SUMMARY
The nucleotide (p)ppGpp mediates bacterial stress responses, but its targets and underlying mechanisms of action vary among bacterial species and remain incompletely understood. Here we characterize the molecular interaction between (p)ppGpp and guanylate kinase (GMK) revealing the importance of this interaction in adaptation to starvation. Combining structural and kinetic analyses, we show that (p)ppGpp binds the GMK active site and competitively inhibits the enzyme. The (p)ppGpp-GMK interaction prevents the conversion of GMP to GDP, resulting in GMP accumulation upon amino acid downshift. Abolishing this interaction leads to excess (p)ppGpp and defective adaptation to amino acid starvation. A survey of GMKs from phylogenetically diverse bacteria shows that the (p)ppGpp-GMK interaction is conserved in members of Firmicutes, Actinobacteria, and Deinococcus-Thermus, but not in Proteobacteria where (p)ppGpp regulates RNA polymerase (RNAP). We propose that GMK is an ancestral (p)ppGpp target and RNAP evolved more recently as a direct target in Proteobacteria.
INTRODUCTION
In evolution, survival and fitness depend on the ability of an organism to adapt to environmental changes. Two molecules that bacteria utilize to deal with these changes are guanosine tetraphosphate and guanosine pentaphosphate, collectively referred to as (p)ppGpp (Potrykus and Cashel, 2008). (p)ppGpp is produced from GTP (or GDP) and ATP and rapidly accumulates in response to stresses (Srivatsan and Wang, 2008). While first discovered in the Gram-negative bacterium Escherichia coli starved for amino acids (Cashel and Gallant, 1969), (p)ppGpp was later found to exist in almost all bacterial species (Atkinson et al., 2011). This broad conservation of (p)ppGpp implies its antiquity and importance in bacteria. Indeed, (p)ppGpp is required not only for adaptation to nutrient limitation, but also for persistence, antibiotic tolerance, and virulence (Dalebroux et al., 2010; Maisonneuve and Gerdes, 2014; Potrykus and Cashel, 2008), suggesting that (p)ppGpp regulates many aspects of cellular physiology. How (p)ppGpp performs such diverse tasks remains incompletely understood.
In E. coli, (p)ppGpp directly binds to RNA polymerase (RNAP) (Mechold et al., 2013; Ross et al., 2013; Zuo et al., 2013) and regulates transcription initiation in concert with the transcription factor DksA (Paul et al., 2004; Paul et al., 2005) (Figure 1A). Depending on the promoter sequence, (p)ppGpp can either activate or repress transcription: it down-regulates transcription of genes encoding translation machinery and up-regulates transcription of amino acid biosynthesis and stress response genes, thus facilitating cellular adaptation to amino acid starvation and other stresses (Magnusson et al., 2005; Murphy and Cashel, 2003; Ross et al., 2013; Srivatsan and Wang, 2008). However, in the Gram-positive bacterium Bacillus subtilis, (p)ppGpp does not directly regulate RNAP (Krasny and Gourse, 2004; Ross et al., 2013), but instead controls GTP levels to indirectly regulate transcription initiation (Krasny and Gourse, 2004; Krasny et al., 2008; Kriel et al., 2014; Tojo et al., 2008). (p)ppGpp controls GTP levels through both consumption of GTP (GDP) to synthesize (p)ppGpp and direct inhibition of several enzymes along the GTP biosynthesis pathways (Figure 1A and B). One of these enzymes, guanylate kinase (GMK), was discovered in our previous work as a new target of (p)ppGpp (Kriel et al., 2012). It is an essential enzyme that catalyzes the phosphorylation of guanosine monophosphate (GMP) to form guanosine diphosphate (GDP) (Figure 1B), the precursor of GTP (Kobayashi et al., 2003; Kriel et al., 2012). Regulation of GMK allows (p)ppGpp to control GTP biosynthesis through both de novo and salvage pathways (Figure 1B). Our discovery raises several questions: What is the molecular mechanism by which (p)ppGpp inhibits GMK? How important is this regulation to cellular physiology? Finally, is this regulation also conserved in species beyond B. subtilis? (p)ppGpp is indispensable to virulence and survival of antibiotic insults in pathogens, including Gram-positive pathogens like Listeria monocytogenes (Bennett et al., 2007), Enterococcus faecalis (Abranches et al., 2009; Gaca et al., 2013), and methicillin-resistant Staphylococcus aureus (MRSA) (Dordel et al., 2014), but the responsible (p)ppGpp targets remain to be determined. Identification of direct (p)ppGpp targets in these pathogens would become the first critical step to elucidate the pivotal roles of (p)ppGpp in bacterial pathogenesis and may aid the development of effective antimicrobial strategies in the future.
Figure 1. Inhibition of GMK by pppGpp in selected firmicutes.
(A) Proposed alternative mechanisms by which (p)ppGpp regulates transcription in E. coli and B. subtilis to adapt to stresses (e.g. amino acid starvation). (p)ppGpp directly targets RNAP in E. coli, and GMK, HPRT, and IMPDH in B. subtilis. (B) (p)ppGpp inhibits multiple enzymes in the GTP biosynthesis pathways of B. subtilis. The white dots indicate the unoccupied (p)ppGpp binding sites. PRPP: phosphoribosylpyrophosphate; IMP: inosine monophosphate; XMP: xanthosine monophosphate; GMP: guanosine monophosphate; IMPDH: inosine monophosphate dehydrogenase; GMK: guanosine monophosphate kinase (guanylate kinase); HPRT: hypoxanthine-guanine phosphoribosyltransferase. Broken lines indicate multiple steps. (C) Dose-dependent inhibition of firmicute GMKs by pppGpp. Data was fitted into the equation , where y is the relative enzyme activity, x is the inhibitor concentration, and s is the slope factor. GMK activity was assayed with increasing concentration of pppGpp. Error bars represent standard error of the mean from three replicates. All the tested GMKs contain an N-terminus 6xHis tag, but the tag does not affect the sensitivity of GMK to pppGpp (Figure S1A). Compared to pppGpp, GTP only slightly inhibits firmicute GMKs (Figure S1C). (D) Sequence alignment of GMKs from selected firmicutes using MEGA (Tamura et al., 2011). Residues that interact with pppGpp only and both pppGpp and GMP are highlighted in yellow and light blue, respectively. Domains are assigned as previously described with minor modification (Hible et al., 2005). S. aureus GMK serves as the reference and identical residues are shown as dots. See also Figure S1.
Here, combining structural and enzyme kinetic analyses, we show that (p)ppGpp binds the GMK active site and acts as a competitive inhibitor. Furthermore, we demonstrate that regulation of GMK by (p)ppGpp blocks GMP to GDP conversion to curtail GTP biosynthesis upon nutrient downshift in B. subtilis. Abolishing this regulation leads to enhanced (p)ppGpp synthesis to drain excess GTP (GDP) and impairs cellular adaptation to nutrient downshift. Remarkably, the GMK regulation is broadly conserved in phylogenetically diverse groups of bacteria, including many Gram-positive pathogens. Our work highlights the molecular details, broad conservation, and physiological importance of the (p)ppGpp-GMK regulation in bacteria.
RESULTS
(p)ppGpp competitively inhibits GMK activity across Firmicutes
(p)ppGpp inhibits the activity of B. subtilis GMK both in vivo and in vitro (Kriel et al., 2012). B. subtilis belongs to a large phylum of low GC Gram-positive bacteria, Firmicutes, which includes human pathogens such as B. anthracis, E. faecalis, L. monocytogenes, S. aureus, and S. mutans. To examine whether inhibition of GMK by (p)ppGpp was conserved in these bacteria, we purified their GMKs as recombinant proteins and tested their sensitivity to (p)ppGpp in vitro. We found that pppGpp and ppGpp inhibited all GMKs tested (Figure 1C and Figure S1D). The IC50 values of pppGpp (concentration required for 50% inhibition, at 50 μM GMP and 4 mM ATP) for S. aureus, L. monocytogenes, S. mutans GMKs, and E. faecalis GMK-1 were ~9–24 μM, comparable to that for B. subtilis GMK (~16 μM) (Table S1). GMK from B. anthracis, a close relative of B. subtilis, was less sensitive to pppGpp (IC50 ~120 μM) (Figure 1C and Table S1), despite its high sequence similarity to B. subtilis GMK (78% identity) (Figure 1D). Interestingly, E. faecalis has a second GMK, annotated here as GMK-2, whose sequence is more diverse (Figure S2G); GMK-2 was only mildly inhibited by pppGpp (IC50 ~460 μM) (Gaca et al., 2013).
