Abstract
Background:
Orthodontic wires are the corner stones of the science and art of orthodontics and they remain in the patient’s mouth for a prolonged period of 18-24 months. It is but natural to expect that they will undergo some biodegradation when in the oral environment during that period. This study aims to compare the biodegradation characteristics of four different orthodontic wires, stainless steel, nickel titanium (NiTi), titanium molybdenum alloy (TMA), and copper NiTi and to assess whether these biodegradation products, are within acceptable limits.
Materials and Methods:
This study involved the incubation of four different wires in artificial saliva and analyzing the amount of metal released from them at the end of a 28 days study period. The metals analyzed for where nickel, chromium, copper, cobalt, manganese, iron, molybdenum, and titanium. The artificial saliva was changed on days 7, 14, and 21 to prevent the saturation of metals in the artificial saliva. At the end of 28 days, these four samples of artificial saliva of each wire were mixed together and analyzed for the eight metals using an inductively coupled plasma spectroscope.
Results:
The results showed only the release of nickel, chromium, and iron from stainless steel wire, nickel from NiTi wire, nickel, and chromium from copper NiTi and none from TMA wire.
Conclusion:
The metals released from arch wires are of such minute quantities to be of any biologic hazard. The amount of metals released is well within acceptable biocompatible limits. Though this study has analyzed the biodegradation of various orthodontic wires, orthodontic wires are never used alone in mechanotherapy. Orthodontic wires along with multiband appliance system with which it is always used and in combination with accessories like face bows may release more metals.
Keywords: Arch wires, corrosion, inductively coupled plasma spectroscope
Introduction
Orthodontic wires are the corner stones on which the science and art of orthodontics is based. These wires remain the mouth for a prolonged period of 18-24 months. It is natural to expect that these wires undergo some sort of biodegradation in the constantly changing oral environment.
Stainless steel wires have for long been the main stay of clinical orthodontics due to their outstanding combination of mechanical properties, bioinertness, and corrosion resistance in the oral environment. Stainless steel is an iron chromium nickel alloy with chromium content 18% and nickel content 8%.1
Nickel titanium (NiTi) has been widely used clinically due to its properties of shape memory and super elasticity. The general composition of these wire alloys are approximately 53.5% nickel, 44.9% titanium, and 1.6% cobalt.1
A beta titanium alloy titanium molybdenum alloy (TMA) is another commonly used alloy. TMA has nominal composition of 77.8% titanium, 11.3% molybdenum, 6.6% zirconium, and 4.3% tin. A noteworthy characteristic of these wires is that beta titanium is the only alloy to possess true weldability and formability.
Copper NiTi is a new quaternary alloy with distinct advantages over formerly available NiTi alloys. This thermally activated alloy wire contains nickel, titanium, copper, and chromium.
Metals leaching out of orthodontic wires can cause toxicity reactions if they exceed the maximum recommended daily intake levels. The daily intake of nickel should not exceed 300 µg, chromium 0.2 mg, manganese 5 mg, copper 3 mg, molybdenum 0.5 mg, iron 18 mg, and cobalt in minute traces. Average dietary intake for titanium is 300-2000 µg/day.2
Hence, this study was conducted with the following aims and objectives:
To compare in-vitro biodegradation characteristics of four different orthodontic wires stainless steel, NiTi, TMA, copper NiTi alloy
To assess whether the corrosion products were within acceptable biocompatible limits.
Materials and Methods
The sample comprised of four orthodontic archwires of uniform dimension 17 mil × 25 mil and length of 100 mm. The archwires used were stainless steel: Preform arch wires, Ortho-Organizers, Inc, Sanmarcos, California; NiTi: Nitinol XL, 3M Unitek, Monrovia, California; TMA: TMA, ormco corporation, Glendora, California; copper NiTi: Copper NiTi, Ormco Corporation. Glendora, California (Figure 1a-d).
Figure 1.
Four different orthodontics wires used in the study. (a) Stainless steel wire, (b) nickel titanium (NiTi) wire, (c) titanium molybdenum wire, (d) copper NiTi wire.
Each orthodontic wire sample was immersed completely in 100 ml of artificial saliva which was measured accurately by the measuring cylinders and was stored in air tight clear poly ethylene container. All measuring containers used in the experimental study were first immersed in soap water over night and then thoroughly rinsed with tap water they were then dried by keeping them in an incubator at 37°C overnight. They were then finally rinsed with distilled, de-ionized obtained from the distillation unit (milli-pore corporation, Bedford) (Figure 2) and kept in an incubator at 37°C overnight for drying them. This was routinely done for all the measuring cylinders used in the experiment.
