Abstract
Background & Aims
By binding to T-cell immunoglobulin mucin-3 (TIM-3) on activated Th1 cells, Galectin-9 (Gal-9) negatively regulates Th1-type alloimmunity. Although T cells contribute to hepatic ischemia-reperfusion injury (IRI), it is unknown whether negative T cell-dependent TIM-3 costimulation may rescue IR-stressed orthotopic liver transplants (OLT) from innate immunity-driven inflammation.
Methods
We used WT and TIM-3Tg mice (C57BL6) as liver donors and recipients in a clinically-relevant model of hepatic cold storage (20h at 4°C in UW solution) and syngeneic OLT.
Results
OLTs in WT or TIM-3Tg->TIM-3Tg groups were resistant against IR- stress, evidenced by preserved hepatocellular function (sALT levels) and liver architecture (Suzuki’s score). In contrast, OLTs in WT or TIM-3Tg->WT groups were susceptible to IRI. TIM-3 induction in recipient circulating CD4+ T cells: 1/ depressed Tbet/IFN-γ, while amplifying GATA3 and IL-4/IL-10 expression in OLTs; 2/ promoted T cell exhaustion (PD-1, LAG-3) phenotype; and 3/ depressed neutrophil and macrophage infiltration/function in OLTs. In parallel studies, we have documented, for the first time that Gal-9, a natural TIM-3 ligand, was produced primarily by and released from IR-stressed hepatocytes, both in-vivo and in-vitro. Moreover, exogenous rGal-9 potentiated liver resistance against IRI by depressing T cell activation and promoting apoptosis of CD4+ T cells.
Conclusion
Harnessing TIM-3–Gal-9 signaling at T cell–hepatocyte interface facilitates homeostasis in IR-stressed OLTs. Enhancing anti-oxidant hepatocyte Gal-9 potentiates liver IR-resistance. Negative regulation by recipient TIM-3+CD4+ cells provides evidence for cytoprotective functions of a discrete T cell subset, which should be spared when applying T cell-targeted immunosuppression in transplant recipients.
Keywords: Liver, ischemia-reperfusion injury, orthotopic liver transplantation, innate immunity, TIM-3, Galectin-9
Introduction
Orthotopic liver transplantation (OLT) is the gold standard of care in patients with end-stage liver disease and those with tumors of hepatic origin [1]. The organ shortage has prompted use of extended criteria organs from older, steatotic, or non-heart beating donors, as well as those that have been subjected to prolonged periods of cold storage. These “marginal” organs are particularly susceptible to ischemia-reperfusion injury (lRI) during procurement and preservation [2]. Indeed, IRI not only contributes to the donor organ shortage but may also lead to graft failure and primary non-function. The cellular damage surrounding organ removal and storage impacts the outcomes because it represents a major risk factor for acute and chronic rejection. Despite its obvious significance, the mechanisms that account for organ IRI are only partially understood [2,3]. Liver IRI represents a continuum of immune processes, including endothelial activation, increased expression of adhesion molecules, macrophage/neutrophil activation, and cytokine release, followed by hepatocyte death. We have been advancing a concept that liver IRI represents the case for an innate immunity-dominated TLR4-driven inflammation [4]. However, although IR-hepatocellular damage develops ex-vivo, in the absence of exogenous antigens, and in syngeneic grafts, we [5] and others [6,7] have documented a crucial role for activated T cells, particularly of CD4 phenotype, in the pathogenesis of liver IRI.
T cell Immunoglobulin Mucin (TIM) family (−1, −2, −3, and −4) of co-stimulatory molecules constitute a relatively new T lymphocyte–macrophage signaling network central at regulation of the innate–adaptive interface in organ transplantation [8]. The blockade of interaction between TIM-3 and its ligand, Galectin-9 (Gal-9), induces cell death, exacerbates autoimmunity, enhances alloreactivity, and abrogates tolerance in experimental models [9,10]. Thus, TIM-3-Gal-9 pathway represents a negative regulatory mechanism between activated Th1-type cells and monocytes, consistent with our finding that disruption of TIM-3 signaling has worsened tissue damage in livers subjected to warm IR-stress [11]. However, in addition to T cells, TIM-3 has been also associated with other immune cell types [12–14], and little is known about Gal-9 distribution during liver inflammation. Hence, to focus on the role of T cell-specific TIM-3 signaling in the mechanism of liver IRI, we have now employed mice, which express TIM-3 transgene (Tg) selectively on their T cells [15]. We used these animals as either liver donors or transplant recipients in a clinically-relevant murine model of extended hepatic cold storage (20h at 4°C) and OLT [16]. Our novel findings provide evidence for important role of negative regulation of innate immune responses by host circulating TIM-3+CD4+ T cells in OLTs. Moreover, our data are the first to identify IR-stressed hepatocytes are the principal source of Gal-9, which by itself may potentiate IRI resistance by impairing TIM-3+ T cell function, and promoting apoptosis. Hence, harnessing TIM-3–Gal-9 signaling at T cell–hepatocyte interface is essential for combating IR-hepatocellular damage, and to maintain homeostasis in OLTs.
Materials and Methods
Mice
Male C57BL/6 wild-type (WT) and B6.129S7-Rag1tm1Mom/J (C57BL/6, RAG−/−) mice at 8–12 weeks of age were used (Jackson Laboratory, Bar Harbor, ME). TIM-3 transgenic (TIM-3Tg) mice (C57BL/6 background) provided by Dr. Vijai Kuchroo (Harvard University, Boston, MA), were generated by expressing the full-length TIM-3 cDNA under the control of human CD2 promoter [15]. Animals were housed in the UCLA facility under pathogen-free conditions and received humane care according to the criteria in Guide for the Care and Use of Laboratory Animals (prepared by the National Academy of Sciences; NIH publication 86–23, revised 1985).