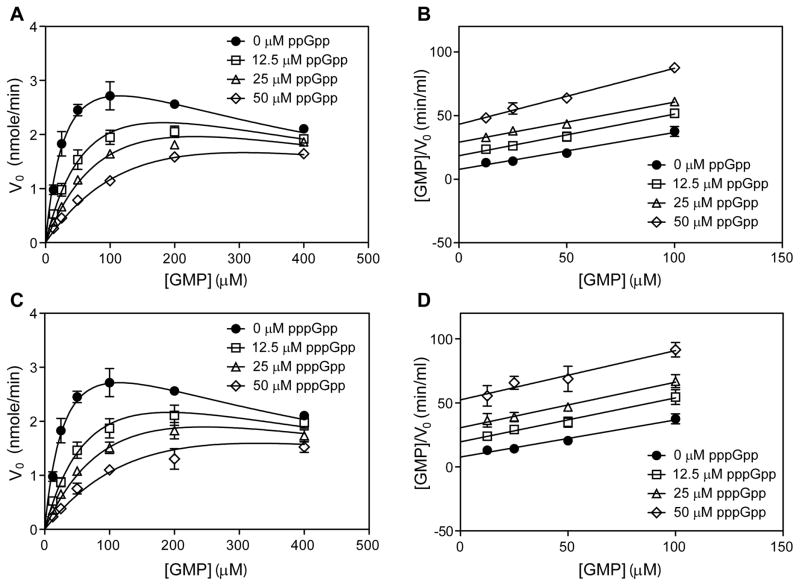
We next investigated the mechanism underlying inhibition of GMK by (p)ppGpp using steady-state kinetic assays. GMK catalyzes the formation of GDP from GMP using ATP as the phosphate donor. To determine the kinetic mechanism of (p)ppGpp inhibition, the effects of varying levels of (p)ppGpp on the initial velocities of the GMK-catalyzed reaction were measured as a function of GMP concentration (Figure 2A and C, and Figure S2A-F), with saturating ATP (4 mM, Figure S1E). Kinetic parameters (kcat, Km, and Ki) were determined by globally fitting the data to a modified competitive inhibition model that accounted for substrate inhibition (Table 2). The dissociation constants (Ki) of pppGpp in general correlated with the IC50 values determined in Figure 1C (Table S1). Kinetic data were also examined graphically using the Hanes-Woolf analysis, in which competitive inhibition yields parallel lines with y-intercepts that depend on the concentration of inhibitor. As shown for S. aureus GMK with ppGpp (Figure 2B) and pppGpp (Figure 2D), parallel lines in the Hanes-Woolf transformation were observed, supporting a competitive inhibition mode. We also characterized GMKs from other firmicutes and found that pppGpp competitively inhibited all of them, except for E. faecalis GMK-2 that appeared to be noncompetitively inhibited by pppGpp (Figure S2A–F and Table 2).
Figure 2. (p)ppGpp competitively inhibits GMK activity.
(A and B) ppGpp competitively inhibits S. aureus GMK. Data is fitted into a modified competitive inhibition equation: to account for substrate (GMP) inhibition (Supplemental Experimental Procedures) (A). Selected data points ([GMP] ≤ 100 μM) are transformed into the Hanes-Woolf equation: , to minimize the effect of substrate inhibition on the linear regression (B). (C and D) pppGpp competitively inhibits S. aureus GMK. Data fitting and transformation are described as in (A and B). See also Figure S2.
Table 2.
Summary of kinetic parameters for GMKs, see also Figure S2
| Species | kcat (sec−1)a | Km (μM)a | Ki (μM)b | Modec | Phylum/Class |
|---|---|---|---|---|---|
| B. anthracis | 57.6 ± 21.9 | 78.1 ± 39.7 | 42.5 ± 7.3 | Comp | Firmicutes |
| B. subtilis | 23.0 ± 1.0 | 24.6 ± 3.4 | 13.5 ± 2.1 | Comp | Firmicutes |
| E. faecalis-1 | 37.3 ± 4.0 | 80.3 ± 14.3 | 5.1 ± 0.5 | Comp | Firmicutes |
| E. faecalis-2 | 23.4 ± 0.6 | 42.3 ± 3.2 | 355.0 ± 26.0 | Noncomp | Firmicutes |
| L. monocytogenes | 54.9 ± 5.6 | 84.7 ± 14.2 | 12.0 ± 1.1 | Comp | Firmicutes |
| S. aureus | 72.9 ± 7.2 | 35.1 ± 7.1 | 8.2 ± 0.4/7.0 ± 0.9 | Comp | Firmicutes |
| S. mutans | 68.9 ± 24.8 | 147.4 ± 68.6 | 16.6 ± 2.4 | Comp | Firmicutes |
| A. tumefaciens | 24.8 ± 0.5 | 18.0 ± 2.1 | 53.5 ± 8.9 | Comp | α-Proteobacteria |
| S. meliloti | 19.5 ± 0.3 | 14.1 ± 1.3 | 81.6 ± 11.8 | Comp | α-Proteobacteria |
| D. radiodurans | 44.5 ± 4.4 | 17.8 ± 5.4 | 1.6 ± 0.4 | Comp | Deinococcus-Thermus |
| T. thermophilus | 4.8 ± 0.4 | 17.5 ± 4.1 | 2.9 ± 0.5 | Comp | Deinococcus-Thermus |
| C. gilvus | 25.0 ± 2.7 | 22.5 ± 7.3 | 1.7 ± 0.5 | Comp | Actinobacteria |
| S. coelicolor | 25.5 ± 0.7 | 29.7 ± 3.4 | 61.5 ± 9.6 | Comp | Actinobacteria |
Values are kcat and Km for GMP with 4 mM ATP at 25 °C and were determined by globally fitting the kinetic data into the modified competitive inhibition equation (Supplemental Experimental Procedures).
Values are Ki for pppGpp. For S. aureus GMK, reported here are Ki for ppGpp and pppGpp, respectively.
Mode of inhibition was determined relative to GMP. Comp and noncomp stand for competitive and noncompetitive, respectively.
Given the competitive nature, the efficacy of (p)ppGpp in inhibiting GMK depends not only on (p)ppGpp, but also GMP concentration. Applying liquid chromatography-mass spectrometry (LC-MS) to B. subtilis, we measured the basal levels of GMP during exponential growth in rich medium to be ~10 μM. (p)ppGpp concentration is estimated to be 10–20 μM during exponential growth, but reaches up to millimolar level during amino acid starvation in B. subtilis (Kriel et al., 2012). If similar levels of (p)ppGpp and GMP are attained in other firmicutes, GMKs tested here should be strongly inhibited during amino acid starvation and should also be regulated by basal levels of (p)ppGpp during exponential growth.
Crystal structure of pppGpp-bound S. aureus GMK
To map the (p)ppGpp binding site on GMK, we obtained a 1.67 Å resolution X-ray crystal structure of S. aureus GMK in complex with pppGpp (Figure 3 and Table 1). The structure was determined by molecular replacement using the GMP-bound S. aureus GMK structure (El Omari et al., 2006) as a search model followed by rounds of model building and structure refinement. The asymmetric unit contained four GMK monomers, three of which had electron density for a pppGpp ligand (Figure 3A) with the fourth monomer free of ligand. The three pppGpp-bound monomers were superimposable but each differed from the apo GMK monomer in the asymmetric unit (RMSD = 1.3–1.5 Å for 178 common Ca atoms). The difference between the two states was due to an apparent closure of GMK to clamp onto pppGpp (Figure 3B). Consistent with the competitive nature of pppGpp, the positions of common atoms in pppGpp- and GMP-bound GMK matched one another closely and both ligand-bound forms were present in a very similar closed state (RMSD = 0.7 Å for 165 common Ca atoms) (Figure 3C).
Figure 3. Structure of pppGpp-bound S. aureus GMK.
