (Fig. 3B). In membrane preparations of NCM460 cells, following ARSB silencing, the membrane-associated BMP4 was increased significantly over the control values (Fig. 3B, C). When treated with chondroitinase ABC, which degrades the glycosidic bonds of chondroitin sulfate, the membrane-sequestered BMP4 declined to the control level. In contrast, treatment with keratanase did not affect the release of the membrane-associated BMP4. The membrane-associated Bmp4 was greater in the ARSB-null mice than in the C57BL/6J control mice, both male and female, as shown by Western blot using the membrane component of an intestinal preparation (Fig. 3D).
Figure 3. BMP4 in culture medium media declined when ARSB was silenced, due to BMP4 sequestration by C4S in the cell membrane.
A BMP4 in the spent media of the NCM460 cells was markedly reduced following ARSB knockdown (p<0.001; one-way ANOVA with Tukey-Kramer post-test; n=6).
B. BMP4 was significantly reduced in the serum of the ARSB-null mice (p<0.01, unpaired t-test, two-tailed; n=5).
C. Following ARSB silencing, the membrane-bound BMP4 increased significantly (p<0.001, one-way ANOVA with Tukey-Kramer post-test; n=3). Treatment with chondroitinase ABC reduced the membrane-bound BMP4 (p<0.001, one-way ANOVA with Tukey-Kramer post-test; n=3). In contrast, there was no effect of keratanase treatment.
D. Representative Western blot demonstrates increase in the intestinal membrane-associated Bmp-4 of male and female ARSB-null mice, compared to gender and age-matched C57BL/6J controls.
E. The quantity of BMP4 that co-immunoprecipitated with C4S in the NCM460 cells increased significantly when ARSB was silenced by siRNA (p<0.0001, unpaired t-test, two-tailed; n=3 independent experiments).
F. Following treatment with chondroitinase ABC, the BMP4 in the spent media increased significantly when ARSB was silenced (p<0.001), although the cellular BMP4 and total BMP4 were reduced (p<0.001, p<0.01, respectively; one way ANOVA with Tukey-Kramer post-test; n=3).
G. mRNA expression of CHST 11 was unaffected by treatment with chondroitinase ABC (n=6). [ARSB=arylsulfatase B; BMP=bone morphogenetic protein; ChABC=chondroitinase ABC; F=female; CHST11=carbohydrate (chondroitin 4) sulfotransferase 11; Kase=keratanase; M=male; N.D.=no difference]
To address the observed increase in membrane sequestration of C4S following ARSB silencing, the BMP4 that co-immunoprecipitated with C4S was measured following ARSB silencing. The amount of BMP4 that co-immunoprecipitated with C4S increased significantly when ARSB was silenced, in comparison to values in the control and control silenced preparations (Fig. 3C,E). When NCM460 cells were silenced by ARSB siRNA and then were treated with chondroitinase ABC, the BMP4 in the spent media increased significantly, although the cellular and total BMP4 were reduced (Fig. 3F). The increases in membrane-bound BMP4 and in the BMP4 in the spent media post-chondroitinase ABC indicate that BMP4 binds more with the more highly sulfated C4S that is present when ARSB is silenced. Treatment with chondroitinase ABC had no effect on the expression of CHST11 in the NCM460 cells (Fig. 3G).
3.4 BMP4 blocking antibody inhibits, and exogenous BMP4 increases expression of CHST11
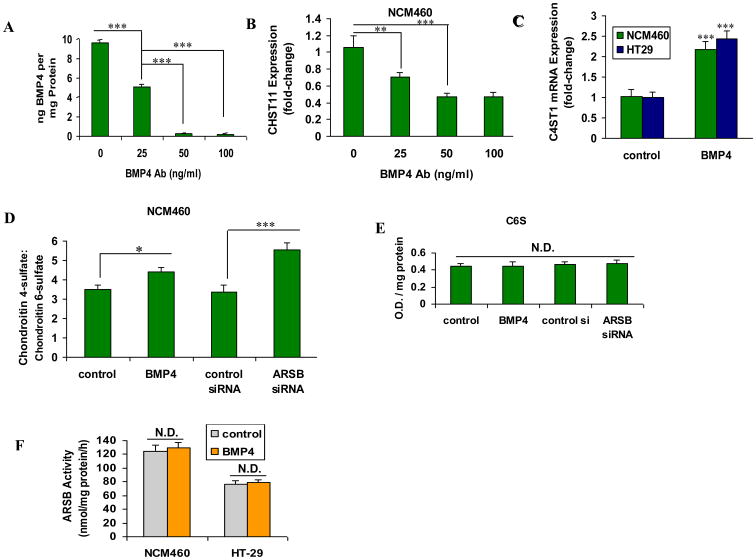
The effectiveness of blocking antibody in inhibiting the effect of BMP4 was demonstrated by BMP4 ELISA assay following exposure to increasing concentrations of blocking antibody (Fig. 4A). When NCM460 cells were treated with BMP4 blocking antibody, the mRNA expression of CHST11 declined (Fig. 4B). In contrast, exogenous recombinant human BMP4 increased CHST11 expression (Fig. 4C). Both decline in ARSB and exogenous BMP4 significantly increased the ratio of chondroitin 4-sulfate (C4S) to chondroitin 6-sulfate (C6S) in the NCM460 cells (Fig. 4D), consistent with the measured increases in C4S. The values (O.D./mg protein) for C6S were similar for the control, control si, ARSB si, and BMP4-treated preparations (Fig. 4E), indicating that the increasing ratio was due solely to the increase in C4S. Exogenous BMP4 (20 ng/ml × 24 h) had no impact on ARSB activity in the NCM460 or HT-29 cells (Fig. 4F).