Figure 2.
Milli reverse osmosis filtration plant.
The stimulated saliva consisted of 0.4 g Nacl, 1.21 g KCl; 0.78 g NaH2PO4 2H2O, 0.005 g Na2s, 1 g urea [Co(HH2)]2 in 1000 ml distilled water (Gjerdet and Hero, 1987). 100 mg/L of bovine serum albumin was added to artificial saliva. The materials weighed using biochemical balance (Figure 3) and pH adjusted using pH meter (Figure 4).
Figure 3.

Digital pH meter.
Figure 4.

Biochemical weighing balance.
Care was taken to ensure that the clear polyethylene containers, containing the wire sample along with 100 ml artificial saliva were kept air tight at all times. These were kept in an incubator at 37°C (Figure 5) which simulates the temperature in the oral cavity. Along with these four wire samples, 100 ml wire sample without any wire was kept which acted as a control sample.
Figure 5.

Incubator with wire samples and controls.
On days 7, 14, 21, and 28 the entire 100 ml of artificial saliva solution was removed from each flask and replaced with a fresh solution except on day 28. The replacement of solution was performed to avoid saturating the artificial saliva medium with corrosion products.
At the end of 28 days period experimental sample, four samples of 100 ml each for each wire sample were mixed together and analysis were performed for nickel, chromium, copper, cobalt, iron, manganese, molybdenum, and titanium using inductively coupled plasma (ICP) atomic emission spectrometer (Figure 6).
Figure 6.

Inductively coupled plasma spectroscope.
The ICP atomic emission spectrometer has proven to be a highly successful technique at for multi element analysis by atomic emission spectroscopy. The ICP excitation source is electrode less argon plasma operated at atmospheric frequency electromagnetic field. The ICP used for this study was plasma scan 8410, manufactured by Labtam Ltd.; Australia.
Statistical analysis was performed using the Kruskal–Wallis test to find out whether there was any significance in the release of metals from the four different wires and also between the eight metals themselves.
Results
The study showed leaching out of three metals out of the three metals analyzed from the four different wires on the study. The three metals are nickel, chromium, iron. There was no release of copper, manganese, cobalt, molybdenum, titanium from any of the wire during the entire course of this study of 4 weeks. The stainless steel arch wire showed nickel release of 0.006 ppm, chromium release of 0.011 ppm, and iron release of 0.289 ppm over the study period of 28 days (Graph 1). There was absolute no evidence of leaching out of any of the five metals studied.
Graph 1.
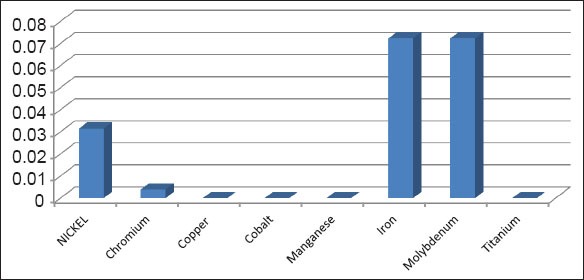
Total metals released.
The NiTi archwire showed only the release 0.002 ppm of nickel over the entire study period. The TMA wire did not show trace of any of the metals for which the analysis were performed. The copper NiTi wire showed nickel release of 0.004 ppm and chromium release of 0.003 ppm over the study period of 28 days.
Discussion
Orthodontic wires are an essential part of any orthodontic appliance system. No matter what the mechanotherapy employed, orthodontic wires are an essential part and parcel of it. Most of the studies that have been done have employed an accelerated corrosion process, either in the form of heat treatment of wires3 or employing a highly corrosive media like 0.1 mol/L lactic acid or 0.1 mol/L NaCl.4 Most of the previous studies have considered the biodegradation of orthodontic wires inartificial saliva medium and simulated oral environment.
The few studies that have been done have focused on the leaching out of mainly two metals nickel and chromium. While it is a fact that nickel is the metal that causes most number of allergic reactions in the human body and that chromium is the second in line,5 other metals such as copper, cobalt, iron, manganese, molybdenum, and titanium which are some of the other constituent metals of orthodontic wires have the potential for causing deleterious effect on the human body if absorbed in sufficient quantities.6 Hence the present study was performed to estimate the amount of the following metals nickel, chromium, copper, cobalt, manganese, iron, molybdenum, and titanium, leaching out of orthodontic wires in artificial saliva over a period of 4 weeks.