(A) Omit electron-density map of one selected pppGpp/Mg2+ contoured at 2 σ with the refined ligand structure shown. (B) Apo (green) and pppGpp-bound (blue) GMK are superimposed to indicate the differences between the two structures. (C) GMP (magenta) (El Omari et al., 2006) and pppGpp-bound GMK (blue) are superimposed. The positions of atoms shared between GMP and pppGpp are nearly identical in the two structures. (D) Detailed view of the pppGpp-binding pocket on GMK. Several residues from GMK recognize the base (Ser39, Glu74, Ile104, and Glu105), sugar (Glu103), 5′ triphosphate (Arg43, Arg46, Tyr55, Tyr78, and Tyr83), and 3′ diphosphate (Lys19, and Tyr78) elements of pppGpp. Yellow residues interact with pppGpp only, whereas blue residues interact with both pppGpp and GMP. (E) Summary of kinetic parameters for GMKs. The dissociation constant (Ki) of pppGpp is shown in boldface.
Table 1.
X-ray data collection and structure determination statistics
| Data Collection | |
| Wavelength, Å | 0.97872 |
| Resolution Range (high resolution bin), Å | 79.56-1.644 (1.686-1.644) |
| Space Group | P21 |
| Unit Cell (a, b, c (Å)) | 69.486, 93.961, 85.259 |
| (α, β, γ (°)) | 90, 111.09, 90 |
| Completeness, % | 99.2 (99.8) |
| Unique Reflections/Unique Collected | 619,843/123,199 |
| Redundancy | 5.0 (4.8) |
| <I/σI> | 32.4 (2.18) |
| Rsyma, % | 6.2 (60.4) |
|
| |
| Refinement
| |
| Resolution, Å | 79.56-1.64 |
| Rwork/Rfreeb, % | 20.1/22.7 |
| Rms deviations | |
| Bonds, Å | 0.006 |
| Angles, ° | 1.17 |
|
| |
| Ramachandran statistics, %
| |
| Favored | 98.5 |
| Allowed | 1.5 |
| Disallowed | 0.0 |
|
| |
| # atoms
| |
| Protein | 6093 |
| pppGpp | 120 |
| Water | 629 |
|
| |
| <B factor>, Å2
| |
| Protein | 28.35 |
| pppGpp | 25.47 |
| Water | 39.20 |
Rsym = ΣΣj|Ij − 〈I〉 |ΣIj, where Ij is the intensity measurement for reflection j and 〈I〉 is the mean intensity for multiply recorded reflections.
Rwork/Rfree = Σ||Fobs| − |Fcalc||/|Fobs|, where the working and free R factors are calculated by using the working and free reflection sets, respectively. The free R reflections (5% of the total) were held aside throughout refinement.
The pppGpp-GMK structure revealed multiple residues that coordinate pppGpp-binding (Figure 3D), all of which should also coordinate ppGpp-binding, if it assumes a similar conformation as pppGpp upon binding GMK. These residues can be classified into two groups: those involved in binding both GMP and pppGpp (Ser39, Arg43, Tyr55, Glu74, Tyr83, Glu103, Ile104, and Glu105) and those specific for binding pppGpp only (Lys19, Arg46, and Tyr78). With one exception, each of the pppGpp-binding residues identified in the S. aureus GMK structure was conserved among the tested firmicute GMKs. The lone exception was Tyr78, which was replaced by Phe78 in B. anthracis GMK (Figure 1D). This Tyr-to-Phe substitution was not specific for the strain tested but was highly conserved among multiple sequenced B. anthracis strains (data not shown). To test whether the differential affinity of (p)ppGpp for B. subtilis and B. anthracis GMK was due to this change, a Phe-to-Tyr variant of B. anthracis GMK and a Tyr-to-Phe variant of B. subtilis GMK were constructed, purified, and tested. When Phe78 was substituted for Tyr (F78Y) in B. anthracis GMK, the Ki for the variant was reduced nearly to the level of B. subtilis GMK; conversely, substitution of Tyr77 for Phe (Y77F) in B. subtilis GMK increased Ki to a level comparable to that of B. anthracis GMK (Figure 3E). Thus, the Tyr78 residue is an important determinant for the affinity of (p)ppGpp to GMK enzymes.
The (p)ppGpp-GMK interaction is conserved in multiple bacterial phyla but not in Proteobacteria
Having shown that regulation of GMK by (p)ppGpp was conserved in Firmicutes, we next asked whether this regulation was shared by other bacterial phyla. Recombinant GMK enzymes from multiple bacterial phyla were purified and tested for their sensitivity to pppGpp (Figure 4 and Figure S4). The first group of GMKs were from the high GC Gram-positive bacterial phylum Actinobacteria including Cellulomonas gilvus, Mycobacterium tuberculosis, and Streptomyces coelicolor. Whereas M. tuberculosis GMK was insensitive to pppGpp, both C. gilvus and S. coelicolor GMKs were competitively inhibited by pppGpp (Figure 4B, Table 2 and S1). The second group of GMKs from the Gram-negative phylum Deinococcus-Thermus, Thermus thermophilus and Deinococcus radiodurans, were strongly and competitively inhibited by pppGpp (Figure 4C, Table 2 and S1). Finally, GMKs from three classes of the Gram-negative phylum Proteobacteria, including E. coli, were tested. Among the three classes, α-Proteobacteria diverged earlier (Figure 4A). GMKs from the α-proteobacteria Agrobacterium tumefaciens and Sinorhizobium meliloti were modestly and competitively inhibited by pppGpp (Figure 4D, Table 2 and S1), but GMK of Rhodobacter sphaeroides was resistant to pppGpp (Figure 4D). Most noticeably, all GMKs tested within β- and γ-Proteobacteria (Neisseria meningitidis, Klebsiella pneumoniae, Pseudomonas syringae, Yersinia enterocolitica, Coxiella burnetii, and E. coli) were completely resistant to pppGpp (Figure 4E). E. coli GMK was also resistant to the tetraphosphate, ppGpp (data not shown).
Figure 4. Conservation of regulation of GMK by pppGpp in bacteria.
(A) A rooted 16S rRNA phylogenetic tree constructed by the neighbor-joining method using MEGA (Tamura et al., 2011), showing bootstrap values higher than 75. Species are colored based on the sensitivity of their GMKs to pppGpp, and S. cerevisiae (18s rRNA) serves as an outgroup. The length of the radius branches indicates the amount of change (substitutions/site) between a pair of nodes. Inhibition curves of GMKs with pppGpp for selected species in (B) Actinobacteria, (C) Deinococcus-Thermus, (D) α-Proteobacteria, and (E) γ- and β-Proteobacteria, as well as S. cerevisiae. Error bars represent standard error of the mean from three replicates, except for S. cerevisiae for which two replicates were performed. Data fitting is as described in Figure 1C. See also Figure S3 and 4.
E. coli GMK has a small substrate binding pocket that may not fit (p)ppGpp
β- and γ-proteobacterial GMKs were insensitive to pppGpp (Figure 4E). To determine whether this insensitivity was due to the inability of pppGpp to bind these GMKs, we performed isothermal titration calorimetry (ITC), which examines interactions between macromolecules through measuring the heat released or absorbed during binding (Wessel et al., 2013). pppGpp binding was measured with E. coli GMK, and B. subtilis GMK was used as a positive control. Complex formation between pppGpp and B. subtilis GMK was detected, whereas binding was not observed in a matched experiment with E. coli GMK (Figure 5A). This suggests that the insensitivity of E. coli GMK to pppGpp is due to the lack of pppGpp binding to the enzyme. The Ki value of pppGpp for B. subtilis GMK was estimated by ITC to be 3.2 ± 0.3 μM, which is comparable to that determined by the enzyme kinetic experiment (13.5 ± 2.1 μM) (Table 2).
Figure 5. E. coli GMK has a small substrate binding pocket that does not fit pppGpp.
