Abstract
Background
Chorea associated with high titers of antiphospholipid antibodies in the absence of antiphospholipid antibody syndrome has been seldom reported.
Case report
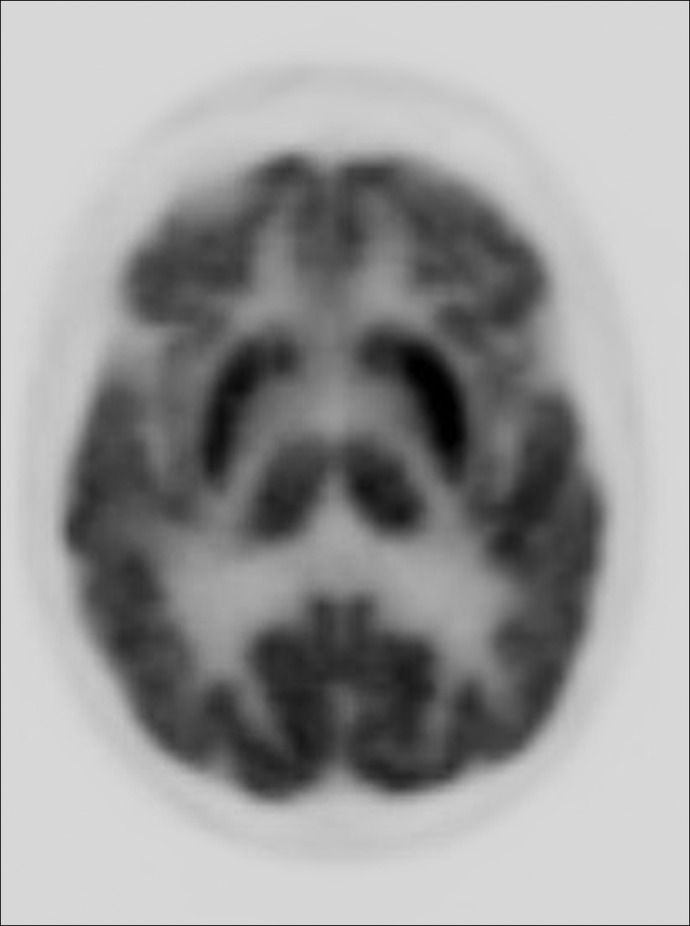
An 89-year-old female developed persistent right side chorea associated with high titers of anticardiolipin antibody (antiphospholipid antibosies immunoglobulin (Ig)M, 45 MPL and 112 IgM aCL (MPL) after 3 months) but normal lupus anticoagulants. Her magnetic resonance imaging (MRI) showed no abnormality, but positron emission tomography (PET) demonstrated increased bilateral striatal metabolic activity, more on the left side. Her MRI showed no cause for chorea. The PET scan demonstrated a marked increase in the metabolic activity of the left basal ganglia.
Discussion
Her chorea remained unchanged over a 9-month follow-up period. The literature on chorea associated with high titers of antiphospholipid antibodies in the absence of antiphospholipid syndrome is reviewed.