Figure 4. BMP4 mediates the mRNA expression of CHST11.
A The effectiveness of BMP4 blocking antibody on the inhibition of BMP4 was demonstrated by measurements of BMP4 protein in the spent medium by ELISA (p<0.001; one-way-ANOVA with Tukey-Kramer post-test; n=3).
B. Addition of BMP4 blocking antibody to the cultured NCM460 cells progressively reduced the mRNA expression of CHST11 (p<0.01, p<0.001; one-way ANOVA with Tukey-Kramer post-test; n=3).
C. In the HT-29 and NCM460 cells, exogenous recombinant human BMP4 increased CHST11 expression significantly, compared to untreated control cell preparations (p<0.001; one-way ANOVA with Tukey-Kramer post-test; n=6).
D. Both exogenous BMP4 and ARSB silencing significantly increased the ratio of chondroitin 4-sulfate to chondroitin 6-sulfate in the NCM460 cells (p<0.05, p<0.001, respectively; one-way ANOVA with Tukey-Kramer post-test; n=3).
E. In contrast to effects on C4S, neither ARSB siRNA nor exogenous BMP4 affected the C6S content in the NCM460 cells (n=3).
F. Exogenous BMP4 (20 ng/ml × 24 h) had no impact on ARSB activity in the NCM460 or HT-29 cells (n=5).
[ARSB=arylsulfatase B; BMP=bone morphogenetic protein; C4S=chondroitin 4-sulfate; C6S=chondroitin 6-sulfate; CHST11=carbohydrate (chondroitin 4) sulfotransferase 11; N.D.=no difference; O.D.=optical density]
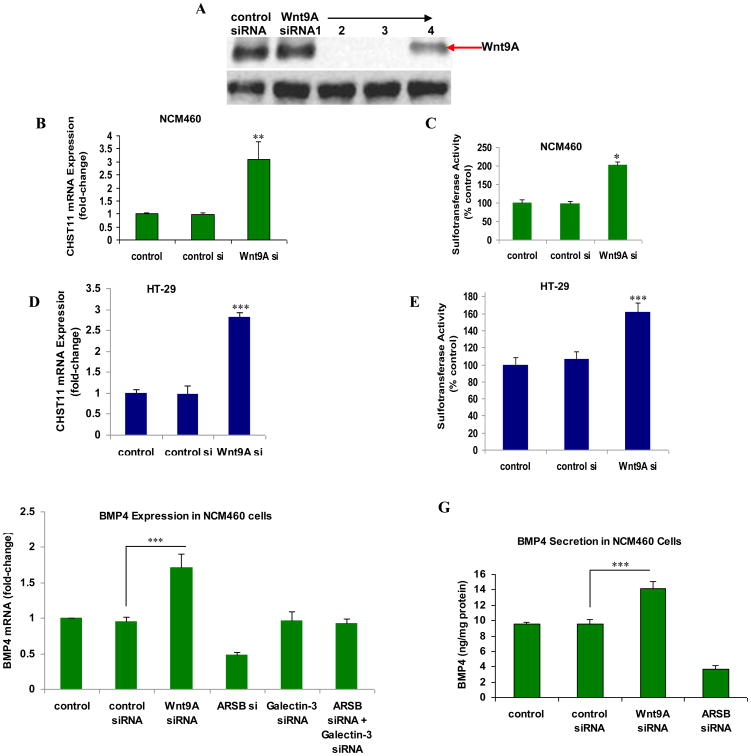
3.5 Wnt9A silencing leads to increased expression of CHST11, increased sulfotransferase activity, and increased expression and secretion of BMP 4
Reciprocal effects on tissue differentiation are triggered by opposing effects of BMP4 and Wnt in multiple tissues, and the underlying mechanisms for these inverse effects have been ascribed to many different mechanisms [27-29]. In the colonic epithelial cells, we have reported that decline in ARSB leads to increased expression of Wnt9A, mediated by Spl and galectin-3 [2]. To further assess the relationship between Wnt9A and CHST11, Wnt9A was effectively silenced by siRNA (Fig. 5A) and the effects on CHST11 mRNA expression, sulfotransferase activity, and BMP4 mRNA and protein secretion were determined. In contrast to the decline in CHST11 expression following suppression of BMP4 by blocking antibody, Wnt9A silencing significantly increased the expression of CHST11 and the sulfotransferase activity in the NCM460 (Fig. 5B, 5C) and the HT-29 (Fig. 5D, 5E) colonic epithelial cells. Wnt9A silencing also increased the BMP4 mRNA expression (Fig. 5F) and the BMP4 protein secretion (Fig. 5G), consistent with their inverse effects on CHST11 expression.