(A) Isothermal titration calorimetry (ITC) graphs of 50 μM B. subtilis GMK and E. coli GMK titrated with 1 mM pppGpp. (B) Structural comparison between pppGpp-bound S. aureus GMK and GMP-bound E. coli GMK (PDB: 2ANB) (Hible et al., 2005) by aligning the latter to the former (residues 1–124). Molecular docking of pppGpp into S. aureus GMK (C) and E. coli GMK (D), using AutoDock Vina (Trott and Olson, 2009). GMKs (the receptors) were prepared from the pppGpp-bound S. aureus GMK structure (Figure 3) and the GMP-bound E. coli GMK structure (PDB: 2ANB) (Hible et al., 2005), respectively. pppGpp (the ligand) was prepared from the pppGpp-bound S. aureus GMK structure (Figure 3). Docked pppGpp with the highest binding affinity to GMK is shown. (E) Schematic of chimeric GMKs. (F) Characterization of chimeric GMKs constructed in (E) with pppGpp. E. coli and S. aureus GMKs are shown here for comparison. (G) Ser13 and Arg149 of E. coli GMK (black sticks) are likely to interfere with pppGpp binding by steric clashes. The GMP-bound E. coli GMK (PDB: 2ANB) (Hible et al., 2005) was aligned to the pppGpp-bound S. aureus GMK as described in (B). For visualization of the steric clashes (yellow circles), residues 16–26, 38, 40–54, and 128–137 are purposefully hidden in the enlarged insets. See also Figure S5.
Why does pppGpp fail to bind E. coli GMK? Variation in the (p)ppGpp binding residues is unlikely to explain it, as these residues are highly conserved between S. aureus and E. coli GMK, except for Tyr78 (Figure S3A). E. coli GMK has a Val at this position, which could not form hydrogen bonds to pppGpp as Tyr does. However, such a change was also observed in several pppGpp-sensitive GMKs (Figure S3A and Figure 4), therefore it should not completely prevent binding. Close examination revealed that its adjacent residue is a Phe that structurally overlaps with Tyr78 of S. aureus GMK and likely compensates for its function. Substitution of both residues (Tyr78 and Val79) in S. aureus GMK with the corresponding residues (Val77 and Phe78) in E. coli GMK did not abolish pppGpp inhibition (Figure S5G), suggesting that the variations at and near Tyr78 are unlikely to account for the differential sensitivity of S. aureus and E. coli GMK to (p)ppGpp.
Comparison of the pppGpp-bound S. aureus GMK with the published GMP-bound E. coli GMK (PDB: 2ANB) (Hible et al., 2005) offered a possible explanation for why pppGpp fails to bind the latter. E. coli GMK has an apparent “lid” domain that is closed onto the substrate-binding pocket, whereas the lid domain in S. aureus GMK points away from the substrate-binding pocket (Figure 5B). This observation led to a hypothesis that E. coli GMK has a small substrate-binding pocket that could not accommodate pppGpp. In support of this hypothesis, virtual ligand binding using AutoDock Vina (Trott and Olson, 2009) was allowed in the substrate-binding pocket of S. aureus GMK, but was not favorable in E. coli GMK (Figure 5C and D).
We next examined the impact of the lid domain on pppGpp binding, by swapping the lid domains between E. coli and S. aureus GMK. Two sets of chimeric GMKs that differed in the swap boundaries were constructed and their sensitivities to pppGpp were tested (Figure 5E and Figure S5A). Chimeric S. aureus GMKs with E. coli lid domains were more resistant to pppGpp and exhibited significantly lower binding affinity for pppGpp than S. aureus GMK (~20 fold less) (Figure 5F, Figure S5B and F), suggesting that the E. coli lid domain partially impairs pppGpp binding. On the other hand, chimeric E. coli GMKs with S. aureus lid domains were still resistant to pppGpp (Figure 5F and Figure S5B). Combining our structural comparison, molecular docking, and characterization of chimeric GMKs, we propose that E. coli GMK has a small substrate-binding pocket that may not accommodate pppGpp and its lid domain contributes to the exclusion of pppGpp. Interestingly, we found that the pppGpp-insensitive C. burnetii GMK (another γ-proteobacterial GMK; PDB: 3TR0) also has a small substrate-binding pocket as E. coli GMK does (Figure S5C and D), implicating that this may be a common feature of γ-proteobacterial GMKs to prevent pppGpp binding.
Finally, we asked in detail how the substrate-binding pocket of E. coli GMK could exclude pppGpp binding. When pppGpp was modeled into E. coli GMK by superimposing the GMP-bound E. coli GMK (PDB: 2ANB) (Hible et al., 2005) onto the pppGpp-bound S. aureus GMK, Arg149 on the lid domain and to a lesser extent Ser13 on the P-loop would sterically clash with the 5′-β and γ, and 3′-β phosphates of pppGpp, respectively (Figure 5G). The attempt to examine the impact of Arg149 on pppGpp binding was unsuccessful as the Arg149Ala change in E. coli GMK almost completely disrupted its enzymatic activity (data not shown), which is consistent with the proximity of Arg149 to the active site and its high degree of conservation in GMKs across species (Figure 5G and Figure S3A). Similarly, mutating the highly conserved Ser13 would probably also result in substantial loss of enzyme activity. Nonetheless, we propose that the insensitivity of E. coli GMK to pppGpp is likely due to the incompatibility between its substrate-binding pocket and pppGpp, possibly by the steric clashes with Ser13 and Arg149.
A (p)ppGpp-insensitive GMK compromises adaptation to amino acid starvation
(p)ppGpp is critical for cells to adapt to stresses (Potrykus and Cashel, 2008). Although (p)ppGpp regulates GMK, it also has many other direct targets (Dalebroux and Swanson, 2012). This prompted us to ask whether regulation of GMK by (p)ppGpp per se was of physiological importance. To evaluate the physiological impact of abolishing the specific regulation of GMK by (p)ppGpp, we engineered a B. subtilis strain where the endogenous GMK coding sequence was replaced with that of the (p)ppGpp-resistant E. coli GMK. This strain was named ecgmk. The exponential growth rates were similar between wild-type (WT) and ecgmk in rich and minimal media (data not shown). The ecgmk strain was then subjected to several amino acid downshifts. First, when downshifted from rich liquid medium to minimal solid medium, ~40% of the ecgmk population failed to form colonies (Figure S6A). Second, when cells were diluted from rich to minimal liquid medium, ecgmk was defective compared to WT cells in adapting to this nutrient downshift (Figure 6A). Lastly, a competition assay was performed between these two strains (Figure 6B). A lacZ marker was introduced into WT or the ecgmk strain to distinguish them by blue/white colony screening on X-gal plates. To eliminate the impact of lacZ expression on fitness, two combinations were tested: WT versus ecgmk-lacZ and WT-lacZ versus ecgmk. Cells were grown in rich medium, washed and mixed at a 1:1 ratio in rich or minimal medium. Approximately every 10 generations, the mixed cultures were diluted back into fresh media to continue the competition and were also plated on X-gal plates to determine the percentage of each strain in the total population. The ecgmk mutant had a competitive disadvantage compared to WT, and this disadvantage was substantially more prominent in minimal medium during the first 10 generations after amino acid downshift (Figure 6D). Taken together, these results suggest that regulation of GMK by (p)ppGpp is important for adaptation to amino acid starvation in B. subtilis.
Figure 6. Abolishing regulation of GMK by (p)ppGpp leads to defective adaptation to amino acid starvation.
(A) Semi-log plot of growth of WT and ecgmk in different nutrient conditions. Error bars (shown as thin lines) represent standard error of the mean from 6 replicates. Schematic of the competition assay (B), and the amino acid downshift and nucleotide detection by LC-MS (C). (D) Percentage of ecgmk in the total population in different nutrient conditions for three continuous growth cycles. Shown here is the geometric average of the two parallel experiments to eliminate the effects of lacZ and error bars represent standard error of the mean from three replicates. Change of GMP (E), GDP (F), and GTP (G) in WT and ecgmk after amino acid downshift. Average of at least two replicates was plotted with standard error of the mean shown. (H) Change of intracellular GTP and GMP in WT during amino acid starvation. Error bars represent the standard error of the mean from at least three replicates. Quantification of intracellular GTP and GMP were described in the Experimental Procedures. (I) (p)ppGpp levels in WT and ecgmk treated with 0.5 mg/ml arginine hydroxamate for 20 minutes. The abundance is defined as PhosphorImager count/OD600. Two replicates for each strain were performed on the same day and samples were loaded onto the same TLC plate so that the PhosphorImager counts are directly comparable. The p-value was calculated using unpaired two-tailed Student’s t-test. See also Figure S6.
