Abstract
MRL-lpr/lpr mice develop a generalized autoimmune disease which includes increased autoantibody production, glomerulonephritis, and development of lymphadenopathy. The lpr genetic defect has been identified as a mutation in the Fas apoptosis gene that results in low expression of Fas mRNA. To determine the significance of the lpr mutation and T cells in the development of the autoimmune disease, we constructed transgenic MRL-lpr/lpr mice using a full-length murine Fas cDNA under the regulation of the T-cell-specific CD2 promoter and enhancer. Here we show that the early correction of the lpr gene defect in T cells eliminates glomerulonephritis and development of lymphadenopathy and decreases the levels of autoantibodies. In this model, early correction of the lpr defect in T cells is sufficient to eliminate the acceleration of autoimmune disease even in the presence of B cells and other cells that express the mutant lpr gene.
Full text
PDF

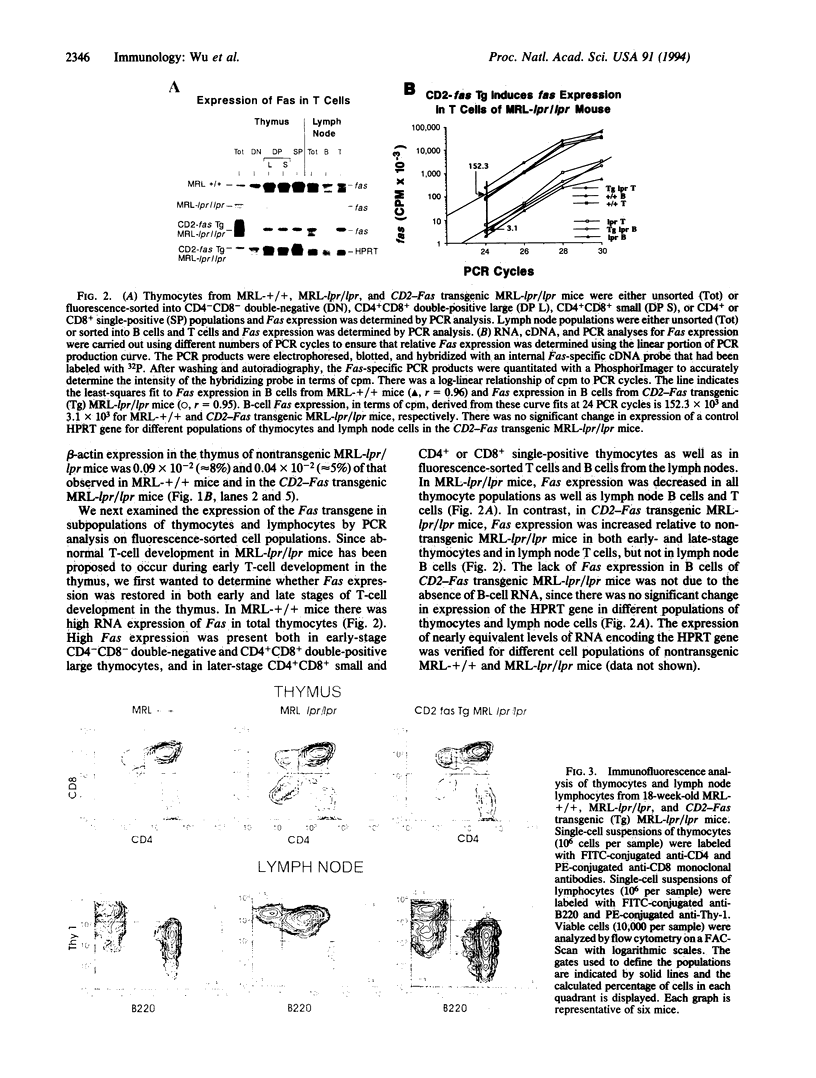
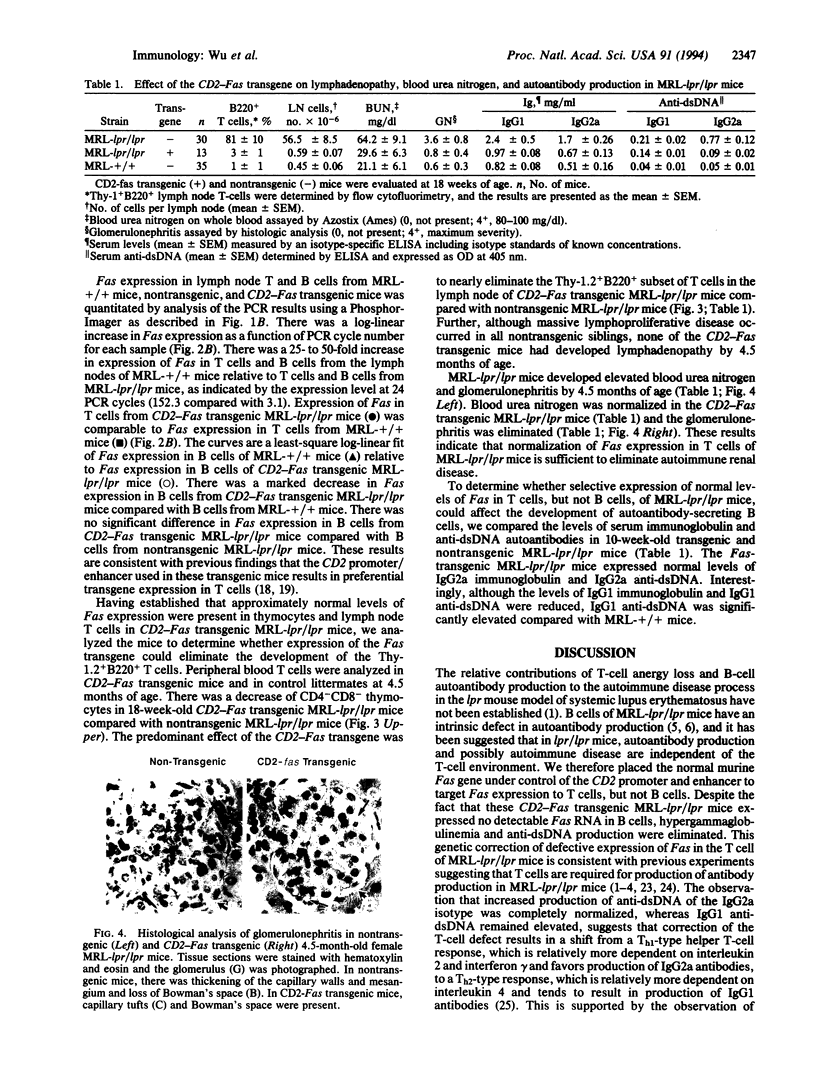

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Watanabe-Fukunaga R., Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carteron N. L., Schimenti C. L., Wofsy D. Treatment of murine lupus with F(ab')2 fragments of monoclonal antibody to L3T4. Suppression of autoimmunity does not depend on T helper cell depletion. J Immunol. 1989 Mar 1;142(5):1470–1475. [PubMed] [Google Scholar]
- Chu J. L., Drappa J., Parnassa A., Elkon K. B. The defect in Fas mRNA expression in MRL/lpr mice is associated with insertion of the retrotransposon, ETn. J Exp Med. 1993 Aug 1;178(2):723–730. doi: 10.1084/jem.178.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. L., Eisenberg R. A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Greaves D. R., Wilson F. D., Lang G., Kioussis D. Human CD2 3'-flanking sequences confer high-level, T cell-specific, position-independent gene expression in transgenic mice. Cell. 1989 Mar 24;56(6):979–986. doi: 10.1016/0092-8674(89)90631-4. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Kotzin B. L., Babcock S. K., Herron L. R. Deletion of potentially self-reactive T cell receptor specificities in L3T4-, Lyt-2- T cells of lpr mice. J Exp Med. 1988 Dec 1;168(6):2221–2229. doi: 10.1084/jem.168.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang G., Mamalaki C., Greenberg D., Yannoutsos N., Kioussis D. Deletion analysis of the human CD2 gene locus control region in transgenic mice. Nucleic Acids Res. 1991 Nov 11;19(21):5851–5856. doi: 10.1093/nar/19.21.