Models of Liver IRI
In our principal model, donor livers were subjected to cold preservation followed by OLT [16]. Livers stored for 20h at 4°C in UW solution were transplanted orthotopically to syngeneic hosts. Anhepatic time was 15–18 min. There were six OLT groups (n=6/group): Group 1&2: WT or TIM-3Tg mice subjected to sham operations; Group 3&4: WT or TIM-3Tg livers ->WT; Group 5&6: WT or TIM-3Tg livers ->TIM-3Tg. Recipients were sacrificed at 6h (the peak of hepatocellular damage in this model) and OLT/sera samples were collected.
In a model of partial (70%) liver warm IRI [4,5,11,17], WT mice (n=4–6/group) were infused 1h prior to hepatic ischemia with a single dose of human stable-form Galectin-9 (rGal-9; 100ug/mouse i.v.; a gift from Prof. Mitsuomi Hirashima, Kagawa University, Japan) [18]. Then, arterial/portal blood supply to the cephalad liver lobes was interrupted for 90min. Animals were sacrificed at 6h of reperfusion; liver/sera samples were collected. Controls were treated with PBS; sham-operated mice underwent the same procedure but without vascular occlusion.
In some experiments, spleen CD4+ T cells from WT or TIM-3Tg mice were negatively selected (>95%) by using a magnetic cell sorting kit (StemCell Technologies, Vancouver, Canada). These cells were infused (5×106 i.v.) into severely T-, B-, and NK T- cell deficient RAG−/− mice (n=3/group). One hour later, RAG−/− test recipients were subjected to 90min of partial local warm ischemia, and liver/sera samples were collected at 6h of reperfusion [19].
OLT Function/Histology
Serum alanine transaminase (sALT) levels were measured in blood samples by IDEXX Laboratories (Sacramento, CA, USA). OLT sections (4-μm) were stained with hematoxylin and eosin (H&E) and analyzed blindly. The histological severity of IRI in OLTs was graded using Suzuki’s criteria with modifications [20].
Lymphocyte Isolation/Flow Cytometry
Liver lymphocytes were isolated by 25%/50% discontinuous Percoll density gradient [21]. To determine TIM-3 expression on CD4+ T cells, spleen lymphocytes were stained with rat antimouse TIM-3 PE (RMT3-23, eBioscience) and FITC-CD4 (GK1.5, eBioscience) before analyzing on a FACS-Calibur cytometer (BD Biosciences, USA). Activated caspase-3/caspase-7 in apoptotic spleen T cells was detected by Flow Cytometry Assay Kit (Life Technologies).
Immunofluorescence
Frozen OLT sections and cultured hepatocytes were stained by immunofluorescence. Primary mAb against neutrophil Ly-6G antigen (1A8, BD Biosciences, USA), macrophage CD68 antigen (FA-11; AbD Serotec, Raleigh, USA), Gal-9 (a gift from Prof. Hirashima, Japan) were used, followed by goat anti-rat Alexa Fluor 555 (Invitrogen, USA) as secondary Ab. For CD4 staining, FITC-CD4 (RM4-5, eBioscience, San Diego, CA) was used. Alexa Fluor 488 or Alexa Fluor 555 actin conjugate (Life Technologies, USA) was employed to stain the cellular skeleton. Slides were mounted by VECTASHIELD medium with DAPI (Vector Labs, USA), and evaluated blindly by counting labeled cells in 10 high-power fields (HPF). The results are expressed as average number of positive cells/HPF (x200 or x400).
Quantitative RT-PCR
Quantitative RT-PCR (qRT-PCR) was performed with platinum SYBR green quantitative PCR kit (Invitrogen, USA) by the Chromo 4 detector (MJ Research, USA). Primers to amplify specific gene fragments were published [22]. Target gene expressions were calculated by their ratios to the housekeeping gene hypoxanthine-guanine phosphoribosyl transferase (HPRT).
In-Vitro Cell Cultures
Spleen T cells were cultured with ConA (2 or 4μg/ml) ± human stable-form rGal-9 (1μg/ml) for 24h. Culture supernatants were assessed for IFN-γ by ELISA (eBioscience, San Diego, CA, USA). Standard curves were performed with serial two-fold dilutions; the optical density (OD) was measured with an ELISA reader. Primary mouse hepatocytes, isolated by a two-stage collagenase perfusion method (viability 95–99%), were cultured in complete L-15 medium ± hydrogen peroxide (H2O2; 0.4 mM; Sigma-Aldrich) for 4h, and processed by immunofluorescence [19,22].
Caspase-3 Activity Assay
Liver tissue samples as well as spleen and hepatic CD4+ T cells (separated by magnetic cell sorting; StemCell Technologies) were assessed for caspase-3 activity (Calbiochem) according to the manufacturer’s instruction [22].
Statistical analysis
All values are expressed as mean±standard deviation (SD). Data were analyzed with an unpaired, two-tailed Student’s-t test; p<0.05 was considered statistically significant.
Results
Recipient TIM-3+CD4+ T Cells Ameliorate IRI in OLTs
First, we confirmed that unlike WT, TIM-3Tg spleen CD4+ T cells overexpressed TIM-3 (2% vs. 55.9%, respectively) by flow cytometry (Fig. 1A). Then, we analyzed as to whether induction of TIM-3 on donor vs. recipient T cells may affect the liver damage in a model of cold storage (20h at 4°C) and isogeneic OLT. As shown in Fig. 1B, by 6h post-OLT, sALT levels (U/L) increased in WT->WT group (8,519±2,236) as compared with controls (35±11 and 26±10 in WT and TIM-3Tg sham groups, respectively; p<0.01). Reduced hepatocellular damage in TIM-3Tg->TIM-3Tg IR-stressed OLTs (sALT: 2,835±902 U/L; p<0.01) indicated a protective role of TIM-3 overexpression. To determine whether this effect was donor or recipient specific, we cross-transplanted livers between WT and TIM-3Tg mice. Indeed, well-preserved hepatocellular function (sALT; U/L) was consistently detected in WT->TIM-3Tg (2,821±870) but not in TIM-3Tg->WT (7,571±2,014; p<0.01) cold-preserved OLTs. This indicated the protective function of TIM-3 in recipient circulating CD4+ T cells. The sALT levels correlated with hepatic histology and Suzuki’s criteria of liver IRI at 6 h post-transplant (Fig. 1C, D). OLTs in WT->WT and TIM-3Tg->WT groups showed lobular edema, widespread hemorrhage, and congestion/hepatocellular necrosis (score: 2.72±0.46 and 2.99±0.72, respectively) in contrast to well-preserved histological detail in TIM-3Tg->TIM-3Tg, and WT->TIM-3Tg OLTs (score: 0.71±0.16 and 0.82±0.27, respectively).