To further investigate how GMK regulation was involved in adaptation to amino acid starvation, we examined the impact of the gmk swap on purine nucleotide levels using liquid chromatography-mass spectrometry (LC-MS) (Figure 6C). First, we found that GTP and ATP levels were highly comparable between ecgmk and WT during exponential growth (Figure S6D and E). When downshifted from rich to minimal medium, WT rapidly accumulated high levels of GMP (~0.3–0.5 mM), which was not observed in ecgmk expressing the (p)ppGpp-insensitive E. coli GMK (Figure 6E and H). This was expected as inhibition of GMK by (p)ppGpp in WT cells reduced the conversion of GMP to GDP, but in ecgmk cells GMP should be freely converted to GDP. The drastic GMP accumulation in WT is quite interesting and might be implicated in adaptation to amino acid starvation.
Based on the above observation, we initially anticipated higher GTP (GDP) levels in ecgmk than WT. However, abolishing the GMK regulation did not appear to impair the cell’s ability to lower GTP (GDP) levels (Figure 6F and G). We next examined whether this was due to the conversion of excess GTP (GDP) to its derivatives, such as (p)ppGpp. Indeed, we found that (p)ppGpp levels were significantly higher in ecgmk than WT during amino acid starvation (Figure 6I). These results suggest that the GMK regulation by (p)ppGpp is one of the several regulatory mechanisms to lower GTP (GDP) levels. In its absence, it appears that excess (p)ppGpp is synthesized to maintain low levels of GTP (GDP).
DISCUSSION
Molecular mechanisms underlying the interaction between (p)ppGpp and GMK
Understanding the functions of (p)ppGpp necessitates examination of the molecular interactions between (p)ppGpp and its targets. High-resolution crystal structures provide insights into these interactions. So far, several (p)ppGpp-bound structures have been solved, including E. coli RNA polymerase (PDB: 4JK1 and 4JKR, ~4 Å resolution) (Mechold et al., 2013; Zuo et al., 2013), lysine decarboxylase (PDB: 3N75, 2.0 Å) (Kanjee et al., 2011), adenylosuccinate synthetase (PDB: 1CH8, 2.5 Å) (Hou et al., 1999), Desulfovibrio vulgaris translation release factor 3 (PDB: 3VR1, 3.0 Å) (Kihira et al., 2012), S. aureus primase (PDB: 4EDT, 2.01Å) (Rymer et al., 2012), Aquifex aeolicus PPX/GPPA phosphatase (PDB: 2J4R, 2.71 Å) (Kristensen et al., 2008), and B. subtilis Obg (PDB: 1LNZ, 2.6 Å) (Buglino et al., 2002). Here, we described the 1.67 Å resolution crystal structure of pppGpp-bound S. aureus GMK, which is the highest resolution achieved for a (p)ppGpp-bound structure to date. pppGpp binds the GMK active site and acts as a competitive inhibitor (Figure 2 and Figure 3C). Similar to other (p)ppGpp-bound structures (Kanjee et al., 2011; Zuo et al., 2013), the (p)ppGpp binding pocket on S. aureus GMK is rich in basic residues to form salt bridges with the 3′ and 5′ phosphates of pppGpp (Lys19, Arg43, and Arg46) (Figure 3D). In addition, three tyrosines (Tyr55, 78, and 83) are involved in hydrogen bonding to the 3′ and 5′ phosphates of pppGpp, and the importance of Tyr78 has been experimentally confirmed (Figure 3E).
Although the (p)ppGpp binding residues are largely conserved in all the GMKs (Figure 1D and Figure S3A), their sensitivities to (p)ppGpp vary significantly (Figure 1C and Figure 4). This variation appears to be primarily determined by changing the conformation of the (p)ppGpp-binding pocket (Figure 5 and Figure S5), instead of varying the (p)ppGpp-binding residues. The constraint to conserve the (p)ppGpp-binding residues is possibly attributable to the fact that (p)ppGpp binds to the active site (Figure 3C) and mutating residues at/near the active site can be extremely detrimental to the enzyme activity (Figure S5E). By contrast, this constraint is less likely to be applicable to RNAP, because ppGpp binds to an allosteric site (Mechold et al., 2013; Ross et al., 2013; Zuo et al., 2013). Without such a constraint, RNAPs in different species appear to diversify their (p)ppGpp-binding residues to modulate their sensitivities to (p)ppGpp (Figure 7A).
Figure 7. Evolution of (p)ppGpp-mediated regulation of GMK and RNAP.
(A) Proposed conservation of (p)ppGpp-GMK/RNAP regulation in bacteria. Residues corresponding to the ppGpp-binding residues identified on the E. coli RNA polymerase (β′ and ω subunits) (Ross et al., 2013; Zuo et al., 2013) are aligned from different species. Presence of DksA/DksA-like protein is proposed based on either published work or BLAST (Basic Local Alignment Search Tool; http://blast.ncbi.nlm.nih.gov/Blast.cgi) search using the E. coli DksA as the query. The R. sphaeroides DksA homolog (RSP2654) has been recently characterized and mechanistically resembles the E. coli DksA (Lennon et al., 2014). The conserved DxxDxA motif has been suggested to be an important determinant of DksA functions (Furman et al., 2012) and is utilized here as a criterion together with sequence homology search to determine the presence or absence of DksA-like proteins. Sequence alignment of DksA/DksA-like proteins is summarized in Figure S3B. (B) Phylogenetic tree and inferred evolutionary trajectory of (p)ppGpp-mediated regulation of GMK and RNAP. The phylogenetic tree was constructed as described in Figure 4A, and shown here as a rectangular tree.
Implication of inhibiting GMK by (p)ppGpp in adapting B. subtilis to amino acid starvation
(p)ppGpp is required for adaptation to amino acid starvation (Potrykus and Cashel, 2008). In B. subtilis, genetic and biochemical studies suggest that reducing GTP levels by (p)ppGpp is a critical regulatory step and is mediated by direct inhibition of GTP biosynthesis enzymes (IMPDH, HPRT, and GMK) (Kriel et al., 2012) and consumption of GTP (GDP) to produce (p)ppGpp. Lowering GTP levels decreases transcription from rRNA operons (Krasny and Gourse, 2004). It also activates transcription of amino acid biosynthesis genes, in part by deactivating CodY, which represses them in response to high GTP levels (Kriel et al., 2014; Molle et al., 2003; Tojo et al., 2008). Additionally, decreased GTP levels are often accompanied by increased ATP levels, which also enhance transcription of amino acid biosynthesis genes as their transcription initiates with ATP (Krasny et al., 2008; Kriel et al., 2014; Tojo et al., 2008). Lastly, hampered transcription of rRNA operons may also lead to the redistribution of RNAP to promoters governing amino acid biosynthesis and stress response genes (Potrykus and Cashel, 2008). These transcriptional reprogrammings allow B. subtilis to curtail consumption and activate production of amino acids, and are important for adaptation to amino acid starvation.
Interestingly, this transcriptional regulation by modulating GTP levels is unlikely to explain the defect observed with ecgmk (Figure 6A and D, and Figure S6A), as its GTP levels change similarly to WT at least up to 180 minutes after nutrient downshift (Figure 6G). The E. coli GMK has similar activities to the B. subtilis enzyme (Figure S6B–D) and it does not seem to be toxic when expressed in B. subtilis (Figure S6F). Although replacing B. subtilis GMK with E. coli GMK might disrupt certain protein interactions and lead to defects, such a possibility does not appear to be consistent with the indistinguishable growth of WT and ecgmk in rich medium (Figure 6A).
Alternatively, based on the differential changes in (p)ppGpp and GMP levels during the downshift, we propose two mechanisms that could potentially account for the defect. First, ecgmk accumulates significantly higher (p)ppGpp than WT under amino acid starvation (Figure 6I). Given that (p)ppGpp inhibits numerous key cellular processes, such as replication, transcription, and translation (Srivatsan and Wang, 2008), it is probably not surprising that induction of (p)ppGpp rapidly hinders growth even in the absence of starvation (Potrykus and Cashel, 2008). Accordingly, it is conceivable that (p)ppGpp levels must decline adequately before cells resume growth. Second, GMP surges to an intracellular concentration ranging from 0.3 to 0.5 mM for at least 20 minutes in starved WT (Figure 6H), but not in ecgmk (Figure 6E). GMP is known to directly regulate many proteins (Deo et al., 1985; Gallant et al., 1971; Naught et al., 2002). It might also affect many GTP-dependent cellular processes (e.g. transcription, translation, and replication), given that its concentration is comparable to or even slightly higher than that of GTP in starved WT cells (Figure 6H). It will be interesting to examine in the future whether accumulation of GMP indeed facilitates adaptation of B. subtilis to amino acid starvation.
