Abstract
Background
Assessing right ventricular (RV) performance is essential for patients with tetralogy of Fallot (TOF). The aim of this study was to investigate the reliability and validity of tricuspid annular plane systolic excursion (TAPSE) against cardiac magnetic resonance imaging measures and cardiopulmonary exercise testing.
Methods
A retrospective study was performed in 125 outpatients with repaired TOF with available protocoldriven echocardiography, cardiac magnetic resonance imaging, and exercise stress testing obtained as part of a cross-sectional study. TAPSE was measured on the two-dimensional apical four-chamber view on echocardiography by two readers. Multivariate linear regression was used to examine the association between TAPSE and measures of RV function and exercise capacity.
Results
The mean age was 12.6 ± 3.3 years, 41 patients (33%) were female, and 104 (83%) were white. TAPSE averaged 1.6 ± 0.37 cm, with an interreader intraclass correlation coefficient of 0.78 (n = 18). TAPSE was significantly associated with cardiac magnetic resonance–based RV stroke volume after adjustment for gender and body surface area (β = 13.8; 95% confidence interval, 2.25–25.30; P = .02). TAPSE was not associated with cardiac magnetic resonance–based RV ejection fraction (P = .77). On exercise testing, TAPSE was not associated with peak oxygen consumption, percentage of predicted oxygen consumption, oxygen pulse, or the ventilatory equivalent for carbon dioxide in patients with maximal exercise stress testing (n = 73 [58%]).
Conclusions
TAPSE is reproducibly measured by echocardiography in patients with TOF. It is not associated with RV ejection fraction or exercise performance, and its association with RV stroke volume may be confounded by body size. On the basis of these results, TAPSE is not representative of global RV performance in patients with TOF.
Keywords: Tricuspid annular plane systolic excursion, Echocardiography, Exercise, Magnetic resonance imaging, Tetralogy of Fallot
Quantification of right ventricular systolic performance is vital given the growing number of survivors with congenital heart disease, in particular those with involvement of the right heart, such as tetralogy of Fallot (TOF). Patients operated on for TOF experience pulmonary insufficiency, which results in RV dilation and ultimately RV failure.1 Therefore, assessment of RV function is of paramount importance in the longitudinal follow-up of this patient population. The assessment of RV function by echocardiography in patients with TOF is challenging and currently is largely qualitative.2
Tricuspid annular plane systolic excursion (TAPSE) is a well-described echocardiographic measure of RV function in the adult population without congenital heart diseases.3,4 Representing the distance of the tricuspid valve annular descent toward the apex of the heart during systole, this measurement reflects the longitudinal component of RV contraction.5 In normal subjects as well as in patients with pulmonary hypertension and other cardiac diagnoses, TAPSE is associated with global measures of RV systolic function, including RV ejection fraction (RVEF).4,6 This likely reflects the fact that longitudinal RV shortening accounts for the majority of total RV contraction in normal subjects.7 Studies in pulmonary hypertension and heart failure have shown that lower TAPSE is associated with a higher risk for death.8,9 Therefore, this measure could similarly be of use in congenital heart disease, in particular because TAPSE depends neither on endocardial border definition nor on geometric assumptions.4 Studies have examined the use of TAPSE in TOF and other congenital heart diseases. Of note, those studies have produced mixed results regarding TAPSE and RVEF; in addition, none have examined its association with maximal exercise capacity.10–14 Therefore, further investigation is needed to determine the usefulness of TAPSE to assess RV function in congenital heart disease.
We sought to examine the reliability of the interpretation of TAPSE and to determine its validity against measures of RV function by cardiac magnetic resonance (CMR) and indices of exercise performance in a large cohort with TOF.
METHODS
Subjects
This was a retrospective study of participants in a cross-sectional protocol conducted at the Children’s Hospital of Philadelphia (RO1-HL74731) that recruited 177 patients and performed echocardiography, CMR, and exercise stress testing (EST). The study sample for this analysis included subjects with surgically treated TOF aged 8 to 19 years who underwent transthoracic echocardiography, CMR, and EST within a maximum of 3 months of one another. TAPSE measurements were retrospectively obtained in this study sample. The study was approved by the Children’s Hospital of Philadelphia Institutional Review Board.
Echocardiography
Echocardiography was performed using a Phillips iE33 machine (Phillips Medical Systems, Andover, MA) using a standard protocol. Briefly, images were acquired with 3-MHz to 8-MHz transducers, suited for patient size and acoustic windows and digitally stored using syngo Dynamics version 3.0 (Siemens Healthcare, Ann Arbor, MI). Although TAPSE was originally measured using two-dimensional (2D) tricuspid annular displacement, TAPSE is currently more commonly measured using M-mode echocardiography, by placing the cursor through the tricuspid annulus and measuring the amount of longitudinal motion of the annulus at peak systole.4,15 Because M-mode imaging was not available in this study population, we performed offline TAPSE measurements using 2D apical four-chamber views. We identified the lateral annulus of the tricuspid valve and measured the distance at end-diastole and end-systole to a point on the screen that was the same for systole and diastole. We measured the maximal excursion and used the electrocardiographic tracing as a guide to ensure that all measurements were obtained at the same time in the cardiac cycle. The distance the annulus travels vertically should be the same whether the point measured is the apex of the heart or the apex of the sector. To corroborate the technique we used in this study, we repeated measurements in 12 patients, comparing the measurement of the annulus with a point on the screen to measurement of the annulus to the apex. We found that the measurements are essentially the same (correlation coefficient = 0.98; P < .0001; intraclass correlation coefficient [ICC] = 0.99; 95% confidence interval [CI], 0.96–1.02).