Figure 1.
TIM-3 overexpressing recipient CD4+ T cells prevent hepatocellular damage in IR-stressed OLTs. (A) TIM-3 expression on spleen CD4+ T cells of TIM-3Tg vs. WT mice (representative flow cytometry data; n=3/group); (B) sALT levels (U/L) at 6h of reperfusion (after 20h cold storage) in mouse recipients of syngeneic OLTs (WT->WT; Tg->WT; WT->Tg; Tg->Tg); (C) Liver histology (representative H&E staining; magnification x100 and x400); (D) Suzuki’s histological score of hepatic IR-damage. **p<0.01, B–D: n=6/group.
TIM-3 Signaling Regulates T Cell Infiltration and Differentiation in IR-Stressed OLTs
At 6h of reperfusion (after 20h of cold storage) CD4+ T cells were readily detectable in IR-stressed OLTs in WT recipients (Fig. 2A: 9.50±1.76 cells/HPF in WT->WT; and 8.67±1.63 cells/HPF in TIM-3Tg->WT). In contrast, only few CD4+ T cells could be found in OLTs transplanted into TIM-3Tg recipients (Fig. 2A: 1.17±0.98 cells/HPF in WT->TIM-3Tg; and 0.83±0.75 cells/HPF in TIM-3Tg->TIM-3Tg). We also found that irrespective of the donor type (WT or TIM-3Tg), increased mRNA levels coding for Th1 transcription factor Tbet/IFN-γ were found in OLTs transplanted to WT, as compared with TIM-3Tg recipients (Fig. 3A: p<0.05). On the contrary, Th2-related transcription factor GATA3 level was enhanced, along with increased IL-4/IL-10 levels in TIM-3Tg recipients of WT or TIM-3Tg liver grafts (Fig. 3B: p<0.05, p<0.01, respectively). In addition, mRNA levels coding for T cell exhaustion markers, PD-1/LAG-3, were elevated exclusively in OLTs of WT->TIM-3Tg and TIM-3Tg->TIM-3Tg groups (Fig. 3C: p<0.05).
Figure 2.
Representative immunofluorescence staining for OLT-infiltrating: (A) CD4+ T cells; (B) Ly-6G+ neutrophils; (C) CD68+ macrophages (6h post-transplantation after 20h of cold ischemia. Magnification x200; n=6/group.
Figure 3.
qRT-PCR-assisted molecular screening of IR-stressed OLTs (6h of reperfusion after 20h cold storage) for: (A) Th1-type factors (Tbet, IFN-γ); (B) Th2-type factors (GATA3, IL-4, IL-10); (C) T cell exhaustion markers (PD-1, LAG-3); (D) TLR4 and chemokines (CCL-2, CXCL-10, CXCL-1); cytokines (TNF-α, IFN-β, IL-1β, and IL-6). Data normalized to HPRT gene expression. *p<0.05, **p<0.01, n=6/group.
TIM-3 Signaling Attenuates Neutrophil and Macrophage Function in IR-Stressed OLTs
The immunofluorescence stains have revealed enhanced accumulation of neutrophils (Ly-6G; 31.25±3.86 cells/HPF in WT->WT; and 33.25±4.92 cells/HPF in Tg->WT; Fig. 2B) and macrophages (CD68; 23.75±3.59 cells/HPF in WT->WT and 23.50±4.43 cells/HPF in Tg->WT; Fig. 2C) in IR-stressed liver transplants. In contrast, OLTs in WT->TIM-3Tg and TIM-3Tg->TIM-3Tg groups were characterized by diminished sequestration (cells/HPF) of neutrophils (10.50±2.64 in WT->Tg and 8.59±2.71 in Tg->Tg; Fig. 2B) and macrophages (9.25±1.70 in WT->Tg and 10.75±2.50 in Tg->Tg; Fig. 2C). In agreement with cell trafficking data, OLTs subjected to cold IR-insult in WT->TIM-3Tg and TIM-3Tg->TIM-3Tg groups were characterized by significantly (p<0.05, p<0.01, respectively) suppressed TLR4 and diminished proinflammatory chemokine (CCL-2, CXCL-10, CXCL-1) and cytokine (TNF-α, IFN-β, IL-1β, IL-6) programs, as compared with WT recipient groups (Fig. 3D, E).
CD4+ T cell-dependent TIM-3 signaling confers IR-hepatoprotection
To further elucidate the role of CD4+ T cell-dependent TIM-3 immunomodulation, we used a model of liver IRI in RAG−/− mice [19]. Indeed, adoptive transfer of spleen CD4+ T cells (5×106 i.v.) from WT (<2% of TIM-3+ cells) recreated classic hepatocellular damage in otherwise IR-resistant RAG−/− test mice, evidenced by sALT levels at 6h of reperfusion (Fig. 4A, 3053±286 vs. 228±84 U/L in controls; p<0.01). In marked contrast, infusion of CD4+ T cells from TIM-3Tg donors (50–60% of TIM-3+ cells) failed to trigger liver IRI in RAG−/− test recipients (Fig. 4A, sALT: 297±85 vs. 228±84 U/L in controls). Moreover, improved liver histology (Fig. 4B) along with diminished TLR4 activation, as well as TNF-α, IL-1β and IL-6 levels (Fig. 4C) were found in RAG−/− mice repopulated with TIM-3Tg but not with WT CD4+ T cells.
Figure 4.
