Summary
In the study of Zhang et al (1), tumor-bearing mice were vaccinated with magnetically labeled, tumor antigen–primed dendritic cells (DCs). After homing of these antigen-presenting cells to the draining lymph node (LN), it was shown that the iron oxide–induced decrease in LN magnetic resonance (MR) imaging signal intensity correlated with the observed tumor growth delay, suggesting that the degree of hypointensity can serve as a surrogate marker for the efficacy of tumor vaccination.
The Setting
According to ClinicalTrials.gov, there are currently about 500 clinical trials registered with the National Institutes of Health that use DC therapy for vaccination against viruses (eg, HIV-1) or cancer. In oncology, this treatment aims to boost the immune system against certain specific antigens that are present on tumor cells—antigens that otherwise cannot effectively be recognized as being foreign—so that the immune system launches a cytotoxic T-cell attack. The clinical outcome of these studies has been extremely variable (2), depending on the type of cancer, number of injected cells, vaccination route, and the patient's innate ability to elicit an adaptive T-cell immune response.
As priming and activation of T cells by DCs takes place primarily in LNs, a critical requirement for successful vaccination is the ability of DCs to home from the injection site to the LN in sufficient numbers. By magnetically labeling DCs with superparamagnetic iron oxide (SPIO), it has previously been shown that this homing process can be noninvasively monitored with MR imaging (3–5). Such cell tracking may be used to evaluate and possibly improve current vaccination regimens—for instance, to study the effects coadministered immunoadjuvants may have on the speed and magnitude of DC antigen delivery (6). In this issue of Radiology, Zhang et al (1) studied the relationship between the LN homing of pancreatic cancer antigen-primed SPIO-labeled DCs and implanted tumor growth and found a direct correlation between the amount of delivered vaccine and tumor immunoprotection.
The Science
DCs are circulating immunosurveillant cells that continuously perform reconnaissance tasks in our bodies, searching for pathogens and foreign antigens. After phagocytosis and digestion of nonself material, they travel to regional LNs where they present immunogenic peptides and glycoproteins at random to B and T lymphocytes. In a successful immune response, when a single histocompatible B or T cell is encountered that has the matching antigen-specific receptor, a clonal lymphocyte expansion will occur, and millions of new cells will seek out to destroy the foreign entity.
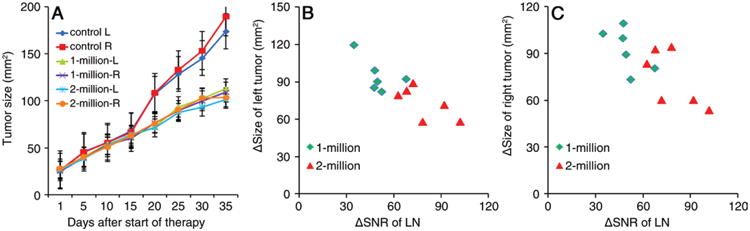
In the study of Zhang and colleagues (1), DCs were isolated from bone marrow and were incubated ex vivo with pancreatic cancer cell lysates to induce capture of the tumor antigens. Cells were subsequently labeled with (fluorescent) SPIO particles to render them magnetic and create hypointensities at MR imaging. Cell viability tests showed no adverse effect of the magnetic labeling procedure. Pancreatic cancer cells were inoculated subcutaneously in C57BL/6 mice in both hind flanks and were allowed to grow until they reached a size of approximately 10 mm. Three groups of mice (nine each) then received a unilateral single footpad injection of either phosphate-buffered saline (PBS) (control mice) or 1 × 106 or 2 × 106 SPIO-labeled pancreatic cancer antigen-primed DCs. T1- and T2-weighted MR images were obtained at 7.0 T before and 6 and 24 hours after vaccination. From these images, the signal-to-noise ratio (SNR) of the ipsilateral popliteal LN was measured (hypointense cells could not be detected in the contralateral LN), and the values were plotted against the size of the developing tumors.
The authors found a good correlation between the decrease in SNR for the ipsilateral draining popliteal LN measured 24 hours after vaccination and the reduced size of subsequent tumor development at 35 days. Importantly, there was systemic immunoprotection, as this correlation was nearly identical for the ipsilateral (R = 0.81) and contralateral (R = 0.76) implanted tumors, even though no DCs were present in the contralateral LN. Furthermore, the immunoprotection was higher for the higher numbers of DCs injected (tumor size: 194–189, 113–109, and 92–90 mm2 for animals injected with PBS, 1 × 106 DCs, and 2 × 106 DCs, respectively). The quantitative MR imaging data were corroborated by counts of Prussian Blue– (SPIO) and CD11c-postitive cells, proving that the decreased SNR was indeed due to the accumulation of the labeled vaccine in the LN.