Implication of regulating GTP levels by (p)ppGpp in bacterial pathogenesis and antibiotic tolerance
In addition to adapting to amino acid starvation, (p)ppGpp is also required for bacterial pathogenesis (Dalebroux et al., 2010) and antibiotic tolerance (Maisonneuve et al., 2013). Deleting the (p)ppGpp synthetase gene relA compromises L. monocytogenes virulence in a murine model (Taylor et al., 2002). In E. faecalis, absence of (p)ppGpp impairs antibiotic tolerance and virulence (Abranches et al., 2009; Gaca et al., 2013). In the methicillin-resistant Staphylococcus aureus (MRSA), RelA appears to be involved in resistance against beta-lactam antibiotics (Dordel et al., 2014). Despite this broad involvement of (p)ppGpp in pathogenesis and antibiotic tolerance, the underlying mechanisms are still not fully understood. Here we found that GMKs from several firmicute pathogens are inhibited by (p)ppGpp (Figure 1C and Figure S1D), suggesting that (p)ppGpp may regulate GTP levels in many firmicute pathogens as it does in B. subtilis. Given the importance of GTP in transcriptional regulation in B. subtilis and the broad conservation of CodY in Firmicutes (Sonenshein, 2005), we propose that (p)ppGpp might reduce GTP levels to regulate virulence gene expression to facilitate pathogenesis. Perhaps like B. subtilis, these firmicute pathogens may exploit (p)ppGpp-modulated GTP levels to regulate the activity of CodY, which may transcriptionally regulate virulence genes by responding directly to GTP (Sonenshein, 2005). In L. monocytogenes, virulence is at least partially attributed to the proper regulation of CodY activity (Bennett et al., 2007; Lobel et al., 2012), perhaps by (p)ppGpp-mediated regulation of GTP levels.
Evolution of the (p)ppGpp-GMK interaction in bacteria
The ubiquity of (p)ppGpp in bacteria (Atkinson et al., 2011) implies that (p)ppGpp possibly existed in the common bacterial ancestor. During the process of evolution, the (p)ppGpp regulatory network has seemingly diversified in different bacteria, as exemplified by E. coli and B. subtilis. This diversity may reflect their divergent evolutionary histories, during which each species evolved the (p)ppGpp regulatory network to meet its needs. On the other hand, species of similar evolutionary histories (i.e. phylogenetically close species) may share (p)ppGpp targets and the corresponding regulatory network. Here, we systematically investigated a large number of species from diverse groups and discovered that the (p)ppGpp-GMK regulation is shared by species not only in Firmicutes, but remarkably also in Actinobacteria, Deinococcus-Thermus, and α-Proteobacteria (Figure 1C and Figure 4B–D). However, it appears to be absent in β- or γ-Proteobacteria (Figure 4E). Interestingly, despite the apparent conservation of GMK regulation, E. faecalis has a second GMK that is less sensitive to pppGpp, the significance of which requires further investigation.
On the other hand, all the species tested from Proteobacteria exhibit strong conservation in the (p)ppGpp binding residues on their RNAPs and they all at least harbor a DksA or DksA-like protein (Figure 7A and Figure S3B). These observations imply that (p)ppGpp may directly regulate RNAP in these species as in E. coli, consistent with a previous study (Ross et al., 2013). In contrast, all the species tested in Firmicutes, Actinobacteria, and Deinococcus-Thermus exhibit substantial variations in the (p)ppGpp binding residues on their RNAPs and they do not harbor a DksA or DksA-like protein, suggesting that in these phyla, (p)ppGpp may not directly regulate transcription initiation as it does in E. coli. Indeed ppGpp does not crosslink to B. subtilis and T. thermophilus RNAPs (Ross et al., 2013) and has little effect on them in in vitro transcription assays (Krasny and Gourse, 2004; Vrentas et al., 2008). In T. thermophilus, (p)ppGpp has been proposed to indirectly regulate rRNA transcription, through direct inhibition of IMPDH to control GTP levels (Kasai et al., 2006). The Ki value of pppGpp for T. thermophilus IMPDH is ~11 μM (Kasai et al., 2006), whereas in our study the Ki value of pppGpp for its GMK is ~3 μM (Table 2). Given that (p)ppGpp is induced to only 10–15 μM in T. thermophilus cells starved for amino acids (Kasai et al., 2006), both targets are likely to be involved in regulating GTP levels.
Lastly, we inferred a possible evolutionary trajectory for the (p)ppGpp-GMK/RNAP regulation in bacteria. Based on the principle of parsimony (fewest evolutionary changes) (Jiang et al., 2014; Yang and Rannala, 2012), we propose that GMK emerged early as a (p)ppGpp target and RNAP evolved later (Figure 7B). The (p)ppGpp-GMK regulation appears to be retained in Firmicutes, Actinobacteria, and Deinococcus-Thermus (Figure 1C, and Figure 4B and C), but is largely lost in Proteobacteria, where RNAP emerged as a (p)ppGpp target. β- and γ-Proteobacteria possibly have completely lost this regulation (Figure 4E). α-Proteobacteria, which diverged earlier than β- and γ-Proteobacteria (Figure 7B), appear to partially retain the GMK regulation (Figure 4D). A recent study showed that GMKs from chloroplasts are inhibited by ppGpp (Nomura et al., 2014). Chloroplasts are proposed to stem from a cyanobacterium-like bacterium (McFadden, 2001), suggesting that the (p)ppGpp-GMK regulation may also have existed in the cyanobacterial ancestor, supporting our hypothesis that GMK is an ancient (p)ppGpp target.
While it is clear that much work is required to understand how diverse (p)ppGpp targets evolved, regulation of GMK appears to be a broadly conserved and ancient alternative to the (p)ppGpp-RNAP regulation to mediate stress resistance in bacteria.
EXPERIMENTAL PROCEDURES
Plasmids, strains, and growth conditions
All primers, plasmids, and strains used in this study are listed in Table S2. Plasmid and strain construction, growth conditions, and assays for growth and competition of WT and ecgmk are described in the Supplemental Experimental Procedures.
Protein purification and in vitro GMK assay
Purification of GMKs and the in vitro GMK assay are described in the Supplemental Experimental Procedures.
Synthesis, purification, and quantification of (p)ppGpp
(p)ppGpp was synthesized in vitro using RelSeq1-385 and GppA, purified, and quantified as described (Mechold et al., 2013).
Co-crystallization of S. aureus GMK with pppGpp and X-ray structure determination
S. aureus GMK was dialyzed in Dialysis Buffer [20 mM Tris-HCl (pH 7.2) and 40 mM KCl] at 4 °C with three buffer changes and protein concentration was determined to be 14 mg/ml by measuring A280. pppGpp was added to S. aureus GMK to a final concentration of 0.272 mM. This mixture was subsequently mixed with the mother liquor [0.1 M Tris-HCl (pH 8.0), 0.2 M Li2SO4, and 24% PEG3350] at a 1:1 ratio and crystallized by hanging drop vapor diffusion at 20 °C. Crystals were transferred to the cryoprotectant solution [0.1 M Tris-HCl (pH 8.0), 0.2 M Li2SO4, 24% PEG3350, and 20% ethylene glycol] and frozen in liquid nitrogen.
X-ray diffraction data were indexed and scaled using HKL2000 (Otwinowski and Minor, 1997). Diffraction data were collected at the Advanced Photon Source, Argonne National Laboratory, LS-CAT, beam line 21ID-F. The structure of S. aureus GMK was determined by molecular replacement using the GMP-bound S. aureus GMK structure (El Omari et al., 2006) as a search model in the program Phaser (McCoy et al., 2007) followed by rounds of manual fitting using Coot (Emsley and Cowtan, 2004) and refinement using REFMAC5 (Winn et al., 2001) and PHENIX (Adams et al., 2010).
Isothermal titration calorimetry
GMKs were dialyzed in 100 mM Tris-HCl (pH 7.5), 100 mM KCl, and 10 mM MgCl2 with three buffer changes at 4 °C. The final concentration of GMK was adjusted to 50 μM and pppGpp to 1 mM. Injections with pppGpp were performed on a MicroCal iTC200 (GE Healthcare) at 23 °C with the following recipe: 1 X 0.4 μl (discarded), 8 X 1 μl, 5 X 2 μl and 5 X 4 μl. Data was fitted into a single-site binding model using the Origin software (MicroCal) when applicable.