RAG−/− mice repopulated with spleen CD4+ T cells from syngeneic WT or TIM-3Tg mice (5×106 i.v.) were subjected 1 hour later to 90min of liver warm ischemia. At 6h of reperfusion, the hepatocellular damage was assessed by: (A) sALT levels; (B) liver histology (representative H&E staining; magnification x100 and x400); (C) qRT-PCR-assisted TLR4, TNF-α, IL-1β, and IL-6 expression. **p<0.01; n=3/group.
Stressed Hepatocytes Express Gal-9
As a natural ligand for TIM-3, Gal-9 is widely distributed throughout tissues, being particularly abundant in the liver, due to the hepatic exposure to diverse antigenic loads [23]. To identify cellular sources of Gal-9, we performed immunofluorescence studies. Fig. 5A shows that unlike in control sham-livers, dense Gal-9 expression (red spots) was detectable in IR-stressed OLTs (WT → WT; 6h of reperfusion), primarily in hepatocytes (arrows) and as secreted protein in sinusoidal liver vessels (arrowheads). Very few mononuclear cells were Gal-9+ at that time-point. A similar labeling pattern was observed at 6h of reperfusion in warm liver IRI model (data not shown). To confirm our in-vivo findings, we then probed for Gal-9 expression mouse primary hepatocyte cultures (at 4h). As shown in Fig. 5B, unlike untreated hepatocytes (blank), those that were H2O2-stressed strongly expressed Gal-9 (arrows). In addition, after its release from hepatocytes, Gal-9 was detected in extracellular spaces (arrowheads). Thus, hepatocytes represent the primary source of Gal-9 in our model.
Figure 5.
Gal-9 - TIM-3 signaling network. Representative immunofluorescence staining for Gal-9 in: (A) IR-stressed OLTs (WT → WT; 6h reperfusion); and (B) H2O2-stressed primary hepatocytes (4h culture). Tissue sections/cultured cells were probed with anti-Gal-9 mAb/fluorescent-labeled secondary Ab. Gal-9 staining (arrows; red spots) in hepatocytes, and secretion to liver sinusoidal vessels (A – arrowheads); and extracellular spaces in hepatocyte cultures (B - arrowheads). DAPI and F-actin identify cell nucleus (blue) and cytoskeleton (green), respectively. Magnification x400, n=6/group,.
Gal-9 Potentiates Liver Resistance Against IR Stress
We reasoned that Gal-9 may manifest itself as an anti-oxidative defense factor contributing to liver IR-resistance in TIM-3-dependent manner. First, we analyzed the kinetics of Gal-9 expression in a mouse model of warm liver ischemia (90min). As shown in Fig. 6A, unlike in sham controls, the expression of hepatic Gal-9 mRNA increased progressively during the reperfusion, peaking at 6h and decreasing thereafter. Then, we have set-up two groups of WT and TIM-3Tg mice that were pre-conditioned with a single dose of human stable-form rGal-9 (100ug/mouse i.v. at -1h). Fig. 6B shows that administration of rGal-9 attenuated IRI, evidenced by sALT levels (764.50±145.98 IU/L vs. 7802.33±1515.33 IU/L in untreated WT; p<0.01) by 6h of reperfusion. Adjunctive rGal-9 further enhanced cytoprotection in IR-stressed TIM-3Tg livers (sALT: 335.27±107.77 IU/L vs. 1368.33±217.82 IU/L in untreated TIM-3Tg; p<0.01). As shown in Fig. 6C, pretreatment with rGal-9 blunted innate immune activation by decreasing mRNA levels coding for TLR4, TNF-α, IL-1β and IL-6 (p<0.05) in IR-stressed WT and TIM-3Tg livers.
Figure 6.
Groups of WT and TIM-3Tg mice, subjected to warm liver IR (90min) −/+ rGal-9 (100ug/mouse i.v. at -1h), were analyzed at 6h of reperfusion for: (A) the kinetics of Gal-9 expression by in IR-stressed livers by qRT-PCR (n=4/group); (B) sALT levels; (C) qRT-PCR-assisted expression of TLR4, TNF-α, IL-1β and IL-6. *p<0.05; **p<0.01; B/C: n=6/group.
Gal-9 Depresses T Cell Activation and Promotes Apoptosis of CD4+ T Cells
To further define the mechanisms by which Gal-9 may exert its biological functions, we first performed in-vitro studies in which spleen T cells from WT or TIM-3Tg mice were cultured for 24h with ConA +/− human stable-form rGal-9 (Fig. 7A). After ConA treatment, T cells from WT mice were readily activated, evidenced by increased IFN-γ levels (pg/ml) [1072.02±213.66 (ConA 2ug/ml), 1609.28±395.72 (ConA 4ug/ml) vs. 10.54±1.34 pg/ml blank group]. In contrast, T cells from TIM-3Tg mice showed lower activation after ConA [IFN-γ: 345.02±64.88 pg/ml (ConA 2ug/ml; p<0.01), 474.19±120.82 pg/ml (ConA 4ug/ml; p<0.01) vs. 9.30±1.35 pg/ml blank group]. Addition of rGal-9 diminished ConA-induced T cell activation in WT and TIM-3Tg cultures, evidenced by decreased IFN-γ levels (pg/ml) [WT+rGal-9: 521.73±84.06 (ConA 2ug/ml); 698.56±113.83 (ConA 4ug/ml) vs. 12.95±1.17 in blank group; TIM-3Tg+rGal-9: 76.34±14.77 (ConA 2ug/ml), 96.49±34.43 (ConA 4ug/ml) vs. 15.36±2.34 pg/ml in blank group]. As TIM-3-Gal-9 signaling negatively regulates T cell function [24], we then focused on the role of Gal-9 in CD4+ T cell apoptosis by assessing caspase-3/7 expression in-vitro by flow cytometry (Fig. 7B). Adjunctive rGal-9 promoted apoptosis of ConA-activated CD4+ WT T cells, compared with untreated ConA-stimulated cells. Indeed, >50% of TIM-3Tg CD4+ T cells cultured with rGal-9 expressed caspase-3/7 markers.