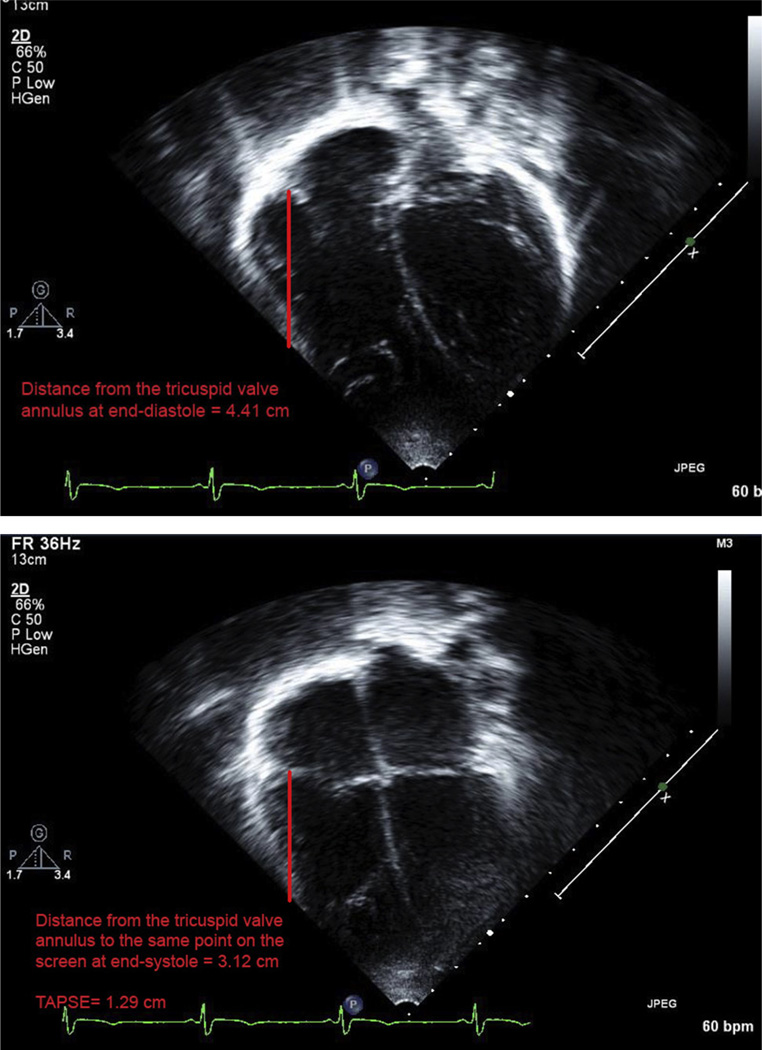
Measurements were performed by two trained readers blinded to clinical information and to CMR and EST results. The tricuspid valve annulus was identified at end-systole and end-diastole using electrocardiographic guidance, and the distance was measured as has been previously reported (Figure 1).4 The average of three TAPSE measurements was used for each patient. We randomly selected 10% of studies for blind reinterpretation by the same reader and 10% for interpretation by the second reader.
Figure 1.
Example of TAPSE measurement by 2D echocardiography on apical four-chamber view from a study patient. (Top) Tricuspid valve annulus at end-diastole (distance to a point on the screen, 4.41 cm). (Bottom) Tricuspid valve annulus in end-systole (distance to the same point on the screen, 3.12 cm). TAPSE = 1.29 cm.
To assess the agreement of 2D TAPSE with M-mode measurements, we compared measurements performed using both techniques performed in a blinded fashion in 20 children without congenital heart disease.
CMR
CMR studies were performed using a 1.5-T Avanto magnetic resonance imaging scanner (Siemens Healthcare, Erlangen, Germany) with a six-channel phased-array body coil using a standard imaging protocol. The magnetic resonance imaging sequences included a steady-state free precession sequence through the cardiac apex. Sedation was used when appropriate according to patient age and ability to lie still for the scan. To assess RV end-systolic volume (RVESV) and RV end-diastolic volume (RVEDV), a cine magnetic resonance sequence in a short-axis view was used (echo time, 2.0 ms; repetition time, 4.5ms; flip angle, 75°–90°; matrix size, 196 × 196). RV stroke volume was calculated by subtracting RVESV from RVEDV. RVESV and RVEDV were indexed to body surface area (BSA), and ventricular size was compared with published normative data.16,17 RVEF was calculated from RV stroke volume divided by RVEDV. RVEF < 50% was defined as abnormal on the basis of prior studies in normal children.16,17 Pulmonary insufficiency was graded as mild if the regurgitant fraction was ≤20%, moderate if it was 20% to 40%, and severe if it was ≥40%. RV regional wall motion abnormalities were assessed qualitatively by examining four-chamber and short-axis views on cine CMR and recorded as present or absent. The RV outflow tract was assessed on the sagittal RV outflow tract view.
EST
Patients exercised to maximal ability using an electronically braked cycle ergometer (SensorMedics, Yorba Linda, CA). Eighteen subjects who were <130 cm tall exercised on a treadmill (Series 2000; Marquette, Milwaukee, WI). Metabolic data were obtained throughout the study and for the first 2 min of recovery on a breath-by-breath basis using a metabolic cart (V29; SensorMedics), including oxygen consumption (VO2), carbon dioxide production (VCO2), maximum work (physical working capacity), oxygen pulse, respiratory exchange ratio, and the ventilatory equivalent of carbon dioxide (VE/VCO2). Anaerobic threshold was measured using the V-slope method. The predicted percentage of maximum VO2 (VO2max%) was calculated for each patient, according to normative values for age, gender, and body size.18 Exercise performance was defined by peak VO2 and VO2max%. Abnormal aerobic performance was defined as VO2max % < 80. Ventilatory efficiency was assessed by VE/VCO2 at the anaerobic threshold and by the slope of the VE/VCO2 relationship from initiation to peak exercise (VE/VCO2 slope). Maximal EST was defined as attaining a respiratory exchange ratio > 1.1.
Statistical Analysis
Continuous variables are presented as mean ± SD or as medians with interquartile ranges, as appropriate. Categorical variables are described using counts and percentages. Reproducibility of TAPSE interpretations and the agreement of 2D TAPSE measurements with M-mode measurements are expressed using ICCs with 95% CIs. Student’s t or Mann-Whitney U tests were used to compare continuous variables according to their distribution.
Multivariate linear regression was used to assess the association of TAPSE (the independent variable) with CMR and EST parameters of interest (the dependent variables). The final models included clinically relevant covariates that were significant (P < .15) on univariate analysis or thought to be confounders, defined as a covariate that resulted in a >15% change in the TAPSE β coefficient. Body size was accounted for as BSA in the multivariate model.19 Receiver operating characteristic curve analysis was used to test the ability of TAPSE to predict RV dysfunction (RVEF < 50%) by CMR.
Analyses of exercise covariates included only those with maximal EST (respiratory exchange ratio > 1.1). All analyses were performed using Stata version 11.0 (StataCorp LP, College Station, TX). Statistical significance was defined as P < .05.
RESULTS
Description of the Study Sample
A total of 177 individuals were recruited for the parent study. One hundred forty-one subjects completed all three studies of interest (echocardiography, CMR, and EST). Of these 141, 125 had interpretable TAPSE plus the other CMR and EST covariates of interest and therefore constituted the study sample. Most echocardiographic (70%) and CMR studies were performed within 2 weeks of each other, and all three studies were performed within 3 months of one another. There were no differences in age, gender, and race between those in the study sample (n = 125) and those excluded (n = 52). The time between echocardiography and CMR averaged 14 ± 25 days (range, 0–96 days). Half of the subjects underwent echocardiography and CMR on the same day (n = 62). Those with echocardiography and CMR performed within 2 weeks of each other were comparable in terms of RV volumes, RVEF, TAPSE, and peak VO2 with those who had studies performed >2 weeks and <3 months apart.