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Yoshikai Y., Asano T., Himeno K., Iwasaki A., Nomoto K. Defect in negative selection in lpr donor-derived T cells differentiating in non-lpr host thymus. J Exp Med. 1991 Jan 1;173(1):127–136. doi: 10.1084/jem.173.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountz J. D., Smith H. R., Wilder R. L., Reeves J. P., Steinberg A. D. CS-A therapy in MRL-lpr/lpr mice: amelioration of immunopathology despite autoantibody production. J Immunol. 1987 Jan 1;138(1):157–163. [PubMed] [Google Scholar]
- Mountz J. D., Smith T. M., Toth K. S. Altered expression of self-reactive T cell receptor V beta regions in autoimmune mice. J Immunol. 1990 Mar 15;144(6):2159–2166. [PubMed] [Google Scholar]
- Mountz J. D., Zhou T., Eldridge J., Berry K., Blüthmann H. Transgenic rearranged T cell receptor gene inhibits lymphadenopathy and accumulation of CD4-CD8-B220+ T cells in lpr/lpr mice. J Exp Med. 1990 Dec 1;172(6):1805–1817. doi: 10.1084/jem.172.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountz J. D., Zhou T., Johnson L. Production of transgenic mice and application to immunology and autoimmunity. Am J Med Sci. 1990 Nov;300(5):322–329. doi: 10.1097/00000441-199011000-00009. [DOI] [PubMed] [Google Scholar]
- Ogasawara J., Watanabe-Fukunaga R., Adachi M., Matsuzawa A., Kasugai T., Kitamura Y., Itoh N., Suda T., Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993 Aug 26;364(6440):806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Perkins D. L., Glaser R. M., Mahon C. A., Michaelson J., Marshak-Rothstein A. Evidence for an intrinsic B cell defect in lpr/lpr mice apparent in neonatal chimeras. J Immunol. 1990 Jul 15;145(2):549–555. [PubMed] [Google Scholar]
- Russell J. H., Rush B., Weaver C., Wang R. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4409–4413. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen J., Rosenberg N., Burakoff S. J. Expression and ontogeny of CD2 on murine B cells. J Immunol. 1990 Apr 15;144(8):2925–2930. [PubMed] [Google Scholar]
- Singer P. A., Balderas R. S., McEvilly R. J., Bobardt M., Theofilopoulos A. N. Tolerance-related V beta clonal deletions in normal CD4-8-, TCR-alpha/beta + and abnormal lpr and gld cell populations. J Exp Med. 1989 Dec 1;170(6):1869–1877. doi: 10.1084/jem.170.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel E. S., Cohen P. L., Eisenberg R. A. lpr T cells are necessary for autoantibody production in lpr mice. J Immunol. 1993 May 1;150(9):4160–4167. [PubMed] [Google Scholar]