Figure 5. Wnt9A silencing increases CHST11 mRNA expression, sulfotransferase activity, and BMP4 expression.
A Two of four siRNAs were highly effective in silencing Wnt9A in the NCM460 cells, as shown by Western blot.
B. Wnt9A silencing in NCM460 cells was associated with significant increase in CHST11 expression (p<0.01; one-way ANOVA with Tukey-Kramer post-test; n=6).
C. Sulfotransferase activity was increased significantly in the NCM460 cells following Wnt9A knockdown (p<0.05; one-way ANOVA with Tukey-Kramer post-test; n=3).
D. Similarly, in the HT-29 cells, Wnt9A silencing led to marked increase in CHST11 mRNA expression (p<0.001; one-way ANOVA with Tukey-Kramer post-test; n=6).
E. Sulfotransferase activity was increased significantly in the HT-29 cells following Wnt9A knockdown (p<0.001; one-way ANOVA with Tukey-Kramer post-test; n=3).
F. Wnt9A silencing produced marked increase in BMP4 mRNA expression in the NCM460 cells (p<0.001; one-way ANOVA with Tukey-Kramer post-test; n=6). Galectin-3 silencing and galectin-3 silencing in combination with ARSB silencing had no impact on the BMP4 expression, although ARSB silencing alone reduced the BMP4 expression, as previously shown (Fig. 2A).
G. Wnt9A silencing led to marked increases in BMP4 secreted protein in the NCM460 cells, in contrast to the effects of control siRNA (p<0.001, one-way ANOVA with Tukey-Kramer post-test; n=3). ARSB silencing again reduced the BMP4 in the culture medium, as previously shown (Fig. 3A).
[ARSB=arylsulfatase B; BMP=bone morphogenetic protein; CHST11=carbohydrate (chondroitin 4) sulfotransferase 11; si=small interfering siRNA[
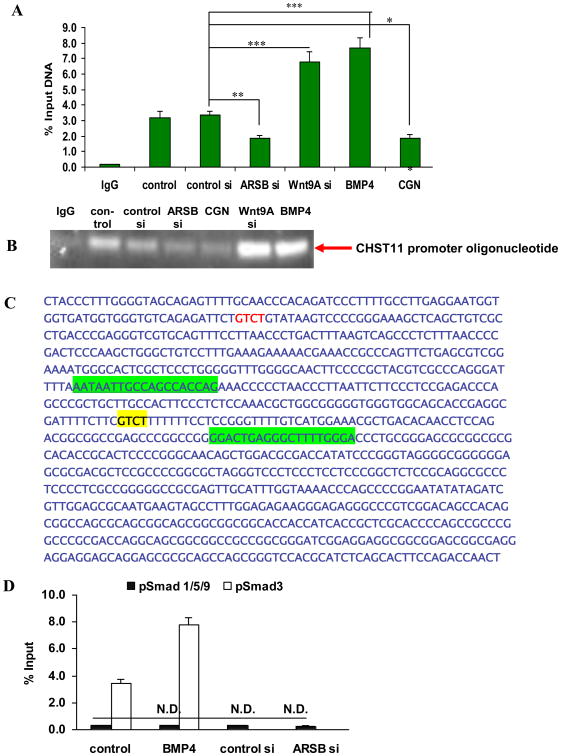
3.6 Chromatin immunoprecipitation (ChIP) assay demonstrates binding of phospho-Smad3 to CHS Til promoter oligonucleotide and inverse effects of BMP 4 and Wnt9A on extent of binding
ARSB was silenced by siRNA and ARSB activity was reduced by the sulfated polysaccharide carrageenan, and the intensity of the binding of phospho-Smad3 to its targeted consensus oligonucleotide in the CHST11 promoter was detected by chromatin immunoprecipitation (ChIP) assay. When ARSB was reduced, the binding of phospho-Smad3 declined markedly, consistent with reduced CHST11 promoter activity (Fig. 6A). In contrast, both Wnt9A silencing and exogenous BMP4 significantly enhanced the binding of phospho-Smad3 to the CHST11 promoter oligonucleotide that encompasses the phospho-Smad3 binding site. Agarose gel confirmed the effects of Wnt9A siRNA, ARSB siRNA, and exogenous BMP4 on the binding of phospho-Smad3 to this oligonucleotide (Fig. 6B). In Fig. 6C, the oligonucleotides used to amplify the promoter region of CHST11 that encompasses the phospho-Smad3 binding consensus sequence (5′-GTCT-3′) are highlighted in green and the consensus sequence is highlighted in yellow.