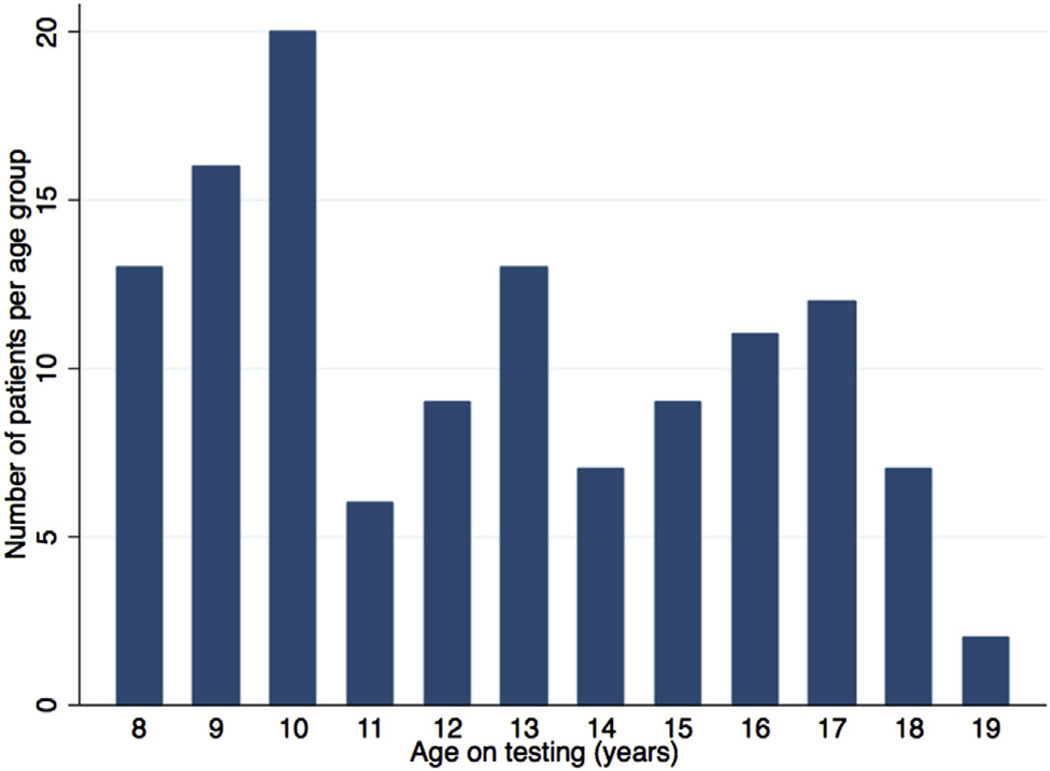
The mean age was 12.5 ± 3.3 years (range, 8–19 years), and 41 patients (33%) were female. All age groups were represented (Figure 2). One hundred four (83%) were non-Hispanic white. Most subjects (76%) were repaired before 1 year of age and presented with pulmonary valve stenosis (79%), followed by atresia and absent pulmonary valve leaflets (15% and 6%, respectively). Surgical repair included a transannular patch in most subjects (67%) (Table 1). No subjects had undergone pulmonary valve replacement at the time of the study.
Figure 2.
Age distribution of the study cohort. The graph displays the number of patients per year of age.
Table 1.
Patient characteristics (n = 125)
| Variable | Value |
|---|---|
| Age (y) | 12.5 ± 3.2 |
| Male | 84 (67.2%) |
| Race/ethnicity | |
| Non-Hispanic white | 104 (83%) |
| African American | 15 (12%) |
| Asian | 6 (5%) |
| Weight (kg) | 42.8 ± 15.7 |
| Height (cm) | 147 ± 17.5 |
| Ideal body weight (kg) | 51.3 ± 11 |
| Body mass index (kg/m2) | 19.8 ± 4.2 |
| BSA (m2) | 1.31 ± 0.3 |
| Original pulmonary valve | |
| Stenosis | 99 (79%) |
| Atresia | 19 (15%) |
| Absent leaflets | 7 (6%) |
| Age at surgical repair (mo) | 4.4 (4.1–4.7) |
| Time elapsed since surgical repair (y) | 7.5 (5.2–10.7) |
| Use of transannular patch | 82 (66%) |
| TAPSE (cm) | 1.6 ± 0.38 |
Data are expressed as mean ± SD, number (percentage), or median (interquartile range).
On CMR, there was significant pulmonary insufficiency and RV dilation. Pulmonary insufficiency was severe in 40% of the subjects (regurgitant fraction > 40%) (Table 2). RV volumes were increased but RV function was preserved, with a mean RVEF of 61 ±8%. RV systolic dysfunction (RVEF < 50%) was present in 10% of patients. Despite overall preserved RVEF, we found average TAPSE to be 1.6 ± 0.38 cm (range, 0.75–2.75 cm), lower than that of children without congenital heart disease. Moreover, TAPSE was decreased in each age group in our study compared with published normal values20 (Table 3). Regional wall motion abnormalities of the right ventricle were noted in most subjects (89%). Most subjects had mild or less than mild tricuspid regurgitation (mean regurgitant fraction, 5.6 ± 5%).
Table 2.