The Practice
Clinical use
In this study, a nonclinical SPIO formulation was used that was prepared in non–good manufacturing practice conditions. Although the first MR imaging cell-tracking study in patients with advanced-stage melanoma (3) used a clinically approved formulation (Feridex, also known as Endorem) as a label, U.S. Food and Drug Administration (FDA)-approved SPIO formulations for MR imaging are no longer available. There are several other ways to label immune cells to make them visible at MR imaging (7); one is to use perfluorocarbons for fluorine 19 (19F)-MR imaging cell tracking, which has recently been introduced into the clinic for imaging of intradermally injected colorectal cancer vaccines (8). It remains to be seen which technique eventually may become mainstream in a hospital setting. While 19F-MR imaging allows straightforward quantification of the number of homing DCs, it has lower sensitivity and resolution than magnetovaccines.
Future opportunities and challenges
In the study by Zhang et al (1), the cancer vaccination did not lead to regression of the tumor, but merely delayed its growth as compared with tumor growth in the unvaccinated control mice. The immunoprotection was thus marginally effective; similar findings have been encountered with the Provenge prostate cancer vaccine, which increases the median life span of patients with advanced-stage prostate cancer by only 4 months (9). Nevertheless, that improvement was significant enough to lead the FDA to grant approval for this form of cell therapy, only the second-ever of its kind.
It is plausible that the sensitivity of DC detection may be further improved. The SPIO labeling protocol followed by Zhang and colleagues (1) resulted in an average iron loading of 0.65 pg iron per cell, which is rather low for phagocytic cells such as DCs. With the increasing interest in MR imaging cell tracking and ever-increasing protocols for efficient cell labeling, we may be able to use more sensitive approaches for monitoring effective vaccination. Little is known about systemic priming outside LNs—whether or not this indeed occurs—and currently available technology lacks the means to investigate such a possibility. In the meantime, it is fair to say that clinically applicable, quantitative monitoring of DC migration patterns will teach us much more about the most effective ways to perform cancer vaccination in larger patient populations. Last, it would be interesting to see if vaccination before tumor induction would lead to better immunoprotection or even prevent the origin of cancer, although the clinical relevance as a preventative measure would be in question.
Figure 1.
Footnotes
Disclosures of Conflicts of Interest: J.W.M.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other relationships: has been issued patent US8236572 on cell tracking; is the founder and owner of SenCEST.
References
- 1.Zhang Z, Li W, Procissi D, et al. Antigen-loaded dendritic cell migration: MR imaging in a pancreatic carcinoma model. Radiology. 2015;274(1):192–200. doi: 10.1148/radiol.14132172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vries IJ, Lesterhuis WJ, Barentsz JO, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23(11):1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 4.Baumjohann D, Hess A, Budinsky L, Brune K, Schuler G, Lutz MB. In vivo magnetic resonance imaging of dendritic cell migration into the draining lymph nodes of mice. Eur J Immunol. 2006;36(9):2544–2555. doi: 10.1002/eji.200535742. [DOI] [PubMed] [Google Scholar]
- 5.Dekaban GA, Hamilton AM, Fink CA, et al. Tracking and evaluation of dendritic cell migration by cellular magnetic resonance imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(5):469–483. doi: 10.1002/wnan.1227. [DOI] [PubMed] [Google Scholar]
- 6.Long CM, van Laarhoven HW, Bulte JW, Levitsky HI. Magnetovaccination as a novel method to assess and quantify dendritic cell tumor antigen capture and delivery to lymph nodes. Cancer Res. 2009;69(7):3180–3187. doi: 10.1158/0008-5472.CAN-08-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahrens ET, Bulte JW. Tracking immune cells in vivo using magnetic resonance imaging. Nat Rev Immunol. 2013;13(10):755–763. doi: 10.1038/nri3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahrens ET, Helfer BM, O'Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med. 2014 Sep 19; doi: 10.1002/mrm.25454. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bass PF., 3rd Life expectancy for men taking Provenge. [Accessed October 2, 2014];Prostate net. http://www.prostate.net/2013/prostate-cancer/provenge-life-expectancy/. Published June 27, 2013.