Keywords: Chorea, antiphospholipid antibody, antiphospholipid syndrome, anticardiolipin antibody
Introduction
Chorea is a movement disorder characterized by involuntary, irregular, non-stereotyped movements of the limbs, trunk, neck, and face parts that can vary in frequency.1 Chorea can be hereditary or non-hereditary. Among the hereditary choreas, Huntington's disease is the most common; other less common hereditary choreas include chorea due to neuroacanthocytosis, Wilson's disease, paroxysmal choreoathetosis, spinocerebellar ataxias, McLeod syndrome, Glucose transporter-1 deficiency syndrome (GLUT1) deficiency, dentatorubropallidoluysian atrophy, Fahr's disease, and Lesch–Nyhan syndrome. Non-hereditary choreas are most commonly caused by drugs and toxins,2 followed by autoimmune disorders such as systemic lupus erythematosus (SLE), Sjögren's syndrome, antiphospholipid antibody syndrome (APS), Sydenham's chorea, chorea gravidarum, vascular and metabolic choreas,1,3,4 and paraneoplastic disorders.5
Low titers of antiphospholipid (aPL) antibodies have been reported in 2–9% of the normal population.6,7 Higher titers can be seen in people affected by certain viral or bacterial infections such as mycoplasma, chlamydia, HIV, Lyme disease, and hepatitis C.8–10 Exposure to certain drugs such as hydralazine and procainamide is another cause of increased aPL titers.11 APS is a hypercoagulable state characterized by the presence of at least one of the following clinical criteria of vascular thrombosis or pregnancy morbidity, in addition to the presence of one of the following antibodies: lupus anticoagulant (LAC or LA), anticardiolipin (aCL), or anti-β2 glycoprotein-I antibody (aβ2GPI).12 Chorea is reported in 4% of the patients with lupus erythematosus 13,14 and can be the first manifestation of lupus in children 15–17 or even in late-onset SLE.18 The aCL antibody is the most frequently detected type of aPL antibodies in SLE, while LAC is the most frequent in SLE patients with chorea.16,19 In one study of 32 lupus patients with chorea, 27 patients (84%) had LAC, 19 (52%) had aCL, and 11 (34%) had aβ2GPI.19
It is the current belief that chorea associated with aPL antibodies occurs in the setting of fully developed APS. In recent years, however, there have been reports of chorea in patients with high titers of aPL antibodies in the absence of autoimmune disease or clinical APS.20,21 In this communication, we report another patient with chorea and high titers of aPL antibodies who does not fulfill the criteria for APS. The literature on aPL antibody titers, pathophysiology, neuroimaging, and treatment is also reviewed.
Case report
An 89- year-old female visited the Yale Movement Disorder clinic on August 2014 for evaluation of involuntary movements of the right side. She first noted subtle involuntary movements of her right hand and right foot in May 2014. The movements gradually increased in intensity but reached a plateau approximately 3 weeks prior to her clinic visit. A magnetic resonance imaging scan performed within a month after the onset of chorea showed mild bilateral microangiopathy in the white matter consistent with her age.
Her past medical history revealed resection of benign polyps in the colon 11 years earlier and a resection of an early stage skin melanoma 9 years previously without recurrence. She had two miscarriages during her youth. Her family history was negative for any neurological disorder or any medical disorder known to cause neurological problems. She did not drink, smoke, or use drugs.
On neurological examination she demonstrated excellent cognition for her age. The cranial nerves were intact. Speech was normal. There were continuous choreiform movements affecting the right upper and lower limbs, which were more prominent distally (Video 1). The face was spared. Subtle chorea was noted intermittently in the left foot. Her muscle strength was 5/5 and normal. There was no hypokinesia or rigidity. Deep tendon reflexes were 2+ and symmetrical. All modalities of sensation were normal. There were no cerebellar findings. Her gait was normal. An extensive blood works including assessment of hepatic and renal functions was normal. She had a normal antinuclear antibody and slightly increased sedimentation rate (28 mm/hour). The aPL antibody assessment revealed abnormal values: aCL IgM: 45 MPL (normal <12 MPL), phosphatidylserine antibody IgM of 54 MPL (normal <25 MPL), β2 glycoprotein IgM was 34 SMU (standard IgM units) (normal <20 SMU). LACs were absent. Repeat magnetic resonance imaging (MRI) was unchanged from the first study. A deoxyglucose positron emission tomography (PET) scan showed markedly increased metabolic activity in the putamen and caudate nuclei bilaterally, more prominent on the left side, contralateral to the patient's chorea (Figure 1). She was treated with aspirin 81 mg/daily. An examination 3 months later showed no change in the pattern and intensity of chorea. The repeat aCL IgM value was 112 and β2 glycoprotein IgM was 36.
Figure 1. Deoxyglucose positron emission tomography shows bilaterally increased metabolic activity (putamen and caudate), more prominent on the left side.
Video 1.
Choreiform movements involving both upper and lower limb, predominantly on the right side.
Discussion
A review of the literature on the subject of chorea associated with a high titer of aPL antibodies without APS (pre-APS syndrome) revealed six previous publications: five single case reports and a small series of 10 patients (Table 1). Age of the reported patients varied from 5 to 21 years. Initial assessment of antibodies revealed an increased titer of aCL antibodies and absent LAC in all 15 patients. The majority of the patients, in addition to chorea, had other central nervous system findings such as seizures, dystonia, and dementia. All patients had normal magnetic resonance imaging. A repeat aPL antibody test demonstrating the persistently elevated titers was documented in only a few patients. PET was reported in one patient with unilateral chorea and showed increased metabolic activity over the contralateral basal ganglia. A variety of medications were tried in patients with moderate to severe chorea (Table 1). Follow-up evaluations beyond 6 months were reported in only three patients.