Figure 7.
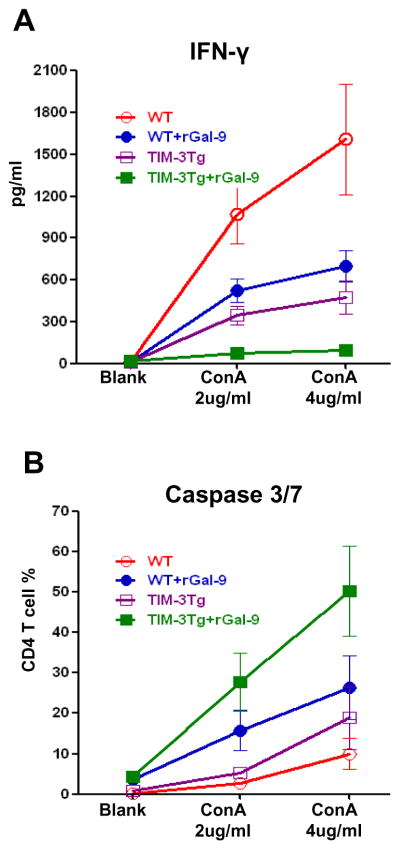
Spleen CD4+ T cells from WT or TIM-3Tg mice cultured with ConA (2μg/ml or 4μg/ml) −/+ rGal-9 (1μg/ml) were assessed at 24h for: (A) IFN-γ (ELISA); and (B) caspase 3/7 (FACS). n=6/group.
Finally, we analyzed as to whether or not Gal-9 may regulate CD4+ T cells apoptosis in-vivo (Fig. 8). Groups of WT and TIM-3Tg mice were pre-treated with rGal-9 followed by warm hepatic ischemia (90min). At 6h of reperfusion, liver and spleen CD4+ T cells were assayed for caspase-3 activity. Increased CD4+ T cell apoptosis in-vivo was detected after rGal-9 treatment in both IR-stressed WT and TIM-3Tg livers (pmol/min/5X10E8 cells; Fig. 8A: 0.26±0.08 in WT+rGal-9 and 0.94±0.12 in TIM-3Tg+rGal-9 group vs. 0.11±0.2 in WT and 0.46±0.07 in TIM-3Tg group, p<0.01). Increased CD4+ T cell caspase-3 activity was also found in recipients’ spleens (pmol/min/5X10E8 cells; Fig. 8B: 1.40±0.36 in WT+rGal-9 and 3.49±0.91 in TIM-3Tg+rGal-9 group vs. 0.43±0.14 in WT and 1.31±0.39 in TIM-3Tg group, p<0.01), indicating systemic Gal-9 effects upon TIM-3+CD4+ T cell function. Thus, TIM-3-Gal-9 cross-linking promotes CD4+ T cell apoptosis in IR-stressed livers.
Figure 8.

Groups of WT and TIM-3Tg mice, subjected to warm liver IR (90min) −/+ rGal-9 (100ug/mouse i.v. at -1h), were sacrificed at 6h of reperfusion. (A) liver-infiltrating CD4+ cells; and (B) spleen-resident CD4+ T cells were assessed for cell apoptosis by analyzing caspase-3 activity (pmol/min/5X10E8 cells). **p<0.01, n=6/group.
Discussion
The importance of TIM-3 – Gal-9 costimulation in anti-oxidative defense became evident in our study in a mouse liver “warm” IRI model, when TIM-3 blockade exacerbated inflammation and dictated its severity in TLR4-dependant manner [11]. However, as TIM-3 molecule is widely distributed, including T cells, DCs, macrophages/monocytes, and NK cells [12–14], the role of T cell-specific TIM-3 in the mechanism of liver IRI remains unknown. Moreover, by targeting distinct signaling pathways in innate and adaptive immune cells, TIM-3 engagement may differentially influence inflammatory cascades by promoting or terminating Th1 immunity [25]. Here, we employed mice that selectively overexpress TIM-3 on their T cells [15] in a clinically-relevant mouse OLT model. Our results show that liver transplants became resistant against cold ischemia-inflicted preservation damage in recipients, which harbored TIM-3+CD4+ T cells. Moreover, we established what we believe to be for the first time, that IR-stressed hepatocytes are the principal source of Gal-9 in our model. Treatment with rGal-9 potentiated resistance against IRI by impairing TIM-3+ T cell function, and promoting apoptosis. Hence, engaging TIM-3 – Gal-9 signaling at the recipient CD4+ T cell – hepatocyte interface, is essential for combating hepatocellular damage, and to maintaining homeostasis in IR-stressed mouse OLTs. No data exists on the role of TIM-3 - Gal-9 pathway in human liver transplants. Published data on TIM-3-Gal-9 signaling in transplant patients are limited. Increased blood concentrations of soluble extracellular TIM-3 before clinical onset of acute GVHD correlated with the severity of GVHD after hematopoietic cell transplantation [26]. It has also been suggested that TIM-3 transcript levels in urine may serve as a rejection biomarker in renal transplant patients [27].
In the current study, we first found that unlike WT livers transplanted into WT mice, those from TIM-3Tg donors remained IRI-resistant in TIM-3Tg recipients, confirming cytoprotective function of systemic TIM-3 overexpression. In analogy with donor-type “passenger leukocyte” migration in the rejection cascade [28], we asked whether donor or recipient TIM-3+CD4+ T cells affect IR-induced OLT damage. IR-resistance was consistently detected in livers (WT or Tg) transplanted into TIM-3Tg, but not WT recipients. Thus, recipient circulating TIM-3+CD4+ T cells promote anti-inflammatory phenotype after migrating into the ischemic liver tissue. We then tested the role of CD4+ T cell-dependent TIM-3 signaling in our adoptive transfer model of liver warm IRI in RAG−/− mice. These test recipients remained resistant against IR-inflammation and damage following infusion of TIM-3 overexpressing but not WT CD4+ T cells, indicating a CD4+ T cell dependent immunomodulatory role of TIM-3. Expressed by terminally-differentiated Th1 cells, TIM-3 signaling has been associated with a number of Th1-dependent diseases [29,30]. In agreement with clinical finding of imbalanced TIM-3 and Tbet expression in the pathogenesis of aplastic anemia [31], we found a decrease of Tbet transcription factor in IR-resistant OLTs in TIM-3Tg recipients. Furthermore, the expression of IFN-γ was depressed in TIM-3Tg recipients, along with enhanced GATA-3, IL-4, and IL-10 mRNA levels. A similar Th2-type polarization, with inhibition of Th1/Th17 cytokine programs was detected in liver IRI after TIM-1 blockade [19]. Thus, our findings are in agreement with the immunomodulatory role of TIM-targeted T cell differentiation/function in the early IRI phase in OLT recipients.
