Abstract
Cocaine abuse represents an immense societal health and economic burden for which no effective treatment currently exists. Among the numerous intracellular signaling cascades impacted by exposure to cocaine, increased and aberrant production of pro-inflammatory cytokines in the CNS has been observed. Additionally, we have previously reported a decrease in retinoid-X-receptor-gamma (RXR-γ) in brains of mice chronically exposed to cocaine. Through obligate heterodimerization with a number of nuclear receptors, RXRs serve as master regulatory transcription factors, which can potentiate or suppress expression of a wide spectrum of genes. Little is known about the regulation of RXR levels, but previous studies indicate cellular stressors such as cytokines negatively regulate levels of RXRs in vitro. To evaluate the mechanism underlying the cocaine-induced decreases in RXR-γ levels observed in vivo, we exposed neurons to cocaine in vitro and examined pathways which may contribute to disruption in RXR signaling, including activation of stress pathways by cytokine induction. In these studies, we provide the first evidence that cocaine exposure disrupts neuronal RXR-γ signaling in vitro by promoting its nuclear export and degradation. Furthermore, we demonstrate this effect may be mediated, at least in part, by cocaine-induced production of TNF-α and its downstream effector c-Jun-NH-terminal kinase (JNK). Findings from this study are therefore applicable to both cocaine abuse and to pathological conditions characterized by neuroinflammatory factors, such as neurodegenerative disease.
Keywords: cocaine, retinoid-X-receptor-γ, neuroinflammation, tumor necrosis factor-α, protein degradation, Jun-N-terminal kinase
INTRODUCTION
Exposure to cocaine induces expression of inflammatory factors, including tumor-necrosis-factoralpha (TNF-α), in both in vitro systems and in vivo [1–3] Induction of inflammatory signaling cascades results in activation of MAP kinase kinases (MKKs) MKK4 and MKK7, which respond to environmental stressors, and in turn activate c-Jun-NH(2)-terminal kinase (JNK) via phosphorylation of Thr183 and Tyr185 [4–6]. In turn, phosphorylated JNK activates a number of pathways through its kinase activity, most notably the c-jun/AP-1 pathway which regulates transcription of a number of genes involved in cellular processes such as apoptosis, differentiation, cell cycle and inflammatory response [6, 7]. More recently, it has been demonstrated that JNK phosphorylates specific nuclear receptor transcription factors, which may impact their function, DNA-binding activity, and/or degradation [6, 8–11]. Among the nuclear receptors identified as substrates for phospho-JNK, the retinoid-X-receptor-alpha (RXR-α) has been demonstrated to be phosphorylated at multiple residues in hepatocytes and COS-1 cells following treatment with pro-inflammatory cytokines such as interleukin 1-beta (IL-1β) or activation of stress pathways through UV irradiation [8, 11]. Additionally, treatment with TNF-α or IL-1 decreases the levels of a number of nuclear receptors, including RXR-α, peroxisome proliferator-activated receptor (PPAR)-α, and PPAR-γ in liver cells in vitro through an uncharacterized mechanism [12]. The IL-1β-induced reduction in liver RXR-α levels was demonstrated in vivo, as well [13]. Despite mounting evidence for a critical role of RXRs in maintaining neuronal health and neurocognitive function, very little literature exists on the regulation of these receptors in response to cellular stress within the CNS. Similarly, very little is known about the regulation of the alternative RXR isoforms, RXR-β and RXR-γ, which share high sequence homology to RXR-α, but differ in their N-terminal activation domains as well as in their tissue-specific expression patterns [14].
Retinoid-X-receptors (RXRs) are required heterodimer partners for a number of ligand-activated transcription factors including the retinoic acid receptor (RAR), thyroid hormone receptor (TR), vitamin D receptor (VDR), and PPAR [14–17]. While whether or not the presence of ligand is required for RXR activity is a topic of controversy, it is well established that other nuclear receptors must be partnered to RXR in order to function as transcriptional regulators [18]. Therefore, alterations in nuclear RXR levels and/or function may impact multiple signaling pathways and result in disrupted expression of a number of target genes. Retinoid-X-receptor-γ (NR2B3) exhibits restricted expression compared to the more ubiquitously found RXR-α and –β, however, it is highly enriched within the CNS and is the only RXR isoform known to be expressed in certain brain regions, including the hypothalamus [19, 20]. Furthermore, RXR-γ is highly expressed in the striatum where it is known to play a critical role in dopaminergic signaling, dopamine-mediated locomotor activity, and reward processing [21–23]. Thus, RXR-γ represents a likely nuclear receptor candidate to be impacted by cocaine. In this context, we have previously demonstrated a significant decrease in RXR-γ expression in brains of cocaine-treated mice [24]. Furthermore, we observed decreased mRNA and protein expression of neurogranin, a synaptic plasticity-related protein shown to be regulated by TR/RXR heterodimers at the transcriptional level [24– 26].
While the degree to which cocaine is directly neurotoxic is a subject of debate, studies demonstrate that cocaine exposure at physiologically relevant doses induces apoptosis via TNF-α-mediated mechanisms in a number of cell types including human brain endothelial cells, fetal rat locus coeruleus neurons, human progenitor neurons, and PC12 cells [1, 27–30]. Additionally, cocaine has been shown to significantly compromise the integrity of the blood-brain-barrier (BBB), allowing inflammatory factors from the periphery to traffic into the CNS [3]. In fact, cocaine has been shown to contribute to these processes via JNK activation [31, 32]. Both cocaine abuse and chronic exposure to cytokines due to infection or disease are associated with significant neuronal dysfunction and cognitive impairment [33–35]. As RXRs are critical regulators of neuronal health and plasticity, cocaine-mediated dysregulation of RXR signaling may contribute to cognitive deficits and neurodegenerative processes commonly observed in drug-abusing populations. Furthermore, as sustained CNS TNF-α expression is a hallmark of a number of neuroinflammatory diseases including HIV-associated encephalitis (HIV-E), multiple sclerosis (MS), Alzheimer’s disease (AD), and Parkinson’s disease (PD), JNK-mediated degradation of RXRs may have a profound impact on the severity of neuronal dysfunction in these diseases [36–38]. Importantly, disrupted signaling of RXR and of nuclear receptors that partner with RXRs has been reported in many of these disorders [39–42].
In this study, we investigated the neuron-specific mechanism(s) underlying the cocaine-mediated decrease in RXR-γ levels in response to chronic cocaine exposure we previously reported in vivo [24]. We hypothesized that cocaine-mediated induction of TNF-α leads to phosphorylation and subsequent nuclear export and proteasome-dependent degradation of RXR-γ in a JNK-dependent manner. We found significantly decreased expression of RXR-γ in neurons in response to cocaine treatment, which could be attenuated by administration of a TNF-α function-blocking antibody or JNK inhibitor, as well as by proteasomal inhibition. Treatment with TNF-α alone mimicked the effects of cocaine, and degradation of RXR-γ appeared to be dependent upon its nuclear export. Taken together, these studies indicate that neuronal RXR signaling is significantly disrupted in response to cocaine and/or inflammatory cytokines. These findings could have significant implications for addressing neuronal dysfunction in drug-abusing populations, and likely extend to a number of neurodegenerative disease states that are characterized by chronic neuroinflammation.
EXPERIMENTAL PROCEDURES
Animals and Tissue Harvest
Wild type C57Bl/6 male mice obtained from Charles Rivers were utilized for this study. All procedures were approved by Temple University IACUC and were conducted accordingly. Mice were housed in a temperature controlled (21–23°C) animal facility with constant airflow and were provided food and water ad libitum. Mice were divided into three groups (n= 6–10 mice per group): control and cocaine as previously described [24, 43]. Control mice received an intraperitoneal injection of 200 µl sterile saline once per day for 10 days. The cocaine groups received intraperitoneal injection of 15mg/kg of cocaine dissolved in 200 µl sterile saline once per day for 5 days or 10 days [24, 43, 44]. Mice were weighed every day before injection. Mice were heavily anesthetized with isoflurane and decapitated immediately after last cocaine injection. Brains were quickly removed and placed into ice-cold 1× PBS. Brains were then sectioned into right and left hemispheres. The left hemispheres was fixed in 10% buffered formalin for 24 h and processed for immunolabeling by standard paraffin embedding and sectioning. Hippocampal tissue was dissected from the right hemisphere and stored at −80°C.
Protein Extraction from Tissue
Brain tissue was homogenized by mechanical dounce disruption on ice in TNN buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.5% NP40, 1 : 200 protease inhibitor cocktail; Calbiochem, San Diego, CA, USA). Tissue homogenates were centrifuged at 13 000 × g at 4°C for 5 min. The supernatant containing proteins was collected and protein concentration was determined by the Bradford assay for Western analyses.
Immunofluorescence Labeling of Tissue
Four µm coronal sections of formalin-fixed, paraffin-embedded tissues were placed on to electromagnetically charged glass slides. Slides were deparaffinized in xylene and rehydrated through descending grades of ethanol up to water. After non-enzymatic antigen retrieval in 0.01 M sodium citrate buffer (pH 6.0) for 30 min at 97°C in a vacuum oven, slides were washed with 1× PBS and placed in blocking solution (5% normal goat or horse serum; Vector Laboratories, Burlingame, CA, USA) for 2 h at room temperature. Primary antibodies consisted of post-synaptic density protein-95 (PSD-95) (1: 200; Abcam), synaptophysin (1: 200, Abcam). Sections were incubated with primary antibody overnight in a dark, humidified chamber at room temperature, rinsed 3 times with 1× PBS, and incubated with fluorescein isothiocyanate (FITC) (1 : 500; Vector Laboratories) or Texas Red (1 : 500; Vector Laboratories)-conjugated secondary antibodies for 1 h at 23°C in the dark. Sections were washed 3 times with 1× PBS, cover-slipped with an aqueous based mounting medium containing DAPI for nuclear labeling (Vectashield; Vector Laboratories), visualized with a Nikon ultraviolet inverted microscope, and processed with deconvolution software (Slidebook 4.0; Intelligent Imaging, Denver, CO, USA). Deconvolution was performed using SlideBook4 software, allowing acquisition of multiple 0.25 µm thick digital sections and 3-D reconstruction of the image. Cell Culture - SH-SY5Y neuroblastoma cells (ATCC® CRL-2266™, Manassas, VA) were maintained in a 1:1 ratio of Dulbecco’s Modified Eagle Medium: Nutrient Mixture F12 (DMEM/F12) (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) (Invitrogen). Twenty-four hours following initial plating, medium was replaced with neurobasal medium containing Glutamax and B27 supplement (Invitrogen) to induce a neuron-like phenotype [45, 46]. Neuro-2a (N2a) mouse neuroblastoma cells (ATCC®CCL-131™) were maintained in DMEM (Invitrogen) containing 10% FBS. SH-SY5Y and N2a cell culture media contained 1% penicillin/streptomycin antibiotic (Invitrogen). Human primary neurons were provided by Temple University’s Comprehensive NeuroAIDS Center. Fetal brain tissue (gestational age 16–18 weeks) was obtained from elective abortion procedures performed in full compliance with National Institutes of Health and Temple University ethical guidelines. The tissue was washed with cold Hanks balanced salt solution (HBSS) and meninges and blood vessels were removed. For primary neuronal isolation, tissue in HBSS was digested with papain (Sigma-Aldrich, St. Louis, MO) for 30 min at 37°C. The tissue was further dissociated to obtain single-cell suspensions by repeated pipetting. Cells were plated at a density of approximately 1.8 × 106 cells/ 60mm dish coated with poly-D lysine in neurobasal media with B27 supplement, horse serum, and gentamicin (Invitrogen). After approximately 2 h, neurons were re-fed with the same neurobasal media. Twenty-four hours later, cultures were re-fed with a complete change of neurobasal media without horse serum. Four days later, one fourth of the media was removed and replaced with neurobasal media supplemented with fluoro-deoxyuridine (FDU) and uridine. Following FDU treatment, neurons were maintained in neurobasal medium containing Glutamax and B27 supplement, with half-media changes every other day. Purity of cell type specific cultures was assessed by immunolabeling for cell-type specific markers.
Plasmid Constructs
For experiments requiring exogenous expression of RXR-γ, N2a cells were transfected with a myc-tagged human RXR-γ expression vector in a pCMV6 entry plasmid (Origene, Rockville, MD). Transfection was carried out using Fugene-mediated gene transfer according to manufacturers’ instructions (Promega, Fitchburg, WI). Vector only (pCMV6)-transfected N2a cells served as controls. For luciferase assays, N2a cells were transfected with a human RXR-γ promoter Renilla luciferase reporter construct (Switchgear Genomics, Menlo Park, CA) or a random control construct (Switchgear Genomics). The random R01 construct contained 1 kb of non-conserved, nongenomic, and non-repetitive fragments from the human genome cloned upstream of the luciferase reporter in place of the RXR-γ promoter to serve as an optimal background control. Additionally, a human GAPDH promoter luciferase reporter construct (Switchgear Genomics) was utilized as a transfection efficiency control and to assess positive luciferase expression.