Figure 6. Chromatin immunoprecipitation (ChiP) assay demonstrated increase in phospho-Smad3 binding to the CHST11 promoter oligonucleotide following exogenous BMP4 or Wnt9A silencing in NCM460 cells.
A ChIP assay demonstrated increased binding to the CHST11 promoter oligonucleotide that encompasses the Smad3 binding site following exogenous BMP4 (20 ng/ml × 24 h) and Wnt9A silencing (150 ng × 24 h) (p<0.001, one-way ANOVA with Tukey-Kramer post-test; n=6). In contrast, followed ARSB siRNA (150 ng × 24 h) or exposure to the common food additive carrageenan (1 μg/ml × 24 h) which inhibits ARSB activity, the % of DNA binding declined (p<0.01, p<0.05, respectively; one-way ANOVA with Tukey-Kramer post-test; n=6).
B. Agarose gel confirmed the measurements from the ChIP assay, showing increased intensity of bands following Wnt9A silencing by siRNA or following exogenous human recombinant BMP4, and decline following ARSB silencing or carrageenan exposure.
C. Oligonucleotides used to amplify the phospho-Smad3 binding site in the CHST11 promoter are highlighted in green and the phospho-Smad3 binding consensus sequence is highlighted in yellow.
D. Phospho-Smad1,5,9 binding to the CHST11 promoter oligonucleotide was not increased in the NCM460 cells following ARSB silencing or exposure to exogenous BMP4 (n=6). As a positive control, exposure to exogenous BMP4 increased the CHST11 promoter oligonucleotide binding to the Smad binding site.
E. Agarose gel confirmed the lack of binding of pSmad1,5,9 to the CHST11 promoter oligonucleotide following ARSB silencing, exogenous BMP4, or exposure to carrageenan, an ARSB inhibitor. In contrast, increased binding of pSmad3 was again demonstrated. DNA ladder indicates band was present at the 100 bp marker.
[ARSB=arylsulfatase B; BMP=bone morphogenetic protein; CGN=carrageenan; N.D.=no difference; si=small interfering siRNA]
When ChIP was performed with phospho-Smad1,5,9 antibody, no increased binding to the CHST11 promoter oligonucleotide occurred (Fig. 6D), in contrast to the increased binding evident with pSmad2,3 antibody. Agarose gel confirmed the lack of binding by the pSmad1,5,9 antibody (Fig. 6E), although positive control showed enhanced binding of pSmad3 following exposure to exogenous BMP4. DNA ladder indicates binding position at ∼100 bp.
3.7 Schematic illustration of interactions among ARSB, CHST11, Wnt9A, BMP4, and C4S
Schematic illustration of interactions between ARSB and CHST11 presents the major transcriptional mechanisms by which decline in ARSB affects CHST11 expression through opposing effects of BMP4 and Wnt9A. In addition to increasing expression of Wnt9A, ARSB silencing also leads to enhanced sequestration of BMP4 by more sulfated C4S, thereby inhibiting the effect of BMP4 (and phospho-Smad3) on the CHST11 promoter and reducing CHST11 expression (Fig. 7). ARSB silencing also increased Wnt9A expression, due to reduced binding of galectin-3 with more highly sulfated C4S. Thus, changes in ARSB can lead to more or to relatively less expression of Wnt9A. Silencing of Wnt9A increased the expression of CHST11, demonstrating inverse effects of BMP4 and Wnt9A on expression of CHST11, mediated through ARSB and C4S.
Figure 7. Schematic illustration of interactions between ARSB, CHST11, BMP4, and Wnt9A.
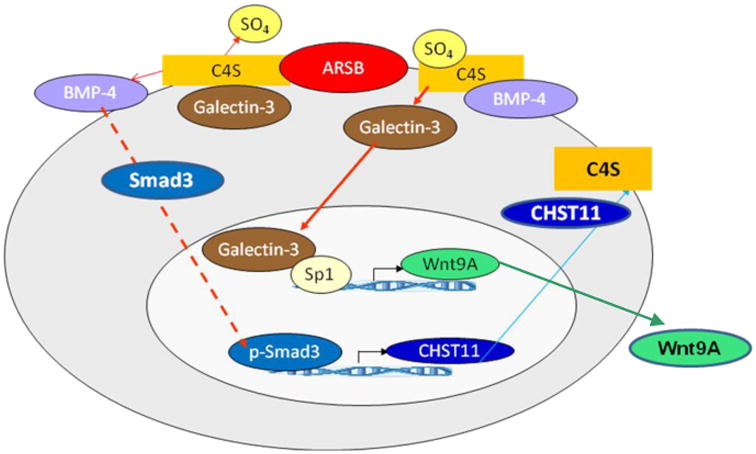
The ARSB-initiated signaling regulates the CHST11 expression. ARSB silencing increased chondroitin-4-sulfation and reduced binding of galectin-3 to the more highly sulfated C4S. Wnt expression increased, due to interaction of nuclear galectin-3 with Sp1 [2]. BMP4 secretion declined, due to increased sequestration of BMP4 with more highly sulfated C4S, leading to less nuclear phospho-Smad3 and reduced CHST11 expression. The increase in C4S present when ARSB is reduced inhibited the BMP4 secretion and increased Wnt9A expression, which was mediated by galectin-3. Inverse effects of decline in ARSB on Wnt9A expression and CHST11 expression are mediated by the opposing effects of increased chondroitin 4-sulfation on binding with galectin-3 and BMP4.