Table 1. Reported Cases of Chorea with Increased Antiphospholipid Antibody and Normal Lupus Anticoagulant Titers in Absence of Antiphospholipid Antibody Syndrome.
| Reference | N | Age (years) | Chorea Description | Other Symptoms | aPL Antibodies | MRI Findings | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Kiechl-Kohlendorfer et al.29 | 1 | 5 | Isolated left-side hemichorea | No | LAC – | Normal | None | Chorea improved, aCL normalized |
| aCL IgM 28 | ||||||||
| aCL IgG 18 | ||||||||
| Usugi et al.30 | 1 | 6 | Chorea started at age 4 months. At age 3 years, continuous bilateral arrhythmic rapid purposeless jerks of the arms and legs were noted | Myoclonic seizures developed at age 2 years | aCL (IgG) aCL +(99) | Infarction in left MCA distribution at 3 months. Atrophy of basal ganglia, at age 3 years and additional atrophy of cerebellum at after 5 years | Methylprednisolone sodium succinate (10 mg/kg) for 3 days caused temporary improvement | Chorea and general condition gradually worsened |
| IgM –, LAC – | ||||||||
| IgG level was decreased to 17 at age 6 years | ||||||||
| Biernacka-Zielinska et al.31 | 1 | 16 | Left upper limb chorea | ? | Marked increase in aCL and aβ2GPI | Not reported | Acetylsalicylic acid (75 mg/day) and risperidone | Chorea disappeared after 9 months |
| LAC first absent but present a year later | ||||||||
| Shimomura et al.32 | 1 | 21 | Onset at age 17, in the left hand, progressed to both hands, mouth/tongue | Unsteady gait and wild gyrations of the arms on walking | LAC – | MRI and CT NL | Not reported | Not reported |
| aCL + | ||||||||
| Sundén-Cullberg et al.33 | 1 | 20 | Chorea started in right side of the body and face, later evolved into generalized chorea | Inability to eat and dress and dramatically impaired speech | LAC – | MRI NL | Pimozide and then after DC of pimozide, IV methylprednisolone | Deterioration after pimozide discontinued. Chorea ceased after IV methylprednisolone |
| aCL + (>100) | ||||||||
| ANA – | PET scan increased metabolism bilaterally more prominent in the lentiform nucleus and caudate and more on the left side. Repeat PET scan was normal after chorea subsided. | |||||||
| ANCA – | ||||||||
| Orzechowski et al.34 | 10 | N/A | Mild six, moderate two, severe two | One generalized ataxia + seizures | 10 patients hadLAC – and aCL + | MRI was normal in all patients | Four patients received tetrabenazine-carbidopa/levodopa,respiridone-fluphenazine/quitiapen | Partial response in seven and a complete response in three |
| Bilateral six | One epilepsy | |||||||
| Four patients: more than one episode | Two dysarthria + seizures | 71% of the non-APS patients had positive IgM aCL. | ||||||
| One dementia | IgM aCL in all patients with definite APS: all – | Three received warfarin/heparin and four treated with aspirin. Three had immunosuppression | ||||||
| Two dystonia |
Abbreviations: aβ2GPI, anti-β2 glycoprotein-I antibody; aCL, Anticardiolipin; ANA, Antinuclear Antibody; ANCA, Antineutrophilic Cytoplasmic Antibodies; aPL, Antiphospholipid; Ig, Immunoglobulin; IV, Intravenous; LAC, Lupus Anticoagulant; MRI, Magnetic Resonance Imaging; NL, normal; PET, Positron Emission Tomography. DC, Discontinuation.