In Vitro Drug Treatment
Cocaine hydrochloride (Sigma-Aldrich) was dissolved in sodium citrate buffer (pH 5.0) to increase stability [28]. Ten millimolar stock solution and subsequent dilutions were prepared daily and added to cell culture medium. A physiologically relevant dose of 5 µM was utilized for all experiments based on our previous experiments and those of others [24, 28, 31, 47–50]. Specifically, Sodium citrate buffer was used as the control vehicle during cocaine treatments. Cells were exposed to 10 µm forskolin or 30 nm dopamine (Calbiochem). (Human recombinant TNF-α (Cell Signaling, Danvers, MA) was dissolved in phosphate-buffered saline (PBS) containing 5% FBS and added to cell culture medium at a final concentration of 10 to 100 ng/ml, depending on the cell type being treated. Tumor necrosis factor-α neutralizing antibody (TNF-α nAb) (Cell Signaling) was diluted in sterile water and added to cell culture medium at a concentration of 0.5 µg/ml. Cells were pre-treated with TNF-α nAb for 2 h prior to cocaine treatments. The JNK inhibitor SP600125 (Cell Signaling) was dissolved in dimethyl sulfoxide (DMSO) and added to cell culture medium at a concentration of 10 µM. Cells were pre-treated with SP600125 for 16 h prior to cocaine or TNF-α treatment. Leptomycin B (LMB) (Cell Signaling) was dissolved in ethanol and added to cell culture medium at a concentration of 1 nM. Cells were pre-treated with LMB for 3 h prior to cocaine or TNF-α treatment. Bortezomib (Bort) (Toronto Research Chemicals, Ontario, Canada) was dissolved in DMSO and added to cell culture medium at a final concentration of 100 pM. Cells were pre-treated with Bort for 16 h prior to cocaine or TNF-α treatment.
Cell Harvest and Protein Extraction
Following treatment, cells were washed in 1× PBS, then scraped and collected in ice-cold PBS. Cell suspension was centrifuged at 13,000 × g for 1 min and the supernatant was discarded. The cell pellet was then re-suspended in ice-cold HEPES lysis buffer (50 mM HEPES (pH 7.4), 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1% Triton X-100) containing protease and phosphatase inhibitor cocktails (Calbiochem, San Diego, CA), vortexed, and incubated on ice for 10 min to complete cell lysis. Samples were then centrifuged at 13,000 × g for 10 min to separate insoluble material. The supernatant containing protein was collected and placed into clean, pre-chilled Eppendorf tubes and samples were stored at −80°C until protein analysis was performed. For experiments requiring nuclear/cytoplasmic fractionation, cells were collected similarly but lysed and fractionated using the NE-PER nuclear and cytoplasmic extraction kit according to the manufacturer’s protocol (Thermo-Scientific, Waltham, MA). Following fractionation, nuclear and cytoplasmic lysates were stored at −80°C until analysis.
Western Blotting
Equal amounts of protein were loaded (20 µg per lane unless otherwise indicated) onto pre-cast midi-gels (4–12% Bis–Tris; Invitrogen), separated by electrophoresis and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were blocked in 5% non-fat milk or bovine serum albumin (BSA) in Tris-buffered saline, 0.1% Tween-20 (TBST) for 30min before incubation with primary antibodies. Primary antibodies included: RXR-γ (1:1,000; Abcam), GAP-43 (1:1000; Abcam), phospho-JNK (1:1,000; Cell Signaling), total JNK (1:1,000; Cell Signaling), phospho-serine (1:1,000; Sigma) and the loading controls tubulin (1:5,000; Sigma-Aldrich) and lamin A/C (1:1,000; Cell Signaling). Membranes were incubated for either 2h at 23°C or overnight at 4°C, washed in 1× TBST, incubated with appropriate secondary anti-mouse or -rabbit antibodies (1:10,000; Thermo Scientific) for 1h, and developed with ECL Prime (Amersham Pharmacia Biotech, Piscataway, NJ). Band intensities were calculated using ImageJ software and normalized to the loading control [51].
RNA Extraction and quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR)
Following treatment, cells were harvested in PBS as described above. Total RNA was extracted using the Qiagen RNeasy® kit according to manufacturers’ instructions (Qiagen, Valencia, CA). Complementary DNA (cDNA) was generated using the iScript™ cDNA synthesis kit following the manufacturer’s protocol (Bio-Rad). Generation of a cDNA product was verified using primers for the housekeeping gene GAPDH. The PCR reaction was performed using a 96-well thermocycler and a standard amplification program. PCR products were separated through 1% agarose gel and visualized under UV light. Once a quality PCR product was confirmed for all samples, qRT-PCR analyses of RXR-γ and TNF-α were performed. Primer sequences utilized were: RXR-γ (F: 5’-aggcaggtttcggaagcttctg-3’; R: 5’-ggagtgtctccaatgagcttga-3’), TNF-α (F: 5’-ctgggcaggtctactttggg-3’; R: 5’-ctggaggccccagtttgaat-3’), and GAPDH (F: 5’-gtctcctctgacttcaacagc-3’; R: 5’-accaccctgttgctgtagccaa-3’). Reaction mixtures for qRT-PCR consisted of LightCycler® 480 SYBR Green I Master Mix (Bio-Rad), forward and reverse primers for RXR-γ, TNF-α, or GAPDH (housekeeping), 2 µL of cDNA (diluted 1 : 10) and H2O to bring the reaction volume up to 20 µL. Samples were placed in a 96-well plate and the qRT-PCR was performed on a Roche LightCycler® 480 (Roche, Indianapolis, IN). All reactions were performed in triplicate and amplification curves and Cp values were obtained for analysis. Results were normalized against GAPDH using the ΔΔCt quantification method and expressed as mean ± SEM.
Enzyme-Linked Immunosorbent Assay (ELISA)
Cell culture supernatant from cocaine-treated SH-SY5Y cells and human primary neurons was analyzed by ELISA for levels of secreted TNF-α. Cells were treated with 5 µM cocaine and the cell culture medium was collected 30 min following treatment. Any non-adherent cells were removed by centrifugation and the remaining supernatants were analyzed using a human TNF-α ELISA kit according to manufacturers’ instructions (BD Biosciences, San Jose, CA). One-hundred microliters of each sample (diluted 1:5 in sample diluent) was measured in duplicate and TNF-α levels were assessed by absorbance at 450 nm. Values were obtained and compared to the standard curve to extrapolate TNF-α concentration.
Luciferase Assay
Neuro-2a cells transfected with the human RXR-γ promoter, GAPDH promoter, or a random 1 kb sequence (R01) driving a luminescent reporter gene (RenSP) were treated with TNF-α and assayed 24 h later according to the manufacturer’s protocol (Switchgear Genomics). Briefly, cells were plated at a density of 10,000 cells per well of a 96-well cell culture plate and transfected with the appropriate promoter reporter constructs 24 h later. Following 36 h of transfection, cells were treated with 50 ng/ml of TNF-α for 24 h. Following treatment, the cell culture plate was frozen overnight at −80°C and thawed the following day to increase cell lysis and enhance luciferase signal. Samples were incubated in Lightswitch assay reagent (Switchgear) for 30 min in the dark and read on a luminometer (Perkin Elmer, Waltham, MA). Relative luciferase units were obtained for each sample and normalized to the untreated average value. Data were compared to R01 and GAPDH promoter activity to assess background luminescence and ensure luciferase expression from control promoter constructs was not affected by treatment.
Immunocytochemistry
Following the appropriate treatment, cells plated on chamber slides were washed once with 1× PBS and fixed with 4% formaldehyde for 15 min at 23°C. Following fixation, slides were washed with 1× PBS and cells were permeabilized with 0.2% Triton X-100 for 15 min, washed again, and placed in blocking solution (5% normal goat or horse serum; Vector Laboratories, Burlingame, CA) for 30 min. Slides were then incubated in primary antibody for 2 hr at 23°C. Primary antibodies consisted of RXR-γ (1:200; Abcam), myc (1:200; Origene), and phospho-JNK (1:200; Cell Signaling). Following incubation with primary antibody, slides were rinsed 3× with PBS, and incubated with fluorescein isothiocyanate (FITC) (1:500; Vector Laboratories) or Texas Red isothiocyanate (TRITC) (1:500; Vector Laboratories)-conjugated secondary antibodies for 1 h at 23°C in the dark. Sections were again washed 3× with PBS, cover-slipped with an aqueous based mounting medium containing DAPI for nuclear labeling (Vectashield; Vector Laboratories), and visualized with Leica Advanced Widefield imaging system (Leica Microsystems; Buffalo Grove, IL).
Immunoprecipitation
For immunoprecipitation (IP) experiments, cells were harvested and lysed in RIPA buffer (20 mM Tris (pH 8.0), 150 mM NaCl, 1% nonidet P-40 (NP-40), 2 mM EDTA, 10% glycerol 0.5% deoxycholic acid) containing protease inhibitor cocktail (1:100; Calbiochem), phosphatase inhibitor cocktail (1:100; Calbiochem), and 20 mM N-ethylmaleimide (Sigma) for 20 min on ice. Samples were centrifuged at 13,000 × g for 10 min to remove insoluble material, and supernatant containing protein was collected. One-hundred fifty µg of total protein was incubated with 1 µg/ml of IP antibody overnight at 4°C with end-over-end rotation. After incubation, 50 µL of Protein-A or -G bead slurry (Abcam), depending on antibody host species, was added and samples were incubated with end-over-end rotation at 4°C for 1 h. The beads with protein conjugates were washed 5 times with PBS and centrifuged to remove supernatant following each wash. After the final wash, protein was eluted from the beads by addition of 50 µL of 1× NuPAGE LDS sample buffer and reducing agent (Invitrogen) and boiling at 90°C for 5 min. Samples were then centrifuged at 13,000 × g at 23°C for 5 min and the supernatant was collected for western blotting, as described above.
Statistical analysis
All data were analyzed using student’s t-test or one-way analysis of variance (ANOVA) with post hoc testing where appropriate using GraphPad Prizm® (GraphPad Software Inc., San Diego, CA). Results were expressed as mean ± SEM, n ≥ 3. Values of p ≤ 0.05 were considered statistically significant.
RESULTS
Cocaine administration decreases RXR-γ and its target protein, GAP-43 in the hippocampus
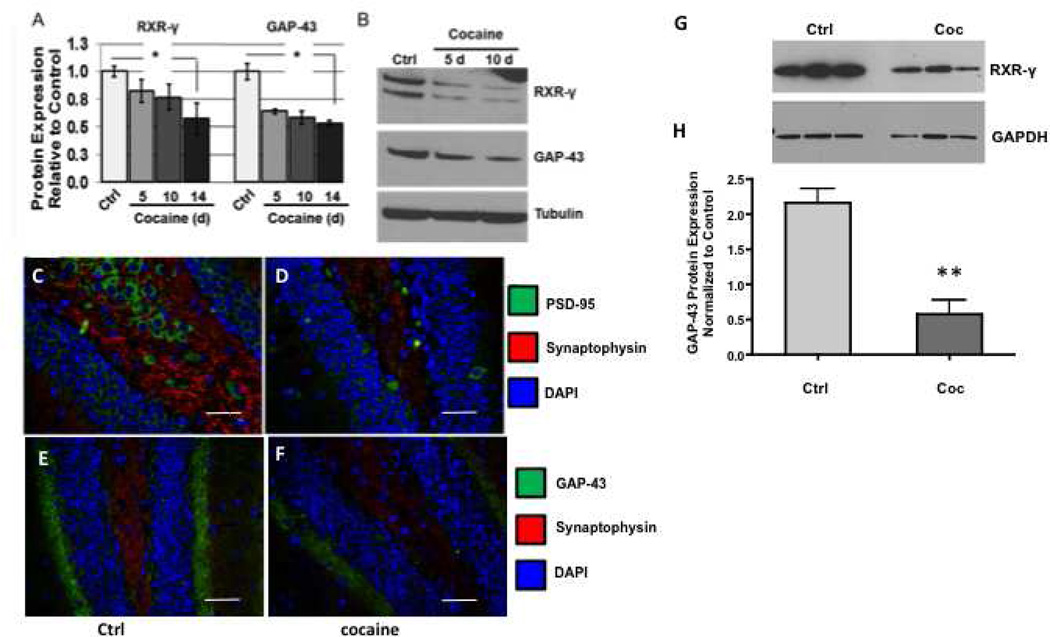
We have previously reported decreased levels of RXR-γ and one of its targets genes, neurogranin in specific brain regions following cocaine exposure in a murine model of chronic administration [22, 24]. Neurogranin (Ng) is a post-synaptic calmodulin-binding protein kinase C substrate that is regulated by retinoic acid. In this context, its pre-synaptic counterpart, growth-associated protein-43 (GAP-43) is also a calmodulin binding PCK substrate and is regulated by retinoic acid. Specifically, the GAP-43 promoter contains a retinoic acid responsive element (RARE), requiring RA for its transcriptional activation. Thus, to determine if like Ng as we previously reported [24], GAP-43 is also affected by cocaine, we measured GAP-43 levels in the mouse hippocampus. Data show that GAP-43 is similarly decreased in the hippocampus by chronic cocaine exposure (Fig. 1A, B, E, F) and in neurons treated with cocaine (Fig. 1G, H). Thus, our observation that cocaine down-regulates hippocampal GAP-43 expression supports our previous data that cocaine leads to dysregulated RA signaling that affects synaptodendritic connections. To illustrate further synaptodendritic dysregulation in mice administered cocaine, two commonly utilized pre- and post-synaptic markers, synaptophysin and PSD-95, respectively were assessed (Figure 1C, D). As expected, immunofluorescence labeling indicated that along with GAP-43, synaptophysin and PSD-95 were decreased as well, indicating loss of synaptodendritic markers in the hippocampus of cocaine treated mice. These results may be significant in that PSD-95 anchors and organizes NMDA receptors and other post-synaptic proteins necessary for proper connections [52]. In addition, synaptophysin is a well-established marker of synaptic density [53]. Taken together, these data confirm that chronic cocaine administration results in loss of synaptodendritic density in the hippocampus.