Amino acid downshift and LC-MS detection and quantification of nucleotides
WT and ecgmk were grown in S7 + CAS at 37 °C until OD600 ~0.2–0.3. Cultures were passed through sterile 0.2 μm nylon filters (EMD Millipore), washed with prewarmed S7, and then resuspended in equal volume prewarmed S7. Immediately before and 2, 5, 10, 20, 60, 120, 180, and 240 minutes after the downshift, 5 ml cultures were removed and passed through the nylon filters. Metabolism was quenched and metabolites were extracted by submerging the filters into 1.5 ml of −20 °C 40:40:20 acetonitrile/methanol/water. Cell extracts were dried with nitrogen gas and resuspended in HPLC-grade water. Samples were analyzed using HPLC-MS consisting of a Dionex UHPLC coupled by electrospray ionization (ESI; negative mode) to a Q Exactive Orbitrap mass spectrometer (Thermo Scientific, San Jose, CA) operated in full scan mode for detection of targeted compounds based on their accurate masses. Liquid chromatography (LC) was performed with a Synergi Fusion-RP 100A column (100 x 2 mm, 2.5 μm particle size, Phenomenex, Torrance, CA). Total run time was 25 minutes with a flow rate of 200 μl/min, using 97:3 water/methanol with 10 mM tributylamine and acetic acid as Solvent A, and methanol as Solvent B. The gradient was: 0 min, 5% B; 2.5 min, 5% B; 5 min, 20% B; 7.5 min, 20% B; 13 min, 55% B; 15.5 min, 95% B; 18.5 min, 95% B; 19 min, 0% B; 25 min, 0% B. Universally labeled 13C-GTP (Sigma-Aldrich) was used as an internal standard for quantifying GTP. Intracellular GTP levels were calculated by the following equation, , where CGTP is the average intracellular GTP concentration, ICGTP the ion count of GTP from the sample, CGTP(13) and ICGTP(13) the concentration and ion count of the 13C-GTP standard, respectively, V the total volume of cell extract (1.5 ml), N CFUs/5 ml culture, and Vcell the median volume of a B. subtilis cell during exponential phase (1.13 μm3) (Maass et al., 2011). Since CGMP/CGTP is almost identical to ICGMP/ICGTP (data not shown), the intracellular GMP concentration was estimated by the following equation, , where CGMP and ICGMP are the concentration and ion count of GMP, respectively.
Detection and quantification of (p)ppGpp
(p)ppGpp was detected and quantified using thin layer chromatography as previously described with minor modifications (Bittner et al., 2014).
Supplementary Material
Acknowledgments
We thank Adel Talaat, Alexei Savchenko, Amy Gehring, Michael Cashel, Robert Gennis, Robert Heinzen, and Timothy Donohue for the generous gifts of genomic DNAs/plasmids/strains, Liya Hu and B.V. Venkataram Prasad for early crystallographic efforts, and David Stevenson for technical support with LC-MS. We thank Richard Gourse, Wilma Ross, and the Wang lab for critical reading of the manuscript and Yves Brun for helpful discussion.
This work was supported by NIGMS R01GM084003 to JDW. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817).
Footnotes
ACCESSION NUMBERS
The coordinate and structural factor files were deposited into the Protein Data Bank with the ID code 4QRH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abranches J, Martinez AR, Kajfasz JK, Chavez V, Garsin DA, Lemos JA. The molecular alarmone (p)ppGpp mediates stress responses, vancomycin tolerance, and virulence in Enterococcus faecalis. J Bacteriol. 2009;191:2248–2256. doi: 10.1128/JB.01726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr Sect D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson GC, Tenson T, Hauryliuk V. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS One. 2011;6:e23479. doi: 10.1371/journal.pone.0023479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett HJ, Pearce DM, Glenn S, Taylor CM, Kuhn M, Sonenshein AL, Andrew PW, Roberts IS. Characterization of relA and codY mutants of Listeria monocytogenes: identification of the CodY regulon and its role in virulence. Mol Microbiol. 2007;63:1453–1467. doi: 10.1111/j.1365-2958.2007.05597.x. [DOI] [PubMed] [Google Scholar]
- Bittner AN, Kriel A, Wang JD. Lowering GTP level increases survival of amino acid starvation but slows growth rate for Bacillus subtilis cells lacking (p)ppGpp. J Bacteriol. 2014;196:2067–2076. doi: 10.1128/JB.01471-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buglino J, Shen V, Hakimian P, Lima CD. Structural and biochemical analysis of the Obg GTP binding protein. Structure. 2002;10:1581–1592. doi: 10.1016/s0969-2126(02)00882-1. [DOI] [PubMed] [Google Scholar]
- Cashel M, Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969;221:838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS. ppGpp conjures bacterial virulence. Microbiol Mol Biol Rev. 2010;74:171–199. doi: 10.1128/MMBR.00046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalebroux ZD, Swanson MS. ppGpp: magic beyond RNA polymerase. Nat Rev Microbiol. 2012;10:203–212. doi: 10.1038/nrmicro2720. [DOI] [PubMed] [Google Scholar]
- Deo SS, Tseng WC, Saini R, Coles RS, Athwal RS. Purification and characterization of Escherichia coli xanthine-guanine phosphoribosyltransferase produced by plasmid pSV2gpt. Biochim Biophys Acta. 1985;839:233–239. doi: 10.1016/0304-4165(85)90003-0. [DOI] [PubMed] [Google Scholar]
- Dordel J, Kim C, Chung M, Pardos de la Gandara M, Holden MT, Parkhill J, de Lencastre H, Bentley SD, Tomasz A. Novel determinants of antibiotic resistance: identification of mutated loci in highly methicillin-resistant subpopulations of methicillin-resistant Staphylococcus aureus. MBio. 2014;5 doi: 10.1128/mBio.01000-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Omari K, Dhaliwal B, Lockyer M, Charles I, Hawkins AR, Stammers DK. Structure of Staphylococcus aureus guanylate monophosphate kinase. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006;62:949–953. doi: 10.1107/S174430910603613X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr Sect D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Furman R, Tsodikov OV, Wolf YI, Artsimovitch I. An insertion in the catalytic trigger loop gates the secondary channel of RNA polymerase. J Mol Biol. 2012;425:82–93. doi: 10.1016/j.jmb.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaca AO, Kajfasz JK, Miller JH, Liu K, Wang JD, Abranches J, Lemos JA. Basal levels of (p)ppGpp in Enterococcus faecalis: the magic beyond the stringent response. MBio. 2013;4 doi: 10.1128/mBio.00646-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J, Irr J, Cashel M. The mechanism of amino acid control of guanylate and adenylate biosynthesis. J Biol Chem. 1971;246:5812–5816. [PubMed] [Google Scholar]
- Hible G, Renault L, Schaeffer F, Christova P, Zoe Radulescu A, Evrin C, Gilles AM, Cherfils J. Calorimetric and crystallographic analysis of the oligomeric structure of Escherichia coli GMP kinase. J Mol Biol. 2005;352:1044–1059. doi: 10.1016/j.jmb.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Hou Z, Cashel M, Fromm HJ, Honzatko RB. Effectors of the stringent response target the active site of Escherichia coli adenylosuccinate synthetase. J Biol Chem. 1999;274:17505–17510. doi: 10.1074/jbc.274.25.17505. [DOI] [PubMed] [Google Scholar]
- Jiang C, Brown PJ, Ducret A, Brun YV. Sequential evolution of bacterial morphology by co-option of a developmental regulator. Nature. 2014;506:489–493. doi: 10.1038/nature12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjee U, Gutsche I, Alexopoulos E, Zhao B, El Bakkouri M, Thibault G, Liu K, Ramachandran S, Snider J, Pai EF, Houry WA. Linkage between the bacterial acid stress and stringent responses: the structure of the inducible lysine decarboxylase. EMBO J. 2011;30:931–944. doi: 10.1038/emboj.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Nishizawa T, Takahashi K, Hosaka T, Aoki H, Ochi K. Physiological analysis of the stringent response elicited in an extreme thermophilic bacterium, Thermus thermophilus. J Bacteriol. 2006;188:7111–7122. doi: 10.1128/JB.00574-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihira K, Shimizu Y, Shomura Y, Shibata N, Kitamura M, Nakagawa A, Ueda T, Ochi K, Higuchi Y. Crystal structure analysis of the translation factor RF3 (release factor 3) FEBS Lett. 2012;586:3705–3709. doi: 10.1016/j.febslet.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Ehrlich SD, Albertini A, Amati G, Andersen KK, Arnaud M, Asai K, Ashikaga S, Aymerich S, Bessieres P, et al. Essential Bacillus subtilis genes. Proc Natl Acad Sci U S A. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasny L, Gourse RL. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J. 2004;23:4473–4483. doi: 10.1038/sj.emboj.7600423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasny L, Tiserova H, Jonak J, Rejman D, Sanderova H. The identity of the transcription +1 position is crucial for changes in gene expression in response to amino acid starvation in Bacillus subtilis. Mol Microbiol. 2008;69:42–54. doi: 10.1111/j.1365-2958.2008.06256.x. [DOI] [PubMed] [Google Scholar]