Our patient was considerably older than previously reported patients with pre-APS syndrome, but primary APS has been reported in elderly females with an age ranging from 65 to 79 years.14,22,23 Moderately raised titers of aPL have been reported in elderly patients with chorea and drug exposure, and immune and infectious disorders.20 Our patient was in excellent health and did not have history of drug exposure or infection. She did not meet the criteria for APS due to absence of associated clinical conditions. Her miscarriages occurred almost 60 years ago. Her repeat aPL antibodies 3 months later showed further elevation of the titers. Her MRI showed no findings to explain the right-sided chorea. Although Huntington's disease is a consideration at any age, her excellent cognition, lack of family history and pattern of her chorea (predominantly unilateral over a follow up period of 9 months and sparing the face and tongue) made this diagnosis very unlikely.
At least two mechanisms have been proposed to account for the central nervous system damage caused by aPL antibodies. The first mechanism is that the antibodies have an affinity for the high phospholipid content of the endothelium of the brain vessels. They bind to the endothelium, which changes the permeability of the vessels. This results in breakdown of the blood–brain barrier, which contributes to hypercoagulation and thrombosis.24–26 The second mechanism is due to aPL antibody damage to the nigro-striatal pathway due to high binding affinity of aPL antibodies for phospholipid-rich neurons in dopaminergic pathway. The aPL-mediated depolarization causes dysfunction of neurons.27 Breakage of the blood–brain barrier by aPL antibodies also makes them more available to directly bind to neurons.
When aPL antibodies are detected but other criteria for definite diagnosis of APS are lacking, chorea and/or other findings such as livido reticularis, thrombocytopenia, valvular heart disease, and nephropathy (not currently included in the definition of APS) have been termed by some as a “Pre-APS” condition.20,21 Some authors suggest that pre-APS should be treated more aggressively in order to prevent thrombosis.21 The mechanism of chorea in pre-APS may be different from that seen in APS. While in APS (for example APS secondary to lupus) chorea is associated with thrombosis and elevated titers of the LAC antibody, pre-APS (our case and those listed in the table) is associated with an increase of aCL antibodies and a normal titer of LAC (Table 1). Regarding antibody subtypes, IgM was the most common subtype of aCL antibodies elevated in pre-APS patients (including our case). This suggests that high titers of the IgM subtype of aCL develop in pre-APS patients, and when the condition progresses to clinical APS, an increase in the IgG subtype occurs. Hence in the pre-APS condition, chorea is more likely the result of direct damage to the basal ganglia by aPL antibodies than occlusion of blood vessels, the latter being common in APS and often associated with an abnormal MRI. On the other hand, deoxyglucose PET scan shows an increased metabolic rate in the basal ganglia in pre-APS (our patient and that of Sundén-Cullberg et al.33 in Table 1), and, hence, has higher diagnostic value than MRI in pre-APS patients with chorea. The relation between severity of chorea and aPL antibodies is not known. In fact, there have been patients with prominent chorea and low levels of aPL antibodies14 and even patients in whom aPL antibody levels remained high even 26 months after total remission from APS symptoms.28
In APS, anticoagulants are typically used to reduce the risk of thrombosis, and several reports have indicated a reduction or even cessation of choreiform movements after such treatments.3,14 Since estrogen enhances dopaminergic sensitivity and can cause chorea in patients with APS, discontinuation of birth control pills high in estrogen is also recommended.14 In our patient, we chose to be conservative and treated her with aspirin 81 mg daily due to her advanced age. She developed no further symptoms but the chorea remained unchanged over a period of 9 months.
Although our patient's chorea and raised level of aPL antibodies could be coincidental, absence of other causes of chorea suggests that chorea resulted from direct action of aCL antibodies on the neural tissue (basal ganglia) similar to the previously reported patients with pre-APS (Table 1). We believe our patient and the current literature support the view that chorea can result from direct action of aCL antibodies on neural tissue of the basal ganglia in the absence of clinical APS. The challenge for the clinician is to decide how aggressively to treat patients with pre-APS syndrome in order to prevent thrombosis and other complications of APS. More data are needed on the natural history of patients with pre-APS and the evolution of pre-APS to APS in order to implement the best preventive strategy.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflict of Interests: The authors report no conflict of interest.