- Sobel E. S., Katagiri T., Katagiri K., Morris S. C., Cohen P. L., Eisenberg R. A. An intrinsic B cell defect is required for the production of autoantibodies in the lpr model of murine systemic autoimmunity. J Exp Med. 1991 Jun 1;173(6):1441–1449. doi: 10.1084/jem.173.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg A. D., Roths J. B., Murphy E. D., Steinberg R. T., Raveche E. S. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J Immunol. 1980 Aug;125(2):871–873. [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Balderas R. S., Shawler D. L., Lee S., Dixon F. J. Influence of thymic genotype on the systemic lupus erythematosus-like disease and T cell proliferation of MRL/Mp-lpr/lpr mice. J Exp Med. 1981 Jun 1;153(6):1405–1414. doi: 10.1084/jem.153.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadwell E. L., Cohen P., Williams D., O'Brien K., Volkman A., Eisenberg R. MRL mice produce anti-Su autoantibody, a specificity associated with systemic lupus erythematosus. J Immunol. 1993 Jan 15;150(2):695–699. [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Itoh N., Yonehara S., Copeland N. G., Jenkins N. A., Nagata S. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol. 1992 Feb 15;148(4):1274–1279. [PubMed] [Google Scholar]
- Watson M. L., Rao J. K., Gilkeson G. S., Ruiz P., Eicher E. M., Pisetsky D. S., Matsuzawa A., Rochelle J. M., Seldin M. F. Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J Exp Med. 1992 Dec 1;176(6):1645–1656. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Zhou T., He J., Mountz J. D. Autoimmune disease in mice due to integration of an endogenous retrovirus in an apoptosis gene. J Exp Med. 1993 Aug 1;178(2):461–468. doi: 10.1084/jem.178.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita H., Nakamura T., Karasuyama H., Okumura K. Monoclonal antibodies specific for murine CD2 reveal its presence on B as well as T cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):645–649. doi: 10.1073/pnas.86.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Bluethmann H., Eldridge J., Berry K., Mountz J. D. Origin of CD4-CD8-B220+ T cells in MRL-lpr/lpr mice. Clues from a T cell receptor beta transgenic mouse. J Immunol. 1993 Apr 15;150(8 Pt 1):3651–3667. [PubMed] [Google Scholar]
- Zhou T., Bluethmann H., Eldridge J., Brockhaus M., Berry K., Mountz J. D. Abnormal thymocyte development and production of autoreactive T cells in T cell receptor transgenic autoimmune mice. J Immunol. 1991 Jul 15;147(2):466–474. [PubMed] [Google Scholar]
- Zhou T., Bluethmann H., Zhang J., Edwards C. K., 3rd, Mountz J. D. Defective maintenance of T cell tolerance to a superantigen in MRL-lpr/lpr mice. J Exp Med. 1992 Oct 1;176(4):1063–1072. doi: 10.1084/jem.176.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]