[ARSB=arylsulfatase B; BMP=bone morphogenetic protein; C4S=chondroitin 4-sulfate; CHST11=carbohydrate (chondroitin 4) sulfotransferase 11; Sp=specificity protein]
Changes in C4S sulfation inversely affect galectin-3 and BMP4 binding to C4S. When ARSB is reduced, galectin-3 binds less to the more sulfated C4S and enhances the transcription of Wnt9A through an Sp1-mediated process [2]. Inversely, BMP4 binds more to more highly sulfated C4S, leading to reduced phospho-Smad3 mediated activation of CHST11 expression. When ARSB activity is higher, C4S is less sulfated and binds less BMP4, leading to increased CHST11 expression.
4.0 Discussion
4.1 New approach to interactions of Wnt and BMP
These study findings present a new framework for examining the signaling interactions between Wnt and BMP through chondroitin 4-sulfation and involving ARSB and CHST11. Decline in ARSB leads to: 1) increase in Wnt9A expression attributable to reduced binding of galectin-3 to more highly sulfated C4S [1]; 2) decline in BMP4 expression; 3) enhanced binding of BMP4 to more highly sulfated C4S; and 4) decline in CHST11 expression. The expression of CHST11 is inhibited due to decline in the activation of phospho-Smad3 when BMP4 binds more tightly to C4S, leading to reduced activation of the CHST11 promoter and reduced expression of CHST11.
In other work has shown that ARSB activity is reduced when ambient oxygen is less [15], suggesting that the ARSB-mediated regulation of Wnt-BMP interactions in developmental and regenerative processes may be affected by oxygen gradations within the tissue. Depending on availability of more or less oxygen, the ARSB activity is modified, thereby affecting the extent of chondroitin-4-sulfation, the degradation of C4S, and critical binding interactions of molecules including BMP4 and galectin-3 with C4S. Galectin-3 binds less to the more highly sulfated C4S present when ARSB is reduced, whereas BMP-4 binds more tightly, thereby reducing the BMP4 secretion. Reduced binding of galectin-3 to C4S leads to increased galectin-3 nuclear translocation and effects on transcription, as shown by the increased expression of Wnt9A when ARSB was reduced [2]. Thus, BMP4 and Wnt9A are inversely regulated by ARSB activity due to effects on chondroitin 4-sulfation.
4.2 Both reduced production and reduced degradation of C4S when ARSB is reduced
The ratio of C4S to C6S in the colonic epithelial cells increased both by exposure to exogenous BMP4 and by ARSB silencing (Fig. 4D). ARSB silencing is anticipated to mediate the BMP4 effect, due to reduced binding of BMP4 with the more highly sulfated C4S present when ARSB is reduced. Thus, the impact on the C4S:C6S ratio when ARSB was silenced reflected both reduced production and reduced degradation of C4S, whereas exogenous BMP4 increased production of C4S. No change in C6S content was evident following either ARSB silencing or exogenous BMP4 exposure.
4.3 Increased sequestration of BMP4 by C4S when ARSB is reduced
A major conclusion of this report is that: decline in ARSB leads to increased sequestration of BMP4 by C4S and thereby reduces BMP4 signaling, leading to reduced expression of CHST11. These events may comprise a reactive mechanism, responsive to small changes in ARSB activity, either due to changes in oxygen or perhaps other inhibitors of ARSB activity, such as chloride [30]. With accumulation of C4S when ARSB-initiated degradation is reduced, less new production of C4S is required, and the BMP4 secretion is blocked.