FIGURE 1. Cocaine administration decreases RXR-γ and GAP-43 levels in a timedependent manner in the adult mouse hippocampus and in vitro.
A, Densitometric analysis and (B) representative western blot of RXR-γ and GAP-43 expression in the hippocampi of control and cocaine-treated mice following a cocaine time course. Retinoid X receptor-γ and GAP-43 expression were significantly reduced within 5 d of daily cocaine administration and expression of both proteins decreased in parallel. Data were analyzed by one-way ANOVA with Tukey’s multiple comparisons post hoc test. n ≥ 6 mice per group. C–D, Double-immunofluorescence labeling demonstrates decreased expression of PSD-95 (green) and synaptophysin (red) in the hippocampus of (D) cocaine-treated compare to (C) saline (ctrl) mice. E–F, Double-immunofluorescence labeling demonstrates decreased expression of GAP-43 (green) and synaptophysin (red) in the hippocampus of (F) cocaine-treated mice compared to (E) saline (ctrl). Nuclei are labeled with DAPI (blue). Magnification 40×, scale bar = 40 µm. RXR-γ, retinoid X receptor-γ; GAP-43, growth-associated protein-43; PSD-95, post-synaptic density protein-95; DAPI, 4',6-diamidino-2-phenylindole; Ctrl, control. *p < 0.01. G–H, human primary neurons were treated with cocaine (5 µm) for 24 hours and GAP-43 protein expression was assessed by western blot. G, Representative western blot of GAP-43 expression in control and cocaine treated neurons compared to tubulin loading control. H, Densitometric quantification of GAP-43 levels in control and cocaine treated neurons. n = 3; ** p= 0.0056. Data were analyzed by unpaired t-test. GAPDH, loading control.
Cocaine Exposure Induces Proteasome-Dependent Degradation of Neuronal RXR-γ in Vitro
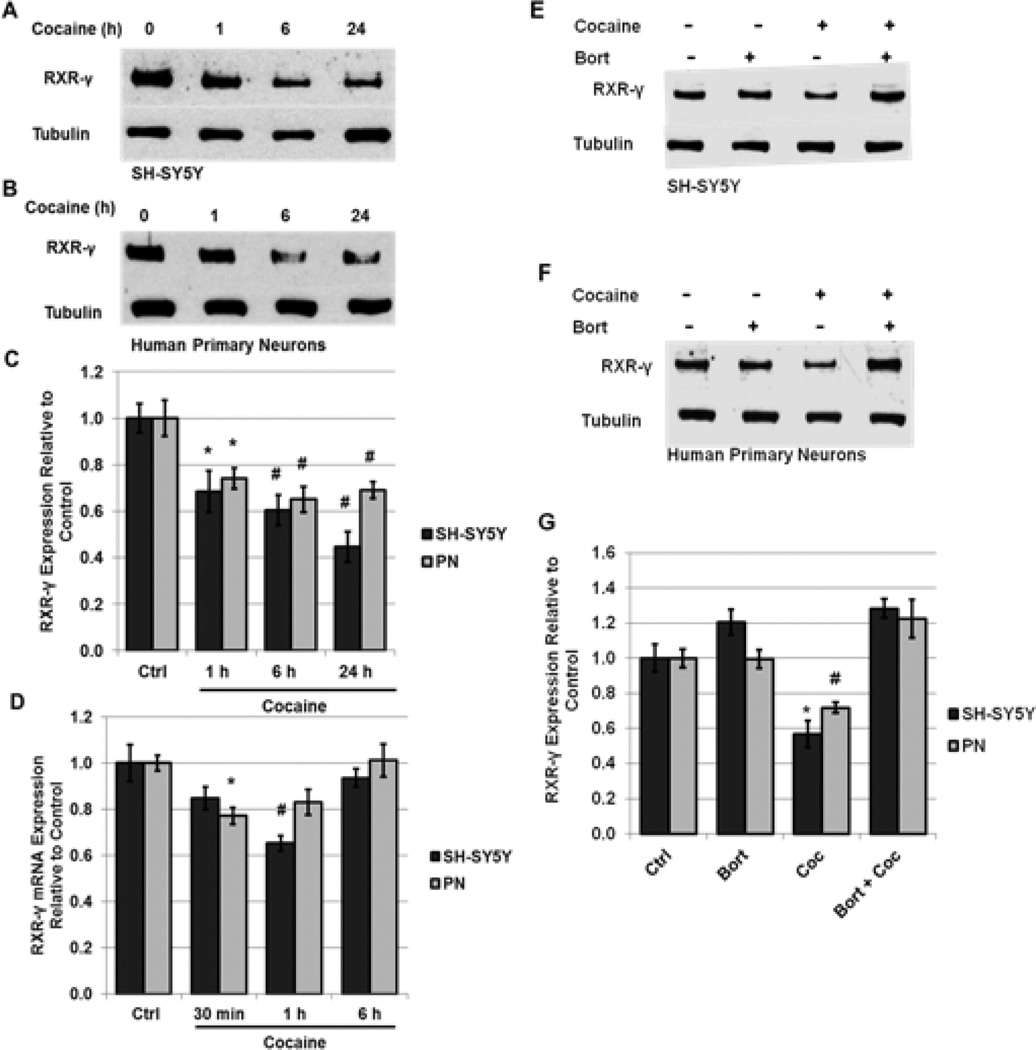
To determine the mechanism(s) underlying this down-regulation in a cell-specific context, we exposed SHSY5Y human neuroblastoma cells and human primary neurons to a physiologically relevant dose of cocaine (5 µM) in vitro [47]. SH-SY5Y cells resemble striatal catecholaminergic neurons and, similarly to striatal neurons, readily express RARs and RXRs [54, 55]. Additionally, SH-SY5Y cells produce TNF-α in response to cellular stressors and are sensitive to a number of compounds known to induce toxicity in dopaminergic neurons [45, 56, 57]. These properties make them a relevant model neuronal system in which to evaluate neurodegenerative processes. Significantly decreased levels of RXR-γ were observed in both SH-SY5Y cells (69% of control) and human primary neurons (74% of control) within 1 h of cocaine treatment (Fig. 2, A–C). The levels of RXR-γ declined in a time-dependent manner, with maximal effect being observed at 24 h of cocaine treatment in SH-SY5Y cells (45% of control levels) and at 6 h of treatment in human primary neurons (65% of control levels) (Fig. 2, A–C).
FIGURE 2. Cocaine induces proteasome-dependent degradation of RXR-γ in neurons.
A–C, SH-SY5Y neuroblastoma cells and human primary neurons (PN) were treated with 5 µM cocaine for 1 h to 24 h. A and B, representative immunoblot of RXR-γ expression in (A) SH-SY5Y cells and (B) human primary neurons compared to tubulin loading control. C, densitometric analysis of A and B. D, mRNA levels of RXR-γ following 30 min to 6 h of cocaine treatment in SH-SY5Y cells and human primary neurons as assessed by qRT-PCR and normalized to the housekeeping gene GAPDH. E–G, SH-SY5Y cells and human primary neurons were treated with 5 µM cocaine for 6 h in the absence or presence of the 26-S proteasome inhibitor, Bortezomib (Bort) (100 pM, 16 h pre-treatment). E and F, representative immunoblot of RXR-γ expression in (E) SH-SY5Y cells and (F) human primary neurons compared to tubulin loading control. G, densitometric analysis of E and F. All experiments were performed in triplicate (n ≥ 3) and bar graphs depict mean ± S.E.M. *p < 0.05; #p < 0.01 by one-way ANOVA.
To evaluate whether decreased protein expression of RXR-γ following exposure to cocaine was accompanied by a reduction in mRNA levels, qRT-PCR analysis of message levels of RXR-γ was conducted in SH-SY5Y cells and in human primary neurons in response to cocaine. In both cell types, message levels of RXR-γ were transiently reduced but recovered to baseline. In primary neurons, RXR-γ mRNA levels were significantly decreased at 30 min of cocaine treatment (77% of control), but recovered to baseline shortly afterwards (Fig. 2D). In SH-SY5Y cells, RXR-γ mRNA levels were significantly reduced at 1 h of cocaine treatment (65% of control), but not at 30 min or 6 h of treatment (Fig. 2D). After 24 h exposure to cocaine, no changes were observed. These findings suggest that cocaine may regulate RXR-γ expression by multiple mechanisms. While transcriptional repression may contribute to the reduction in protein levels, decreased mRNA production does not likely account for the rapid and sustained decrease in protein levels observed up to 24 h following treatment.
Because mRNA levels of RXR-γ decrease initially in response to cocaine treatment but then quickly recover to near baseline levels (Fig. 2D), we sought to determine whether the observed rapid sustained decrease in protein levels was due to protein degradation. Inhibition of the 26 S proteasome with Bortezomib (Bort) (100 pM; 16 h pre-treatment) completely blocked the decrease in RXR-γ protein levels at 1 h of cocaine treatment in both SH-SY5Y cells and human primary neurons (Fig. 2E–G). Treatment with Bort alone did not induce a significant change in RXR-γ levels, suggesting little protein is degraded under normal, non-stressed cellular conditions. These findings are in line with previous studies, which demonstrated that cytokine-induced repression of nuclear receptor signaling is due, in part, to increased protein degradation [10, 13, 58].
Cocaine Exposure Induces TNF-α Expression and Phosphorylation of JNK in Neuronal Cells
While RXRs are known to play a critical role in neuronal signaling, little information exists regarding how their levels are regulated. Our studies demonstrate that RXR-γ levels do not change in response to treatment with either dopamine or forskolin (Supplementary Fig. 1), which mimics dopamine 1 receptor activation by activating adenylyl cyclase and raising levels of intracellular cAMP. Therefore, the decrease observed in RXR-γ levels in response to cocaine is likely mediated primarily through a dopamine-independent mechanism.
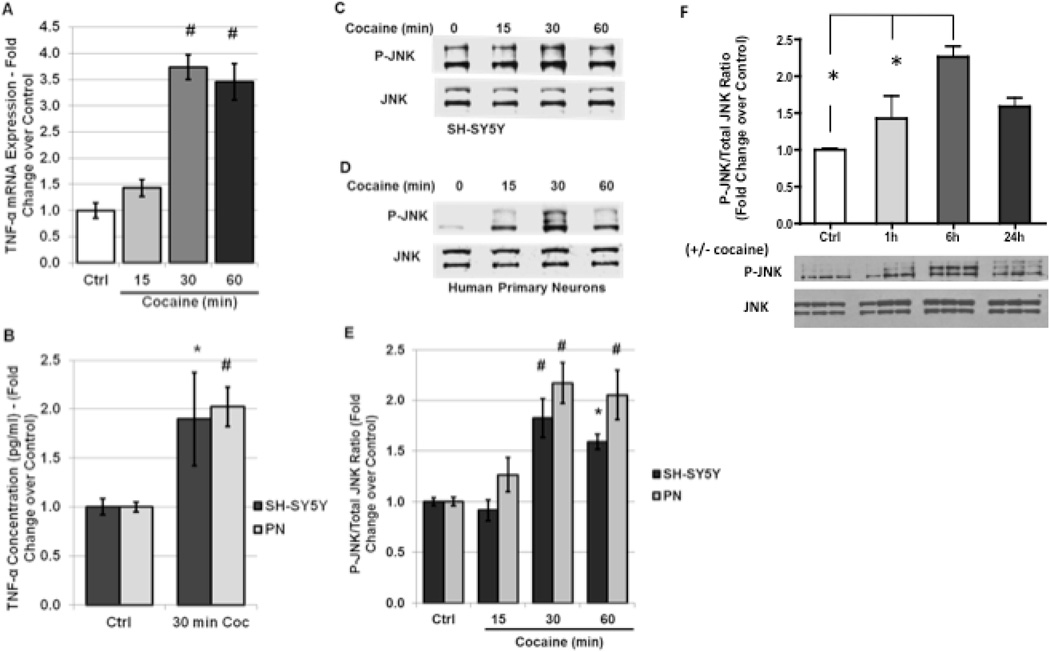
Studies in other systems including cardiomyocytes, liver cells and lung carcinoma cells suggest that inflammatory cytokines such as IL-1β and TNF-α negatively regulate both retinoic acid signaling and levels of nuclear receptors [10–12, 59, 60]. Evidence exists particularly for the TNF-α downstream effector JNK to play a role in nuclear receptor regulation in response to stress [6, 9–11]. For example, RXR-α is a known substrate of phospho-JNK and JNK-mediated phosphorylation at specific serine residues regulates the subcellular localization and ubiquitination state of RXR-α in hepatocytes and cardiomyocytes [9, 11, 60]. Furthermore, previous studies demonstrate that exposure to cocaine induces production and secretion of TNF-α both in vivo and in vitro [2, 28, 30]. Therefore, we hypothesized that cocaine-induced production of TNF-α may contribute to alterations in RXR-γ levels. To confirm these findings in our neuronal culture system, and to characterize the time course of JNK activation in response to cocaine treatment, we assessed TNF-α mRNA levels following a time course of cocaine treatment by qRT-PCR, and the amount of secreted TNF-α by ELISA following 30 min of cocaine exposure. Our results demonstrate that cocaine induced a 3.7-fold induction in TNF-α mRNA in SH-SY5Y cells within 30 min of treatment, and levels remain significantly elevated for up to 1 h (Fig. 3A). Because message levels of TNF-α were significantly increased following 30 min of cocaine administration in SH-SY5Y cells, and ELISA analysis in previous studies demonstrates an increase in secreted TNF-α at 30 min of cocaine treatment in rat primary neurons [28], we examined levels of secreted TNF-α in the cell culture supernatant of SH-SY5Y and human primary neurons by ELISA at 30 min of cocaine exposure. Our results demonstrate significantly increased TNF-α secretion at 30 min of cocaine treatment in both SHSY5Y cells (185.1% of untreated control) and in human primary neurons (202.3% of untreated control) (Fig. 3B). These findings are in accordance with previous studies demonstrating TNF-α induction and release in cell cultures in response to cocaine treatment [28, 30].