One of principal mechanisms by which TIM-3 may induce T cell dysfunction is via “exhaustion”. Several lines of evidence suggest TIM-3 molecule as a marker of T cell exhaustion phenotype in cancer immune surveillance and autoimmune disorders [24]. In addition, targeting TIM-3 effectively induced anti-tumor/virus T cell immunity [32,33]. In our study, high TIM-3 expression by recipient CD4+ T cells protected liver grafts from IRI, accompanied by enhanced levels of PD-1 and LAG-3 T cell exhaustion markers. Indeed, we have demonstrated the essential role of PD-1/B7-H1 pathway in liver inflammation, when stimulating PD-1 ameliorated IR-stress, at least in part by inhibiting T cell activation [17]. Consistent with co-expression of TIM-3 and PD-1 identifying T cell exhaustion phenotype [34], our results suggest that harnessing negative TIM-3 and PD-1 signaling dysregulates T cell function with resultant protection against hepatic IRI.
We have identified T cell – macrophage crosstalk via TLR4-downstream IRF-3 signaling, instrumental in IR-induced inflammation/hepatocellular damage [4]. In our current study, decreased TLR4 expression along with impaired activation/recruitment of CD68+ macrophages were observed in IR-stressed OLTs in TIM-3Tg recipients. Indeed, unlike in sham controls, elevated neutrophil infiltration was found in TIM-3Tg liver grafts of WT recipients. In contrast, TIM-3 overexpressing recipient CD4+ T cells that migrated into IR-stressed OLTs were essential in blunting neutrophil infiltration, along with proinflammatory cytokine profiles. The inability of TIM-3 overexpressing T cells to mount IR-response is in accord with exacerbated local inflammation after disruption of TIM-3 signaling [11], emphasizing the essential function of TIM- 3+ CD4+ T cells in acquiring hepatic homeostasis.
Gal-9, originally identified as a T cell-derived factor with eosinophil chemotactic activity [35], plays important albeit diverse regulatory functions [15,36]. On one hand, as proinflammatory factor, Gal-9 induces DC maturation to enhance Th1-type immunity [37]. On the other hand, Gal-9 may limit Th1 activation to protect against allo-specific T cell responses [38] and restrain allograft rejection [39]. Gal-9 may also serve as a key regulator in viral and inflammatory liver disease states [40,41]. Our findings are the first to document that in addition to well-documented Kupffer cell/macrophage expression [42,] hepatocytes are the major Gal-9 producers in livers subjected to IR-insult. Indeed, abundant Gal-9 expression was detected in IR-stressed hepatic cells in-vivo and in H2O2-stressed hepatocyte cultures, with Gal-9 protein released from the cytoplasm into liver sinusoids to directly exert its regulatory functions. Our finding of Gal-9 normally at low levels, and oxidative stress inducing its predominantly hepatocyte expression, both in-vivo and in-vitro, along with the ability of rGal-9 to halt IRI in WT and enhance protection in TIM-3Tg livers, suggest Gal-9 as a novel hepatoprotective mechanism against IR-inflammation. This is in agreement with Gal-9-mediated negative feedback mechanism to trigger T cell apoptosis through engagement/stimulation of TIM-3 [43]. Others have suggested that higher Gal-9 expression by HCV-infected human hepatocytes may drive CD4+CD25+ Foxp3+ regulatory T-cell development through TIM-3-Gal-9 pathway [44].
Gal-9-TIM-3 signaling suppresses T-cell mitogenic stimulation, whereas Gal-9 may directly induce Th1 cell apoptosis and down-regulate Th1 responses [45]. We found that cultured TIM-3+CD4+ T cells were susceptible to rGal-9-induced apoptosis via caspases, along with diminished ConA-mediated activation. In addition, exogenous rGal-9 influenced early features of hepatic IRI, evidenced by amelioration of parenchymal cell apoptosis (caspase-3) and inhibition of Kupffer cell (TNF-α/IL-1β) and endothelial cell (E-selectin/P-selectin/ICAM-1) activation as early as at 1h of reperfusion (Suppl. Fig. 1A–C). Hence, hepatocyte-derived Gal-9 may negatively regulate immune responses in divergent IR-stressed liver cell types. As Gal-9 can be released to the circulation, it is plausible that CD4+ T cell apoptosis may occur in secondary lymphoid organs (e.g., spleen) with resultant depressed intrahepatic CD4+ T cell sequestration.
In conclusion, by providing new insights into the pathogenesis hepatic damage in IR-stressed OLTs, our results have important clinical implications. First, negative regulation by recipient TIM-3+CD4+ cells provides evidence for cytoprotective functions by a discrete T cell subset, which should be spared when applying T cell-targeted immunosuppression in transplant recipients. Second, enhancing anti-oxidant hepatocyte-derived Gal-9 depresses T cell activation and promotes apoptosis of pathogenic CD4+T cells to potentiate liver IR-resistance. Thus, harnessing TIM-3–Gal-9 signaling at the recipient CD4+T cell–hepatocyte interface may lead to the development of novel strategies to induce durable homeostasis in OLTs.