CMR and EST characteristics (n = 125)
| Variable | Value |
|---|---|
| CMR | |
| RVEF (%) | 60.6 ± 8.3 |
| RVEF | |
| <50% (n = 13) | 46 ± 4 |
| ≥50% (n = 112) | 62 ± 7 |
| RV cardiac output (L/min) | 7.2 ± 2.5 |
| RV cardiac index (L/min/m2) | 5.5 ± 1.5 |
| RV stroke volume (mL) | 92.8 ± 37 |
| RV stroke volume index (mL/m2) | 70.3 ± 19.3 |
| RVEDV (mL) | 155.2 ± 63 |
| Indexed RVEDV (mL/m2) | 117.7 ± 34.4 |
| RVESV (mL) | 62.4 ± 31.2 |
| Indexed RVESV (mL/m2) | 47 ± 20 |
| Pulmonary regurgitant fraction (%) | 34.1 ± 16.73 |
| RV mass (g) | 91 (67–127) |
| RV mass (g/m2) | 76 (58–93) |
| Pulmonary insufficiency* | |
| Mild or less | 20 (16%) |
| Moderate | 49 (39%) |
| Severe | 50 (40%) |
| Regional wall motion abnormalities† | |
| Yes | 111 (89%) |
| No | 13 (10%) |
| Left ventricular ejection fraction (%) | 68.4 ± 7.1 |
| EST | Overall (n = 125) | RER > 1.1 (n = 73) | RER ≤ 1.1 (n = 52) |
|---|---|---|---|
| Peak VO2 (mL/kg/min) | 31.7 ± 8.41 | 33. 5 ± 7.8 | 29.2 ± 8.4 |
| VO2 (L/min) | 1.34 ± 0.56 | 1.56 ± 0.53 | 0.96 ± 0.38 |
| VO2max% | 76 ± 18.4 | 80 ± 18 | 70 ± 18 |
| Maximum work (W) | 114 ± 48.6 | 128.1 ± 50 | 84.3 ± 29 |
| Oxygen pulse (mL/beat) | 7.4 ± 3 | 8.58 ± 2.9 | 5.63 ± 2.1 |
| RER | 1.14 ± 0.13 | 1.22 ± 0.9 | 1.02 ± 0.07 |
| VE/VCO2 at anaerobic threshold | 39 ± 6.9 | 37.8 ± 6.61 | 42.3 ± 6.73 |
| VE/VCO2 slope | 37.9 ± 8.23 | 35.7 ± 6 | 41.6 ± 10.1 |
| Measured anaerobic threshold | 89 (68%) | 65 (86%) | 24 (48%) |
RER, Respiratory exchange ratio.
Data are expressed as mean ± SD, number (percentage), or median (interquartile range).
Not assessed in six subjects.
Not assessed in one subject.
Table 3.
Comparison of TAPSE measurements with normal published values
| Patients with TOF | Normal controls20 | ||||||
|---|---|---|---|---|---|---|---|
| Age (y) | n | Mean | SD | n | Mean | SD | P |
| 8 | 15 | 1.45 | 0.2720 | 23 | 1.97 | 0.1525 | <.0001 |
| 9 | 17 | 1.48 | 0.3456 | 20 | 2.01 | 0.1425 | <.0001 |
| 10 | 20 | 1.51 | 0.3518 | 27 | 2.05 | 0.13 | <.0001 |
| 11 | 8 | 1.79 | 0.4137 | 25 | 2.1 | 0.1325 | <.0001 |
| 12 | 10 | 1.45 | 0.2424 | 18 | 2.14 | 0.1475 | <.0001 |
| 13 | 15 | 1.58 | 0.2554 | 20 | 2.2 | 0.1725 | <.0001 |
| 14 | 9 | 1.60 | 0.4491 | 35 | 2.26 | 0.195 | <.0001 |
| 15 | 9 | 1.74 | 0.3479 | 25 | 2.33 | 0.205 | <.0001 |
| 16 | 11 | 1.72 | 0.5198 | 34 | 2.39 | 0.2 | <.0001 |
| 17 | 12 | 1.93 | 0.4984 | 27 | 2.45 | 0.21 | <.0001 |
| 18 | 7 | 1.62 | 0.1502 | 21 | 2.47 | 0.215 | <.0001 |
On EST, the overall cohort had decreased aerobic performance (mean VO2max%, 76 ± 18.4%). Seventy-three subjects (58%) achieved maximum effort. In those, the peak VO2 was 33.7 ± 7.9 mL/kg/min (VO2max%, 80 ± 17%) compared with 29.3 ± 8.4 mL/kg/min (VO2max%, 70 ± 18%) in subjects who achieved submaximal EST (P < .001).
Comparisons among TAPSE, CMR, and EST
TAPSE measurements showed excellent intrareader reliability and interreader reliability (intrareader 1 ICC = 0.85 [95% CI, 0.68–1]; intrareader 2 ICC = 0.66 [95% CI, 0.27–1]; interreader ICC = 0.78 [95% CI, 0.52–1]). In addition, we found excellent agreement between 2D and M-mode TAPSE measured in a sample of 20 patients without congenital heart disease (ICC = 0.90, P < .001), confirming that measurements by these two different methods are essentially identical.
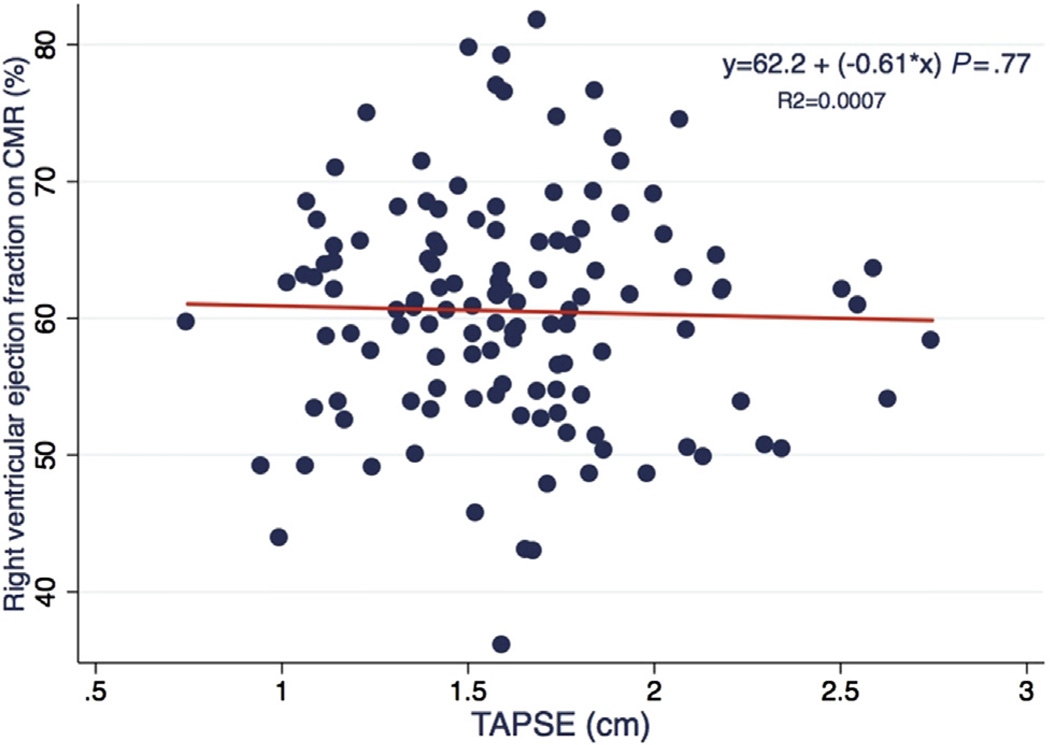
TAPSE was associated with stroke volume after adjustment for sex and BSA (β = 13.8; 95% CI, 2.25–25.30; P = .02; Table 4). There also appeared to be direct associations between TAPSE and RVEDV and RVESV. There was no significant association between TAPSE and RVEF (Figure 3). Similarly, subanalysis limited to patients with pulmonary valve stenosis demonstrated no association of TAPSE and RVEF (β = −0.78, P = .72).