FIGURE 3. Cocaine exposure induces neuronal TNF-α expression and JNK activation in vitro.
A, SH-SY5Y cells were treated with 5 µM cocaine for 15 min to 1 h and mRNA levels of TNF-α were assessed by qRT-PCR and normalized to the housekeeping gene GAPDH. Quantification is shown as fold-change over untreated controls. B, SH-SY5Y cells and human primary neurons were treated with 5 µM cocaine for 30 min, then cell culture media was collected and assessed for levels of TNF-α by ELISA. Quantification is shown as fold-change over untreated control. C–E, SH-SY5Y cells and human primary neurons were treated with 5 µM cocaine for 15 min to 1 h and the ratio of p-JNK to total JNK was determined by western blot analysis. C and D, representative immunoblot of p-JNK expression in (C) SH-SY5Y cells and (D) human primary neurons compared to total JNK levels in response to cocaine exposure. E, densitometric quantification of C and D. F) pJNK ratios to total JNK in SH-SY5Y neurons exposed to cocaine up to 24 h. All experiments were performed in triplicate (n ≥ 3) and bar graphs depict mean ± S.E.M. *p < 0.05; #p < 0.01 by one-way ANOVA.
Increased extracellular levels of TNF-α typically result in activation of TNF receptors on nearby neurons, in turn triggering downstream signaling cascades including the phosphorylation of JNK. To confirm this signaling pathway is intact in our cell culture systems and to determine the time course of JNK activation in response to cocaine exposure as previously reported [31]SH-SY5Y cells and primary neurons were treated with cocaine and then harvested at various time points. Western blotting was performed to assess the ratio of activated JNK (P-JNK) to total JNK. Our results demonstrated that the PJNK/ total JNK ratio was significantly increased within 30 min of cocaine treatment in SH-SY5Y cells (2.0-fold induction over untreated control) and in human primary neurons (2.2-fold induction over untreated control) (Fig. 3C–E). Expanded time course studies in SHSY neurons indicated that the pJNK/total JNK ratios remained elevated up to 24 h after exposure to cocaine (Fig. 3F). These findings demonstrate that TNF-α release and phosphorylation of JNK shortly precede the decrease in RXR-γ protein levels (Fig. 2). Interestingly, untreated SH-SY5Y cells expressed low levels of P-JNK constitutively, indicating these cells may produce low amounts of TNF-α under normal conditions, and/or that culture of SH-SY5Y cells in neurobasal medium activates JNK pathways during differentiation.
In Vitro Treatment with TNF-α Alone Mimics the Effects of Cocaine on RXR-γ Levels, while Inhibiting TNF-α Signaling During Cocaine Treatment Blocks the Effects on RXR-γ Levels
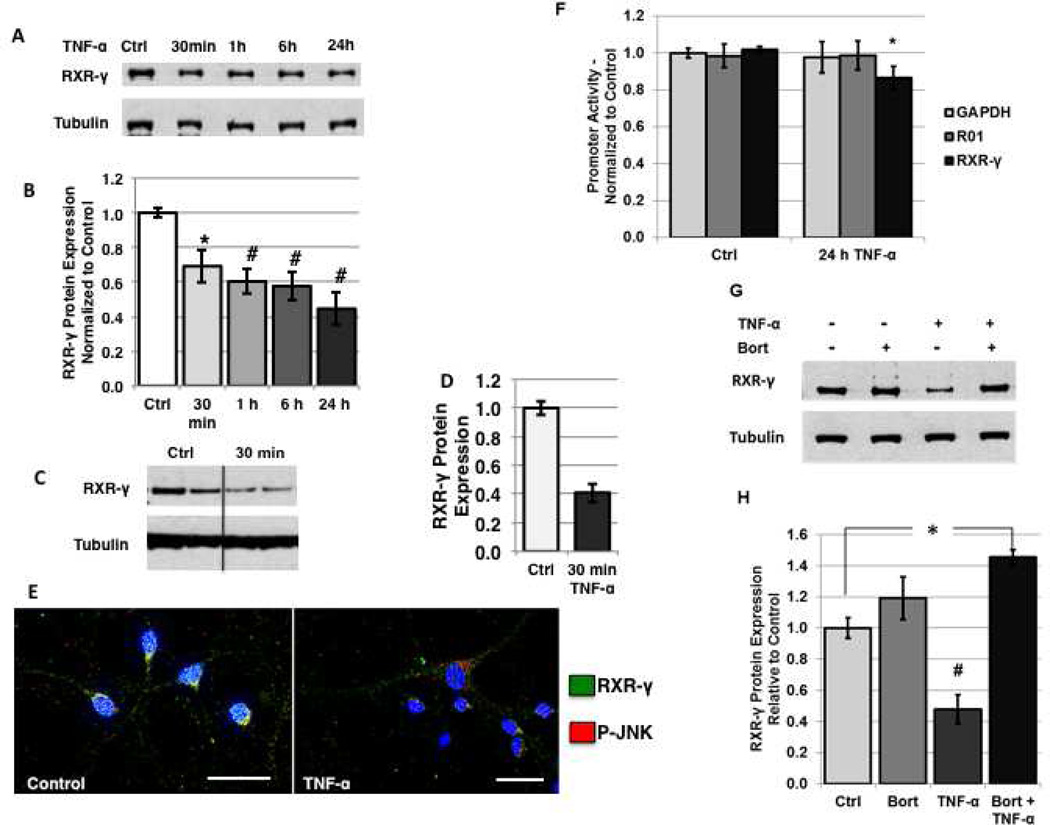
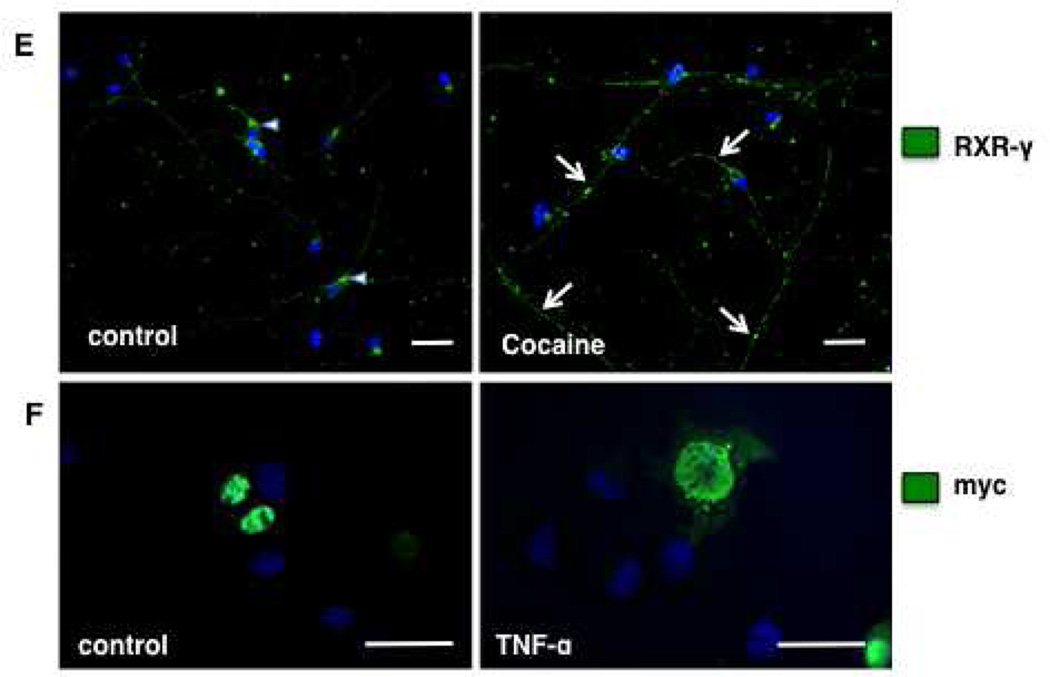
Since our findings demonstrate that cocaine treatment induces TNF-α expression and JNK activation at time points that shortly precede changes noted in RXR-γ levels in response to cocaine exposure, we evaluated whether TNF-α treatment alone would mimic the effects of cocaine on neuronal RXR-γ levels. In SH-SY5Y cells, TNF-α treatment induced a time-dependent decrease in whole cell RXR-γ protein levels as assessed by Western blot (Fig. 4A, B). Levels of RXR-γ were significantly decreased in SH-SY5Y by 30 min of treatment (70% of control), and remained decreased at 1, 6, and 24 h (Fig. 4A, B). Because a significant reduction in RXR-γ was observed as early as 30 min in SH-SY5Y cells, we evaluated protein levels in human primary neurons following 30 min of TNF-α treatment. Levels of RXR-γ were significantly decreased by TNF-α treatment within 30 min (44% of control) (Fig. 4C, D). Immunolabeling of untreated and TNF-α-treated primary neurons demonstrated barely detectable nuclear expression of RXR-γ in the nuclei of TNF-α-treated cells, which coincided with increased expression of P-JNK (Fig. 4E).
FIGURE 4. Treatment with TNF-α alone mimics the effects of cocaine on neuronal RXR-γ levels in vitro.
A and B, SH-SY5Y cells were treated with 100 ng/ml human recombinant TNF-α for 30 min to 24 h and levels of RXR-γ were assessed by western blotting. A, representative immunoblot of RXR-γ expression compared to tubulin loading control. B, densitometric quantification of A. C–E, human primary neurons were treated with 10 ng/ml TNF-α for 30 min. C, representative immunoblot of RXR-γ expression in human primary neurons compared to tubulin loading control. D, densitometric quantification of C. E, double-immunoflourescence labeling of RXR-γ (green) and phosphorylated JNK (p-JNK) in untreated (left panel) and TNF-α-treated (right panel) human primary neurons. Nuclei are labeled blue with DAPI. Magnification = 1,000×, scale bar 40 µm. F, N2a cells were transfected with a renilla luciferase reporter construct being driven by the human RXR-γ promoter, human GAPDH promoter (housekeeping), or random non-coding 1 kb sequence (R01) (background control). Luciferase signal from untreated and TNF-α-treated (50 ng/ml; 24 h) cells was determined and represented graphically. G and H, SH-SY5Y cells were treated with TNF-α (100 ng/ml; 1 h) in the absence or presence of Bort (100 pM; 16 h pre-treatment). G, representative immunoblot of RXR-γ expression compared to tubulin loading control. H, quantification of G. All experiments were performed in triplicate (n ≥ 3) and bar graphs depict mean ± S.E.M. *p < 0.05; #p < 0.01 by one-way ANOVA.
Similarly to the cocaine-mediated effects on RXR-γ mRNA levels, TNF-α treatment induced a transient decrease in message levels followed by a return to baseline in both SH-SY5Y cells and human primary neurons (data not shown). To evaluate whether decreased promoter activity accounts for the decrease in RXR-γ mRNA levels in response to TNF-α, N2a cells were transfected with a human RXR-γ promoter luciferase construct and treated with TNF-α for 24 h. Promoter activity was slightly but significantly reduced in TNF-α treated cells compared to untreated controls (87% of control) (Fig. 4F). Luciferase expression in GAPDH- and R01-transfected cells did not change following TNF-α treatment, demonstrating specificity of TNF-α action at the RXR-γ promoter (Fig. 4F). Similar to the effect seen in cocaine-treated cells, proteasomal inhibition with Bort (100 pM; 16 h pre-treatment) attenuated the TNF-α-mediated decrease in RXR-γ levels at 1 h of treatment in SH-SY5Y cells (Fig. 4G, H). These findings suggest there are likely multiple mechanisms regulating the effects of TNF-α on RXR-γ levels, including a slight repression of promoter activity in addition to the robust increase in protein degradation.