4.4 Integration of BMP4 and Wnt signaling
Decline in ARSB was shown to lead to increased expression of Wnt9A [1, 2], thereby providing a ligand for initiation of Wnt/β-catenin signaling. Wnt/β-catenin signaling has been reported to affect the expression of BMP4 by a transcriptional mechanism, although published findings have shown both reduced BMP4 and increased BMP4, depending on the experimental systems studied [31-34]. In this report, Wnt9A silencing by siRNA increased the BMP4 mRNA expression and protein secretion (Fig. 5F, 5G), consistent with the increased CHST11 expression which followed Wnt9A silencing (Fig. 5B, 5D). We hypothesize that when ARSB activity is greater, the mRNA expression of Wnt9A is less, paracrine Wnt signaling is reduced, and an inhibitory effect on BMP4 transcription is relieved. Higher ARSB activity permits BMP4 release from the cell membrane and stimulation of new C4S production by increased CHST11. These interactions among ARSB, C4S, BMP4, and Wnt9A provide a reciprocal mechanism, whereby CHST11 expression is regulated based on the inverse pattern of C4S binding between galectin-3 and BMP4. Reduced ARSB, and the resulting increased sulfation of C4S, lead to reduced galectin-3 binding with C4S and increased Wnt9A expression, as well as increased sequestration of BMP4 by C4S and reduced expression of CHST11.
The functional integration of Wnt and BMP has been described in multiple ways, including global transcriptional programs associated with epithelial differentiation and activation of β-catenin/TCF/Lef responsive genes in development and in tissue repair. Further analysis of the impact of decline in ARSB on chondroitin sulfate structure may lead to better definition of the specific chemical associations of BMP and Wnt with both chondroitin sulfate and heparin/heparan sulfate. N-acetylgalactsoamine 4-sulfate residues at the non-reducing terminus of C4S may also be sulfated by carbohydrate (chondroitin 4-sulfate 6-O) sulfotransferase (CHST)15, leading to the doubly sulfated CSE disaccharide [35,36]. The presence of CSE would help to reconcile the observations of interactions of Wnt and BMP4 with 6-sulfate groups [5-10]. Also, further analysis of the distinct functional roles in different cell types of the heparin sulfotransferases (hs6st) and sulfatases (sulf1) vs. the chondroitin sulfotransferases (CHST11, CHST12, and CHST15) and sulfatases [ARSB and N-acetylgalactosamine 6-sulfatase (GALNS)], as well as the associated proteoglycans, in the regulation of BMP/Wnt signaling may yield improved understanding of tissue morphology and the programmatic requirements for regeneration and differentiation [37]. Post-translational modifications, such as the palmitoylation of Wnt [38], may also contribute to the complex interactions among these vital mediators of development and repair which occur between extracellular matrix and cells at the cell membrane. Clarification of the important role of ARSB in the regulation of critical transcriptional and signaling events will contribute to improved understanding of these essential mechanisms.
The impact of other related molecules, including BMP2 and TGF-β, on the expression of CHST11 is not addressed in the current studies. Phospho-Smad1,5,9 (Smad9 is also known as Smad8) did not affect the activation of the CHST11 promoter at the pSmad3 binding site, indicating a specific effect of phospho-Smad3 on the CHST11 expression. The impact of Wnt antagonists, such as members of the Dickkopf (Dkk), secreted Frizzled-related proteins (sFRP), Noggin, and Wnt inhibitory factor (WIF) families [39], is anticipated to have similar effects to those observed with Wnt9A silencing in our studies, thereby leading to increased expression of BMP4 and CHST11. Elucidation of specific, direct interactions of these and other Wnt antagonists with sulfatases, sulfotransferases, or chondroitin sulfates may lead to better understanding of the complex pathways involved in development, tissue repair, and disease pathogenesis.
Highlights.
Decline in ARSB reduces chondroitin 4-sulfotransferase (CHST11) expression
Effect of ARSB on CHST11 expression is mediated by BMP-4 and pSmad3
BMP-4 binds more tightly with chondroitin 4-sulfate when ARSB is reduced
Silencing Wnt9A increases CHST11 and BMP4 expression
Acknowledgments
Funding was provided by ULI RR 029879 to JKT from the UIC CCTS.