Table 4.
Associations among TAPSE, CMR, and EST adjusted for sex and BSA
| Dependent variable | Parameter estimate (β) | SE | P |
|---|---|---|---|
| CMR | |||
| RVEF | −0.61 | 2.1 | .77 |
| RV stroke volume | 13.8 | 5.82 | .02 |
| RV cardiac output | 0.31 | 0.46 | .51 |
| RVEDV | 25.7 | 10.3 | .01 |
| RVESV | 11.93 | 6.15 | .055 |
| RV mass | 2.91 | 8.17 | .72 |
| Pulmonary regurgitation | 7.72 | 4.2 | .07 |
| EST* | |||
| Peak VO2 | 2.2 | 2.0 | .28 |
| Percentage predicted VO2† | 8.15 | 4.96 | .10 |
| Oxygen pulse | 0.72 | 0.48 | .13 |
| Maximum work | 11.2 | 8.9 | .21 |
| VE/VCO2 slope | −1.14 | 1.69 | .50 |
Figure 3.
Scatterplot with linear regression line demonstrating the relationship of TAPSE and RVEF.
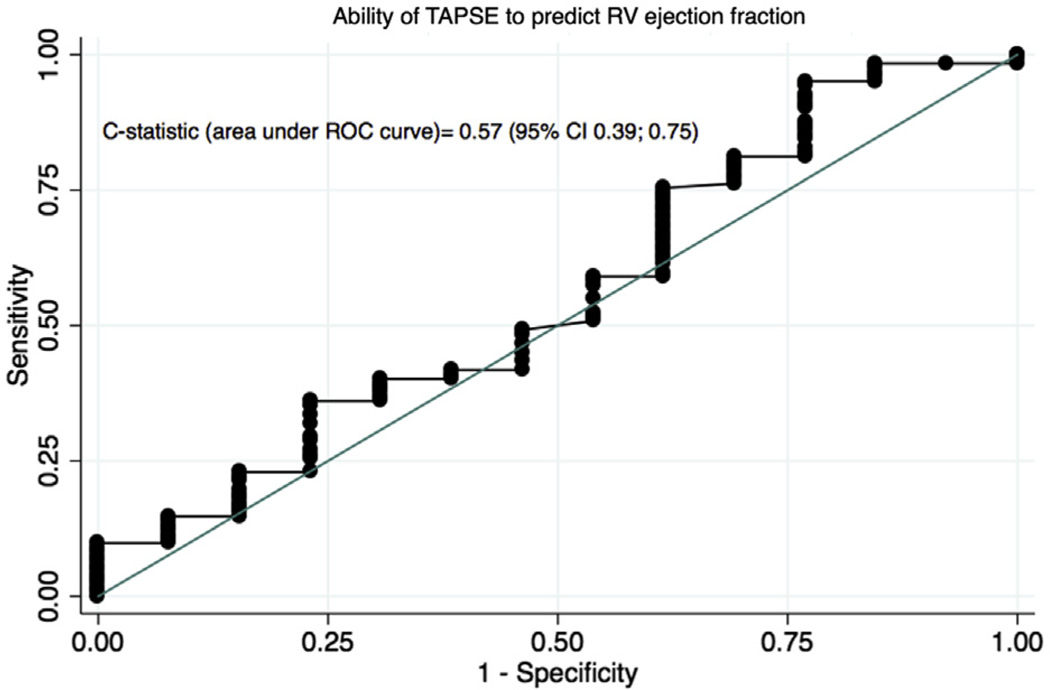
The ability of TAPSE to discriminate those with systolic dysfunction (RVEF < 50%) from those with preserved RV function was no better than chance (c-statistic = 0.57; 95% CI, 0.39–0.75; Figure 4). Using a TAPSE value of 1.6 (the median in our study population), TAPSE had only 49% sensitivity and 54% specificity to detect abnormal RV function on CMR. There was no association among TAPSE, RV cardiac output, and RV mass after adjustment for sex and BSA.
Figure 4.
Receiver operating characteristic (ROC) curve displaying the ability of TAPSE to identify decreased RVEF (<50%) on CMR. C-statistic = 0.57 (95% CI, 0.39–0.75).
TAPSE was not associated with any measures of exercise performance (peak VO2, VO2max%, oxygen pulse, or VE/VCO2 slope) after adjustment for sex and BSA.
DISCUSSION
We have shown that TAPSE may be reproducibly measured from echocardiography in patients with TOF offline using 2D imaging. Higher TAPSE was associated with greater RV stroke volume on CMR after adjustment for sex and body size. There also appeared to be direct associations between TAPSE and RVEDV and RVESV. TAPSE was not associated with EF or any other CMR parameters. TAPSE did not predict maximal exercise performance in patients operated for TOF.
RVEF assessed by CMR is considered the gold standard measure of RV systolic function.21–23 RVEF and RV volumes from CMR are particularly useful in the follow-up of patients with TOF to assist in determining the timing for pulmonary valve replacement.23–25 Although RVEF by CMR offers a more robust measure of function than most echocardiographic indices, ejection fraction is load dependent and does not offer a measure of contractility. In addition, CMR is limited by cost, inconvenience, and potential requirement for sedation. Therefore, echocardiographic indices that predict RV function by CMR would be clinically useful.
Prior studies in normal and diseased hearts have demonstrated the reproducibility of TAPSE with high intrareader and interreader agreements, including recently in patients with TOF.8,9,12,26,27 We have confirmed the reliability of the measurement of TAPSE in TOF, but we failed to demonstrate an association of TAPSE and RVEF on CMR. These findings are in contrast to those of some studies but in agreement with the results of others.11,12,27 Van der Zwaan et al.27 studied patients with heart failure and affected right and/or left ventricles and found a modest correlation of TAPSE with RVEF on CMR (correlation coefficient = 0.4). In TOF, Koestenberger et al.12 found a modest correlation (correlation coefficient = 0.47) between TAPSE and RVEF measured on CMR. Their study included patients with a wide age range, so it is possible that they examined a different population with TOF. However, a smaller study of TOF in an age group comparable with ours also found that TAPSE failed to predict RVEF on CMR.11 In agreement with this smaller study, our data suggest that TAPSE is not discriminative, sensitive, or specific for identifying patients with TOF with reduced RVEFs.