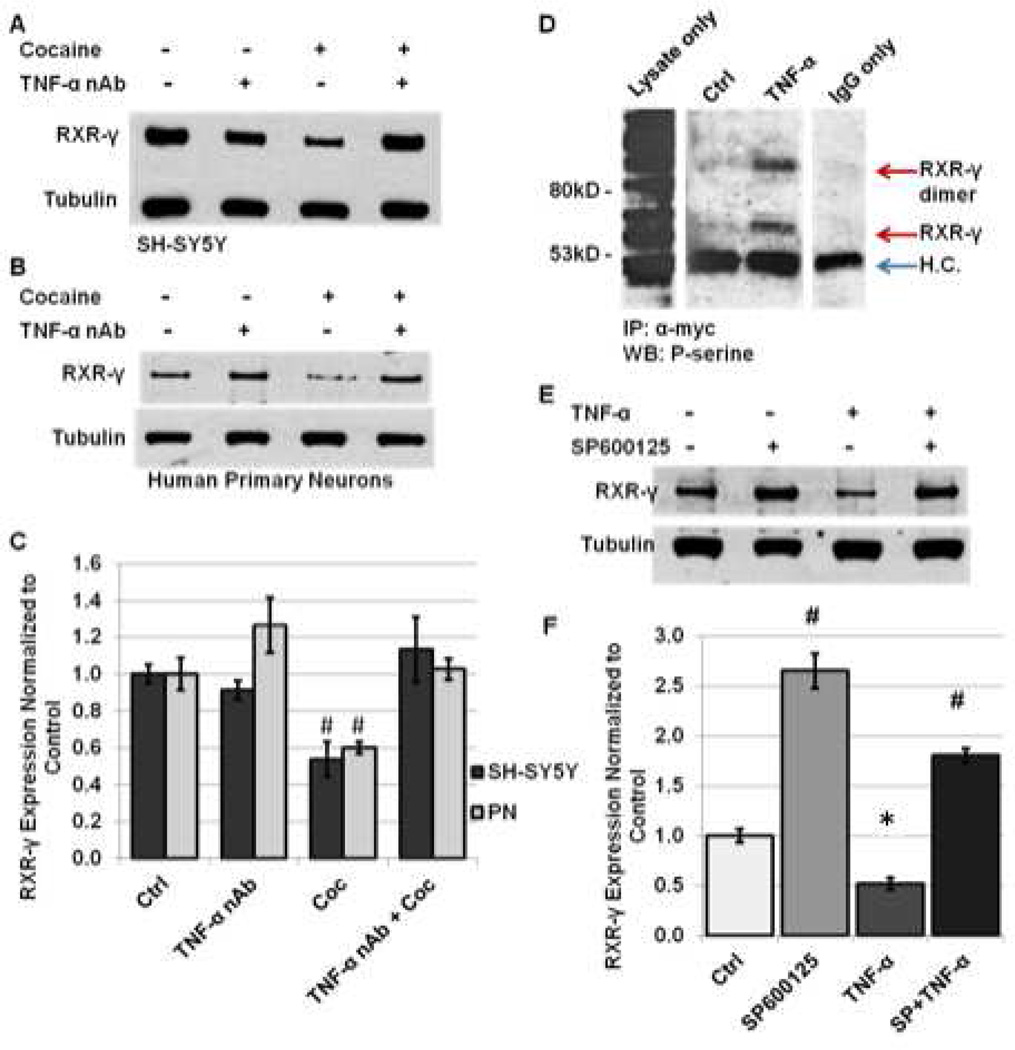
To evaluate whether cocaine induces changes in RXR-γ levels through induction of TNF-α signaling, SH-SY5Y cells and human primary neurons were exposed to 1 h of cocaine treatment in the absence or presence of a TNF-α neutralizing antibody (TNF-α nAb) (0.5 µg/mL; 2 h pre-treatment). Following treatment with TNF-α nAb and cocaine, RXR-γ levels were not significantly different from control levels in either cell type (113% of control in SH-SY5Y cells and 103% of control in human primary neurons), while cocaine treatment alone induced an approximate 40% reduction, as previously noted (Fig. 5A–C). A number of previous studies suggest that cytokines exert their effects on nuclear receptor signaling primarily through activation of JNK and subsequent P-JNK-mediated phosphorylation at specific residues of target nuclear receptors [6, 8–10]. Therefore, we evaluated whether RXR-γ is phosphorylated in response to TNF-α treatment, and whether inhibition of JNK signaling with the specific pharmacological inhibitor of P-JNK downstream kinase activity, SP600125, could attenuate the decrease of RXR-γ in response to TNF-α exposure. Immunoprecipitation experiments demonstrated that RXR-γ is phosphorylated at serine residues in response to TNF-α treatment (Fig. 5D). Neuro-2a cells, which do not express endogenous RXR-γ, were transfected with myc-tagged RXR-γ plasmid DNA and treated with vehicle or TNF-α for 15 min. Because N2a cells do not respond to cocaine treatment, likely due to lack of appropriate receptors and endogenous TNF-α production, studies performed in N2a cells examined the effects of TNF-α treatment only. We confirmed phosphorylation of JNK in response to TNF-α but not to cocaine treatment in N2a cells in preliminary studies (data not shown). Following treatment with vehicle or TNF-α for 15 min, cells were harvested and RXR-γ was immunoprecipitated using an antibody directed against the myc-tag. Western blotting with a phospho-serine primary antibody demonstrated presence of phosphorylated serine residues of RXR-γ in TNF-α-treated cells only (Fig. 5D). Two distinct bands were present, correlating to RXR-γ and a second band approximately double the molecular weight that likely represents a phosphorylated RXR homodimer or heterodimer (Fig. 5D). No phospho-serine reactivity was observed in the IP lane from untreated cells. The first lane demonstrates western blot of lysate only (no IP) showing detection of many phosphorylated proteins. The last lane shows IP with IgG only and expresses heavy chain (H.C.) immunoreactivity upon western blot analysis (Fig. 5D).
FIGURE 5.
Inhibition of TNF-α signaling prevents cocaine-induced decrease in RXR-γ protein levels. A–C, SH-SY5Y cells and human primary neurons were treated with 5 µM cocaine for 1 h in the absence or presence of a TNF-α neutralizing antibody (TNF-α nAb) (0.5 µg/ml; 2 h pretreatment) and harvested for protein extraction. A and B, representative immunoblot of RXR-γ expression in (A) SH-SY5Y and (B) human primary neurons compared to tubulin loading control. C, densitometric quantification of A and B. All experiments were performed in triplicate (n ≥ 3) and bar graphs depict mean ± S.E.M. *p < 0.05; #p < 0.01. D, RXR-γ is phosphorylated at serine residues in response to TNF-α exposure. Immunoblot demonstrates phospho-serine immunoreactivity in lysate following immunoprecipitation using anti-myc antibody. Lanes shown represent results obtained from the same membrane. Some lanes were excluded for clarity. No phosho-serine reactivity was observed in IP lane from vehicle-treated cells. H.C.; heavy chain. E–F, human primary neurons were treated with TNF-α (10 ng/ml; 30 min) in the absence or presence of SP600125 pretreatment (10 µM; 16 h pre-treatment). E, representative western blot of RXR-γ expression compared to tubulin loading control. F, densitometric quantification of (E). #p < 0.01 (SP600125 and SP600125 with TNF-α compared to control and TNF-α), *p < 0.05 (control compared to TNF-α). All experiments were performed in triplicate (n ≥ 3) and bar graphs depict mean ± S.E.M by one-way ANOVA.
We next investigated whether inhibiting the kinase activity of P-JNK with SP600125 during TNF-α treatment would prevent decreases in RXR-γ protein levels. Our findings revealed that SP600125 (10 µM; 16 h pretreatment) attenuated the TNF-α mediated decrease in RXR-γ protein levels in human primary neurons following 30 min of treatment (Fig. 5E, F). Furthermore, treatment with SP600125 alone significantly increased the basal expression of RXR-γ in human primary neurons by more than twofold (Fig. 5E, F). These findings suggest that induction of TNF-α signaling and resultant P-JNK activation are key mediators of cocaine-induced reduction in RXR-γ protein levels.
A Shift in Subcellular Localization of RXR-γ Occurs in Response to Cocaine and TNF-α Treatment
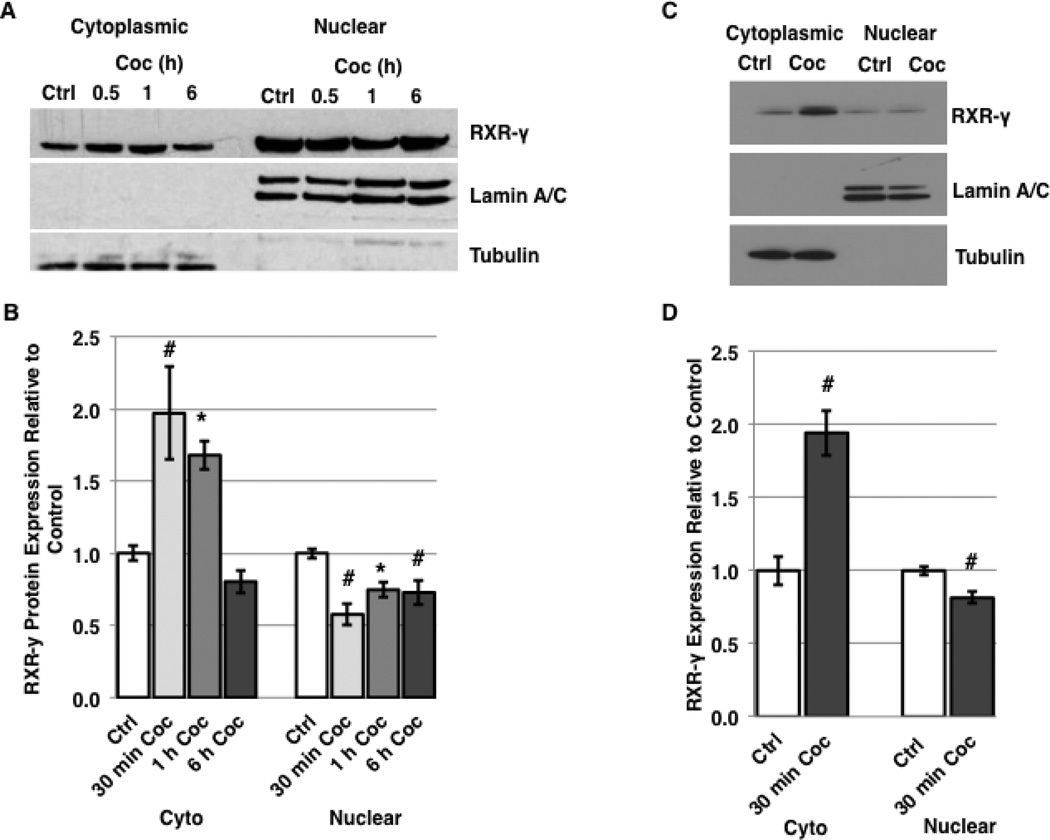
In hepatocytes, the inflammatory cytokine IL-1β induces nuclear export of RXR-α in a JNKdependent manner, which leads to proteasomal degradation of the protein in the cytosol [11]. A shift in subcellular localization of RXRs to the cytoplasm suggests a loss of function, since these predominantly nuclear proteins act as DNA-binding transcription factors which bind to both target DNA recognition elements and to other nuclear receptors within the nuclear compartment [14, 16]. To assess whether exposure to cocaine induces nuclear export of RXR-γ in neuronal cells, we treated SH-SY5Y cells with cocaine for time intervals of 30 min, 1 h, and 6 h and assessed the subcellular localization of RXR-γ by nuclear/cytoplasmic fractionation followed by western blotting. Our results demonstrate the cocaine induces a decrease in nuclear levels of RXR-γ within 30 minutes (57.6% of control), and a concomitant significant increase in cytoplasmic levels of RXR-γ compared to untreated cells (197% of control) (Fig. 6A, B). The cytoplasmic levels of RXR-γ remain significantly increased compared to controls for up to 1 h of treatment, while nuclear levels remain significantly decreased. By 6 h of cocaine treatment, cytoplasmic levels of RXR-γ returned to baseline while nuclear levels remained significantly lower than those of controls (Fig. 6A, B).
FIGURE 6.
Cocaine induces nuclear export of RXR-γ. A–B, SH-SY5Y cells were treated with 5 µM cocaine for 30 min, 1 h, and 6 h and harvested for nuclear and cytoplasmic fractions. A, representative western blot of RXR-γ expression compared to Lamin A/C (nuclear) and tubulin (cytoplasmic) loading controls. B, densitometric quantification of (A). C–D, human primary neurons were treated with 5 µM cocaine for 30 min and harvested for nuclear and cytoplasmic fractions. C, representative western blot of RXR-γ expression compared to Lamin A/C (nuclear) and tubulin (cytoplasmic) loading controls. D, densitometric quantification of (C). E, immunofluorescent labeling of RXR-γ in control and cocaine-treated human primary neurons. Arrows in the cocaine-treated panel point to detectable RXR-γ expression in non-nuclear compartments. Nuclei are labeled blue with DAPI. Magnification = 400×. F, immunofluorescent labeling of myc-tagged-RXR-γ in transfected N2a cells treated with vehicle or TNF-α (50 ng/ml; 15 min). Nuclei are labeled blue with DAPI. Magnification = 1,000×. Scale bars = 40 µm. All experiments were performed in triplicate (n ≥ 3) and bar graphs depict mean ± S.E.M by one-way ANOVA.
Because of the similarities in signaling in response to cocaine we have observed thus far between SH-SY5Y cells and human primary neurons, we elected to evaluate the subcellular localization of RXR-γ in human primary neurons following 30 min of cocaine treatment. Our results revealed a significant decrease in nuclear levels (83% of control) along with a significant increase in cytoplasmic levels (194% of control) (Fig. 6C, D). Likewise, immunolabeling experiments in human primary neurons following 30 min of cocaine treatment revealed detectable expression of RXR-γ in cell bodies and processes compared to untreated neurons, where RXR-γ expression was predominantly nuclear (Fig. 6E). Furthermore, N2a cells transfected with myc-tagged RXR-γ plasmid DNA showed increased cytoplasmic expression of myc-RXR-γ following 15 min of TNF-α treatment in immunolabeling experiments (Fig. 6F). These findings suggest RXR-γ may be exported to the cytoplasm following exposure to cocaine or TNF-α prior to degradation, and that genes regulated by RXR heterodimers binding to promoter recognition elements may be disrupted by decreased nuclear localization of RXR even prior to degradation events.