Supplementary Material
Exogenous rGal-9 regulates early hepatic IRI (1h of reperfusion after 90min of warm ischemia). (A) Liver tissue caspase-3 activity; (B) qRT-PCR -assisted detection of hepatic TNF-α/IL-1β; (C) hepatic endothelial cell activation, assessed by E-/P-selectin and ICAM-1 gene expression. **p<0.01; n=6/group.
Cold preservation time affects IR-stressed OLT function. sALT levels at 6h of reperfusion of liver grafts subjected to 0.5h vs. 20h of cold storage. **p<0.01; n= 6/group.
Acknowledgments
Financial Support: NIH Grant RO1 DK062357 (JWKW) ; W.M. Keck Foundation; The Dumont Research Foundation. HJ is a recipient of the Pilot and Feasibility Grant from CURE Digestive Diseases Research Center and Clinical/Translational Science Institute (CTSI) at UCLA.
Abbreviations
- IRI
ischemia-reperfusion injury
- OLT
orthotopic liver transplantation
- TIM-3
T cell immunoglobulin mucin-3
- Tg
transgenic
- Gal-9
Galectin-9
- sALT
serum alanine aminotransferase
Footnotes
Conflict of interest: None
Author’s contribution: Yuanxing Liu: conception/design of the work, in-vitro experiments, data analysis; Haofeng Ji: conception/design of the work, immunofluorescence studies, data analysis; Yu Zhang: some in-vitro/molecular studies; Xiuda Shen: animal surgery; Feng Gao: animal surgery; Xiangyi He: in-vitro experiments; Gabriella A. Li: in-vitro experiments; Ronald W. Busuttil: discussant, approval of the final manuscript, financial support; Vijay K. Kuchroo: discussant, provided TIM-3Tg mice; Jerzy W. Kupiec-Weglinski: conception/design of the work, manuscript revisions, financial support.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wertheim JA, Petrowsky H, Saab S, Kupiec-Weglinski JW, Busuttil RW. Major challenges limiting liver transplantation in the United States. Am J Transplant. 2011;11:1773–1784. doi: 10.1111/j.1600-6143.2011.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563–1569. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 5.Zhai Y, Shen XD, Hancock WW, Gao F, Qiao B, Lassman C, et al. CXCR3+CD4+ T cells mediate innate immune function in the pathophysiology of liver ischemia/reperfusion injury. J Immunol. 2006;176:6313–6322. doi: 10.4049/jimmunol.176.10.6313. [DOI] [PubMed] [Google Scholar]
- 6.Pommey S, Lu B, McRae J, Stagg J, Hill P, Salvaris E, et al. Liver grafts from CD39-overexpressing rodents are protected from ischemia reperfusion injury due to reduced numbers of resident CD4+ T cells. Hepatology. 2013;57:1597–1606. doi: 10.1002/hep.25985. [DOI] [PubMed] [Google Scholar]
- 7.Kuboki S, Sakai N, Tschöp J, Edwards MJ, Lentsch AB, Caldwell CC. Distinct contributions of CD4+ T cell subsets in hepatic ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1054–9. doi: 10.1152/ajpgi.90464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeung MY, McGrath M, Najafian N. The emerging role of the TIM molecules in transplantation. Am J Transplant. 2011;11:2012–2019. doi: 10.1111/j.1600-6143.2011.03727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 10.Veenstra RG, Taylor PA, Zhou Q, Panoskaltsis-Mortari A, Hirashima M, Flynn R, et al. Contrasting acute graft-versus-host disease effects of Tim-3/galectin-9 pathway blockade dependent upon the presence of donor regulatory T cells. Blood. 2012;120:682–690. doi: 10.1182/blood-2011-10-387977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida Y, Ke B, Freitas MC, Yagita H, Akiba H, Busuttil RW, et al. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology. 2010;139:2195–2206. doi: 10.1053/j.gastro.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Moorman JP, et al. Tim-3 regulates pro- and anti-inflammatory cytokine expression in human CD14+ monocytes. J Leukoc Biol. 2012;91:189–196. doi: 10.1189/jlb.1010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel J, Bozeman EN, Selvaraj P. Taming dendritic cells with TIM-3: another immunosuppressive strategy used by tumors. Immunotherapy. 2012;4:1795–1798. doi: 10.2217/imt.12.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol. 2010;52:322–329. doi: 10.1016/j.jhep.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Dardalhon V, Anderson AC, Karman J, Apetoh L, Chandwaskar R, Lee DH, et al. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J Immunol. 2010;185:1383–1392. doi: 10.4049/jimmunol.0903275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen XD, Gao F, Ke B, Zhai Y, Lassman CR, Tsuchihashi S, et al. Inflammatory responses in a new mouse model of prolonged hepatic cold ischemia followed by arterialized orthotopic liver transplantation. Liver Transpl. 2005;11:1273–1281. doi: 10.1002/lt.20489. [DOI] [PubMed] [Google Scholar]
- 17.Ji H, Shen X, Gao F, Ke B, Freitas MC, Uchida Y, et al. Programmed death-1/B7-H1 negative costimulation protects mouse liver against ischemia and reperfusion injury. Hepatology. 2010;52:1380–1389. doi: 10.1002/hep.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moritoki M, Kadowaki T, Niki T, Nakano D, Soma G, Mori H, et al. Galectin-9 Ameliorates Clinical Severity of MRL/lpr Lupus-Prone Mice by Inducing Plasma Cell Apoptosis Independently of Tim-3. PLoS One. 2013;8:e60807. doi: 10.1371/journal.pone.0060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Ji H, Shen X, Cai J, Gao F, Koenig KM, et al. Targeting TIM-1 on CD4 T cells depresses macrophage activation and overcomes ischemia-reperfusion injury in mouse orthotopic liver transplantation. Am J Transplant. 2013;13:56–66. doi: 10.1111/j.1600-6143.2012.04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Shen X, Wang Y, Gao F, Ren F, Busuttil RW, Kupiec-Weglinski JW, et al. CD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury. Hepatology. 2009;50:1537–1546. doi: 10.1002/hep.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji H, Zhang Y, Shen XD, Gao F, Huang CY, Abad C, et al. Neuropeptide PACAP in mouse liver ischemia and reperfusion injury: immunomodulation by the cAMP-PKA pathway. Hepatology. 2013;57:1225–1237. doi: 10.1002/hep.25802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wada J, Kanwar YS. Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin. J Biol Chem. 1997;272:6078–6086. doi: 10.1074/jbc.272.9.6078. [DOI] [PubMed] [Google Scholar]