- Kriel A, Bittner AN, Kim SH, Liu K, Tehranchi AK, Zou WY, Rendon S, Chen R, Tu BP, Wang JD. Direct regulation of GTP homeostasis by (p)ppGpp: a critical component of viability and stress resistance. Mol Cell. 2012;48:231–241. doi: 10.1016/j.molcel.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriel A, Brinsmade SR, Tse JL, Tehranchi AK, Bittner AN, Sonenshein AL, Wang JD. GTP dysregulation in Bacillus subtilis cells lacking (p)ppGpp results in phenotypic amino acid auxotrophy and failure to adapt to nutrient downshift and regulate biosynthesis genes. J Bacteriol. 2014;196:189–201. doi: 10.1128/JB.00918-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen O, Ross B, Gajhede M. Structure of the PPX/GPPA phosphatase from Aquifex aeolicus in complex with the alarmone ppGpp. J Mol Biol. 2008;375:1469–1476. doi: 10.1016/j.jmb.2007.11.073. [DOI] [PubMed] [Google Scholar]
- Lennon CW, Lemmer KC, Irons JL, Sellman MI, Donohue TJ, Gourse RL, Ross W. A Rhodobacter sphaeroides protein mechanistically similar to Escherichia coli DksA regulates photosynthetic growth. MBio. 2014;5:e01105–01114. doi: 10.1128/mBio.01105-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel L, Sigal N, Borovok I, Ruppin E, Herskovits AA. Integrative genomic analysis identifies isoleucine and CodY as regulators of Listeria monocytogenes virulence. PLoS Genet. 2012;8:e1002887. doi: 10.1371/journal.pgen.1002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass S, Sievers S, Zuhlke D, Kuzinski J, Sappa PK, Muntel J, Hessling B, Bernhardt J, Sietmann R, Volker U, et al. Efficient, global-scale quantification of absolute protein amounts by integration of targeted mass spectrometry and two-dimensional gel-based proteomics. Anal Chem. 2011;83:2677–2684. doi: 10.1021/ac1031836. [DOI] [PubMed] [Google Scholar]
- Magnusson LU, Farewell A, Nystrom T. ppGpp: a global regulator in Escherichia coli. Trends Microbiol. 2005;13:236–242. doi: 10.1016/j.tim.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Maisonneuve E, Castro-Camargo M, Gerdes K. (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell. 2013;154:1140–1150. doi: 10.1016/j.cell.2013.07.048. [DOI] [PubMed] [Google Scholar]
- Maisonneuve E, Gerdes K. Molecular mechanisms underlying bacterial persisters. Cell. 2014;157:539–548. doi: 10.1016/j.cell.2014.02.050. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden GI. Chloroplast origin and integration. Plant Physiol. 2001;125:50–53. doi: 10.1104/pp.125.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechold U, Potrykus K, Murphy H, Murakami KS, Cashel M. Differential regulation by ppGpp versus pppGpp in Escherichia coli. Nucleic Acids Res. 2013;41:6175–6189. doi: 10.1093/nar/gkt302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molle V, Nakaura Y, Shivers RP, Yamaguchi H, Losick R, Fujita Y, Sonenshein AL. Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis. J Bacteriol. 2003;185:1911–1922. doi: 10.1128/JB.185.6.1911-1922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy H, Cashel M. Isolation of RNA polymerase suppressors of a (p)ppGpp deficiency. Methods Enzymol. 2003;371:596–601. doi: 10.1016/S0076-6879(03)71044-1. [DOI] [PubMed] [Google Scholar]
- Naught LE, Gilbert S, Imhoff R, Snook C, Beamer L, Tipton P. Allosterism and cooperativity in Pseudomonas aeruginosa GDP-mannose dehydrogenase. Biochemistry. 2002;41:9637–9645. doi: 10.1021/bi025862m. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Izumi A, Fukunaga Y, Kusumi K, Iba K, Watanabe S, Nakahira Y, Weber AP, Nozawa A, Tozawa Y. Diversity in guanosine 3′,5′-bisdiphosphate (ppGpp) sensitivity among guanylate kinases of bacteria and plants. J Biol Chem. 2014;289:15631–15641. doi: 10.1074/jbc.M113.534768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. In: Carter CW Jr, Sweet RM, editors. Methods in enzymology. New York: Academic Press; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- Paul BJ, Barker MM, Ross W, Schneider DA, Webb C, Foster JW, Gourse RL. DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell. 2004;118:311–322. doi: 10.1016/j.cell.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Paul BJ, Berkmen MB, Gourse RL. DksA potentiates direct activation of amino acid promoters by ppGpp. Proc Natl Acad Sci U S A. 2005;102:7823–7828. doi: 10.1073/pnas.0501170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- Ross W, Vrentas CE, Sanchez-Vazquez P, Gaal T, Gourse RL. The magic spot: a ppGpp binding site on E. coli RNA polymerase responsible for regulation of transcription initiation. Mol Cell. 2013;50:420–429. doi: 10.1016/j.molcel.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymer RU, Solorio FA, Tehranchi AK, Chu C, Corn JE, Keck JL, Wang JD, Berger JM. Binding mechanism of metal•NTP substrates and stringent-response alarmones to bacterial DnaG-type primases. Structure. 2012;20:1478–1489. doi: 10.1016/j.str.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein AL. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr Opin Microbiol. 2005;8:203–207. doi: 10.1016/j.mib.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Srivatsan A, Wang JD. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr Opin Microbiol. 2008;11:100–105. doi: 10.1016/j.mib.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CM, Beresford M, Epton HA, Sigee DC, Shama G, Andrew PW, Roberts IS. Listeria monocytogenes relA and hpt mutants are impaired in surface-attached growth and virulence. J Bacteriol. 2002;184:621–628. doi: 10.1128/JB.184.3.621-628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo S, Satomura T, Kumamoto K, Hirooka K, Fujita Y. Molecular mechanisms underlying the positive stringent response of the Bacillus subtilis ilv-leu operon, involved in the biosynthesis of branched-chain amino acids. J Bacteriol. 2008;190:6134–6147. doi: 10.1128/JB.00606-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2009;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrentas CE, Gaal T, Berkmen MB, Rutherford ST, Haugen SP, Vassylyev DG, Ross W, Gourse RL. Still looking for the magic spot: the crystallographically defined binding site for ppGpp on RNA polymerase is unlikely to be responsible for rRNA transcription regulation. J Mol Biol. 2008;377:551–564. doi: 10.1016/j.jmb.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel SR, Marceau AH, Massoni SC, Zhou R, Ha T, Sandler SJ, Keck JL. PriC-mediated DNA replication restart requires PriC complex formation with the single-stranded DNA-binding protein. J Biol Chem. 2013;288:17569–17578. doi: 10.1074/jbc.M113.478156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MD, Isupov MN, Murshudov GN. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr Sect D Biol Crystallogr. 2001;57:122–133. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- Yang Z, Rannala B. Molecular phylogenetics: principles and practice. Nat Rev Genet. 2012;13:303–314. doi: 10.1038/nrg3186. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Wang Y, Steitz TA. The mechanism of E. coli RNA polymerase regulation by ppGpp is suggested by the structure of their complex. Mol Cell. 2013;50:430–436. doi: 10.1016/j.molcel.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.