Degradation of RXR-γ is dependent upon its nuclear export
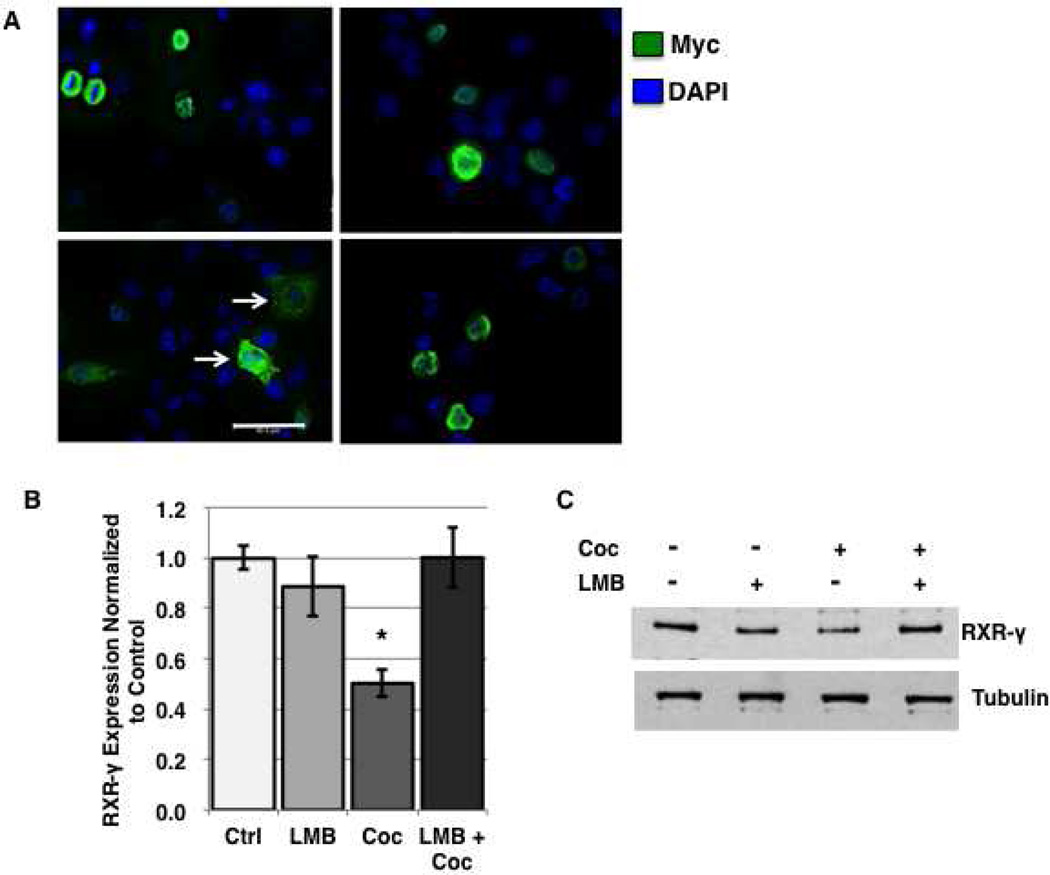
Previous studies demonstrate that JNK-mediated phosphorylation of nuclear receptors may mediate subcellular localization and/or additional post-translational modifications that can signal for alterations in localization, such as nuclear export resulting from mono-ubiquitination [6, 11]. To assess whether the nuclear export of RXR-γ we observed in response to cocaine and TNF-α treatment (Fig. 6) precedes its degradation, we inhibited nuclear export with Leptomycin B (LMB) prior to treatment. First, we demonstrate inhibition of nuclear export of exogenous myc-RXR-γ in response to TNF-α treatment in N2a cells by immunolabeling. Fluorescent labeling using primary antibody directed against myc demonstrates increased cytoplasmic expression of myc-RXR-γ in response to 15 min of TNF-α treatment (Fig. 7A, bottom left panel) compared to vehicle-treated cells where myc-tagged RXR-γ expression is predominantly nuclear (Fig. 7A, top left panel). Leptomycin B alone did not affect normal localization of RXR-γ in vehicle-treated cells (Fig. 7A, top right panel), and blocked export of myc-RXR-γ in response to TNF-α treatment (Fig. 7A, bottom right panel). These findings confirm nuclear export in response to TNF-α exposure, and demonstrate for the first time that RXR-γ is exported from the nucleus in a CRM-1-dependent manner.
FIGURE 7.
Inhibition of nuclear export prevents cocaine and TNF-α-mediated decreases in RXR-γ protein levels. A, N2a cells were transfected with myc-tagged-RXR-γ and treated with TNF-α (50 ng/ml; 15 min) in the absence or presence of the nuclear export inhibitor LMB (1 nM; 3 h pre-treatment). Immunufluorescence labeling against myc antibody. Nuclei are labeled blue with DAPI. Magnification = 1,000×. B and C, SH-SY5Y cells were treated with 5 µM cocaine (1 h) in the absence or presence of LMB (1 nM; 3 h pre-treatment) and RXR-γ levels were assessed by western blot. B, representative western blot of RXR-γ expression compared to tubulin loading control. C, densitometric quantification of (B). *p < 0.05. All experiments were performed in triplicate (n ≥ 3) and bar graphs depict mean ± S.E.M by one-way ANOVA.
To evaluate whether nuclear export of RXR-γ in response to cocaine treatment is critical for protein degradation, we treated SH-SY5Y cells with cocaine in the absence or presence of LMB and evaluated whole cell RXR-γ protein levels. Our results demonstrated that pre-treatment with the nuclear export inhibitor LMB prevents whole cell decreases in RXR-γ protein in response to cocaine treatment (100.1% of control in LMB + Coc group) (Fig. 7B, C). Treatment with LMB alone slightly reduced RXR-γ levels (88.6% of control), which has been observed in prior studies exploring nuclear export of RXR-α [11] and may occur in order to prevent over-stimulation of RXR-dependent signaling pathways.
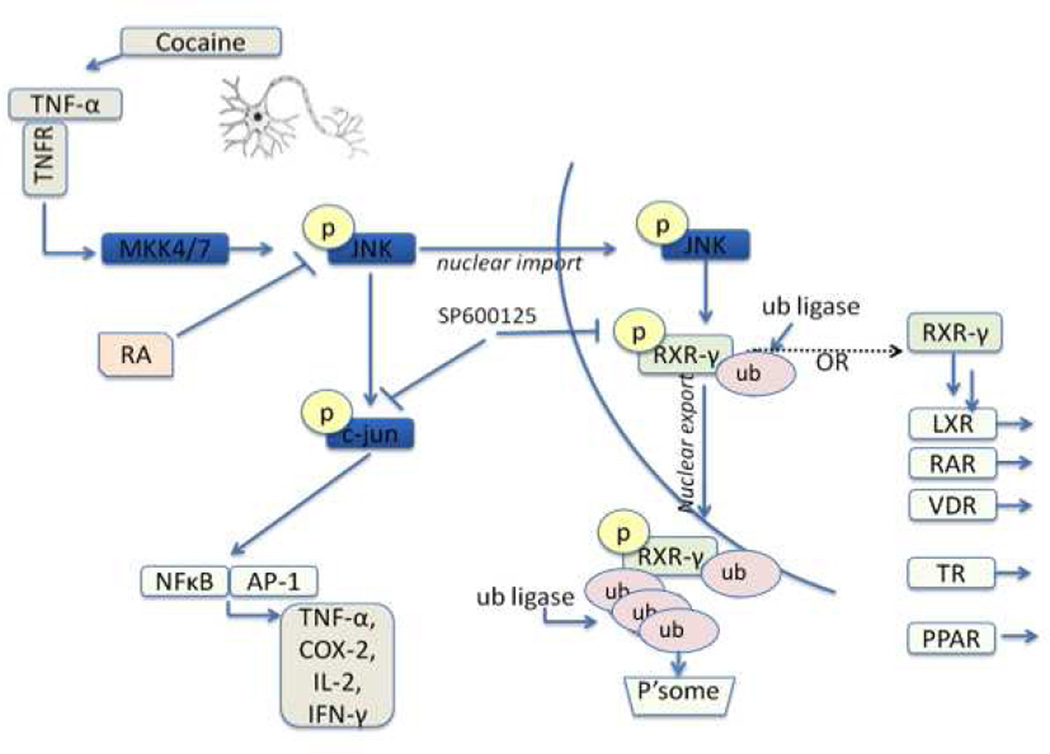
Our overall proposed signaling pathway is depicted in Figure 8. We provide evidence that cocaine induces neuronal expression of TNF-α and subsequent activation (phosphorylation) of JNK. Following activation, JNK may phosphorylate nuclear RXR-γ, leading to its ubiquitination, nuclear export, and proteasome-dependent degradation. Decreased nuclear expression of RXR-γ as a result of cocaine/cytokine exposure leads to reduced availability for heterodimerization with nuclear receptor partners and diminished DNA binding capacity for regulation of target genes.
FIGURE 8.
Proposed signaling pathway. Cocaine induces neuronal TNF-α expression, leading to the phosphorylation of JNK. p-JNK can, in turn, phosphorylate the well-characterized c-jun pathway, and potentially RXR-γ. RXR-γ is exported from the nucleus to the cytosol in response to phosphorylation and potentially subsequent post-translational modification such as ubiquitination. Following export, RXR-γ is degraded in the cytosol in a proteasome-dependent manner. In the absence of cellular stressors, RXR-γ is free to heterodimerize with nuclear receptor partners including RAR, TR, VDR, and PPAR. Ub; ubiquitin, P’some; proteasome.
DISCUSSION
We have previously reported decreased protein and mRNA levels of RXR-γ in the brains of cocaine-treated mice, but underlying mechanisms remain unknown [24]. In the current study, we utilized in vitro neuronal systems to investigate the effects of cocaine on RXR-γ expression in a cell-specific context. While mechanisms underlying the regulation of RXR-γ in the CNS have been vastly unexplored, previous studies have demonstrated significant suppression of RXR-α signaling and levels in other cell types, including liver cells, keratinocytes, and cardiomyocytes in response to cellular stressors such as inflammatory factors and UV irradiation [11, 12, 58, 60]. While RXR-α is ubiquitously expressed and plays a vital role in proper function of a number of organ systems, RXR-γ exhibits much more restricted expression and little is known about its key functions. However, RXR-γ is highly enriched within the CNS including within dopaminergic neurons of the striatum, a key target of cocaine action. Furthermore, RXR-γ expression largely overlaps that of the TR/RXR-regulated synaptic plasticity protein, neurogranin, which we also found to be significantly down regulated in response to chronic cocaine exposure in vivo [24].
In the present study, we report that in vitro cocaine treatment of SH-SY5Y neuroblastoma cells and human primary neurons induces a decrease in RXR-γ protein levels in both cell types, which can be prevented by proteasomal inhibition. Because cocaine is known to induce production of proinflammatory cytokines such as TNF-α both in vivo and in vitro [2, 28, 30], we evaluated whether cocaine-mediated induction of TNF-α was responsible for the observed alterations in RXR-γ expression. In this context, we found that cocaine treatment induced TNF-α secretion and increased mRNA levels, and that inhibition of TNF-α with a function-blocking antibody during cocaine treatment attenuated the decrease in RXR-γ protein levels. Additionally, treatment of neurons with TNF-α alone mimicked the effects of cocaine, while inhibition of a key TNF-α downstream effector, JNK, with SP600125 attenuated TNF-α-mediated alterations in RXR-γ expression.
Retinoid-X-receptor-α is a known substrate of phopsho-JNK [8, 13], and its phosphorylation is believed to induce its nuclear export and degradation in non-CNS cell types, such as hepatocytes [11]. Here, we provide the first evidence to date that RXR-γ may also serve as a substrate of phospho-JNK, and that phosphorylation likely negatively regulates levels of RXR-γ in neurons. Phosphorylation of RXR-γ could impact its DNA binding capacity or impair co-activator recruitment, an effect seen following JNK-mediated phosphorylation of RXR-α [59]. Both cocaine and TNF-α treatment induced a nuclear-to-cytoplasmic shift in RXR-γ subcellular localization, suggesting potential loss of function as a nuclear transcription factor. Whether cocaine or TNF-α-induced nuclear export and degradation of RXR-γ result in altered transcription of retinoid target genes in vitro will be the topic of future studies. Additionally, whether inhibition of P-JNK signaling can abrogate cocaine or TNF-α-mediated changes in gene expression will be investigated. Our findings were consistent between the neuronal cell line SH-SY5Y and human primary neurons, suggesting cocaine and cytokine-mediated down-regulation of RXR-γ is a general property of neuronal populations. There are many studies in the literature that use SHSY as a representative model for neuronal response to disease related factors. In this context, controversy exists as to whether SHSY cells are an appropriate model for studying neuronal response. Given the complex phenotype of SHSY as described by Kovalevich and Langford [45], the fact that SHSY cells show the same responses as human primary neurons is notable. For example, retinoic acid (RA) treatment is a commonly used method to induce differentiation of SHSY cells into a mature neuronal phenotype, but controversy exists regarding the use of supplements or other methods to obtain expression of dopaminergic or cholinergic subtype [57]. In fact, our choice of differentiation method ensured that GAP-43 would not be unintentionally increased [61, 62]. Given the limitation of using RA in the current studies to differentiate SHSY, we have established that the modified approach as described in the methods section by using neurobasal medium containing Glutamax and B27 supplement is sufficient to study RA signaling in these commonly used neurons.