Abbreviations
- ARSB
arylsulfatase B
- BMP
bone morphogenetic protein
- C4S
chondroitin 4-sulfate
- ChABC
chondroitinase ABC
- CHST11
carbohydrate (chondroitin 4) sulfotransferase 11
- CHST12
carbohydrate (chondroitin 4) sulfotransferase 12
- CHST15
carbohydrate (N-acetylgalactosamine 4-sulfate 6-O) sulfotransferase 15
- ChIP
chromatin immunoprecipitation
- CS
chondroitin sulfate
- GAG
glycosaminoglycan
- PAPS
3′-phosphoadenosine-5′-phosphosulfate
- ST
sulfotransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang A, Bhattacharyya S, Linhardt RJ, Tobacman JK. Exposure to common food additive carrageenan leads to reduced sulfatase activity and increase in sulfated glycosaminoglycans in human epithelial cells. Biochimie. 2012;94(6):1309–1316. doi: 10.1016/j.biochi.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharyya S, Feferman L, Tobacman JK. Increased expression of colonic Wnt9A through Sp1-mediated transcriptional effects involving arylsulfatase B, chondroitin 4-sulfate, and galectin-3. J Biol Chem. 2014;289(25):17564–17575. doi: 10.1074/jbc.M114.561589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharyya S, Feferman L, Borthakur S, Tobacman JK. Common food additive carrageenan stimulates Wnt/β-catenin signaling in colonic epithelium by inhibition of nucleoredoxin reduction. Nutr Cancer. 2014;66(1):117–127. doi: 10.1080/01635581.2014.852228. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya S, Borthakur A, Dudeja PK, Tobacman JK. Carrageenan reduces bone morphogenetic protein-4 (BMP4) and activates Wnt/β-catenin pathway in normal human colonic epithelial cells. Dig Dis Sci. 2007;52(10):2766–2774. doi: 10.1007/s10620-006-9531-4. [DOI] [PubMed] [Google Scholar]
- 5.Hintze V, Moeller S, Schnabelrauch M, Bierbaum S, Viola M, Worch H, Schamweber D. Modifications of hyaluronan influence the interaction with human bone morphogenetic protein-4 (hBMP-4) Biomacromolecules. 2009;10(91):3290–3297. doi: 10.1021/bm9008827. [DOI] [PubMed] [Google Scholar]
- 6.Viviano BL, Paine-Saunders S, Gasiunas N, Gallagher J, Saunders S. Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of morphogenetic protein antagonist Noggin. J Biol Chem. 2004;279(7):5604–5611. doi: 10.1074/jbc.M310691200. [DOI] [PubMed] [Google Scholar]
- 7.Salbach-Hirsch J, Ziegler N, Thiele S, Moeller S, Schnabelrauch M, Hintze V, Schweber D, Rauner M, Hofbauer LC. Sulfated glycosaminoglycans support osteoblast functions and concurrently suppress osteoclasts. J Cell Biochem. 2014;115(6):1101–1111. doi: 10.1002/jcb.24750. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki T, Miyauchi S, Tawada A, Anada T, Matsuzaka S, Suzuki O. Oversulfated chondroitin sulfate-E binds to BMP-4 and enhances osteoblast differentiation. J Cell Physiol. 2008;217(3):769–777. doi: 10.1002/jcp.21557. [DOI] [PubMed] [Google Scholar]
- 9.Reichsman F, Smith L, Cumberledge S. Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction. J Cell Biol. 1996;135(3):819–827. doi: 10.1083/jcb.135.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadanaka S, Ishida M, Ikegami M, Kitagawa H. Chondroitin 4-O-sulfotransferase-1 modulates Wnt-3a signaling through control of E disaccharide expression of chondroitin sulfate. J Biol Chem. 2008;283(40):27333–27343. doi: 10.1074/jbc.M802997200. [DOI] [PubMed] [Google Scholar]
- 11.Nadanaka S, Kinouchi H, Taniguchi-Morita K, Tamura J, Kitagawa H. Down-regulation of chondroitin-4-O-sulfotransfearase-1 by Wnt signaling triggers diffusion of Wnt-3a. J Biol Chem. 2011;286(6):4199–4208. doi: 10.1074/jbc.M110.155093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shortkroff S, Yates KE. Alteration of matrix glycosaminoglycans diminishes articular chondrocytes' response to a canonical Wnt signal. Osteoarthritis Cartilage. 2007;15(2):147–154. doi: 10.1016/j.joca.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Rosen SD, Lemjabbar-Alaoui H. SULF-2: an extracellular modulator of cell signaling and a cancer target candidate. Expert Opin Ther Targets. 2010;14(9):935–949. doi: 10.1517/14728222.2010.504718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ai X, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson CP., Jr QSULF1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J Cell Biol. 2003;162:341–351. doi: 10.1083/jcb.200212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharyya S, Tobacman JK. Hypoxia reduces arylsulfatase B activity and silencing arylsulfatase B replicates and mediates the effects of hypoxia. PLoS One. 2012;7(3):e33250. doi: 10.1371/journal.pone.0033250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharyya S, Feferman L, Tobacman JK. Arylsulfatase B regulates versican expression by galectin-3 and AP-1 mediated transcriptional effects. Oncogene. 2013 doi: 10.1038/onc.2013.483. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyer MP, Manzano LA, Merriman RL, Stauffer JS, Tanzer LR. NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell Dev Biol Anim. 1996;32:315–317. doi: 10.1007/BF02722955. [DOI] [PubMed] [Google Scholar]
- 18.http://craniofacial.jax.org/publications/curtainetalnm3775m1J/html