- 24.Zhu C, Anderson AC, Kuchroo VK. TIM-3 and its regulatory role in immune responses. Curr Top Microbiol Immunol. 2011;350:1–15. doi: 10.1007/82_2010_84. [DOI] [PubMed] [Google Scholar]
- 25.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 26.Hansen JA, Hanash SM, Tabellini L, Baik C, Lawler RL, Grogan BM, et al. A novel soluble form of Tim-3 associated with severe graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:1323–1330. doi: 10.1016/j.bbmt.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renesto PG, Ponciano VC, Cenedeze MA, Saraiva Camara NO, Pacheco-Silva A. High expression of Tim-3 mRNA in urinary cells from kidney transplant recipients with acute rejection. Am J Transplant. 2007;7:1661–1665. doi: 10.1111/j.1600-6143.2007.01795.x. [DOI] [PubMed] [Google Scholar]
- 28.Fu F, Li W, Lu L, Thomson AW, Fung JJ, Qian S. Prevention and restoration of second-set liver allograft rejection in presensitized mice: the role of “passenger” leukocytes, donor major histocompatibility complex antigens, and host cytotoxic effector mechanisms. Transplantation. 1999;67:444–450. doi: 10.1097/00007890-199902150-00018. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Shu Q, Gao L, Hou N, Zhao D, Liu X, et al. Increased Tim-3 expression on peripheral lymphocytes from patients with rheumatoid arthritis negatively correlates with disease activity. Clin Immunol. 2010;137:288–295. doi: 10.1016/j.clim.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Kanai Y, Satoh T, Igawa K, Yokozeki H. Impaired expression of Tim-3 on Th17 and Th1 cells in psoriasis. Acta Derm Venereol. 2012;92:367–371. doi: 10.2340/00015555-1285. [DOI] [PubMed] [Google Scholar]
- 31.Shan NN, Hu Y, Liu X, Wang X, Yuan D, Li Y. Imbalanced expression of T-bet and T cell immunoglobulin mucin-3 in patients with aplastic anaemia. J Clin Immunol. 2013;33:809–816. doi: 10.1007/s10875-013-9864-7. [DOI] [PubMed] [Google Scholar]
- 32.Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol. 2012;24:213–216. doi: 10.1016/j.coi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirashima M, Kashio Y, Nishi N, Yamauchi A, Imaizumi TA, Kageshita T, et al. Galectin-9 in physiological and pathological conditions. Glycoconj J. 2004;19:593–600. doi: 10.1023/B:GLYC.0000014090.63206.2f. [DOI] [PubMed] [Google Scholar]
- 36.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 37.Dai SY, Nakagawa R, Itoh A, Murakami H, Kashio Y, Abe H, et al. Galectin-9 induces maturation of human monocyte-derived dendritic cells. J Immunol. 2005;175:2974–2981. doi: 10.4049/jimmunol.175.5.2974. [DOI] [PubMed] [Google Scholar]
- 38.Shimmura-Tomita M, Wang M, Taniguchi H, Akiba H, Yagita H, Hori J. Galectin-9-mediated protection from allo-specific T cells as a mechanism of immune privilege of corneal allografts. PLoS One. 2013;8:e63620. doi: 10.1371/journal.pone.0063620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou FC, Kuo CC, Wang YL, Lin MH, Yen BL, Chang DM, et al. Overexpression of Galectin-9 in Islets Prolongs Grafts Survival via Downregulation of Th1 Responses. Cell Transplant. 2012 doi: 10.3727/096368912X657891. [DOI] [PubMed] [Google Scholar]
- 40.Tang ZH, Liang S, Potter J, Jiang X, Mao HQ, Li Z. Tim-3/galectin-9 regulate the homeostasis of hepatic NKT cells in a murine model of nonalcoholic fatty liver disease. J Immunol. 2013;190:1788–1796. doi: 10.4049/jimmunol.1202814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kared H, Fabre T, Bedard N, Bruneau J, Shoukry NH. Galectin-9 and IL-21 mediate cross-regulation between Th17 and Treg cells during acute hepatitis C. PLoS Pathog. 2013;9:e1003422. doi: 10.1371/journal.ppat.1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, et al. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One. 2010;5:e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jayaraman P, Sada-Ovalle I, Beladi S, Anderson AC, Dardalhon V, Hotta C, et al. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J Exp Med. 2010;207:2343–2354. doi: 10.1084/jem.20100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji XJ, Ma CJ, Wang JM, Wu XY, Niki T, Hirashima M, et al. HCV-infected hepatocytes drive CD4+ CD25+ Foxp3+ regulatory T-cell development through the Tim-3/Gal-9 pathway. Eur J Immunol. 2013;43:458–467. doi: 10.1002/eji.201242768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu LH, Nakagawa R, Kashio Y, Ito A, Shoji H, Nishi N, et al. Characterization of galectin-9-induced death of Jurkat T cells. J Biochem. 2007;141:157–172. doi: 10.1093/jb/mvm019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exogenous rGal-9 regulates early hepatic IRI (1h of reperfusion after 90min of warm ischemia). (A) Liver tissue caspase-3 activity; (B) qRT-PCR -assisted detection of hepatic TNF-α/IL-1β; (C) hepatic endothelial cell activation, assessed by E-/P-selectin and ICAM-1 gene expression. **p<0.01; n=6/group.
Cold preservation time affects IR-stressed OLT function. sALT levels at 6h of reperfusion of liver grafts subjected to 0.5h vs. 20h of cold storage. **p<0.01; n= 6/group.