Cocaine treatment induced TNF-α production, as assessed by ELISA, with a maximal effect being observed at 30 min. This time course coincided with downstream JNK activation, as assessed by ratio of P-JNK to total JNK by western blotting. Our results appeared to be independent of cocaine’s effects on the dopaminergic system, as treatment of SH-SY5Y cells with dopamine alone did not produce a discernible effect on RXR-γ expression (data not shown). Interestingly, it has previously been reported that dopamine treatment induces TNF-α expression in SH-SY5Y cells [56], an effect not observed in our studies. This discrepancy could be attributed to differences in dosage, as we selected to use a physiological dose of dopamine (100 nM), while previous studies instead utilized cytotoxic doses of 100 to 500 µM [56]. Additionally, differences in duration of treatment may have affected the observed outcomes. However, it remains a possibility that excessive synaptic dopamine resulting from cocainemediated inhibition of the dopamine transporter could induce cytotoxicity and inflammatory cascades through reactive breakdown products in vivo [63, 64]. Thus, the mechanism for cocaine-mediated induction of neuronal TNF-α expression is not entirely clear. The effect may be mediated by cocaine’s interactions with proteins other than the dopamine transporter, such as sigma-1 (σ 1) receptors, which are also known to bind cocaine at physiologically relevant doses in vivo and may play a role in behavioral response to psychostimulant drugs [65–67]. Interestingly, σ 1 receptor activation is primarily associated with immunosuppressive effects in peripheral immune cells [68]. However, the role of σ 1 receptor activation on immune response in neurons has yet to be characterized, while a number of studies suggest antagonism of σ 1 receptors could be therapeutically beneficial in the treatment of cocaine addiction and toxicity [69, 70].
Our studies demonstrate multiple levels of cocaine and TNF-α-mediated regulation of RXR-γ levels including decreased mRNA levels and increased protein degradation. As we have demonstrated TNF-α exerts repressive effects on the human RXR-γ promoter, this likely accounts for the decreased production of mRNA levels. However, a potential direct impact of TNF-α on RXR-γ mRNA, such as alterations in mRNA stability, cannot be discounted and may be the topic of future studies. In this context, RA has previously been reported to decrease the stability of TNF-α mRNA [71], and the reciprocal relationship may exist but has not been previously explored. Furthermore, retinoic acid has previously been demonstrated to negatively regulate AP-1 responsive genes, at least in part through suppression of JNK activation [72–74]. Our findings are in line with previous studies demonstrating IL-1β-induced increased proteasome-dependent degradation of RXR-α protein [11]. Whether cocaine and/or TNF-α similarly affect neuronal RXR-α and RXR-β is a topic of future investigation. Interestingly, one previous study suggests phosphorylation of RXR-α can confer resistance to subsequent ubiquitination and degradation [75]. Differences in the effects of post-translational modifications such as phosphorylation likely depend on the residue modified as well as on the cell type. For instance, the prior study was performed in human hepatocellular carcinoma cells [75]. Cancer cells commonly express aberrant signaling pathways and adapt mechanisms to promote survival and tumorigenesis and thus often fail to accurately reflect the processes, which occur in non-malignant cells.
Our current findings may extend to other CNS cell types. For example, it was recently found that RXR-γ serves a critical role in oligodendrocyte maturation and remyelination following white matter lesion [41]. We have previously reported a reduction in white matter protein levels and increased oligodendrocyte cell death in response to cocaine exposure in vivo [43]. Additionally, this loss of white matter was attenuated by administration of the β-lactam antibiotic, Ceftriaxone (Ceft). While the protective effects of Ceft on white matter integrity were attributed primarily to maintenance of glutamate homeostasis via up-regulation of glial glutamate transporters, decreased cocaine-mediated neuroinflammation and subsequent effects on oligodendrocyte RXR-γ expression and signaling cannot be discounted from playing a role in the Ceft-mediated protection.
Results from the current study could yield clinical implications in the treatment of drug abuse disorders, as emerging evidence over the past decade indicates the CNS immune response to drugs of abuse could have profound effects on neuronal signaling pathways and to the development of addiction and sickness behaviors during withdrawal [34, 76, 77]. Particularly psychostimulants like cocaine and methamphetamine are associated with increased striatal expression of TNF-α mRNA [77] and microglial activation [77, 78]. Similarly, a number of neurodegenerative diseases including HIVE, and Alzheimer’s, Parkinson’s and Huntington’s diseases are characterized by chronic neuroinflammation and high levels of cytokines including TNF-α [34, 35, 79, 80]. Additionally, a recent study by Hellmann-Regen et al. describes increased RA degradation by activated microglia [81], further supporting a role for disrupted RA signaling in response to immune activation. Importantly, administration of RA or RAR/RXR agonists has conferred neuroprotection in a number of animal models of neurodegenerative disease. For instance, a 2012 study by Cramer et al. demonstrated that administration of the RXR agonist bexarotene ameliorated pathological and cognitive deficits in an animal model of AD [82].
Overall, our findings describe a novel mechanism whereby cocaine and TNF-α negatively regulate levels of RXR-γ, potentially contributing to aberrant retinoid signaling and neuronal dysfunction. We also demonstrate this effect is mediated, at least in part, by activation of JNK and nuclear export of RXR-γ. The contribution of post-translational modifications such as phosphorylation, ubiquitination, and sumoylation to the regulation of RXR-γ levels and function is the topic of ongoing studies in our laboratory. Additionally, the potential for therapeutic intervention to preserve RXR-mediated signaling is being explored both in vitro and in vivo.
Supplementary Material
Dopamine and forskolin do not affect RXR-γ levels. A, Representative Western blot of RXR-γ in neurons treated with 30 nm dopamine. B, densitometric quantification of A. C, Representative Western blot of RXR-γ in neurons treated with 10 µm forskolin. D, densitometric quantification of C. No significant changes were observed in levels of RXR-γ. Tubulin was used as a loading control.
Acknowledgments
This work was by supported in part by NIH DA029523 to TDL, and Temple University's Comprehensive NeuroAIDS Center P30 MH092177.
The abbreviations used are
- RXR-γ
retinoid-X-receptor-gamma
- JNK
c-Jun-NH-terminal kinase
- p-JNK
phosphorylated JNK
- TNF-α
tumor-necrosis-factor-alpha
- Bort
Bortezomib
Footnotes
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Crawford FC, Wood ML, Wilson SE, Mathura VS, Hollen TR, Geall F, Kolippakkam DN, Mullan MJ. Cocaine induced inflammatory response in human neuronal progenitor cells. Journal of neurochemistry. 2006;97(3):662–674. doi: 10.1111/j.1471-4159.2006.03760.x. [DOI] [PubMed] [Google Scholar]
- 2.Ahluwalia MS. 2010 Society for Neuro-Oncology Annual Meeting: a report of selected studies. Expert review of anticancer therapy. 2011;11(2):161–163. doi: 10.1586/era.10.227. [DOI] [PubMed] [Google Scholar]
- 3.Kousik SM, Napier TC, Carvey PM. The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation. Frontiers in pharmacology. 2012;3:121. doi: 10.3389/fphar.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moriguchi T, Toyoshima F, Masuyama N, Hanafusa H, Gotoh Y, Nishida E. A novel SAPK/JNK kinase, MKK7, stimulated by TNFalpha and cellular stresses. The EMBO journal. 1997;16(23):7045–7053. doi: 10.1093/emboj/16.23.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes & development. 2001;15(11):1419–1426. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiology and molecular biology reviews : MMBR. 2006;70(4):1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1996;351(1336):127–134. doi: 10.1098/rstb.1996.0008. [DOI] [PubMed] [Google Scholar]
- 8.Adam-Stitah S, Penna L, Chambon P, Rochette-Egly C. Hyperphosphorylation of the retinoid X receptor alpha by activated c-Jun NH2-terminal kinases. The Journal of biological chemistry. 1999;274(27):18932–18941. doi: 10.1074/jbc.274.27.18932. [DOI] [PubMed] [Google Scholar]
- 9.Lee HY, Suh YA, Robinson MJ, Clifford JL, Hong WK, Woodgett JR, Cobb MH, Mangelsdorf DJ, Kurie JM. Stress pathway activation induces phosphorylation of retinoid X receptor. The Journal of biological chemistry. 2000;275(41):32193–32199. doi: 10.1074/jbc.M005490200. [DOI] [PubMed] [Google Scholar]
- 10.Srinivas H, Juroske DM, Kalyankrishna S, Cody DD, Price RE, Xu XC, Narayanan R, Weigel NL, Kurie JM. c-Jun N-terminal kinase contributes to aberrant retinoid signaling in lung cancer cells by phosphorylating and inducing proteasomal degradation of retinoic acid receptor alpha. Molecular and cellular biology. 2005;25(3):1054–1069. doi: 10.1128/MCB.25.3.1054-1069.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmerman TL, Thevananther S, Ghose R, Burns AR, Karpen SJ. Nuclear export of retinoid X receptor alpha in response to interleukin-1beta-mediated cell signaling: roles for JNK and SER260. The Journal of biological chemistry. 2006;281(22):15434–15440. doi: 10.1074/jbc.M508277200. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Sweeney TR, Shigenaga JK, Chui LG, Moser A, Grunfeld C, Feingold KR. Tumor necrosis factor and interleukin 1 decrease RXRalpha, PPARalpha, PPARgamma, LXRalpha, and the coactivators SRC-1, PGC-1alpha, and PGC-1beta in liver cells. Metabolism: clinical and experimental. 2007;56(2):267–279. doi: 10.1016/j.metabol.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosters A, White DD, Sun H, Thevananther S, Karpen SJ. Redundant roles for cJun-N-terminal kinase 1 and 2 in interleukin-1beta-mediated reduction and modification of murine hepatic nuclear retinoid X receptor alpha. Journal of hepatology. 2009;51(5):898–908. doi: 10.1016/j.jhep.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 17.de Urquiza AM, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science (New York, NY. 2000;290(5499):2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 18.Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugawara A, Yen PM, Qi Y, Lechan RM, Chin WW. Isoform-specific retinoid-X receptor (RXR) antibodies detect differential expression of RXR proteins in the pituitary gland. Endocrinology. 1995;136(4):1766–1774. doi: 10.1210/endo.136.4.7895689. [DOI] [PubMed] [Google Scholar]
- 20.Zetterstrom RH, Lindqvist E, Mata de Urquiza A, Tomac A, Eriksson U, Perlmann T, Olson L. Role of retinoids in the CNS: differential expression of retinoid binding proteins and receptors and evidence for presence of retinoic acid. The European journal of neuroscience. 1999;11(2):407–416. doi: 10.1046/j.1460-9568.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- 21.Krezel W, Ghyselinck N, Samad TA, Dupe V, Kastner P, Borrelli E, Chambon P. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science (New York, NY. 1998;279(5352):863–867. doi: 10.1126/science.279.5352.863. [DOI] [PubMed] [Google Scholar]
- 22.Krzyzosiak A, Szyszka-Niagolov M, Wietrzych M, Gobaille S, Muramatsu S, Krezel W. Retinoid×receptor gamma control of affective behaviors involves dopaminergic signaling in mice. Neuron. 2010;66(6):908–920. doi: 10.1016/j.neuron.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Samad TA, Krezel W, Chambon P, Borrelli E. Regulation of dopaminergic pathways by retinoids: activation of the D2 receptor promoter by members of the retinoic acid receptor-retinoid X receptor family. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14349–14354. doi: 10.1073/pnas.94.26.14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovalevich J, Corley G, Yen W, Kim J, Rawls S, Langford D. Cocaine decreases expression of neurogranin via alterations in thyroid receptor/retinoid X receptor signaling. Journal of neurochemistry. 2012;121(2):302–313. doi: 10.1111/j.1471-4159.2012.07678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iniguez MA, Rodriguez-Pena A, Ibarrola N, Aguilera M, Munoz A, Bernal J. Thyroid hormone regulation of RC3, a brain-specific gene encoding a protein kinase-C substrate. Endocrinology. 1993;133(2):467–473. doi: 10.1210/endo.133.2.8344193. [DOI] [PubMed] [Google Scholar]
- 26.Husson M, Enderlin V, Alfos S, Boucheron C, Pallet V, Higueret P. Expression of neurogranin and neuromodulin is affected in the striatum of vitamin Adeprived rats. Brain research Molecular brain research. 2004;123(1–2):7–17. doi: 10.1016/j.molbrainres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Snow DM, Smith JD, Booze RM, Welch MA, Mactutus CF. Cocaine decreases cell survival and inhibits neurite extension of rat locus coeruleus neurons. Neurotoxicology and teratology. 2001;23(3):225–234. doi: 10.1016/s0892-0362(01)00137-4. [DOI] [PubMed] [Google Scholar]
- 28.Dey S, Snow DM. Cocaine exposure in vitro induces apoptosis in fetal locus coeruleus neurons through TNF-alpha-mediated induction of Bax and phosphorylated c-Jun NH(2)-terminal kinase. Journal of neurochemistry. 2007;103(2):542–556. doi: 10.1111/j.1471-4159.2007.04750.x. [DOI] [PubMed] [Google Scholar]