The wires studied were all preformed and of uniform dimension of 0.017 × 0.025 inches which was in accordance with the study done by Barrett et al.2 The wires are of uniform length of 100 mm. The total tooth material in the upper arch from distal of first molar on one side to distal of first molar on the other side. According to Ashley and Howe, is in the range of 85-98 mm the higher limit of this range was taken and 1 mm was added on either side to allow for synch back. Hence, the total length of the wire chosen was 100 mm.
The artificial saliva used in the study was the one recommended by Barrett et al.2 This artificial saliva is a modification of the one used by Gjerdet and Hero,3 the difference being that of calcium chloride was substituted the use potassium chloride. This was done because the calcium ions interfered with the accurate measurement of chromium. Potassium chloride was preferred to sodium chloride since it has similar corrosive action as calcium chloride.
Artificial saliva was changed on days 7, 14, and 21 in order to avoid the super saturation of metals released from the wires in the saliva preventing further release of metals.7 Artificial saliva was freshly prepared on the day the experiment started and on days 7, 14, and 21. Saliva was freshly prepared on those days to avoid storage of saliva with protein over a period of time which could induce bacterial growth and hence increase the protein level of artificial saliva.
All the storing containers were made of polyethylene because containers made of polyethylene do not contain any metals and hence the chances of metals going into solution from the container were nil. All these containers were thoroughly washed with soap rinsed with tap water and left to dry in an incubator at 37°C overnight. Again, the containers were rinsed thoroughly with de-ionized water and left over night in an incubator at 37°C. All water used in the study was de-ionized by five stage milli-o-plus to remove any potential metal contaminants. The measuring cylinders used in the study were made of glass (Borosil Ltd.). All measuring cylinders and glass funnels used for transferring solutions were carefully washed as previously described.
The amount of metal released was analyzed by means of an ICP spectroscope. The instrument vaporizes the saliva sample and metals released combines with argon plasma at 6000-10,000°K. The amount of each metal is calculated at certain predetermined wavelengths by the instrument from the argon plasma. Nickel was estimated at a wavelength of 221.647 nm, chromium at 205.55 nm, cobalt at 238.892 nm, copper at 324.754 nm, iron at 238.207 nm, manganese at 257.610 nm, molybdenum at 202.032 nm, and titanium at 323.452 nm. Three readings were taken for each sample. If three readings were within the range of 10% of each other than the mean of all these three were calculated if not a fourth reading was calculated (Graph 2).
Graph 2.
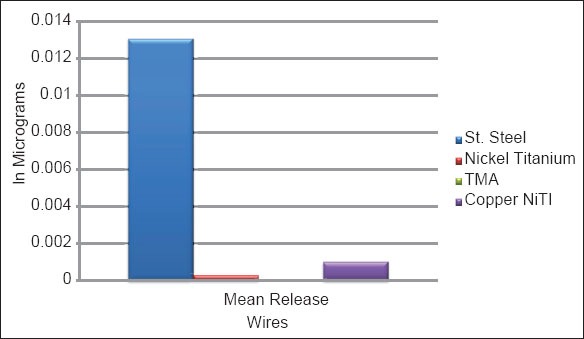
Mean release of metals from the four different wires.
Relatively significant amount of metals were found to be leaching out of stainless steel wires. It showed leaching out of nickel - 0.006 ppm, chromium - 0.011 ppm, and iron - 0.289 ppm. Analysis performed for the other metals in this sample proved negative. Corrosion of stainless steel occurs mainly due to breakdown of the passivating layer of chromium8 as the results showed there is relatively significant amount of chromium which has leached out of the wire in the 4 weeks study period which is much more than the nickel leached out. Significant leaching out of iron is to be expected because the content of iron in stainless steel wire is to the tune of around 70%.9,10
NiTi arch wire showed only very minute quantities of nickel released and no release of any other metals in the 28 days study period. The amount of nickel which leached out was 0.002 ppm for the 4 weeks study period. This was in contrast to the finding of a study done by Grimsdottir et al. in which no nickel was released from Unitek Nitinol at the end of 14 days after incubation in 0.9% Nacl at 23°C. The value obtained for the release of nickel in the present study was also very minute, even though the total concentration of nickel in this wire is around 55%. This may be because these alloys contain significant amount of titanium (around 45%) which is highly corrosion resistant and it appears to bind with nickel making the alloy corrosion resistant.11,12 However, Dunlap13 reported a case of severe allergic reaction after insertion of NiTi arch wire in a nickel sensitive patient. This may be due to contact hypersensitivity of the patient to the nickel in the arch wire.14
The TMA wire which was tested showed no release of any of the eight metals which were tested for. This may be because the main constituent of the wire that is titanium and molybdenum are highly inert and thus highly corrosion resistant. Also as stated earlier, the presence of titanium may bind the constituent metals, thus making the whole alloy highly corrosion resistant.