There are some possible explanations for why TAPSE performed less well in our population with TOF than in other patients with pulmonary hypertension or congestive heart failure.8,9,28 The right ventricle in TOF poses a particular challenge in the assessment of systolic function because of its native complex shape and the fact that patients with TOF usually have RV outflow tract patches that can be hypocontractile. Although TAPSE measures the longitudinal motion of the RV body, ejection fraction calculated on CMR combines the RV body and outflow tract. Therefore, TAPSE may not represent global RV systolic function in patients with dysfunctional RV outflow tracts. Prior studies have shown that in the normal heart, RV infundibular and outflow tract contraction contributes only a minor degree (~13%) to the overall stroke volume generated by the right ventricle, so the RVEF is preserved in this young population, although TAPSE values are abnormal.29,30 To corroborate this hypothesis, a recent study by Puranik et al.31 found similar TAPSE (measured on CMR) in patients with TOF compared with those with dilated right ventricles from pulmonary regurgitation after pulmonary valvuloplasty. In patients without congenital heart disease or in those with congenital heart disease but normal RV outflow tracts, the contractility of the RV outflow tract may reflect RV function. However, such methods may be less useful in TOF given the dysfunctional outflow tract seen in this setting.32
Patients with TOF have diminished regional longitudinal deformation properties of the RV free wall.14,33–35 It is possible that wall motion abnormalities (present in most patients in our study) could have contributed to the lack of association between TAPSE and RVEF and could explain abnormal TAPSE values.
In our study, the mean RVEF was normal at approximately 60%, yet TAPSE was lower than that of children without congenital heart disease.20 In adults, TAPSE is consistently reported at >2.0 cm.7,36,37 These findings suggest that there is a difference in the RV contractile pattern of patients with TOF compared with non–congenital heart disease states, such as acquired valvular heart disease, cardiomyopathy, and pulmonary hypertension. Therefore, longitudinal contraction may not be the principal determinant of RVEF in patients with certain types of RV disease. Pettersen et al.10 demonstrated predominant circumferential over longitudinal free wall shortening in systemic right ventricles after the Senning operation for patients with D-transposition of the great arteries. Even though the right ventricles in patients with TOF (supporting the pulmonary circulation) and in those with D-transposition of the great arteries (supporting the systemic circulation) are pathophysiologically different, it is possible that the contractile pattern changes in certain disease states, including TOF. A recent study in patients with pulmonary hypertension also showed a better correlation between transverse wall motion and baseline RVEF than longitudinal wall motion.38
We found borderline associations of TAPSE with RV volumes. Although higher TAPSE tracked with greater RVEDV, RVESV, and RV stroke volume, residual confounding by body size could explain these findings, and the association of TAPSE with RV stroke volume should be interpreted with caution.
We found decreased exercise performance in our study patients overall. This is in keeping with other studies demonstrating that patients with TOF have exercise intolerance over time, with decreased peak VO2 compared with healthy subjects.39–44 Because TAPSE was not an adequate indicator of RV systolic function in TOF (and RV function is an important determinant of exercise performance), it is not surprising that TAPSE did not track with measures of exercise performance, including VE/VCO2, as it does in other cardiac diseases.45
Limitations
There were certain limitations to this study. We performed retrospective, offline measurements of TAPSE from 2D images on echocardiograms, and M-mode measurements were not possible. We applied a different technique from the standard 2D and M-mode methods. However, we found good agreement between readers, supporting at least the reliability of this technique. Moreover, we found a high correlation between 2D and M-mode measurements in a set of echocardiograms without congenital heart disease, supporting the use of this technique for offline TAPSE measurements when M-mode is not available. We also highlight that the technique used in this study must be taken into consideration when interpreting differences from normal values, acquired using standard methodology.
Our study population constitutes one of the largest cohorts of children and teenagers with TOF that has ever been studied with concurrent echocardiography, EST, and CMR, and this study was one of the first to examine the association of TAPSE with measures of exercise performance. Although it is possible that potentially “sicker” patients were not included in this sample if they had hardware (such as pulmonary artery stents) that precluded their undergoing CMR testing, we found that excluded patients were comparable with the study sample in terms of demographics and anthropometrics, making selection bias less likely. Because patients included in the study were not limited by original pulmonary valve anatomy, type of surgical repair, genetic status, place of follow-up, gender, race, or age at surgical repair, generalizability is a particular strength of this study.
A prolonged time interval between tests and resultant biologic variability could have biased our results toward the null hypothesis, such that there could have been associations we did not detect. However, tests were performed within a maximum of 3 months to avoid this type of bias. In addition, in the clinical setting, methods for the evaluation of RV function are typically performed non-simultaneously. Moreover, we believe that pulmonary regurgitation and ejection fraction are not factors that have major variability in stable patients with TOF.
Measurement error in CMR parameters could have occurred, biasing our results toward or away from the null; nonetheless, CMR studies were reviewed by a single experienced reader who was blinded to other test results, making systematic measurement error less likely.
Most subjects in the study had normal RVEFs, but that should not affect the ability of TAPSE to detect RV dysfunction. A greater number of patients with decreased RVEF would lead to greater precision of the c-statistic but would be unlikely to change the study results.
We were not able to compare TAPSE and RVEF according to regional wall dysfunction, because the number of patients without regional dysfunction was too small to allow a meaningful comparison. Likewise, we did not study the effect of tricuspid regurgitation, because it was present only in a small number of patients.
CONCLUSIONS
We demonstrated that TAPSE is a reliable measure by echocardiography in patients with TOF. TAPSE was not associated with RVEF or other CMR parameters. In addition, TAPSE was not associated with exercise performance. These findings suggest that longitudinal shortening is not the dominant contributor to global RV systolic function in TOF. In addition, TAPSE is not representative of global RV performance in TOF and may not be useful as a single measure of RV function in this patient population. Future studies should explore other novel measures of RV function in TOF.
Acknowledgments
This work was in part supported by grants P50-HL74731 and K24-HL103844 from the National Institutes of Health (Bethesda, MD).
