Abstract
We present evidence that virus-specific RNA is present in polyribosomes of transformed cells replicating the murine sarcoma-leukemia virus complex and that it serves as messenger RNA for the synthesis of viral-coded proteins. Both virus-specific RNA (detected by hybridization with the [3H]DNA product of the viral RNA-directed DNA polymerase) and nascent viral polypeptides (measured by precipitation with antiserum to purified virus) were found in membrane-bound and free polyribosomes. Membrane-bound polyribosomes contained a higher content of both virus-specific RNA and nascent viral polypeptides. From 60 to 70% of viral RNA sequences were released from polyribosomes with EDTA, consistent with a function as messenger RNA. Maximum amounts of both virus-specific RNA and nascent viral polypeptides were found in the polyribosome region sedimenting at about 350 S.
Keywords: hybridization, RNA-directed DNA polymerase, membrane-bound polysomes, immunoprecipitation
Full text
PDF
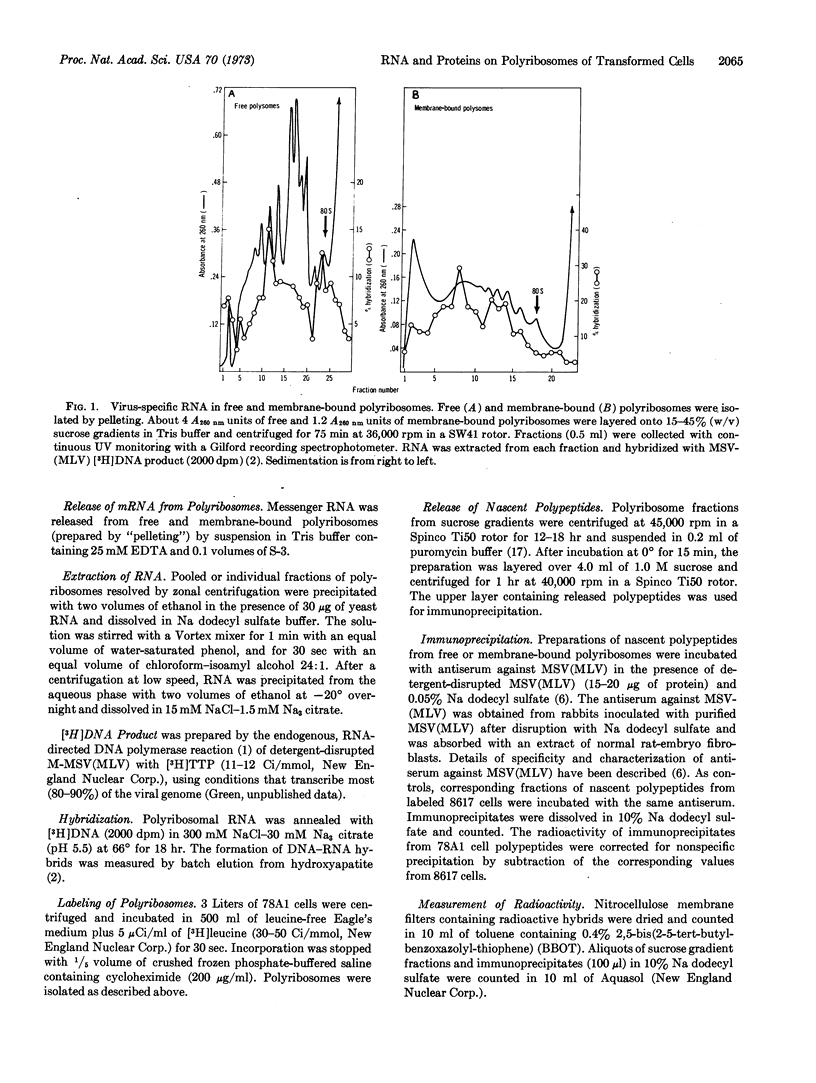
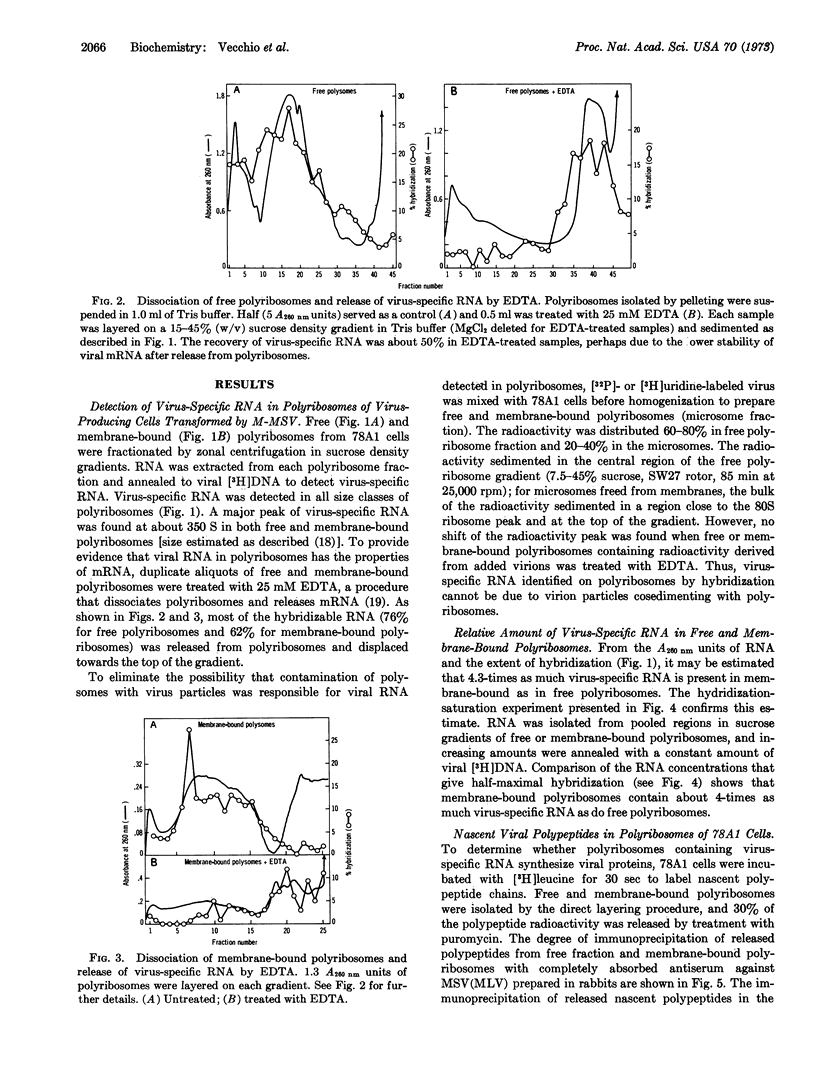
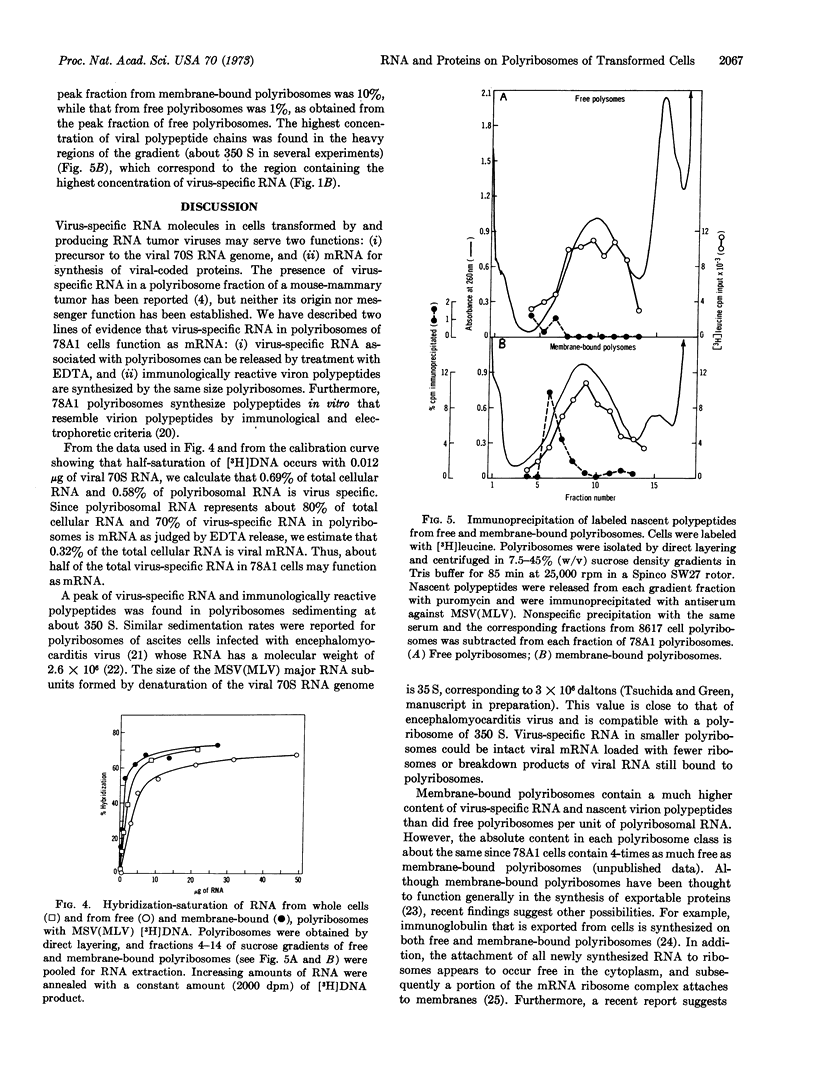

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Schlom J., Spiegelman S. Evidence for translation of viral-specific RNA in cells of a mouse mammary carcinoma. Proc Natl Acad Sci U S A. 1972 Mar;69(3):535–538. doi: 10.1073/pnas.69.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C., Bleiberg I., Zauderer M. Assembly of membrane-bound polyribosomes. Nat New Biol. 1971 Jul 7;232(27):8–12. doi: 10.1038/newbio232008a0. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Vesco C., Jacobs-Lorena M. The role of ribosomal subunits in mammalian cells. Cold Spring Harb Symp Quant Biol. 1969;34:555–565. doi: 10.1101/sqb.1969.034.01.063. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Ribosomes in rat liver: an estimate of the percentage of free and membrane-bound ribosomes interacting with messenger RNA in vivo. J Mol Biol. 1967 Sep 28;28(3):539–542. doi: 10.1016/s0022-2836(67)80103-7. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Temin H. M. Hybridization of Rous sarcoma virus deoxyribonucleic acid polymerase product and ribonucleic acids from chicken and rat cells infected with Rous sarcoma virus. J Virol. 1972 May;9(5):766–775. doi: 10.1128/jvi.9.5.766-775.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgarno L., Cox R. A., Martin E. M. Polyribosomes in normal Krebs 2 ascites tumor cells and in cells infected with encephalomyocarditis virus. Biochim Biophys Acta. 1967 Apr 18;138(2):316–328. doi: 10.1016/0005-2787(67)90492-3. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson W. S. Nucleic acid and proteins isolated from the Rauscher mouse leukemia virus (MLV). Proc Natl Acad Sci U S A. 1966 Jan;55(1):219–227. doi: 10.1073/pnas.55.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by DNA mammalian viruses. VII. Viral genes transcribed in adenovirus type 2 infected and transformed cells. Proc Natl Acad Sci U S A. 1970 Feb;65(2):375–382. doi: 10.1073/pnas.65.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielkens A. L., Berns T. J., Bloemendal H. An efficient procedure for the isolation of polyribosomes from tissue culture. Eur J Biochem. 1971 Oct 26;22(4):478–484. doi: 10.1111/j.1432-1033.1971.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Green M., Rokutanda H., Rokutanda M. Virus specific RNA in cells transformed by RNA tumour viruses. Nat New Biol. 1971 Apr 21;230(16):229–232. doi: 10.1038/newbio230229a0. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowska-Bernstein B., Lamm M. E., Vassalli P. Synthesis of immunoglobulin heavy and light chains by the free ribosomes of a mouse plasma cell tumor. Proc Natl Acad Sci U S A. 1970 Jun;66(2):425–432. doi: 10.1073/pnas.66.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA-protein complexes and newly synthesized ribosomal subunits: analysis of free particles and components of polyribosomes. J Mol Biol. 1968 Jul 14;35(1):37–59. doi: 10.1016/s0022-2836(68)80035-x. [DOI] [PubMed] [Google Scholar]
- Rosbash M. Formation of membrane-bound polyribosomes. J Mol Biol. 1972 Apr 14;65(3):413–422. doi: 10.1016/0022-2836(72)90198-2. [DOI] [PubMed] [Google Scholar]
- SIEKEVITZ P., PALADE G. E. A cytochemical study on the pancreas of the guinea pig. 5. In vivo incorporation of leucine-1-C14 into the chymotrypsinogen of various cell fractions. J Biophys Biochem Cytol. 1960 Jul;7:619–630. doi: 10.1083/jcb.7.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam G., Vecchio G., Attardi D., Green M. Immunological studies on viral polypeptide synthesis in cells replicating murine sarcoma-leukemia virus. J Virol. 1972 Sep;10(3):447–455. doi: 10.1128/jvi.10.3.447-455.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Robin M. S., Green M. Viral RNA subunits in cells transformed by RNA tumor viruses. Science. 1972 Jun 30;176(4042):1418–1420. doi: 10.1126/science.176.4042.1418. [DOI] [PubMed] [Google Scholar]
- Van Ventrooij W. J., Henshaw E. C., Hirsch C. A. Nutritional effects on the polyribosome distribution and rate of protein synthesis in Ehrlich ascites tumor cells in culture. J Biol Chem. 1970 Nov 25;245(22):5947–5953. [PubMed] [Google Scholar]
- Vassart G. Specific synthesis of thyroglobulin on membrane bound thyroid ribosomes. FEBS Lett. 1972 Apr 15;22(1):53–56. doi: 10.1016/0014-5793(72)80217-5. [DOI] [PubMed] [Google Scholar]