- 29.Lepsch LB, Munhoz CD, Kawamoto EM, Yshii LM, Lima LS, Curi-Boaventura MF, Salgado TM, Curi R, Planeta CS, Scavone C. Cocaine induces cell death and activates the transcription nuclear factor kappa-B in PC12 cells. Molecular brain. 2009;2:3. doi: 10.1186/1756-6606-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YW, Hennig B, Fiala M, Kim KS, Toborek M. Cocaine activates redox-regulated transcription factors and induces TNF-alpha expression in human brain endothelial cells. Brain research. 2001;920(1–2):125–133. doi: 10.1016/s0006-8993(01)03047-5. [DOI] [PubMed] [Google Scholar]
- 31.Yao H, Allen JE, Zhu X, Callen S, Buch S. Cocaine and human immunodeficiency virus type 1 gp120 mediate neurotoxicity through overlapping signaling pathways. Journal of neurovirology. 2009;15(2):164–175. doi: 10.1080/13550280902755375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao H, Duan M, Buch S. Cocaine-mediated induction of platelet-derived growth factor: implication for increased vascular permeability. Blood. 2011;117(8):2538–2547. doi: 10.1182/blood-2010-10-313593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Washton AM, Tatarsky A. Adverse effects of cocaine abuse. NIDA research monograph. 1984;49:247–254. [PubMed] [Google Scholar]
- 34.Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neuroscience and biobehavioral reviews. 2008;32(5):883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rojo LE, Fernandez JA, Maccioni AA, Jimenez JM, Maccioni RB. Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer's disease. Archives of medical research. 2008;39(1):1–16. doi: 10.1016/j.arcmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nature reviews. 2001;2(10):734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 37.Achim CL, Heyes MP, Wiley CA. Quantitation of human immunodeficiency virus, immune activation factors, and quinolinic acid in AIDS brains. The Journal of clinical investigation. 1993;91(6):2769–2775. doi: 10.1172/JCI116518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. Journal of neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Connor MJ, Sidell N. Retinoic acid synthesis in normal and Alzheimer diseased brain and human neural cells. Molecular and chemical neuropathology / sponsored by the International Society for Neurochemistry and the World Federation of Neurology and research groups on neurochemistry and cerebrospinal fluid. 1997;30(3):239–252. doi: 10.1007/BF02815101. [DOI] [PubMed] [Google Scholar]
- 40.Theodosiou M, Laudet V, Schubert M. From carrot to clinic: an overview of the retinoic acid signaling pathway. Cellular and molecular life sciences : CMLS. 2010;67(9):1423–1445. doi: 10.1007/s00018-010-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, Kagechika H, Bauer J, Zhao C, Evercooren AB, et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nature neuroscience. 2011;14(1):45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karter DL, Karter AJ, Yarrish R, Patterson C, Kass PH, Nord J, Kislak JW. Vitamin A deficiency in non-vitamin-supplemented patients with AIDS: a cross-sectional study. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1995;8(2):199–203. [PubMed] [Google Scholar]
- 43.Kovalevich J, Corley G, Yen W, Rawls SM, Langford D. Cocaine-Induced Loss of White Matter Proteins in the Adult Mouse Nucleus Accumbens Is Attenuated by Administration of a beta-Lactam Antibiotic during Cocaine Withdrawal. The American journal of pathology. 2012;181(6):1921–1927. doi: 10.1016/j.ajpath.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao H, Kim K, Duan M, Hayashi T, Guo M, Morgello S, Prat A, Wang J, Su TP, Buch S. Cocaine hijacks sigma1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS. J Neurosci. 2011;31(16):5942–5955. doi: 10.1523/JNEUROSCI.5618-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovalevich J, Langford D. Considerations for the Use of SH - SY5Y Neuroblastoma Cells in Neurobiology. In: Amini S, White MK, editors. Neuronal Cell Culture: Methods and Protocols, Methods in Molecular Biology. Volume 1078. 1st edn. New York: Springer; 2013. pp. 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borland MK, Trimmer PA, Rubinstein JD, Keeney PM, Mohanakumar K, Liu L, Bennett JP., Jr. Chronic, low-dose rotenone reproduces Lewy neurites found in early stages of Parkinson's disease, reduces mitochondrial movement and slowly kills differentiated SH-SY5Y neural cells. Molecular neurodegeneration. 2008;3:21. doi: 10.1186/1750-1326-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans SM, Cone EJ, Henningfield JE. Arterial and venous cocaine plasma concentrations in humans: relationship to route of administration, cardiovascular effects and subjective effects. The Journal of pharmacology and experimental therapeutics. 1996;279(3):1345–1356. [PubMed] [Google Scholar]
- 48.Yao H, Bethel-Brown C, Buch S. Cocaine Exposure Results in Formation of Dendritic Varicosity in Rat Primary Hippocampal Neurons. American journal of infectious diseases. 2009;5(1):26–30. doi: 10.3844/ajidsp.2009.26.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costa BM, Yao H, Yang L, Buch S. Role of endoplasmic reticulum (ER) stress in cocaine-induced microglial cell death. J Neuroimmune Pharmacol. 2013;8(3):705–714. doi: 10.1007/s11481-013-9438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee CT, Chen J, Hayashi T, Tsai SY, Sanchez JF, Errico SL, Amable R, Su TP, Lowe RH, Huestis MA, et al. A mechanism for the inhibition of neural progenitor cell proliferation by cocaine. PLoS medicine. 2008;5(6):e117. doi: 10.1371/journal.pmed.0050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rasband WS. ImageJ. 1.37 edn. Bethesda: National Institues of Health; 1997–2009. [Google Scholar]
- 52.Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from midgestation into early adulthood. Neuroscience. 2007;149(3):582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masliah E, Terry RD, Alford M, DeTeresa R. Quantitative immunohistochemistry of synaptophysin in human neocortex: an alternative method to estimate density of presynaptic terminals in paraffin sections. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1990;38(6):837–844. doi: 10.1177/38.6.2110586. [DOI] [PubMed] [Google Scholar]
- 54.Hewson QC, Lovat PE, Pearson AD, Redfern CP. Retinoid signalling and gene expression in neuroblastoma cells: RXR agonist and antagonist effects on CRABP-II and RARbeta expression. Journal of cellular biochemistry. 2002;87(3):284–291. doi: 10.1002/jcb.10310. [DOI] [PubMed] [Google Scholar]
- 55.Biedler JL, Helson L, Spengler BA. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer research. 1973;33(11):2643–2652. [PubMed] [Google Scholar]
- 56.Gomez-Santos C, Francisco R, Gimenez-Xavier P, Ambrosio S. Dopamine induces TNFalpha and TNF-R1 expression in SH-SY5Y human neuroblastoma cells. Neuroreport. 2007;18(16):1725–1728. doi: 10.1097/WNR.0b013e3282f0d3db. [DOI] [PubMed] [Google Scholar]
- 57.Presgraves SP, Ahmed T, Borwege S, Joyce JN. Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotoxicity research. 2004;5(8):579–598. doi: 10.1007/BF03033178. [DOI] [PubMed] [Google Scholar]
- 58.Boudjelal M, Wang Z, Voorhees JJ, Fisher GJ. Ubiquitin/proteasome pathway regulates levels of retinoic acid receptor gamma and retinoid X receptor alpha in human keratinocytes. Cancer research. 2000;60(8):2247–2252. [PubMed] [Google Scholar]
- 59.Macoritto M, Nguyen-Yamamoto L, Huang DC, Samuel S, Yang XF, Wang TT, White JH, Kremer R. Phosphorylation of the human retinoid X receptor alpha at serine 260 impairs coactivatoRs) recruitment and induces hormone resistance to multiple ligands. The Journal of biological chemistry. 2008;283(8):4943–4956. doi: 10.1074/jbc.M707517200. [DOI] [PubMed] [Google Scholar]
- 60.Singh AB, Guleria RS, Nizamutdinova IT, Baker KM, Pan J. High glucose-induced repression of RAR/RXR in cardiomyocytes is mediated through oxidative stress/JNK signaling. Journal of cellular physiology. 2012;227(6):2632–2644. doi: 10.1002/jcp.23005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanchez S, Jimenez C, Carrera AC, Diaz-Nido J, Avila J, Wandosell F. A cAMP-activated pathway, including PKA and PI3K, regulates neuronal differentiation. Neurochemistry international. 2004;44(4):231–242. doi: 10.1016/s0197-0186(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 62.Kume T, Kawato Y, Osakada F, Izumi Y, Katsuki H, Nakagawa T, Kaneko S, Niidome T, Takada-Takatori Y, Akaike A. Dibutyryl cyclic AMP induces differentiation of human neuroblastoma SH-SY5Y cells into a noradrenergic phenotype. Neuroscience letters. 2008;443(3):199–203. doi: 10.1016/j.neulet.2008.07.079. [DOI] [PubMed] [Google Scholar]
- 63.Miyazaki I, Asanuma M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta medica Okayama. 2008;62(3):141–150. doi: 10.18926/AMO/30942. [DOI] [PubMed] [Google Scholar]
- 64.Masserano JM, Baker I, Venable D, Gong L, Zullo SJ, Merril CR, Wyatt RJ. Dopamine induces cell death, lipid peroxidation and DNA base damage in a catecholaminergic cell line derived from the central nervous system. Neurotoxicity research. 2000;1(3):171–179. doi: 10.1007/BF03033288. [DOI] [PubMed] [Google Scholar]
- 65.Sharkey J, Glen KA, Wolfe S, Kuhar MJ. Cocaine binding at sigma receptors. European journal of pharmacology. 1988;149(1–2):171–174. doi: 10.1016/0014-2999(88)90058-1. [DOI] [PubMed] [Google Scholar]
- 66.Matsumoto RR, McCracken KA, Pouw B, Zhang Y, Bowen WD. Involvement of sigma receptors in the behavioral effects of cocaine: evidence from novel ligands and antisense oligodeoxynucleotides. Neuropharmacology. 2002;42(8):1043–1055. doi: 10.1016/s0028-3908(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 67.Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: potential medications development target for anti-cocaine agents. European journal of pharmacology. 2003;469(1–3):1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- 68.Roth MD, Whittaker KM, Choi R, Tashkin DP, Baldwin GC. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. Journal of leukocyte biology. 2005;78(6):1198–1203. doi: 10.1189/jlb.0405219. [DOI] [PubMed] [Google Scholar]
- 69.Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neuroscience and biobehavioral reviews. 2002;26(4):499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 70.Matsumoto RR, Hewett KL, Pouw B, Bowen WD, Husbands SM, Cao JJ, Hauck Newman A. Rimcazole analogs attenuate the convulsive effects of cocaine: correlation with binding to sigma receptors rather than dopamine transporters. Neuropharmacology. 2001;41(7):878–886. doi: 10.1016/s0028-3908(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 71.Motomura K, Ohata M, Satre M, Tsukamoto H. Destabilization of TNF-alpha mRNA by retinoic acid in hepatic macrophages: implications for alcoholic liver disease. American journal of physiology Endocrinology and metabolism. 2001;281(3):E420–E429. doi: 10.1152/ajpendo.2001.281.3.E420. [DOI] [PubMed] [Google Scholar]
- 72.Schule R, Rangarajan P, Yang N, Kliewer S, Ransone LJ, Bolado J, Verma IM, Evans RM. Retinoic acid is a negative regulator of AP-1-responsive genes. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(14):6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee HY, Walsh GL, Dawson MI, Hong WK, Kurie JM. All-trans-retinoic acid inhibits Jun N-terminal kinase-dependent signaling pathways. The Journal of biological chemistry. 1998;273(12):7066–7071. doi: 10.1074/jbc.273.12.7066. [DOI] [PubMed] [Google Scholar]
- 74.Li J, Molkentin JD, Colbert MC. Retinoic acid inhibits cardiac neural crest migration by blocking c-Jun N-terminal kinase activation. Developmental biology. 2001;232(2):351–361. doi: 10.1006/dbio.2001.0203. [DOI] [PubMed] [Google Scholar]
- 75.Adachi S, Okuno M, Matsushima-Nishiwaki R, Takano Y, Kojima S, Friedman SL, Moriwaki H, Okano Y. Phosphorylation of retinoid X receptor suppresses its ubiquitination in human hepatocellular carcinoma. Hepatology (Baltimore, Md. 2002;35(2):332–340. doi: 10.1053/jhep.2002.31164. [DOI] [PubMed] [Google Scholar]
- 76.Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain, behavior, and immunity. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clark KH, Wiley CA, Bradberry CW. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotoxicity research. 2013;23(2):174–188. doi: 10.1007/s12640-012-9334-7. [DOI] [PubMed] [Google Scholar]
- 78.Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: relationship to methamphetamine-induced nerve ending damage. Annals of the New York Academy of Sciences. 2006;1074:31–41. doi: 10.1196/annals.1369.003. [DOI] [PubMed] [Google Scholar]
- 79.Farooqui T, Farooqui AA. Lipid-mediated oxidative stress and inflammation in the pathogenesis of Parkinson's disease. Parkinson's disease. 2011;2011:247467. doi: 10.4061/2011/247467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tai YF, Pavese N, Gerhard A, Tabrizi SJ, Barker RA, Brooks DJ, Piccini P. Microglial activation in presymptomatic Huntington's disease gene carriers. Brain. 2007;130(Pt 7):1759–1766. doi: 10.1093/brain/awm044. [DOI] [PubMed] [Google Scholar]
- 81.Hellmann-Regen J, Kronenberg G, Uhlemann R, Freyer D, Endres M, Gertz K. Accelerated degradation of retinoic acid by activated microglia. Journal of neuroimmunology. 2013;256(1–2):1–6. doi: 10.1016/j.jneuroim.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 82.Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, et al. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science (New York, NY. 2012;335(6075):1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dopamine and forskolin do not affect RXR-γ levels. A, Representative Western blot of RXR-γ in neurons treated with 30 nm dopamine. B, densitometric quantification of A. C, Representative Western blot of RXR-γ in neurons treated with 10 µm forskolin. D, densitometric quantification of C. No significant changes were observed in levels of RXR-γ. Tubulin was used as a loading control.