Abbreviations
- BSA
Body surface area
- CI
Confidence interval
- CMR
Cardiac magnetic resonance
- EST
Exercise stress testing
- ICC
Intraclass correlation coefficient
- RV
Right ventricular
- RVEDV
Right ventricular end-diastolic volume
- RVEF
Right ventricular ejection fraction
- RVESV
Right ventricular end-systolic volume
- TAPSE
Tricuspid annular plane systolic excursion
- TOF
Tetralogy of Fallot
- 2D
Two-dimensional
- VCO2
Carbon dioxide production
- VE/VCO2
Ventilatory equivalent of carbon dioxide
- VO2
Oxygen consumption
- VO2max%
Predicted percentage of maximum oxygen consumption
REFERENCES
- 1.Bouzas B, Kilner PJ, Gatzoulis MA. Pulmonary regurgitation: not a benign lesion. Eur Heart J. 2005;26:433–439. doi: 10.1093/eurheartj/ehi091. [DOI] [PubMed] [Google Scholar]
- 2.Redington AN. Determinants and assessment of pulmonary regurgitation in tetralogy of Fallot: practice and pitfalls. Cardiol Clin. 2006;24:631–639. doi: 10.1016/j.ccl.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107:526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson M, Ugander M, Heiberg E, Arheden H. The quantitative relationship between longitudinal and radial function in left, right, and total heart pumping in humans. AmJ Physiol Heart Circ Physiol. 2007;293:H636–H644. doi: 10.1152/ajpheart.01376.2006. [DOI] [PubMed] [Google Scholar]
- 6.Saxena N, Rajagopalan N, Edelman K, Lopez-Candales A. Tricuspid annular systolic velocity: a useful measurement in determining right ventricular systolic function regardless of pulmonary artery pressures. Echocardiography. 2006;23:750–755. doi: 10.1111/j.1540-8175.2006.00305.x. [DOI] [PubMed] [Google Scholar]
- 7.Brown SB, Raina A, Katz D, Szerlip M, Wiegers SE, Forfia PR. Longitudinal shortening accounts for the majority of right ventricular contraction and improves after pulmonary vasodilator therapy in normal subjects and patients with pulmonary arterial hypertension. Chest. 2011;140:27–33. doi: 10.1378/chest.10-1136. [DOI] [PubMed] [Google Scholar]
- 8.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 9.Ghio S, Recusani F, Klersy C, Sebastiani R, Laudisa ML, Campana C, et al. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 2000;85:837–842. doi: 10.1016/s0002-9149(99)00877-2. [DOI] [PubMed] [Google Scholar]
- 10.Pettersen E, Helle-Valle T, Edvardsen T, Lindberg H, Smith HJ, Smevik B, et al. Contraction pattern of the systemic right ventricle shift from longitudinal to circumferential shortening and absent global ventricular torsion. J Am Coll Cardiol. 2007;49:2450–2456. doi: 10.1016/j.jacc.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 11.Bonnemains L, Stos B, Vaugrenard T, Marie PY, Odille F, Boudjemline Y. Echocardiographic right ventricle longitudinal contraction indices cannot predict ejection fraction in post-operative Fallot children. Eur J Echocardiogr. 2012;13:235–242. doi: 10.1093/ejechocard/jer263. [DOI] [PubMed] [Google Scholar]
- 12.Koestenberger M, Nagel B, Ravekes W, Everett AD, Stueger HP, Heinzl B, et al. Systolic right ventricular function in pediatric and adolescent patients with tetralogy of Fallot: echocardiography versus magnetic resonance imaging. J Am Soc Echocardiogr. 2011;24:45–52. doi: 10.1016/j.echo.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Koestenberger M, Nagel B, Ravekes W, Everett AD, Stueger HP, Heinzl B, et al. Tricuspid annular plane systolic excursion and right ventricular ejection fraction in pediatric and adolescent patients with tetralogy of Fallot, patients with atrial septal defect, and age-matched normal subjects. Clin Res Cardiol. 2011;100:67–75. doi: 10.1007/s00392-010-0213-z. [DOI] [PubMed] [Google Scholar]
- 14.Morcos P, Vick GW, III, Sahn DJ, Jerosch-Herold M, Shurman A, Sheehan FH. Correlation of right ventricular ejection fraction and tricuspid annular plane systolic excursion in tetralogy of Fallot by magnetic resonance imaging. Int J Cardiovasc Imaging. 2009;25:263–270. doi: 10.1007/s10554-008-9387-0. [DOI] [PubMed] [Google Scholar]
- 15.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Sarikouch S, Peters B, Gutberlet M, Leismann B, Kelter-Kloepping A, Koerperich H, et al. Sex-specific pediatric percentiles for ventricular size and mass as reference values for cardiac MRI: assessment by steady-state free-precession and phase-contrast MRI flow. Circ Cardiovasc Imaging. 2010;3:65–76. doi: 10.1161/CIRCIMAGING.109.859074. [DOI] [PubMed] [Google Scholar]
- 17.Robbers-Visser D, Boersma E, Helbing WA. Normal biventricular function, volumes, and mass in children aged 8 to 17 years. J Magn Reson Imaging. 2009;29:552–559. doi: 10.1002/jmri.21662. [DOI] [PubMed] [Google Scholar]
- 18.Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol. 1984;56:628–634. doi: 10.1152/jappl.1984.56.3.628. [DOI] [PubMed] [Google Scholar]
- 19.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 20.Koestenberger M, Ravekes W, Everett AD, Stueger HP, Heinzl B, Gamillscheg A, et al. Right ventricular function in infants, children and adolescents: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of Z score values. J Am Soc Echocardiogr. 2009;22:715–719. doi: 10.1016/j.echo.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Alfakih K, Plein S, Bloomer T, Jones T, Ridgway J, Sivananthan M. Comparison of right ventricular volume measurements between axial and short axis orientation using steady-state free precession magnetic resonance imaging. J Magn Reson Imaging. 2003;18:25–32. doi: 10.1002/jmri.10329. [DOI] [PubMed] [Google Scholar]
- 22.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–329. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 23.Helbing WA, Rebergen SA, Maliepaard C, Hansen B, Ottenkamp J, Reiber JH, et al. Quantification of right ventricular function with magnetic resonance imaging in children with normal hearts and with congenital heart disease. Am Heart J. 1995;130:828–837. doi: 10.1016/0002-8703(95)90084-5. [DOI] [PubMed] [Google Scholar]
- 24.Dorfman AL, Geva T. Magnetic resonance imaging evaluation of congenital heart disease: conotruncal anomalies. J Cardiovasc Magn Reson. 2006;8:645–659. doi: 10.1080/10976640600721544. [DOI] [PubMed] [Google Scholar]