Very minute amounts of nickel and chromium were released from the copper NiTi alloy during the course of this study. Nickel released was only 0.004 ppm, whereas chromium was only 0.003 ppm.
In a study of corrosion of different orthodontic alloys Grimsdottir and Hensten-Pettersen15 found that the least amount of metals were released from orthodontic wire alloys when compared to face bows, molar bands, and brackets.
The present study showed that the minute quantities of metals released from as-received Stainless steel, NiTi, TMA,16 and Copper NiTi alloy wires were insignificant. However, a study by Gjerjet and Hero3 showed that orthodontics wires when heat treated release 15-60 times more metals than those wires received in as - Received state. Heating of orthodontic wire alloys initiated an accelerated corrosion process, which account for the higher metal release from them. This factor should be taken into consideration in procedures involving application of heat to orthodontic wires. Furthermore, when comparing orthodontic wires in an as received state to silver soldered ones, the silver soldered wire showed more corrosion than the other wires. This was demonstrated in a study by Berge et al.17 This is obviously due to the fact that “silver solders” are themselves eutectic alloys and very highly prone to corrosion.
As far as the toxicity reactions of the metals analyzed are concerned, the release of theses metals are in such minute quantities that there is no danger of them exceeding the maximum daily intake levels.
The maximum recommended daily intake level for nickel is 300 µg. The amount of nickel released in the 28 days period from stainless steel is 0.006 µg, from NiTi is 0.002 µg and copper NiTi is 0.004 µg. Hence, if we calculate the daily intake of nickel from stainless steel wire it is 0.002 µg, from NiTi, it is 0.00007 µg and from copper NiTi, it is 0.0001 µg which is if no significance at all when compared to the daily dietary intake of nickel.18 Although the amount of nickel released is not sufficient to cause any systemic toxic reactions, the amount of the metal released may cause allergic reactions in a previously sensitized patient.19,20
Orthodontic wires stay in the patient’s mouth for a prolonged period of 18-24 months. Though this study has demonstrated how minute quantities of metals are actually released from the wires, this study was done only over a period of 28 days. All the more, orthodontic wires are never used alone in any mechanotherapy. Orthodontic wires along with the multiband appliance system with which it is always used and accessories like face bows is likely to show release of more metals as shown in the study by Barrett et al.2
Even though care was taken to replicate the conditions that exist in the oral cavity as far as possible, constant washing action of saliva, sudden changes in temperature and in pH could not be duplicated.21 These factors may increase the corrosion rate of orthodontic wires especially during a longer duration of time as shown in the study by Baswa and Surendrashetty.22 A study of orthodontic wires in conjunction with a multibanded/bracket appliance system simulating the oral conditions in more detail and over a longer duration of time is recommended.
Conclusion
The metals released from archwires are of such minute quantities to be of any biologic hazard. The amount of metals released is well within acceptable biocompatible limits.
Though this study has analyzed the biodegradation of various orthodontic wires, orthodontic wires are never used alone in mechanotherapy. Orthodontic wires along with multibanded appliance system, with which it is always used and in combination with accessories like face bows may release more metals.