References
- 1.Walker RH. Differential diagnosis of chorea. Curr Neurol Neurosci Rep. 2011;11:385–395. doi: 10.1007/s11910-011-0202-2. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaki JM. Chorea caused by toxins. Handb Clin Neurol. 2011;100:335–346. doi: 10.1016/B978-0-444-52014-2.00026-4. [DOI] [PubMed] [Google Scholar]
- 3.Pandey S. Chorea. J Assoc Physicians India. Review. 2013;61:471–4, 483. [PubMed] [Google Scholar]
- 4.Bhidayasiri R, Truong DD. Chorea and related disorders. Postgrad Med J. 2004;80:527–534. doi: 10.1136/pgmj.2004.019356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vigliani MC, Honnorat J, Antoine JC, et al. PNS EuroNetwork. Chorea and related movement disorders of paraneoplastic origin: The PNS EuroNetwork experience. J Neurol. 2011;258:2058–2068. doi: 10.1007/s00415-011-6074-1. [DOI] [PubMed] [Google Scholar]
- 6.Shi W, Krilis SA, Chong BH, Gordon S, Chesterman CN. Prevalence of lupus anticoagulant and anticardiolipin antibodies in a healthy population. Aust NZ J Med. 1990;20:231–236. doi: 10.1111/j.1445-5994.1990.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 7.Vila P, Hernández MC, López-Fernández MF, Batlle J. Prevalence, follow-up and clinical significance of the anticardiolipin antibodies in normal subjects. Thromb Haemost. 1994;72:209–213. [PubMed] [Google Scholar]
- 8.Vaarala O, Palosuo T, Kleemola M, Aho K. Anticardiolipin response in acute infections. Clin Immunol Immunopathol. 1986;41:8–15. doi: 10.1016/0090-1229(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Snowden N, Wilson PB, Longson M, Pumphery RS. Antiphospholipid antibodies and Mycoplasma pneumoniae infection. Postgrad Med J. 1990;66:356–362. doi: 10.1136/pgmj.66.775.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avcin T, Toplak N. Antiphospholipid antibodies in response to infection. Curr Rheumatol Rep. 2007;9:212–218. doi: 10.1007/s11926-007-0034-x. [DOI] [PubMed] [Google Scholar]
- 11.Bick RL, Baker WF. Antiphospholipid and thrombosis syndrome. Semin Thromb Hemost. 1994;20:3–15. doi: 10.1055/s-2007-1001885. [DOI] [PubMed] [Google Scholar]
- 12.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanna G, Bertolaccini ML, Cuadrado MJ, et al. Neuropsychiatric manifestations in systemic lupus erythematosus: Prevalence and association with antiphospholipid antibodies. J Rheumatol. 2003;30:985–992. [PubMed] [Google Scholar]
- 14.Cervera R, Asherson RA, Font J, et al. Chorea in the antiphospholipid syndrome. Clinical, radiologic, and immunologic characteristics of 50 patients from our clinics and the recent literature. Medicine (Baltimore) 1997;76:203–212. doi: 10.1097/00005792-199705000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Albishri JA. Chorea as a first manifestation in young patients with systemic lupus erythematosus who was initially diagnosed with rheumatic fever. Clin Med Insights Case Rep. 2012;5:19–21. doi: 10.4137/CCRep.S9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iqbal S, Sher MR, Good RA, Cawkwell GD. Diversity in presenting manifestations of systemic lupus erythematosus in children. J Pediatr. 1999;135:500–505. doi: 10.1016/S0022-3476(99)70174-5. [DOI] [PubMed] [Google Scholar]
- 17.Arisaka O, Obinata K, Sasaki H, Arisaka M, Kaneko K. Chorea as an initial manifestation of systemic lupus erythematosus. A case report of a 10-year-old girl. Clin Pediatr (Phila) 1984;23:298–300. doi: 10.1177/000992288402300514. [DOI] [PubMed] [Google Scholar]