- 25.Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95:779–782. doi: 10.1016/j.amjcard.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Nunez-Gil IJ, Rubio MD, Carton AJ, Lopez-Romero P, Deiros L, Garcia-Guereta L, et al. Determination of normalized values of the tricuspid annular plane systolic excursion (TAPSE) in 405 Spanish children and adolescents. Rev Esp Cardiol. 2011;64:674–680. doi: 10.1016/j.recesp.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 27.van der Zwaan HB, Geleijnse ML, McGhie JS, Boersma E, Helbing WA, Meijboom FJ, et al. Right ventricular quantification in clinical practice: two-dimensional vs. three-dimensional echocardiography compared with cardiac magnetic resonance imaging. Eur J Echocardiogr. 2011;12:656–664. doi: 10.1093/ejechocard/jer107. [DOI] [PubMed] [Google Scholar]
- 28.Ghio S, Klersy C, Magrini G, D’Armini AM, Scelsi L, Raineri C, et al. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2010;140:272–278. doi: 10.1016/j.ijcard.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 29.Geva T, Powell AJ, Crawford EC, Chung T, Colan SD. Evaluation of regional differences in right ventricular systolic function by acoustic quantification echocardiography and cine magnetic resonance imaging. Circulation. 1998;98:339–345. doi: 10.1161/01.cir.98.4.339. [DOI] [PubMed] [Google Scholar]
- 30.Alghamdi MH, Mertens L, Lee W, Yoo SJ, Grosse-Wortmann L. Longitudinal right ventricular function is a better predictor of right ventricular contribution to exercise performance than global or outflow tract ejection fraction in tetralogy of Fallot: a combined echocardiography and Magnetic Resonance Study. Eur Heart J Cardiovasc Imaging. 2013;14:235–239. doi: 10.1093/ehjci/jes137. [DOI] [PubMed] [Google Scholar]
- 31.Puranik R, Tsang V, Lurz P, Muthurangu V, Offen S, Frigiola A, et al. Long-term importance of right ventricular outflow tract patch function in patients with pulmonary regurgitation. J Thorac Cardiovasc Surg. 2012;143:1103–1107. doi: 10.1016/j.jtcvs.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 32.Asmer I, Adawi S, Ganaeem M, Shehadeh J, Shiran A. Right ventricular outflow tract systolic excursion: a novel echocardiographic parameter of right ventricular function. Eur Heart J Cardiovasc Imaging. 2012;13:871–877. doi: 10.1093/ehjci/jes055. [DOI] [PubMed] [Google Scholar]
- 33.Weidemann F, Eyskens B, Mertens L, Dommke C, Kowalski M, Simmons L, et al. Quantification of regional right and left ventricular function by ultrasonic strain rate and strain indexes after surgical repair of tetralogy of Fallot. Am J Cardiol. 2002;90:133–138. doi: 10.1016/s0002-9149(02)02435-9. [DOI] [PubMed] [Google Scholar]
- 34.Niezen RA, Helbing WA, van der Wall EE, van der Geest RJ, Rebergen SA, de Roos A. Biventricular systolic function and mass studied with MR imaging in children with pulmonary regurgitation after repair for tetralogy of Fallot. Radiology. 1996;201:135–140. doi: 10.1148/radiology.201.1.8816534. [DOI] [PubMed] [Google Scholar]
- 35.Smith JL, Bolson EL, Wong SP, Hubka M, Sheehan FH. Three-dimensional assessment of two-dimensional technique for evaluation of right ventricular function by tricuspid annulus motion. Int J Card Imaging. 2003;19:189–197. doi: 10.1023/a:1023655705807. [DOI] [PubMed] [Google Scholar]
- 36.Hammarstrom E, Wranne B, Pinto FJ, Puryear J, Popp RL. Tricuspid annular motion. J Am Soc Echocardiogr. 1991;4:131–139. doi: 10.1016/s0894-7317(14)80524-5. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Candales A, Dohi K, Rajagopalan N, Edelman K, Gulyasy B, Bazaz R. Defining normal variables of right ventricular size and function in pulmonary hypertension: an echocardiographic study. Postgrad Med J. 2008;84:40–45. doi: 10.1136/pgmj.2007.059642. [DOI] [PubMed] [Google Scholar]
- 38.Kind T, Mauritz GJ, Marcus JT, van de Veerdonk M, Westerhof N, Vonk-Noordegraaf A. Right ventricular ejection fraction is better reflected by transverse rather than longitudinal wall motion in pulmonary hypertension. J Cardiovasc Magn Reson. 2010;12:35. doi: 10.1186/1532-429X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kipps AK, Graham DA, Harrild DM, Lewis E, Powell AJ, Rhodes J. Longitudinal exercise capacity of patients with repaired tetralogy of fallot. Am J Cardiol. 2011;108:99–105. doi: 10.1016/j.amjcard.2011.02.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahle WT, McBride MG, Paridon SM. Exercise performance in tetralogy of Fallot: the impact of primary complete repair in infancy. Pediatr Cardiol. 2002;23:224–229. doi: 10.1007/s00246-001-0054-7. [DOI] [PubMed] [Google Scholar]
- 41.Rowe SA, Zahka KG, Manolio TA, Horneffer PJ, Kidd L. Lung function and pulmonary regurgitation limit exercise capacity in postoperative tetralogy of Fallot. J Am Coll Cardiol. 1991;17:461–466. doi: 10.1016/s0735-1097(10)80116-0. [DOI] [PubMed] [Google Scholar]
- 42.Sutton NJ, Peng L, Lock JE, Lang P, Marx GR, Curran TJ, et al. Effect of pulmonary artery angioplasty on exercise function after repair of tetralogy of Fallot. Am Heart J. 2008;155:182–186. doi: 10.1016/j.ahj.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Fredriksen PM, Therrien J, Veldtman G, Ali Warsi M, Liu P, Thaulow E, et al. Aerobic capacity in adults with tetralogy of Fallot. Cardiol Young. 2002;12:554–559. doi: 10.1017/s1047951102001002. [DOI] [PubMed] [Google Scholar]
- 44.Giardini A, Specchia S, Tacy TA, Coutsoumbas G, Gargiulo G, Donti A, et al. Usefulness of cardiopulmonary exercise to predict long-term prognosis in adults with repaired tetralogy of Fallot. Am J Cardiol. 2007;99:1462–1467. doi: 10.1016/j.amjcard.2006.12.076. [DOI] [PubMed] [Google Scholar]
- 45.Methvin AB, Owens AT, Emmi AG, Allen M, Wiegers SE, Dries DL, et al. Ventilatory inefficiency reflects right ventricular dysfunction in systolic heart failure. Chest. 2011;139:617–625. doi: 10.1378/chest.10-0318. [DOI] [PubMed] [Google Scholar]
- 46.Cooper DM, Weiler-Ravell D. Gas exchange response to exercise in children. Am Rev Respir Dis. 1984;129:S47–S48. doi: 10.1164/arrd.1984.129.2P2.S47. [DOI] [PubMed] [Google Scholar]