- 19.Borthakur A, Bhattacharyya S, Dudeja PK, Tobacman JK. Carrageenan-induced Interleukin-8 production through BCL10 pathway in normal human colonic epithelial cells. AJP: Gastrointest Liver Physiol. 2007;292:G829–G838. doi: 10.1152/ajpgi.00380.2006. [DOI] [PubMed] [Google Scholar]
- 20.http://primer3.wi.mit.edu
- 21.Klüppel M, Vallis KA, Wrana JL. A high-throughput induction gene trap approach defines C4ST as a target of BMP signals. Mech Dev. 2002;118:77–89. doi: 10.1016/s0925-4773(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 22.Prokova V, Mavridou S, Papakosta P, Kardassis D. Characterization of a novel transcriptionally active domain in the transforming growth factor beta-regulated Smad3 protein. Nuclei Acids Res. 2005;33(12):2708–21. doi: 10.1093/nar/gki679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upton PD, Davies RJ, Tajsic T, Morrell NW. Transforming Growth Factor-B1 represses bone morphogenetic protein-mediated Smad signaling in pulmonary artery smooth muscle. Am J Respir Cell Mol Biol. 201349(6):1135–1146. doi: 10.1165/rcmb.2012-0470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G, Long J, Matsuura I, He D, Liu F. The Smad3 linker region contains a transcriptional activation domain. Biochem J. 2005;386:29–34. doi: 10.1042/BJ20041820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inman GJ. Commentary: Linking Smads and transcriptional activation. Biochem J. 2005;386:e1–e3. doi: 10.1042/BJ20042133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhattacharyya S, Solakyildirim K, Zhang Z, Linhardt RJ, Tobacman JK. Cell-bound IL-8 increases in bronchial epithelial cells following Arylsulfatase B silencing. Am J Respir Cell Mol Biol. 2010;42(1):51–61. doi: 10.1165/rcmb.2008-0482OC. [DOI] [PubMed] [Google Scholar]
- 27.Soshnikova N, Zechner D, Huelsken J, Mishina Y, Behringer RR, Taketo MM, Crenshaw EB, Birchmeier W. Genetic interaction between Wnt/beta-catenin and BMP receptor signaling during formation of the AER and the dorsal-ventral axis in the limb. Genes Dev. 2003;17(16):1963–1966. doi: 10.1101/gad.263003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin EJ, Erickson CA, Takada S, Burrus LW. Wnt and BMP signaling govern lineage segregation of melanocytes in the avian embryo. Dev Biol. 2001;233(1):22–37. doi: 10.1006/dbio.2001.0222. [DOI] [PubMed] [Google Scholar]
- 29.Hashiguchi M, Mullins MC. Anteroposterior and dorsoventral patterning are coordinated by an identical patterning clock. Development. 2013;140(9):1970–1980. doi: 10.1242/dev.088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotlo K, Bhattacharyya S, Yang B, Feferman L, Tejaskumar S, Linhardt R, Danziger R, Tobacman JK. Impact of salt exposure on N-acetylgalactosamine-4-sulfatase (arylsulfatase B) activity, glycosaminoglycans, kininogen, and bradykinin. Glycoconj J. 2013;30(7):667–676. doi: 10.1007/s10719-013-9468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker JC, Beddington RS, Harland RM. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 1999;13(23):3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuroda K, 1, Kuang S, Taketo MM, Rudnicki MA. Canonical Wnt signaling induces BMP-4 to specify slow myofibrogenesis of fetal myoblasts. PLoS Genet. 2012;8(11):e1003031. doi: 10.1186/2044-5040-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shu W, Guttentag S, Wang Z, Andi T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal distal patterning in the lung. Dev Biol. 2005;283(1):226–239. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Oyajobi BO, Harris SE, Chen D, Tsao C, Deng HW, Zhao M. Wnt/beta-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone. 2013;52(1):145–156. doi: 10.1016/j.bone.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohtake S, Kimata K, Habuchi O. A unique nonreducing terminal modification of chondroitin sulfate by N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase. J Biol Chem. 2003;278(40):38443–52. doi: 10.1074/jbc.M306132200. [DOI] [PubMed] [Google Scholar]
- 36.Ohtake S, Kondo S, Morisaki T, Matsumura K, Kimata K, Habuchi O. Expression of sulfotransferases involved in the biosynthesis of chondroitin sulfate E in the bone marrow derived mast cells. BBA. 2008;1780:687–695. doi: 10.1016/j.bbagen.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Dani N, Nahm M, Lee S, Broadie K. A targeted glycan-related gene screen reveals heparan sulfate proteoglycan sulfation regulates WNT and BMP trans-synaptic signaling. Bone. 2013;52(1):145–156. doi: 10.1371/journal.pgen.1003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doubravska L, Krausova M, Gradl D, Vojtechova M, Tumova L, Lukas J, Valenta T, Pospichalova V, Fafilek B, Plachy J, Sebesta O, Korinek V. Fatty acid modification of Wnt1 and Wnt3a at serine is prerequisite for lipidation at cysteine and is essential for Wnt signalling. Cell Signal. 2011;23(5):837–848. doi: 10.1016/j.cellsig.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Cruciat CM, Niehrs C. Secreted and transmembrane Wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 2013;5:a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]