- 18.Ariizumi Y, Ozawa T, Tokutake T, et al. Chorea as the first sign in a patient with elderly-onset systemic lupus erythematosus. Case Rep Neurol Med. 2012;2012:317082. doi: 10.1155/2012/317082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarr T, Lakos G, Bhattoa HP, et al. Clinical thrombotic manifestations in SLE patients with and without antiphospholipid antibodies: A 5-year follow-up. Clin Rev Allergy Immunol. 2007;32:131–137. doi: 10.1007/s12016-007-0009-8. [DOI] [PubMed] [Google Scholar]
- 20.Peluso S, Antenora A, De Rosa A, et al. Antiphospholipid-related chorea. Front Neurol. 2012;3:150. doi: 10.3389/fneur.2012.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asherson RA. New subsets of the antiphospholipid syndrome in 2006: “PRE-APS” (probable APS) and microangiopathic antiphospholipid syndromes (“MAPS”) Autoimmun Rev. 2006;6:76–80. doi: 10.1016/j.autrev.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Carecchio M, Comi C, Varrasi C, et al. Complex movement disorders in primary antiphospholipid syndrome: A case report. J Neurol Sci. 2009;281:101–103. doi: 10.1016/j.jns.2009.03.011. Jun 15. [DOI] [PubMed] [Google Scholar]
- 23.Gelosa G, Tremolizzo L, Galbussera A, et al. Narrowing the window for ‘senile chorea’: A case with primary antiphospholipid syndrome. J Neurol Sci. 2009;284:211–213. doi: 10.1016/j.jns.2009.05.009. Sep 15. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt R, Auer-Grumbach P, Fazekas F, Offenbacher H, Kapeller P. Anticardiolipin antibodies in normal subjects. Neuropsychological correlates and MRI findings. Stroke. 1995;26:749–754. doi: 10.1161/01.STR.26.5.749. [DOI] [PubMed] [Google Scholar]
- 25.Asherson RA, Hughes GRV. Antiphospholipid antibodies and chorea. J Rheumatol. 1988;15:377–379. [PubMed] [Google Scholar]
- 26.Levine SR, Brey LR. Neurological aspects of antiphospholipid antibody syndrome. Lupus. 1996;5:347–353. doi: 10.1177/096120339600500503. [DOI] [PubMed] [Google Scholar]
- 27.Chapman J, Cohen-Armon M, Shoenfeld Y, Korczyn AD. Antiphospholipid antibodies permeabilize and depolarize brain synaptoneurosomes. Lupus. 1999;8:127–133. doi: 10.1191/096120399678847524. [DOI] [PubMed] [Google Scholar]
- 28.Wu SW, Graham B, Gelfand MJ, Gruppo RE, Dinopolous A, Gilbert DL. Clinical and positron emission tomography findings of chorea associated with primary antiphospholipid syndrome. Movement disorders. 2007;22:1813–1815. doi: 10.1002/mds.21657. [DOI] [PubMed] [Google Scholar]
- 29.Kiechl-Kohlendorfer U, Ellemunter H, Kiechl S. Chorea as the presenting clinical feature of primary antiphospholipid syndrome in childhood. Neuropediatrics. 1999;30:96–98. doi: 10.1055/s-2007-973468. [DOI] [PubMed] [Google Scholar]
- 30.Usugi T, Nakano K, Nakayama T, Ishii K, Osawa M. Familial antiphospholipid antibody in a child with involuntary movement and deterioration. Pediatr Int. 2007;49:238–241. doi: 10.1111/j.1442-200X.2007.02346.x. [DOI] [PubMed] [Google Scholar]
- 31.Biernacka-Zielinska M, Lipinska J, Szymanska-Kaluza J, Stanczyk J, Smolewska E. Recurrent arterial and venous thrombosis in a 16-year-old boy in the course of primary antiphospholipid syndrome despite treatment with low-molecular-weight heparin: A case report. J Med Case Rep. 2013;7:221. doi: 10.1186/1752-1947-7-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimomura T, Takahashi S, Takahashi S. Chorea associated with antiphospholipid antibodies. Rinsho Shinkeigaku. 1992;32:989–93. [in Japanese] [PubMed] [Google Scholar]
- 33.Sundén-Cullberg J, Tedroff J, Aquilonius SM. Reversible chorea in primary antiphospholipid syndrome. Mov Disord. 1998;13:147–149. doi: 10.1002/mds.870130127. Review. [DOI] [PubMed] [Google Scholar]
- 34.Orzechowski NM, Wolanskyj AP, Ahlskog JE, Kumar N, Moder KG. Antiphospholipid antibody-associated chorea. J Rheumatol. 2008;35:2165–2170. doi: 10.3899/jrheum.080268. [DOI] [PubMed] [Google Scholar]