Footnotes
Conflict of Interest: None
Source of Support: Nil
References
- 1.Petoumenou E, Arndt M, Keilig L, Reimann S, Hoederath H, Eliades T, et al. Nickel concentration in the saliva of patients with nickel-titanium orthodontic appliances. Am J Orthod Dentofacial Orthop. 2009;135(1):59–65. doi: 10.1016/j.ajodo.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Barrett RD, Bishara SE, Quinn JK. Biodegradation of orthodontic appliances Part I. Biodegradation of nickel and chromium in vitro. Am J Orthod Dentofacial Orthop. 1993;103(1):8–14. doi: 10.1016/0889-5406(93)70098-9. [DOI] [PubMed] [Google Scholar]
- 3.Gjerdet NR, Herø H. Metal release from heat-treated orthodontic archwires. Acta Odontol Scand. 1987;45(6):409–14. doi: 10.3109/00016358709096365. [DOI] [PubMed] [Google Scholar]
- 4.Leung VW, Darvell BW. Artificial salivas for in vitro studies of dental materials. J Dent. 1997;25(6):475–84. doi: 10.1016/s0300-5712(96)00068-1. [DOI] [PubMed] [Google Scholar]
- 5.Epidemiology of contact dermatitis in North America: 1972. Arch Dermatol. 1973;108:537–40. [PubMed] [Google Scholar]
- 6.Kazantzis G. Role of cobalt, iron, lead, manganese, mercury, platinum, selenium, and titanium in carcinogenesis. Environ Health Perspect. 1981;40:143–61. doi: 10.1289/ehp.8140143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramanyan M, Lal B. 12th ed. New Delhi: Ratan Prakashan Mandir; 1997. Electricity and Magnetism. [Google Scholar]
- 8.Phillips RW. 10th ed. London: WB Saunders; 2004. Science of Dental Materials. [Google Scholar]
- 9.Obrien WJ. Chicago, IL: Quintessence Pubishing Co. Inc; 1989. Dental Materials: Properties and Selection. [Google Scholar]
- 10.Sfondrini MF, Cacciafesta V, Maffia E, Massironi S, Scribante A, Alberti G, et al. Chromium release from new stainless steel, recycled and nickel-free orthodontic brackets. Angle Orthod. 2009;79(2):361–7. doi: 10.2319/042108-223.1. [DOI] [PubMed] [Google Scholar]
- 11.Grimsdottir MR, Gjerdet NR, Hensten-Pettersen A. Composition and in vitro corrosion of orthodontic appliances. Am J Orthod Dentofacial Orthop. 1992;101(6):525–32. doi: 10.1016/0889-5406(92)70127-V. [DOI] [PubMed] [Google Scholar]
- 12.Matos de Souza R, Macedo de Menezes L. Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances. Angle Orthod. 2008;78(2):345–50. doi: 10.2319/111806-466.1. [DOI] [PubMed] [Google Scholar]
- 13.Dunlap CL, Vincent SK, Barker BF. Allergic reaction to orthodontic wire: Report of case. J Am Dent Assoc. 1989;118:449–50. doi: 10.14219/jada.archive.1989.0174. [DOI] [PubMed] [Google Scholar]
- 14.Blanco-Dalmau L. The nickel problem. J Prosthet Dent. 1982;48(1):99–101. doi: 10.1016/0022-3913(82)90054-3. [DOI] [PubMed] [Google Scholar]
- 15.Grimsdottir MR, Hensten-Pettersen A. Cytotoxic and antibacterial effects of orthodontic appliances. Scand J Dent Res. 1993;101(4):229–31. doi: 10.1111/j.1600-0722.1993.tb01110.x. [DOI] [PubMed] [Google Scholar]
- 16.Verstrynge A, Van Humbeeck J, Willems G. In-vitro evaluation of the material characteristics of stainless steel and beta-titanium orthodontic wires. Am J Orthod Dentofacial Orthop. 2006;130(4):460–70. doi: 10.1016/j.ajodo.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Berge M, Gjerdet NR, Erichsen ES. Corrosion of silver soldered orthodontic wires. Acta Odontol Scand. 1982;40(2):75–9. doi: 10.3109/00016358209041118. [DOI] [PubMed] [Google Scholar]
- 18.Robinson CH, Lawler MR. New York: Macmillan Publishing Company Inc; 1982. Normal and Therapeutic Nutrition. [Google Scholar]
- 19.Kao CT, Ding SJ, He H, Chou MY, Huang TH. Cytotoxicity of orthodontic wire corroded in fluoride solution in vitro. Angle Orthod. 2007;77(2):349–54. doi: 10.2319/0003-3219(2007)077[0349:COOWCI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Pazzini CA, Pereira LJ, Carlos RG, de Melo GE, Zampini MA, Marques LS. Nickel: Periodontal status and blood parameters in allergic orthodontic patients. Am J Orthod Dentofacial Orthop. 2011;139(1):55–9. doi: 10.1016/j.ajodo.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 21.Kuhta M, Pavlin D, Slaj M, Varga S, Lapter-Varga M, Slaj M. Type of archwire and level of acidity: Effects on the release of metal ions from orthodontic appliances. Angle Orthod. 2009;79(1):102–10. doi: 10.2319/083007-401.1. [DOI] [PubMed] [Google Scholar]
- 22.Baswa VK, Shetty S. Biodegradation of orthodontic appliances, an atomic absorption spectrophotometry assessment. J Indian Orthod Soc. 1998;31:207–11. [Google Scholar]


